Abstract
Background
In the present study, we sought to determine whether opening a persistently occluded infarct-related artery (IRA) by percutaneous coronary intervention (PCI) in patients beyond the acute phase of myocardial infarction (MI) improves patency and indices of left ventricular (LV) size and function.
Methods and Results
Between May 2000 and July 2005, 381 patients with an occluded native IRA 3 to 28 days after MI (median 10 days) were randomized to PCI with stenting (PCI) or optimal medical therapy alone. Repeat coronary and LV angiography was performed 1 year after randomization (n=332, 87%). Coprimary end points were IRA patency and change in LV ejection fraction. Secondary end points included change in LV end-systolic and end-diastolic volume indices and wall motion. PCI was successful in 92%. At 1 year, 83% of PCI versus 25% of medical therapy–only patients had a patent IRA (P<0.001). LV ejection fraction increased significantly (P<0.001) in both groups, with no between-group difference: PCI 4.2±8.9 (n=150) versus medical therapy 3.5±8.2 (n=136; P=0.47). Median change (interquartile range) in LV end-systolic volume index was −0.5 (−9.3 to 5.0) versus 1.0 (−5.7 to 7.3) mL/m2 (P=0.10), whereas median change (interquartile range) in LV end-diastolic volume index was 3.2 (−8.2 to 13.3) versus 5.3 (−4.6 to 23.2) mL/m2 (P=0.07) in the PCI (n=86) and medical therapy–only (n=76) groups, respectively.
Conclusions
PCI with stenting of a persistently occluded IRA in the subacute phase after MI effectively maintains long-term patency but has no effect on LV ejection fraction. On the basis of these findings and the lack of clinical benefit in the main Occluded Artery Trial, routine PCI is not recommended for stable patients with a persistently occluded IRA after MI.
Keywords: myocardial infarction, occlusion, angioplasty, remodeling, cardiac volume, stents
CLINICAL PERSPECTIVE
Nearly a third of patients diagnosed with ST-elevation myocardial infarction receive no reperfusion therapy for acute coronary occlusion, many because of late presentation. Half of these patients will have persistent occlusion of the infarct-related artery. The Total Occlusion Study of Canada (TOSCA)--2, an angiographic substudy of the 2166-patient Occluded Artery Trial (OAT), randomized 381 stable patients with persistent infarct-related artery occlusions days to weeks after MI to percutaneous coronary intervention (PCI) or medical therapy alone, to determine if PCI would improve left ventricular (LV) function and attenuate post MI adverse (LV) remodeling. Despite excellent patency 1 year after PCI, no salutary effect on LV ejection fraction was observed. PCI appeared, however, to be independently predictive of a modest reduction in adverse remodeling in a subgroup of patients with LV volume measurement, an observation that may or may not apply to the full cohort. This benefit did not translate into an effect on clinical events either in TOSCA-2 (not powered for clinical events), or in the well-powered OAT study, which conversely suggested excess reinfarction in PCI-assigned patients. These discordant observations imply competing effects of PCI in this setting: a potential modest beneficial effect on LV remodeling counterbalanced by excess reinfarction over an average 3-year follow-up in OAT. Thus, there is no indication for PCI of persistently occluded IRAs in stable patients in the days to weeks after MI. Whether the clinical balance between a potential remodeling benefit and an adverse effect of excess reinfarction will sway toward a significant difference in favor of either medical therapy alone or PCI over the long-term is unknown.
INTRODUCTION
Late reperfusion has long been thought to be potentially beneficial for the many patients who present beyond the time window proven effective for reperfusion therapy for acute coronary occlusion.1 Experimental studies have suggested that late reperfusion may reduce adverse left ventricular (LV) remodeling, with resultant better indices of LV size and function, independent of myocardial salvage,2 through improved healing or recovery of function of hibernating myocardium. Because LV size, function, and survival are closely linked, this effect could underpin improvements in clinical outcome. Randomized studies examining this hypothesis have been inconclusive.3-7 Post hoc analyses suggesting marked improvement in LV function if infarct-related artery (IRA) patency is maintained6 do not prove an effect of late reperfusion; both patency and function may be preserved because of less microvascular dysfunction or more viable myocardium. Thus, whether to revascularize occluded coronary arteries in the subacute phase of myocardial infarction (MI) continues to be debated.
The Occluded Artery Trial (OAT) was an international randomized trial funded by the National Institutes of Health that tested the hypothesis that a strategy of percutaneous coronary intervention (PCI)– based reperfusion would reduce adverse clinical events over 3 years in stable high-risk patients with an occluded IRA identified 3 to 28 days after MI.8 The Total Occlusion Study of Canada (TOSCA)–2 trial, also funded by the National Institutes of Health, is a mechanistic ancillary study of OAT, with 2 primary specific aims: (1) to determine whether PCI with stenting in addition to optimal medical therapy in the subacute phase after MI compared with optimal medical therapy alone yields superior long-term IRA patency, and (2) to determine whether this strategy improves LV function and size at 1 year.
Methods
Study Design
TOSCA-2 enrolled participants between May 2000 and July 2005 in 33 of 217 OAT sites with ethics board approval separate from OAT. (See the Appendix for a listing of participating centers.) TOSCA-2 patients were OAT participants who provided additional informed consent. Randomization to PCI plus optimal medical therapy (PCI) or to optimal medical therapy alone (MED) was by a voice-response system at the Data Coordinating Center that used a randomization sequence that was separate from OAT and stratified by site.
Patient Sample
Eligibility criteria for TOSCA-2 mirror those for OAT.8 Patients had a documented MI and underwent cardiac catheterization within 3 to 28 days, with day 1 being the date of symptom onset. Angiographic criteria included IRA occlusion judged suitable for stenting and at least 1 high-risk criterion: proximal coronary occlusion subtending at least 25% of the LV (Figure 1) or LV ejection fraction (LVEF) <50%. IRA occlusion was defined as 100% stenosis with Thrombolysis In Myocardial Infarction (TIMI) grade 0 or 1 antegrade flow.9 All patients were required to (1) have a baseline LV angiogram suitable for quantitative analysis and (2) agree to follow-up cardiac catheterization. Patients with clinical instability, left main or 3-vessel disease, or severe inducible ischemia were excluded. Retrospective enrollment of OAT participants (which was allowed provided that in a given TOSCA-2–approved site, consecutive patients were enrolled to minimize selection bias) occurred in 37 cases.
Figure 1.
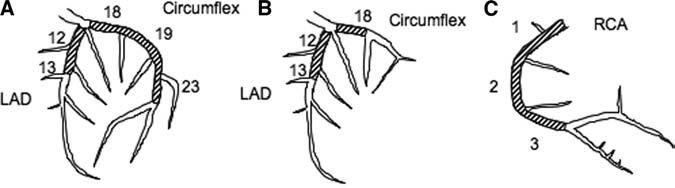
IRA segment study eligibility criteria with LVEF ≥50% for (A) a left-dominant system, (B) a right-dominant system, and (C) right coronary artery in a right-dominant system. Eligibility when LVEF was >50% required that the occluded artery supply at least 25% of the LV.
Study Procedures
Patients assigned to PCI were to undergo the procedure within 24 hours of randomization; whenever possible, 1 or more locally approved coronary stents were to be deployed, and a glycoprotein IIb/IIIa inhibitor was to be used. Drug-eluting stents were deployed in 29 cases. Thienopyridine therapy was recommended to commence before PCI and to continue for 2 to 4 weeks after PCI and to be used for 1 year in both groups after publication of the efficacy of longer-duration clopidogrel therapy.10 Angiographic follow-up was obtained 12±3 months after randomization.
Angiographic Analysis
Sites were certified after core laboratory review of angiograms of PCI of total coronary occlusions and procedural statistics of proposed TOSCA-2 operators.8 Guidelines for angiographic images included reproduction of baseline views, extended cine runs for antegrade and collateral flow assessment, and use of intracoronary nitroglycerin. Whenever possible, a standardized calibration ball was used to allow calculation of LV volume end points.
Angiographic images were analyzed at a core laboratory with the ImageComm system (Quinton, Bothell, Wash). Coronary luminal diameter, including 5-mm-long perilesional segments, was measured to obtain in-stent and in-lesion minimal luminal diameter. Restenosis was defined as >50% diameter stenosis at the point of in-lesion minimal luminal diameter with proximal-only reference segments. Antegrade and collateral flow was scored with standard criteria,9,11 with late patency defined as antegrade flow grades 2 or 3 irrespective of lumen dimensions. The area-length method was used to measure LVEF and LV volumes when calibration images were provided. Regional wall motion was measured by the centerline method.12
Study End Points
Two coprimary end points were specified: (1) follow-up IRA patency and (2) change in LVEF. Secondary end points included change in LV end-systolic volume index (LVESVI), LV end-diastolic volume index (LVEDVI), and regional wall-motion score. With Data Safety Monitoring Board approval, the Steering Committee changed the original protocol-defined coprimary end point of LVESVI to LVEF after 1 year of enrollment,13 to avoid systematic exclusion of patients with study-qualifying angiograms performed at non-OAT sites without routine calibration. LVESVI and LVEDVI were retained as secondary end points. Clinical events were adjudicated by an independent Mortality and Morbidity Classification Committee blinded to treatment assignment.8
Statistical Analysis
An intention-to-treat principle was used for all treatment-related analyses unless otherwise specified. Patency was evaluated with α=0.01 for a χ2 test of proportions and LVEF with α=0.04 for a 2-sided, 2-group t test comparing change in LVEF from baseline to 1 year between the 2 treatment groups. As in the main OAT trial, a probability value of 0.01 for secondary analyses was considered strong evidence of association. The planned study size was 380 patients (190 patients per group), which would provide 90% power to detect a difference in the absolute change in LVEF between the study groups of 4%, based on observations of LVEF change and SD of change among similar subjects enrolled in prior studies6,14 (common SD of 8.0%) and the following assumptions: 25% crossovers (PCI in MED patients and no attempt at PCI or failed PCI, which are biological crossovers, in the PCI-assigned patients) and 15% loss of ventriculogram data. A total of 381 patients were enrolled; 332 (87%) had paired angiographic data, and 286 (75%) had paired ventriculographic data (for LVEF assessment), of which 162 (42%) were calibrated (for LVESVI and LVEDVI measurement). The observed percent crossovers were 14 (8% with unsuccessful PCI in the PCI group and 6% with PCI in the MED group). With an observed SD of 8.6% for LVEF change, TOSCA-2 had 94% power to detect a difference of 4.0% and 81% power to detect a difference of 3.25% in the change in LVEF between the 2 study groups. The study also had 78% power to detect a difference in change in mean LVESVI of 12 mL/m2 assuming the observed SD of 20.8. For patency, TOSCA-2 had ≥85% power to detect an absolute difference of 25% in patency between the 2 treatment groups. Change in LVEF was normally distributed and suitable for the 2-group t test. Changes in LVESVI and in LVEDVI were not normally distributed and were analyzed with the nonparametric Wilcoxon rank-sum test.
For multivariable models, we used stepwise linear regression for continuous outcomes and logistic models for binary outcomes. The prespecified variables tested in each stepwise model were as follows: PCI group, age, sex, race, days from MI to randomization, prior MI, hypertension, diabetes mellitus, multivessel disease, collateral arteries, ST-segment elevation MI (STEMI) on ECG (ST elevation and/or new Q waves and/or loss of R waves), left anterior descending coronary artery culprit, β-blockers, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, thrombolysis, and baseline LVEF, plus baseline LVESVI for the LVESVI model and baseline LVEDVI for the LVEDVI model. Because models of change in LVESVI and LVEDVI had normally distributed residuals that exhibited homoscedasticity, these measures were suitable for analysis with linear regression. We also performed a sensitivity analysis using these variables in a multiple imputation procedure for missing LVEF data.
The authors had full access to the data and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
A total of 381 patients were enrolled, representing 67% of all contemporaneous OAT enrollments at TOSCA-2 centers (18% of the 2166 OAT patients). Follow-up angiography was performed in 332 patients (87%) at a median 379 days (interquartile range, 363 to 417 days) after randomization (Figure 2).
Figure 2.
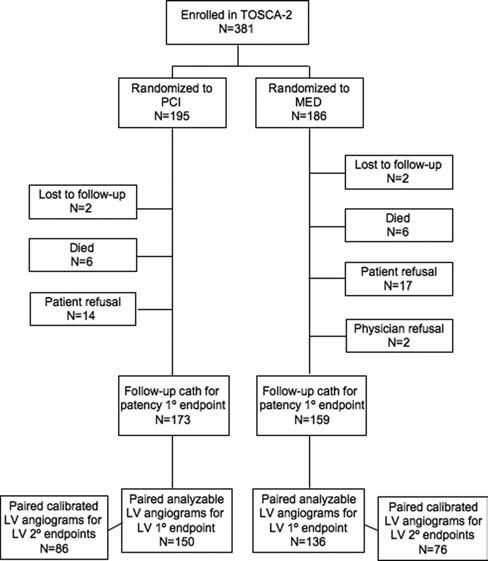
TOSCA-2 study flow
Baseline Characteristics
The PCI and MED groups were similar in most respects (clinical [Tables 1 and 2] and angiographic [Table 3]) and were similar to other OAT patients with the exception of Killip class ≥II during the index MI (12.3% versus 20.7%). A STEMI ECG was found in 86%. Fewer than one fourth of all patients had received fibrinolytic therapy. Median interval from MI to randomization was 10 days. The 49 patients who did not undergo follow-up angiography were more likely to be diabetic, to have a lower baseline LVEF, and to have a higher index MI Killip class than those with follow-up angiograms. Characteristics of these patients were similar across treatment groups.
Table 1.
Baseline Clinical Characteristics
| Variable | PCI (n=195) | MED (n=186) |
|---|---|---|
| Mean age (range), y | 57.3 (34–80) | 57.8 (33–81) |
| Female, n (%) | 33 (16.9) | 33 (17.7) |
| Race, n (%) | ||
| White | 160 (82.1) | 151 (81.2) |
| Black | 6 (3.1) | 5 (2.7) |
| Other | 29 (14.9) | 30 (16.1) |
| No. (%) with history of: | ||
| Diabetes* | 32 (16.4) | 47 (25.3) |
| Hypertension | 89 (45.6) | 92 (49.5) |
| Hyperlipidemia | 108 (55.4) | 111 (59.7) |
| Prior MI | 25 (12.8) | 17 (9.1) |
| Cerebrovascular disease† | 11 (5.6) | 1 (0.5) |
| PVD | 5 (2.6) | 3 (1.6) |
| CHF | 4 (2.1) | 3 (1.6) |
| Prior PCI | 8 (4.1) | 4 (2.2) |
| Current smoker, n (%) | 66 (33.8) | 65 (34.9) |
| NYHA class at randomization, n (%) | ||
| Class I | 182 (93.3) | 170 (91.4) |
| Class II | 13 (6.7) | 16 (8.6) |
| Class III or IV | 0 | 0 |
| Body mass index,† mean±SD | 27.5±4.2 (n=195) | 28.8±5.1 (n=186) |
| Heart rate, bpm, mean±SD | 69.2±12.2 (n=195) | 69.8±12.4 (n=186) |
| Systolic blood pressure, mm Hg, mean±SD | 116.6±16.0 (n=195) | 117.1±17.0 (n=186) |
| Diastolic blood pressure, mm Hg, mean±SD | 71.0±11.2 (n=195) | 70.8±12.1 (n=186) |
| Serum creatinine, mg/dL, mean±SD | 1.0±0.2 (n=191) | 1.0±0.2 (n=184) |
| Creatinine clearance, mL/min, mean±SD | 83.1±19.8 (n=191) | 82.3±21.4 (n=184) |
PVD indicates peripheral vascular disease; CHF, congestive heart failure.
P=0.03,
P=0.01.
Table 2.
Index MI Characteristics
| Variable | PCI (n=195) |
MED (n=186) |
|---|---|---|
| Confirmatory evidence of index MI | ||
| Chest pain or equivalent | 189 (96.9) | 185 (99.5) |
| Any positive serum marker | 187 (95.9) | 177 (95.2) |
| ST-T changes, new LBBB | 159 (81.5) | 158 (84.9) |
| ST-elevation or new Q-waves or loss of R-waves | 163 (83.6) | 165 (88.7) |
| High serum marker,* n/N (%) | 101/187 (54.0) | 88/177 (49.7) |
| Therapy during first 24 hours of MI | ||
| Thrombolytic agent only | 46 (23.6) | 39 (21.0) |
| GP IIb/IIIa inhibitor | 7 (3.6) | 10 (5.4) |
| Combination | 4 (2.1) | 1 (0.5) |
| None of the above | 136 (69.7) | 134 (72.0) |
| Unknown | 2 (1.0) | 2 (1.1) |
| Interval from MI to randomization | ||
| ≤7 d | 64 (32.8) | 70 (37.6) |
| 8–14 d | 62 (31.8) | 53 (28.5) |
| 15–28 d | 67 (34.4) | 62 (33.3) |
| ≥29 d | 2 (1.0) | 1 (0.5) |
| Median, d | 10.0 | 10.0 |
LBBB indicates left bundle-branch block; GP, glycoprotein. Values are n (%), unless otherwise indicated.
Above-median value of the ratio of the peak serum marker to the upper limit of normal.
Table 3.
Baseline Angiographic Characteristics: Angiographic Core Laboratory Data
| Variable | PCI (n=195) |
MED (n=186) |
|---|---|---|
| IRA, n (%) | ||
| LAD | 60 (30.8) | 79 (42.5) |
| Circumflex | 28 (14.4) | 22 (11.8) |
| RCA | 107 (54.9) | 85 (45.7) |
| IRA TIMI flow grade, n (%) | ||
| Grade 0 | 155 (79.9) | 155 (83.3) |
| Grade I | 37 (19.1) | 31 (16.7) |
| Grade II | 2 (1.0) | 0 (0.0) |
| Missing | 1 | 0 |
| Collateral grade in IRA, n (%) | ||
| Grade 0 | 21 (10.9) | 17 (9.2) |
| Grade I | 138 (71.5) | 135 (73.0) |
| Grade II | 34 (17.6) | 33 (17.8) |
| Missing | 2 | 1 |
| No. of diseased vessels >70%, n (%) | ||
| Single | 159 (81.5) | 158 (84.9) |
| Double | 34 (17.4) | 28 (15.1) |
| Triple | 2 (1.0) | 0 (0.0) |
| LVEF <50%, n/N (%) | 95/183 (51.9) | 87/170 (51.2) |
| Proximal occlusion, n (%) | 179 (91.8) | 175 (94.1) |
| LVEF <50% or proximal occlusion, n/N (%) | 192/195 (98.5) | 184/186 (98.9) |
| LVEF ≥50% and not proximal | 3/195 (1.5) | 2/186 (1.1) |
| LV function and volume baseline values, mean±SD | ||
| LVEF, % (PCI, MED: n=183, 170) | 48.2±10.6 | 47.5±10.7 |
| LVESVI, ml/m2 (n=105, 97) | 34.9±19.5 | 33.9±15.2 |
| LVEDVI, ml/m2 (n=105, 97) | 67.9±32.1 | 64.1±24.2 |
| Target-region wall motion (SD/chord) (n=182, 170) | −2.99±0.91 | −3.08±0.81 |
LAD indicates left anterior descending coronary artery; RCA, right coronary artery.
Postrandomization Medical Therapy
The utilization of proven medical therapies was high and similar in the 2 groups with the exception of thienopyridines, taken by all patients with successful PCI but by fewer than one third of MED patients (Table 4).
Table 4.
Utilization of Medical Therapies at Discharge
| Discharge |
1 Year |
|||
|---|---|---|---|---|
| Agent | PCI (n=195) | MED (n=186) | PCI (n=184) | MED (n=172) |
| Aspirin | 99.0 | 96.8 | 94.6 | 94.2 |
| Thienopyridine (clopidogrel or ticlopidine) | 92.8 | 30.6* | 33.1 | 23.3 |
| Aspirin or thienopyridine | 100.0 | 99.5 | 96.2 | 97.7 |
| Aspirin plus thienopyridine | 91.8 | 28.0* | 22.3 | 12.8† |
| Warfarin | 8.7 | 16.1 | NC | NC |
| One or more of ASA, warfarin, thienopyridine | 100.0 | 100.0 | … | … |
| Two or more of ASA, warfarin, thienopyridine | 93.3 | 41.9* | … | … |
| β-Blocker | 88.7 | 94.1 | 85.3 | 93.6† |
| ACE inhibitor or ARB | 86.7 | 89.8 | 87.5 | 90.1 |
| Spironolactone | 4.1 | 7.5 | 9.2 | 11.0 |
| Lipid-lowering agent | 83.6 | 85.5 | 88.6 | 90.7 |
ASA indicates aspirin; NC, not collected; ACE, angiotensin-converting enzyme; and ARB, angiotensin receptor blocker. All values are percentages.
P<0.001 at discharge;
P<0.05 at 1 year.
Revascularization: PCI Group
All PCI-assigned patients underwent target-vessel intervention, 76% within 24 hours and 96% within 32 hours of randomization. Glycoprotein IIb/IIIa inhibitors were used in 81%. Core laboratory–adjudicated procedural success was present in 91.8%, and stents were deployed in all but 1 successful case. TIMI grade 3 flow was achieved in 85.1% and grade 2 in 5.6% (Table 5). Four patients (2.1%) with <50% residual epicardial stenosis but post-PCI TIMI grade 1 flow presumed due to microvascular obstruction were adjudicated as PCI successes. Among successful procedures, immediate postprocedural diameter stenosis was 4.6% instent and 28.7% in-lesion. There were few procedural complications (6 complications, 5 PCI-related: 1 cardiogenic shock, 1 coronary dissection, 1 limb ischemia, and 2 Mortality and Morbidity Classification Committee–adjudicated MIs). In addition, within 48 hours of study entry, site-reported reelevation of serum myocardial biomarkers was present in 17 (9.2%) of 184 of the PCI group and 7 (4.5%) of 154 of the MED group (P=0.09). Four patients (2.1%) underwent non-IRA intervention at the time of index PCI. By 1 year, performance of nonprotocol (including non-IRA) PCI occurred in 9 PCI-assigned patients (4.6%), repeat PCI of the IRA in 4 (2.1%), and coronary artery bypass grafting in 4 (2.1%) patients.
Table 5.
IRA PCI Procedural and 1-Year Results
| PCI |
MED |
||||
|---|---|---|---|---|---|
| Variable | Baseline (n=195) |
Post-PCI (n=195) |
1 Year (n=173) |
Baseline (n=186) |
1 Year (n=159) |
| TIMI grade flow† | |||||
| Grade 0 | 155 (79.9) | 12 (6.2) | 22 (12.7) | 155 (83.3) | 57 (35.8) |
| Grade 1 | 37 (19.1) | 6 (3.1) | 8 (4.6) | 31 (16.7) | 62 (39.0) |
| Grade 2 | 2 (1.0) | 11 (5.6) | 13 (7.5) | 0 (0.0) | 20 (12.6) |
| Grade 3 | 0 (0.0) | 166 (85.1) | 130 (75.1) | 0 (0.0) | 20 (12.6) |
| TIMI grade 2 or 3† | 2 (1.0) | 177 (90.8) | 143 (82.7) | 0 | 40 (25.2) |
| Missing | 1 | 0 | 22 | 0 | 27 |
| In-lesion | |||||
| MLD, mm* | 0.002±0.02 | 2.1±0.8 | 1.5±0.9 | 0 | 0.3±0.6 |
| % Diameter stenosis* | 99.8±2.6 | 34.3±23.6 | 52.5±26.6 | 100±0.4 | 90.2±17.4 |
| Late loss index | … | … | 0.27±0.36 | … | … |
| In-stent | |||||
| MLD, mm | … | 2.7±0.6 | 1.7±0.9 | … | … |
| % Diameter stenosis | … | 15.9±16.4 | 45.9±26.8 | … | … |
| Restenosis rate % | … | … | 45.7 | … | … |
MLD indicates minimal luminal diameter.
P<0.001,
P<0.0001 for 1-year PCI vs MED comparison.
Revascularization: MED Group
Fourteen MED-assigned patients (7.5%) underwent PCI of the IRA within 1 year of randomization. Reasons for crossover included unstable angina, a positive stress test, ventricular arrhythmia, silent ischemia, and physician preference. During this period, PCI of non-IRA vessels was performed in 10 patients (5.4%) and coronary artery bypass grafting in 2 (1.1%).
Primary End Point
Late patency was observed in 143(83%) of 173 PCI and 40 (25%) of 159 MED patients (P<0.0001; Table 5). TIMI 3 flow was present in 130 PCI patients (75%) and 20 MED patients (13%; P<0.0001). Among those with initially successful interventions, late patency was observed in 89% and TIMI 3 flow in 82%.
Analyzable, paired LV angiograms were available in 150 PCI patients (77%) and 136 MED patients (73%), with another 23 PCI and 23 MED angiograms being technically inadequate and 22 PCI and 27 MED not performed. LVEF improved significantly in both groups, but there was no between-groups difference: mean change in LVEF was 4.2±8.9% in the PCI group and 3.5±8.2% in the MED group (P=0.47; Figure 3; Table 6). As-treated and imputed secondary analyses of LVEF change by treatment yielded similar results. On multivariable analyses of change in LVEF, left anterior descending coronary artery culprit (P=0.02) was a positive predictor, and age (P=0.03) was a negative predictor. Presence of a STEMI ECG (P=0.07) was a weak positive predictor. There was no effect of PCI (P=0.38) or time from MI to randomization. Regardless of initial treatment assignment, patients with a patent IRA at follow-up had a greater increase in LVEF than those with an occluded artery (absolute difference of 3.0%; P=0.003).
Figure 3.
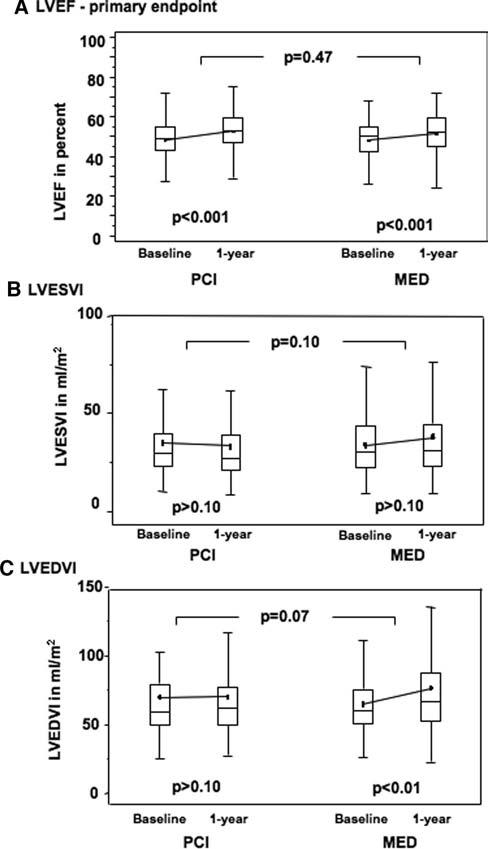
Comparison of (A) baseline and follow-up LVEF (primary LV function end point; between group P=0.47), (B) LVESVI, and (C) LVEDVI in the 2 study groups. Box-and-whiskers plots indicate medians, means, and interquartile ranges. Probability values indicate baseline to 1-year comparisons within each group and between groups as indicated.
Table 6.
Change in LVEF, Volumes, and Regional Wall Motion
| PCI Group |
MED Group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | 1 Year | Absolute Change |
% Change | Baseline | 1 Year | Absolute Change |
% Change | Absolute Change, P |
% Change, P |
| LVEF | 48.3±10.2 | 52.5±10.1 | 4.2±8.9 | 11.1±21.7 | 48.0±10.3 | 51.4±10.1 | 3.5±8.2 | 9.3±21.2 | 0.47 | 0.47 |
| LVESVI, mL/m2* | 29.7 | 27.1 | −0.5 | −1.7 | 30.4 | 31.5 | 1.0 | 4.5 | 0.10 | 0.07 |
| IQR | 23.3, 39.5 | 21.0, 38.7 | −9.3, 5.0 | −30.3, 14.9 | 22.4, 43.3 | 23.1, 44.5 | −5.7, 7.3 | −17.0, 20.8 | … | … |
| LVEDVI, mL/m2* | 58.9 | 61.8 | 3.2 | 5.3 | 60.4 | 66.6 | 5.3 | 8.7 | 0.07 | 0.09 |
| IQR | 49.3, 79.5 | 49.8, 77.2 | −8.2, 13.3 | −15.1, 21.5 | 50.6, 75.8 | 52.2, 87.4 | −4.6, 23.2 | −7.1, 39.0 | … | … |
| Target RWM score, SD/chord | −2.98±0.90 | −2.31±1.09 | 0.67±1.00 | 20.3±38.9 | −3.11±0.83 | −2.63±1.07 | 0.49±0.89 | 14.8±33.3 | 0.10 | 0.20 |
IQR indicates interquartile range; RWM, regional wall motion.
For LVEF, n=150 PCI, 136 MED; for LVESVI and LVEDVI, n=86 PCI, 76 MED; for RWM, n=149 PCI, 133 MED.
P values are for between group-comparisons.
Unindexed median absolute changes for PCI and MED groups are: LVESV −1.0 vs 2.0 mL; LVEDV 6.0 vs 10.0 mL. Unindexed mean absolute changes for PCI and MED groups are: LVESV −4.4±28.5 vs 8.1±48.0 mL; LVEDV 0.4±43.0 vs 22.7±64.2 mL.
Secondary End Points
Univariate analyses demonstrated a trend favoring PCI in attenuating absolute and percentile diastolic and systolic volume increases (LVEDVI: absolute change P=0.07, percent change P=0.09; LVESVI: absolute change P=0.10, percent change P=0.07; Figure 3; Table 6). Multivariable analyses showed assignment to PCI to be an independent predictor of smaller absolute increase (P=0.02) and percent change (P=0.04) in LVEDVI. Other independent predictors of smaller LVEDVI increase were baseline LVEF (P=0.002), baseline LVEDVI (P<0.001), and left anterior descending coronary artery culprit (P=0.04). There was no association with time from MI to randomization. PCI also independently predicted a smaller percent increase in LVESVI (P=0.04). Baseline LVEF (P=0.004), LVESVI (P=0.005), and a left anterior descending coronary artery culprit (P=0.02) were other independent predictors of a smaller percent increase in LVESVI. There was a weak trend toward STEMI ECG predicting a greater percent increase (P=0.09). No interaction was observed between the effect of PCI and STEMI ECG, large infarct size by serum marker elevation, below-median LVEF (49%), or left anterior descending coronary artery culprit on change in LV volumes. Regardless of treatment assignment, patients with an occluded IRA at follow-up had more LV dilation than those with a patent artery (absolute change in LVESVI P=0.003, and absolute change in LVEDVI P=0.03).
Both groups demonstrated significant improvement in target-region wall-motion score; the change over 1 year tended to be greater in the PCI group. The angiographic restenosis rate in the PCI group was 45.7% (Table 5).
The 12-month cumulative event rate for the Mortality and Morbidity Classification Committee–adjudicated primary OAT end point of death, MI or New York Heart Association class IV congestive heart failure was 6.2% in the PCI group and 6.5% in the MED group. The adjudicated MI cumulative event rate was 2.6% and 3.8% in these 2 groups. The study was not powered to detect differences in event rates.
Discussion
A strategy of stent-based PCI for persistently occluded IRA diagnosed in the subacute post-MI period resulted in IRA patency in four fifths of patients at 1 year, triple the rate observed in medically treated patients. This did not result in significant improvement in LVEF. In the 42% subset with volumetric measures, PCI appeared to be independently predictive of somewhat less LV dilation over 1 year.
Small randomized studies examining this issue in the balloon angioplasty era were negative or inconclusive.4,6,7 In spite of the efficacy of stenting of coronary occlusions,15,16 studies using routine stenting and showing excellent long-term patency have continued to show mixed or inconclusive results.3,5
The postulated mechanisms underpinning the potential benefits of late opening of the IRA center on improvement of LV function and reduction of LV remodeling. The present study had excellent statistical power to address the former but less so for the latter, and only in a subgroup of patients. The TOSCA-2 results show that late PCI of an occluded IRA does not improve LVEF at 1 year compared with medical therapy alone. Significant improvement in LVEF was observed in both groups.
LV dilation begins early in the course of acute MI as a result of necrosis and myofibril slippage and is associated with microvascular dysfunction.17 Dilation portends a poor long-term outcome. Prior evidence for an association between persistent IRA occlusion and late-phase LV remodeling,18 characterized by increased contractile segment length and change in shape toward the spherical,19 is more robust than the evidence for the association of IRA status and global LV systolic function.
The present trial was able to assess the effect of late PCI of an occluded IRA on LV remodeling only as a secondary end point and only in a subgroup of patients selected on the basis of technical features of the LV angiogram and on patient compliance with the second catheterization. We found a trend toward a smaller increase in LVEDVI in the PCI group compared with the MED group; on multivariable analysis, including adjustment for baseline imbalances, there was an independent effect of PCI leading to less LV dilation over 1 year. Whether the strength of the observed association would be greater or less if the full cohort were evaluated is unclear.
At least moderately retained viability in the infarct zone was found at baseline in 69% of 124 patients in an OAT ancillary study.20 In TOSCA-2, both treatment groups had significant improvement in target-region wall motion, and there was a trend toward greater improvement in the PCI group. The heterogeneity of the response implies that mechanisms other than vessel patency are operative.
The results of TOSCA-2 must be interpreted in the context of OAT. TOSCA-2 confirms that PCI resulted in 1-year patency exceeding 80%, yet OAT showed that PCI did not reduce the combined end point of death, recurrent MI, or New York Heart Association class IV congestive heart failure.20 Prior studies of pharmacotherapy suggest that attenuation of LV remodeling to the degree observed in the present study, if it were generalizable to all OAT patients, should have had clinical importance.21 OAT and TOSCA-2 patients received angiotensin-converting enzyme inhibitors and β-blockers, agents known to attenuate the maladaptive neurohumoral response that contributes to late-phase LV remodeling.22 This background therapy may have diminished the therapeutic potential of late revascularization. We confirmed that patients with low LVEF were at greatest risk for LV dilation. The absolute effect of PCI was greatest in those patients; however, the same relative effect was seen across the range of LVEFs. Indeed, in OAT, there was no interaction between the effect of PCI and LVEF on clinical outcomes.20
Interestingly, the modest potential PCI-related benefit in ventricular volumes suggested in TOSCA-2, coupled with the trend toward excess reinfarction observed in OAT, is consistent with PCI as a therapy that presents competing effects. LV remodeling may be attenuated, but the same procedure is associated with excess risk of reinfarction, seen in the overall OAT cohort. Extended clinical follow-up of the OAT cohort to better define the balance between a potential long-term benefit resulting from improved LV remodeling versus the adverse effect of the trend toward excess reinfarction20 is being planned.
Study Limitations
Although the angiographic follow-up rate up was at least comparable to that in other angiographic studies, TOSCA-2 was limited by a small proportion of technically inadequate LV angiograms and lack of calibration in almost half of the analyzable paired LV angiograms, which decreased power for the volume end points. Follow-up angiography reflects bias, with sicker patients not returning. It is unknown whether the apparent beneficial effect of PCI on LV remodeling observed in a subset extends to the entire cohort.
Conclusions
A strategy of PCI to recanalize persistently occluded IRAs in stable patients 3 to 28 days after MI is effective in establishing and maintaining long-term patency of the IRA but does not improve LVEF. Progressive LV dilation over 1 year appears to be modestly attenuated in the subset with available data. On the basis of these findings and the lack of clinical benefit over an average 3-year follow-up in OAT, routine PCI is not recommended for persistent IRA occlusion in stable patients days to weeks after MI.
Acknowledgments
The authors wish to express their appreciation for the excellent work by Staci Abramsky and Ana Mon.
Appendix
Executive Committee: V. Džavík (Principal Investigator), C.E. Buller (Coprincipal Investigator), J.S. Hochman, G.A. Lamas, and G.L. Knatterud. Global TOSCA-2 Coordinator: D. Atchison. Steering Committee: Executive Committee members and G.B. Mancini, W. Cantor, E.A. Cohen, R.G. Carere, T.J. Anderson, A. Glanz, D. Almond, W.J. Rankin, J. Renkin, and J. Col. Angiographic Core Laboratory: CIRCL, University of British Columbia (G.B. Mancini, C.E. Buller, and E. Yeoh). Data Coordinating Center: Maryland Medical Research Institute (G.L. Knatterud, S. Forman, and B. Barton). Participating Centers (number of patients enrolled): Argentina: Hemodinamia Rosario—C.R. Vozzi, M. Cardonna (14). Australia: Royal Perth Hospital—J.M. Rankin, A.M. Tunesi (15). Belgium: Cliniques Universitaires UCL St. Luc—J.P.M. Renkin, J. Col, R. Lauwers (4). Brazil: Hospital Socor—J.A. Saad, M.C. Machado (11); Hospital Sao Lucas—P.R.A. Caramori, R. Lasevitch, P. Hickmann (3). Canada: Vancouver General Hospital—C.E. Buller, R.S. Fox (49); Toronto General Hospital—J.R. Ross, A.R. Patel (41); St. Michael's Hospital—W.J. Cantor, B. Strauss, A. Fry, A. DiMarco (32); St. Paul's Hospital—R.G. Carere, J.G. Webb, T. Kot, L. Milosavljevi (23); Hotel Dieu Grace Hospital—A. Glanz, R. Chetty, C. Vilag (16); Sunnybrook Hospital—E.A. Cohen, L. Balleza (16); University of Alberta Hospital—J.R. Burton, N. Hogg, L. Lindholm (11); Royal Alexandra Hospital—N. Brass, C. Buck (9); London Health Sciences Centre, Victoria Campus, London—D. Almond, P. Teefy, D. Wiseman (9); Queen Elizabeth II H.S.C—L.M. Title, N. Fitzgerald (8); L'Institut de Cardiologie de Montreal—L. Bilodeau, N. St. Jean, N. Hardy (7); Peterborough Regional Health Centre—A. Abdullah, A. Janmohammed, B. Potvin (7); The Scarborough Hospital—J. Cherry, J. Smith (4); University of Ottawa Heart Institute—M. Labinaz, N. Shore, E. Brilliant (4); Foothills Hospital—T.J. Anderson, L. Mann (3); Hospital Maisonneuve-Rosemont—C. Constance, M.-F. Gauthier (2); Trillium Health Centre—C. Lazzam, A. Carter (2); London Health Sciences Centre—W.J. Kostuk, S. Carr (2); St. Boniface General Hospital—A. Miller, M.-L. Wilson (1); CHUM, Notre Dame—F. Reeves, M.-C. Pashko (1); Hospital Sacre Coeur—E. Schampaert, D. Palisaitis, C. Mer-cure, M.A. Capobianco (1). New Zealand: Waikato Hospital—G. Devlin, D. Peek, L. Low (15). Poland: Upper Silezian Cardiac Center—P. Buszman, A. Zurakowski (19); T. Marciniak Hospital—K. Loboz-Grudzien, L. Sokalski (12). Portugal: Hospital Fernando Fonseca—P.F. Abreu, B. Thomas, M. Nedo (18). Slovakia: Central Slovak Institute of Cardiovascular Diseases—P. Meiar, P. Kurray (15); Slovak Institute of Cardiovascular Diseases—V. Fridrich, S. Mizera (5).
Footnotes
Presented in part at the 2006 AHA Scientific Sessions, Chicago, Ill, November 14, 2006.
Clinical trial registration information—URL: http://www.clinicaltrials.gov. Unique identifier: NCT00025766.
Sources of Funding
TOSCA-2 is funded by the National Heart, Lung, and Blood Institute (No. HL67683-01A1 to Dr Džavík). Medtronic Canada, Inc, provided free stents in Canada. Cordis, Johnson & Johnson (Miami Lakes, Fla) provided free stents in Australia, Canada, and Poland. Glycoprotein IIb/IIIa inhibitors were provided by Schering-Plough, Millennium Pharmaceuticals, and Eli Lilly & Co.
Disclosures
Dr Džavík reports research, honorarium, and Advisory Board member funds from Cordis, Johnson & Johnson, and honoraria from Boston Scientific. Dr Rankin reports an educational grant from Cordis, Johnson & Johnson. Dr Buszman owns stock in American Heart of Poland Ltd and NAFIS SA (Poland). Dr Hochman reports advisory board funding from Eli Lilly and Bristol Myers Squibb/Sanofi-Aventis, and Network for Continuing Medical Education speaker's honoraria (funded by Bristol Myers Squibb). The remaining authors have no conflicts to disclose.
References
- 1.White HD, Braunwald E. Applying the open artery theory: use of predictive survival markers. Eur Heart J. 1998;19:1132–1139. doi: 10.1053/euhj.1998.1017. [DOI] [PubMed] [Google Scholar]
- 2.Hochman JS, Choo H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation. 1987;75:299–306. doi: 10.1161/01.cir.75.1.299. [DOI] [PubMed] [Google Scholar]
- 3.Yousef ZR, Redwood SR, Bucknall CA, Sulke AN, Marber MS. Late intervention after anterior myocardial infarction: effects on left ventricular size, function, quality of life, and exercise tolerance: results of The Open Artery Trial (TOAT Study) J Am Coll Cardiol. 2002;40:869–876. doi: 10.1016/s0735-1097(02)02058-2. [DOI] [PubMed] [Google Scholar]
- 4.Topol EJ, Califf RM, Vandormael M, Grines CL, George BS, Sanz ML, Wall T, O'Brien M, Schwaiger M, Aguirre FV. A randomized trial of late reperfusion therapy for acute myocardial infarction: Thrombolysis and Angioplasty in Myocardial Infarction-6 Study Group. Circulation. 1992;85:2090–2099. doi: 10.1161/01.cir.85.6.2090. [DOI] [PubMed] [Google Scholar]
- 5.Steg PG, Thuaire C, Himbert D, Carrie D, Champagne S, Coisne D, Khalife K, Cazaux P, Logeart D, Slama M, Spaulding C, Cohen A, Tirouvanziam A, Montely JM, Rodriguez RM, Garbarz E, Wijns W, Durand-Zaleski I, Porcher R, Brucker L, Chevret S, Chastang C. DECOPI (DEsobstruction COronaire en Post-Infarctus): a randomized multi-centre trial of occluded artery angioplasty after acute myocardial infarction. Eur Heart J. 2004;25:2187–2194. doi: 10.1016/j.ehj.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Dzavik V, Beanlands DS, Davies RF, Leddy D, Marquis JF, Teo KK, Ruddy TD, Burton JR, Humen DP. Effects of late percutaneous transluminal coronary angioplasty of an occluded infarct-related coronary artery on left ventricular function in patients with a recent (<6 weeks) Q-wave acute myocardial infarction (Total Occlusion Post-Myocardial Infarction Intervention Study [TOMIIS]–a pilot study) Am J Cardiol. 1994;73:856–861. doi: 10.1016/0002-9149(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 7.Horie H, Takahashi M, Minai K, Izumi M, Takaoka A, Nozawa M, Yokohama H, Fujita T, Sakamoto T, Kito O, Okamura H, Kinoshita M. Long-term beneficial effect of late reperfusion for acute anterior myocardial infarction with percutaneous transluminal coronary angioplasty. Circulation. 1998;98:2377–2382. doi: 10.1161/01.cir.98.22.2377. [DOI] [PubMed] [Google Scholar]
- 8.Hochman JS, Lamas GA, Knatterud GL, Buller CE, Dzavik V, Mark DB, Reynolds HR, White HD. Design and methodology of the Occluded Artery Trial (OAT) Am Heart J. 2005;150:627–642. doi: 10.1016/j.ahj.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, Dodge HT, Francis CK, Hillis D, Ludbrook P. Thrombolysis in Myocardial Infarction (TIMI) Trial, phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase: clinical findings through hospital discharge. Circulation. 1987;76:142–154. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]
- 10.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 11.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–592. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan FH, Bolson EL, Dodge HT, Mathey DG, Schofer J, Woo HW. Advantages and applications of the centerline method for characterizing regional ventricular function. Circulation. 1986;74:293–305. doi: 10.1161/01.cir.74.2.293. [DOI] [PubMed] [Google Scholar]
- 13.Ahnve S, Gilpin E, Henning H, Curtis G, Collins D, Ross J., Jr Limitations and advantages of the ejection fraction for defining high risk after acute myocardial infarction. Am J Cardiol. 1986;58:872–878. doi: 10.1016/s0002-9149(86)80002-9. [DOI] [PubMed] [Google Scholar]
- 14.Dzavik V, Carere RG, Mancini GB, Cohen EA, Catellier D, Anderson TE, Barbeau G, Lazzam C, Title LM, Berger PB, Labinaz M, Teo KK, Buller CE. Predictors of improvement in left ventricular function after percutaneous revascularization of occluded coronary arteries: a report from the Total Occlusion Study of Canada (TOSCA) Am Heart J. 2001;142:301–308. doi: 10.1067/mhj.2001.116960. [DOI] [PubMed] [Google Scholar]
- 15.Sirnes PA, Golf S, Myreng Y, Molstad P, Emanuelsson H, Albertsson P, Brekke M, Mangschau A, Endresen K, Kjekshus J. Stenting in Chronic Coronary Occlusion (SICCO): a randomized, controlled trial of adding stent implantation after successful angioplasty. J Am Coll Cardiol. 1996;28:1444–1451. doi: 10.1016/s0735-1097(96)00349-x. [DOI] [PubMed] [Google Scholar]
- 16.Buller CE, Dzavik V, Carere RG, Mancini GB, Barbeau G, Lazzam C, Anderson TJ, Knudtson ML, Marquis JF, Suzuki T, Cohen EA, Fox RS, Teo KK. Primary stenting versus balloon angioplasty in occluded coronary arteries: the Total Occlusion Study of Canada (TOSCA) Circulation. 1999;100:236–242. doi: 10.1161/01.cir.100.3.236. [DOI] [PubMed] [Google Scholar]
- 17.Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, Cerisano G, Antoniucci D. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–1126. doi: 10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- 18.Pizzetti G, Belotti G, Margonato A, Cappelletti A, Chierchia SL. Coronary recanalization by elective angioplasty prevents ventricular dilation after anterior myocardial infarction. J Am Coll Cardiol. 1996;28:837–845. doi: 10.1016/s0735-1097(96)00276-8. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell GF, Lamas GA, Pfeffer MA. Ventricular remodeling after myocardial infarction. Adv Exp Med Biol. 1993;346:265–276. doi: 10.1007/978-1-4615-2946-0_25. [DOI] [PubMed] [Google Scholar]
- 20.Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky S, Maggioni A, White HD, Ruzyllo W, Sadowski Z, Carvalho A, Rankin J, Renkin J, Steg PG, Forman S, Mascette A, Sopko G, Mark DB, Knatterud G. Percutaneous intervention for persistent cozronary occlusion after myocardial infarction: the results of the Occluded Artery Trial (OAT) N Engl J Med. In press. [Google Scholar]
- 21.Pfeffer MA, Lamas GA, Vaughan DE, Parisi AF, Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988;319:80–86. doi: 10.1056/NEJM198807143190204. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]


