Abstract
Background
Recent studies have identified a polymorphism in the ECE-1b promoter (−338C/A) that is strongly associated with hypertension in women, and the polymorphism is located in a consensus binding sequence for the E2F family of transcription factors. E2F proteins are crucially involved in cell-cycle regulation, but their roles in cardiovascular function are poorly understood. Here, we investigated the potential role of E2F2 in blood pressure (BP) regulation.
Methods and Results
Tail-cuff measurements of systolic and diastolic BP were significantly higher in E2F2-null (E2F2−/−) mice than in their wild-type (WT) littermates, and in ex vivo ring assays, aortas from the E2F2−/− mice exhibited significantly greater contractility in response to big endothelin-1 (BigET-1). BigET-1 is activated by endothelin converting enzyme-1 (ECE-1), and mRNA levels of ECE-1b, the repressive ECE-1 isoform, were significantly lower in E2F2−/− mice than in WT mice. In endothelial cells, chromatin-immunoprecipitation (ChIP) assays confirmed that E2F2 binds the ECE-1b promoter, and promoter-reporter assays indicated that E2F2 activates ECE-1b transcription. Furthermore, loss or downregulation of E2F2 led to a decline in ECE-1b levels, to higher levels of the membranous ECE-1 isoforms (i.e., ECE-1a, -1c, and -1d), and to deregulated ECE-1 activity. Lastly, Sam68 co-immunopreciptated with E2F2, occupied the ECE-1b promoter (ChIP), and repressed E2F2-mediated ECE-1b promoter activity (promoter-reporter assays).
Conclusions
Our results identify a cell cycle-independent mechanism by which E2F2 regulates endothelial function, arterial contractility, and BP.
Keywords: E2F, Sam68, Endothelium, Endothelin, Blood pressure
Introduction
Endothelin-1 (ET-1) is a potent vessel-constricting peptide1. It is synthesized in endothelial cells (ECs) from a larger preproET-1 precursor that is cleaved into an inactive 38-amino-acid peptide, big-endothelin-1 (BigET-1), and thereafter further processed by endothelin converting enzyme-1 (ECE-1) into the 21-amino-acid active ET-12. Subsequently, ET-1 acts on the smooth muscle cells (SMCs) through two receptors of G-protein coupled receptor family, ETA and ETB3, 4. Ample evidence from genetic mouse models and blockade of ET-1 receptors in humans has demonstrated that the ET-1/ECE-1 system plays a critical role in the regulation of blood pressure (BP) homeostasis5, 6.
ECE-1 is a membrane-bound zinc metalloendopeptidase expressed predominantly in the vascular endothelium7. ECE-1 catalyzes the rate-limiting step during biogenesis of ET-12. The physiological importance of the BigET-1-to-mature ET-1 conversion is demonstrated by observations in ECE-1, ET-1, and ETA knockout animals, which exhibit virtually identical cardiac and craniofacial abnormalities during embryonic development5, 8, and by the ~140-fold greater vasoconstrictive potency of ET-12. There are four isoforms of ECE-1: ECE-1a, -1b, -1c, and -1d, which are expressed via alternative promoters from the same gene located on human chromosome 1 (1p36)9, 10 (Supplemental Figure S1A). These isoforms exist primarily as homodimers and localize to different subcellular regions because of their dissimilar N-termini; ECE-1a, -1c and -1d are located on the plasma membrane, and ECE-1b is intracellular9, 11, 12 (Supplemental Figure S1B). It has been shown that ECE-1b is located intracellularly in the late endosomes/multivesicular bodies13. Its N-terminal Leucine-based motifs are involved in the intracellular retention. Interaction of a plasma membrane isoform with ECE-1b resulted in its intracellular localization and decreased its extracellular activity12, 14, 15. Therefore, the targeting signals specific for ECE-1b constitute a regulatory domain that modulates the localization and activity of other isoforms13.
Recently, two independent clinical studies have revealed a polymorphism in the 5′-regulatory region of the ECE-1b gene (ECE1 C-338A, 338 bp upstream from the translation start site) that is strongly correlated with increases in the systolic, diastolic and mean BP levels in women16, 17. Moreover, the EDN1 K198N polymorphism in the coding region of the preproET-1 gene, previously known to be associated with BP in overweight people18, interacted with the ECE1 C-338A variant to influence the BP levels17. Interestingly, the ECE1 C-338A polymorphism is located in a consensus site for the E2F family of transcription factors and alters its binding affinity specifically to E2F217. However, it is unknown if E2F2 plays a role in the regulation of ECE-1b gene expression and the pathogenesis of hypertension.
The E2F family of transcription factors regulate cell growth, differentiation, and survival19. The classically described mechanism by which E2F family members regulate transcription involves binding to DNA as heterodimers with members of the DP protein family and the recruitment of other regulatory elements19. Accumulating evidence indicates that the eight known E2F proteins (E2F1-8) target both common and unique genes in the genome20, 21, and that each E2F family member has diverse physiological functions that are specific to the tissue type and biological context22–24. The functional diversity of E2F members is exemplified by the strikingly different phenotypes found in the corresponding knockout animals22. The specific roles of E2F family members in regulating the vasculature, however, are poorly characterized.
Recently, we have shown that E2F1 is involved in the regulation of angiogenesis. Genetic deletion of E2F1 enhanced blood flow recovery after ischemic injury in mice24. To determine whether other E2F family members are involved in angiogenesis, we performed experiments in E2F2-null (E2F2−/−) mice. Angiogenesis was unchanged; however, the E2F2−/− mice were hypertensive with altered vasomotion. Here we provide genetic evidence that E2F2 regulates BP via specific regulation of endothelial ECE-1b expression, subcellular localization and hence vascular reactivity. Further, our studies reveal that Sam68, an RNA-binding protein and oncogene, acts as a novel transcription cofactor of E2F2 in the regulation of ECE-1b.
Methods
Mice
The heterozygote E2F2+/− mice were obtained from Dr. Gustavo Leone’s lab (Ohio State University)25 and were bred, maintained, and operated in the Center for Comparative Medicine of Northwestern University following protocols approved by the Institutional Animal Care and Use Committee. All animals were genotyped by PCR of tail DNA. Age- and sex-matched E2F2−/−, E2F2+/−, and their WT littermates were used.
BP measurement
Arterial BP and heart rates from WT and E2F2−/− mice were measured by the standard noninvasive tail cuff method (CODA System, Kent Scientific, Torrington, CT)26. Measurements were performed at day time with previous 5 days of training. On each day of BP determination, 20 measurements were obtained and averaged for each mouse.
Aortic ring assay
The contractile properties of aortic arteries were analyzed by the ring assay27 as described previously and as summarized in the Supplemental Methods.
Measurement of ET-1 peptides
Levels of ET-1 in mouse plasma and in cell culture medium were measured using a commercially available ELISA kit (Biomedica, Vienna, Austria) following manufacturer’s instructions.
Tissue RNA isolation and real-time RT-PCR
Tissue RNA was extracted and real-time PCR performed as described previously24. Primer sequences are listed in Supplemental Figure S2.
Plasmids and siRNA
Human pECE-1b/C-AP and pECE-1b/A-AP plasmids were provided by Dr. Benoit Funalot (INSERM, France)28. pRC/CMV-E2F1 and pRC/CMV-E2F2 plasmids were provided by Dr. Farbio Martelli (IRCSS, Italy). Myc-Sam68 plasmid was provided by Dr. Chi Wai Eric So (The Institute of Cancer Research, Sutton, UK). Human and bovine E2F1 and E2F2 siRNA were synthesized by Dharmacon, Inc. (Lafayette, CO). Mouse ECE-1b promoter-AP reporter plasmid was constructed following standard cloning technique (see Supplemental Methods for details).
Cell culture, plasmid and siRNA transient transfection, and reporter assays
HUVECs and BAECs were obtained from ATCC (Manassas, VA), cultured as described24, and used within passage 5. For plasmid transfection of BAECs, TransFast (Promega, Madison, WI) or Arrest-In (Open Biosystems, Huntsville, AL) was used. For siRNA transfection of HUVECs, Arrest-In or RNAiFect (QIAGEN, Valencia, CA) was used. Control β-Gal-expressing plasmid (pCMV-β-Gal) was co-transfected to normalize the transduction efficiency. Chemiluminescent reporter gene assays for detection of AP and β-Gal activities were performed as described previously24.
Isolation of mouse primary lung ECs
Primary ECs were isolated from mouse lung tissues following the published protocol29 (see Supplemental Methods for details). The cells were used before passage 5 and the EC identity was confirmed before each experiment by staining with FITC-conjugated anti-CD31 antibody24.
Subcellular fractionation, Western blotting, and co-immunoprecipitation
Plasma-membrane and cytosolic proteins were fractionated by using the Pinpoint Cell Surface Protein Isolation Kit (Pierce) (see Supplemental Methods for details) and then analyzed by Western blotting24. The primary antibodies used were anti-E2F2, anti-E2F1, anti-Sam68, anti-HA, anti-Tie-2, anti-actin (Santa Cruz), anti-human ECE-1 (R&D systems), anti-Calnexin (Stressgen, Ann Arbor, MI), and anti-Myc (Cell Signaling Technology). For co-immunoprecipitation, cell lysates were incubated overnight at 4°C with the appropriate antibody, followed by incubation with protein A/G plus-Agarose (Santa Cruz) for 1 h at 4°C. After washing, the immunoprecipitates were eluted by boiling for 5 min, and extracts were analyzed by immunoblotting as described above. Band intensities were determined densitometrically with Image J software.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed in HUVECs by using ChIP assay kit (Upstate) (see Supplemental Methods for details). Primers sequences are listed in Supplemental Figure S2.
Mass spectrometry (MS)
Experiments followed a standard protein identification strategy30 as described in the Supplemental Methods.
Fluorescent immunohistochemistry
Fluorescent immunohistochemical staining was performed following standard techniques31 (see Supplemental Methods for details) and the slides were examined under confocal microscope (Zeiss LSM 510 META).
ECE-1 enzymatic activity in intact primary ECs
Conversion of the exogenous substrate BigET-1 (Alexis, Switzerland) into mature ET-1 was determined in primary lung ECs after incubation with 10−7 M Big ET-1 for 60 min in a 96-well plate at 37°C. Reaction was stopped by addition of 1mM NaEDTA. ET-1 generated was measured in the culture medium with an ELISA kit (Biomedica, Vienna, Austria). The value obtained from the well without cells was considered the ELISA cross-reactivity for BigET-1 and subtracted from all the measurements where BigET-1 had been added. The ET-1 level in each well was normalized to the quantity of cells determined by a crystal violet staining method we described previously31.
Statistical analysis
All values were expressed as mean ± SEM. Comparison between two means was performed with an unpaired Student’s t test, whereas ANOVA with Fisher’s protected least significant differences and Bonferroni–Dunn post hoc analysis were used for comparisons of more than two means. Comparison between concentration–response curves was performed using ANOVA for repeated measures. Significance was defined as P<0.05.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Loss of E2F2 expression in mice results in elevated BP and exaggerated arterial contraction in response to BigET-1
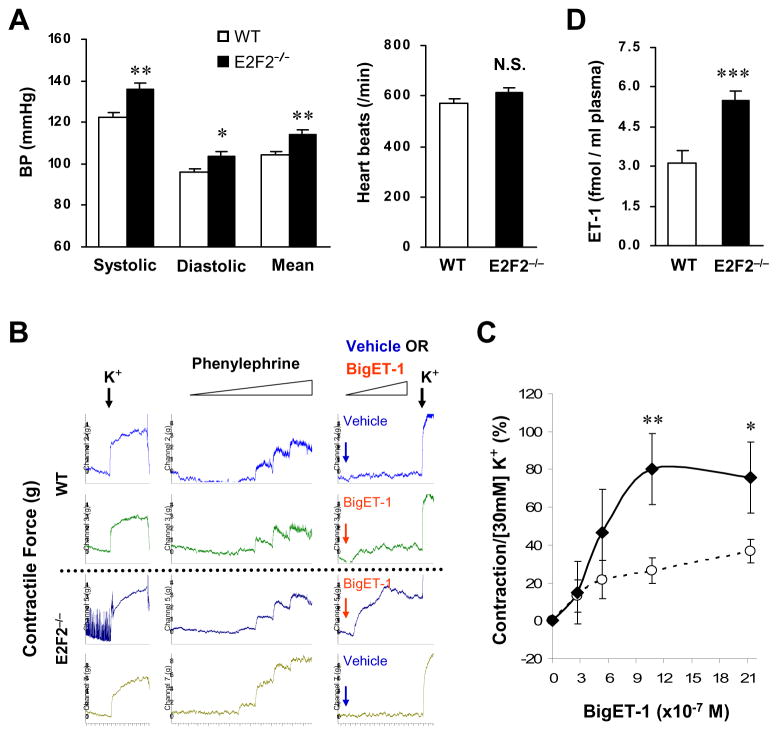
We measured BP in E2F2−/− mice and their wild-type (WT) littermates and found that despite similar heart rates, the E2F2−/− mice displayed significantly higher systolic and diastolic BP (Figure 1A). Using ex vivo aortic ring assays, we compared the contractility of aortas isolated from E2F2−/− mice and their wild-type (WT) littermates in response to BigET-1 and a variety of vasoactive substances. Contraction induced by KCl (30 mM) and PE (1×10−9−3×10−6M) and relaxation in response to Ach (1×10−9−3×10−6M) were similar in the two strains, but aortas from the E2F2−/− mice exhibited significantly greater contractility in response to serial doses of BigET-1 (2.7×10−7 M to 2.1×10−6 M) (Figure 1B and 1C).
Figure 1. Loss of E2F2 expression in mice results in elevated BP and exaggerated arterial contraction in response to BigET-1.
(A) Tail-cuff arterial BP (Left) and heart rates (Right) in E2F2−/− mice and their WT littermates at age of 6 months. Data are means ± SEM. (n=12 per group; **P<0.01, *P<0.05, NS, not significant vs. WT). (B–C) The contractility of endothelium-intact aortas isolated from E2F2−/− mice and their WT littermates were evaluated via aortic ring assay. (B) Representative recordings of the ring assay. Arrows indicate the time points when K+ (black arrow), BigET-1 (red arrow), or vehicle control (blue arrow) was added. K+: 30 mM, Phenylephrine: 1×10−9 −3×10−6 M, BigET-1: 2.7 ×10−7 −21×10−7 M. Contractile response to the addition of K+ at the end of each BigET-1 assessment was similar between groups, indicating that the differences in response to BigET-1 were caused by an altered ECE-1 activity rather than by a general change in contractile function. (C) Quantification of contraction in response to BigET-1 (WT: dotted line, E2F2−/−: solid line). The percentage of BigET-1 induced contraction was calculated with K+ (30 mM)-induced contraction set to 100%. Data are means ± SEM. (n=4 per group; *P<0.05, **P<0.01 vs. WT). (D) Plasma ET-1 levels in the E2F2−/− mice and WT littermates (n=12 per group; ***P<0.001 vs. WT).
Because BigET-1 is converted to active endothelin 1 (ET-1) by endothelin-converting enzyme 1 (ECE-1), thereby increasing vessel contractility, our observations suggest that the influence of E2F2 on contractility could be generated, at least in part, through the regulation of ECE-1 activity. If so, the elevated BP in E2F2−/− mice may be attributable to an increase in ET-1 level and activity. Plasma ET-1 levels were significantly higher in E2F2−/− mice than in WT mice (Figure 1D), and selective antagonism of the ETA receptor with BQ123 dramatically reduced BP in E2F2−/− mice to levels that did not differ significantly from WT (Supplemental Figure S3). In addition, we found that E2F2−/− mice express a higher level of ETA protein in the lung tissues (Supplemental Figure S4). Collectively, these findings indicate that enhanced ET-1 biosynthesis and activity, resulting from increased ECE-1 activity, contribute to the BP elevations observed in E2F2−/− mice.
E2F2 regulates ECE-1b transcription
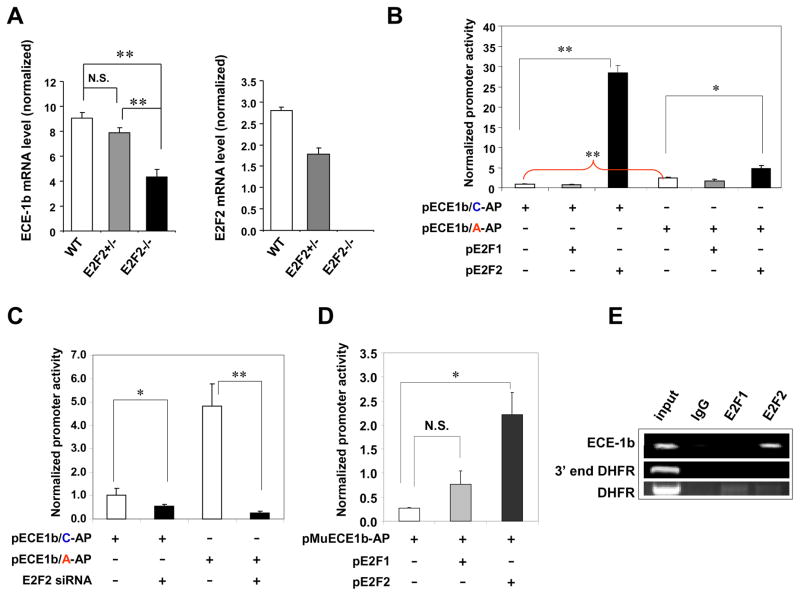
Because recent clinical studies have identified a strong association between an E2F binding-site polymorphism on the ECE-1b promoter (C-338A) and hypertension in women16, 17, our findings may be relevant to human disease. Accordingly, we evaluated whether E2F2 regulates ECE-1b mRNA expression in vivo via real-time RT-PCR analyses. In lung tissues, the levels of ECE-1b mRNA were 50% lower in E2F2−/− mice than in their WT littermates (Figure 2A), suggesting that endogenous E2F2 levels maintain basal ECE-1b expression in WT tissue; the levels of ECE-1a, -1c, and -1d mRNAs, however, were similar between E2F2−/− mice and WT controls (Supplemental Figure S5). Interestingly, ECE-1b mRNA levels were similar in the lung tissues from E2F1−/− and WT mice (Supplemental Figure S6), indicating that the reduced ECE-1b expression observed in E2F2−/− mice was an E2F2-specific effect.
Figure 2. E2F2 regulates ECE-1b transcription.
(A) ECE-1b (Left) and E2F2 (Right) mRNA expression in mouse lung tissue. Real time RT-PCR was performed with RNA freshly isolated from the lung tissues of E2F2−/− mice, E2F2+/− mice, and their WT littermates (n=4). ECE-1b and E2F2 mRNA levels were normalized to GAPDH mRNA level. (B) Effect of E2F2 overexpression on human ECE-1b promoter activity. BAECs were co-transfected with plasmids expressing E2F1 (pE2F1) or E2F2 (pE2F2) and with plasmids expressing alkaline phosphatase (AP) from either the native (−338C) ECE-1b promoter (pECE1b/C-AP) or the polymorphic (−338A) ECE-1b promoter (pECE1b/A-AP) that has been linked to hypertension in women. (n=3). (C) Effect of E2F2 knockdown on human ECE-1b promoter activity. The native or polymorphic human promoter reporter plasmids were transfected with or without bovine E2F2 siRNA. (n=3). (D) Effect of E2F2 overexpression on mouse ECE-1b promoter activity. A mouse ECE-1 proximal promoter (1.6 kb)-AP (pMuECE1b-AP) was co-transfected with pE2F1 or pE2F2. (n=4). (E) ChIP assay showing E2F2 occupation at ECE-1b promoter in vivo. ChIP assays were performed in HUVECs with E2F2 antibody, E2F1 antibody, and control IgG. The promoter region for the dihydrofolate reductase gene (DHFR), which is regulated by both E2F1 and E2F2, was used as a positive control, and the 3′ untranslated region served as a negative control. Data are means ± SEM for (A - D); AP activities were normalized to β-Gal activity (*P<0.05, **P<0.01, N.S., not significant).
To investigate whether E2F2-regulated ECE-1b mRNA expression is allele-specific for the ECE-1b promoter, bovine aortic endothelial cells (BAECs) were co-transfected with an E2F2-expressing plasmid and a reporter plasmid that expressed alkaline phosphatase (AP) from either the native (−338C) human ECE-1b promoter or the polymorphic (−338A) promoter associated with hypertension in women (Figure 2B and Supplemental Figure S1A); cells were harvested 24 h after transfection for AP activity assays. In the absence of E2F2 overexpression, the activity of the polymorphic promoter was significantly higher than native promoter activity (Figure 2B).
Overexpression of E2F2 significantly increased the activity of both the polymorphic and native ECE-1b promoters, but the enhancement of native promoter activity was significantly greater (native: 28.7-fold, C-338A: 1.9-fold) (Figure 2B). These observations suggest that E2F2 activates ECE-1b expression, but this activation is impaired by the C-338A polymorphism. Results from similar experiments indicate that the human ECE-1b promoter is not regulated by E2F1 (Figure 2B). To determine whether endogenous E2F2 regulates ECE-1b promoter activity, we performed promoter-reporter assays in BAECs after knocking down endogenous E2F2 expression with siRNA. Both polymorphic and native ECE-1b promoter activity were significantly reduced in the presence of E2F2 siRNA, but the activity of the polymorphic promoter was reduced to a greater extent (Figure 2C).
To correlate our observations from experiments in E2F2−/− mice with the results from our human ECE-1b promoter-reporter assays, we investigated whether regulation of the mouse ECE-1b promoter is disrupted in E2F2−/− mice. BAECs were co-transfected with a plasmid that expressed AP from a murine ECE-1b proximal (1.6 kb) promoter, which contains 3 putative E2F binding sites, and a plasmid expressing either E2F2 or E2F1. Overexpression of E2F2, but not E2F1, significantly upregulated murine ECE-1b promoter activity (Figure 2D), confirming that E2F2 regulates ECE-1b expression in both mice and humans.
To determine whether E2F2 physically interacts with the ECE-1b promoter, we performed ChIP assays with human umbilical-vein endothelial cells (HUVECs). Antibodies to E2F2, but not E2F1, coprecipitated with the ECE-1b promoter, indicating that endogenous E2F2 occupies the ECE-1b promoter region in vivo and that ECE-1b is a direct transcriptional target of E2F2 (Figure 2E).
Sam68 suppresses E2F2-mediated ECE-1b transcription
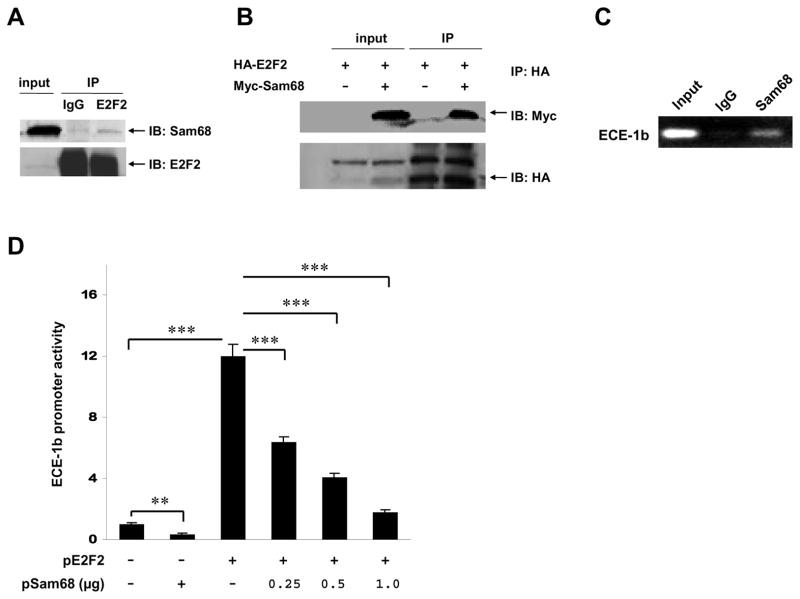
To identify cofactors that may interact with E2F2 and modulate ECE-1b regulation, we isolated nuclear protein extracts from cultured HUVECs, co-immunoprecipitated the extracts with the E2F2 antibody, resolved the E2F2-coprecipitated proteins on a 1-D SDS-PAGE gel, and identified the proteins via mass spectrometry. One of the proteins identified was Sam68, an RNA-binding protein32. Both endogenous and overexpressed Sam68 protein co-immunoprecipitated with E2F2 (Figure 3A and 3B), confirming that Sam68 binds E2F2 in vivo and identifying Sam68 as a possible cofactor involved in E2F2 transactivity. We then performed ChIP assays and confirmed that Sam68, like E2F2, occupies the ECE-1b promoter region in vivo (Figure 3C).
Figure 3. Sam68 suppresses E2F2-mediated ECE-1b transcription.
(A) Interaction between endogenous Sam68 and E2F2 proteins. HUVEC lysates were immunoprecipitated (IP) with anti-E2F2 antibody, followed by immunoblotting (IB) with anti-Sam68 antibody and reblotting with anti-E2F2 antibody. (B) Interaction between overexpressed Sam68 and E2F2 proteins. 293T cells were transfected with HA-tagged E2F2 and Myc-tagged Sam68. Cell lysates were immunoprecipitated with anti-HA antibody, followed by blotting with anti-Myc antibody and reblotting with anti-HA antibody. (C) ChIP assay showing Sam68 occupation of the ECE-1b promoter in vivo in HUVECs. (D) Effect of Sam68 overexpression on basal and E2F2-induced ECE-1b promoter activity. BAECs were co-transfected with pECE1b/C-AP and with plasmids expressing E2F2 (pE2F2), Sam68 (pSam68), or both; AP activity was assessed 24 h after transfection and expressed as the fold-difference from the level observed in the pECE1b/C-AP– only group. Data are means ± SEM; AP activities were normalized to β-Gal activity (n=9 per treatment; **P<0.01, ***P<0.001).
To determine whether Sam68 modulates E2F2 transactivity at the ECE-1b promoter, we performed co-transfection experiments with plasmids expressing E2F2, Sam68, and the native human ECE-1b promoter-AP construct. Overexpression of Sam68 suppressed E2F2-induced ECE-1b promoter activity in a dose-dependent manner as well as basal ECE-1b promoter activity (Figure 3D). Collectively, our data indicate that Sam68 is an E2F2 cofactor that represses E2F2-mediated ECE-1b transcription.
Loss or downregulation of E2F2 expression increases levels of the plasma-membrane ECE-1 isoforms and reduces levels of the intracellular ECE-1 isoforms
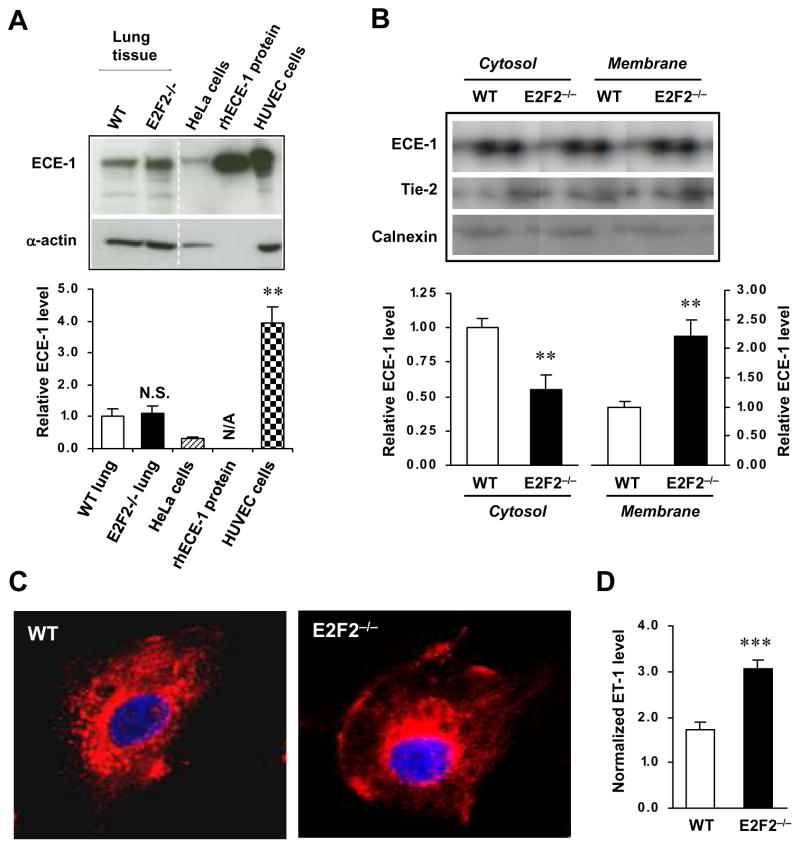
Because E2F2 regulates ECE-1b transcription, we investigated whether ECE-1 protein levels differ in tissues harvested from E2F2−/− mice and their WT littermates. Since the available antibodies cannot distinguish between the isoforms of ECE-1 (i.e., ECE-1a, -1b, -1c, and -1d), we performed Western blotting with a polyclonal antibody that identifies a C-terminal epitope present in all four ECE-1 isoforms. Total ECE-1 protein levels in the lung tissues of E2F2−/− and WT mice were similar (Figure 4A). We then isolated primary ECs from the lung tissues of E2F2−/− and WT mice (Supplemental Figure S7). To differentiate between the plasma-membrane and intracellular ECE-1 isoforms, we labeled the surface of the lung ECs in culture with nonpermeable Sulfo-NHS-SS-Biotin, then isolated the plasma-membrane and cytosolic protein compartments by fractionation. Compared to WT lung ECs, E2F2−/− lung ECs displayed significantly less ECE-1 immunoreactivity in the cytosolic fraction (i.e., ECE-1b immunoreactivity) and significantly greater ECE-1 immunoreactivity in the plasma membrane fraction (i.e., ECE-1a, -1c, and -1d immunoreactivity) (Figure 4B). These results were confirmed via confocal microscopic analyses of cells immunofluorescently stained for by ECE-1 (Figure 4C), and experiments in HUVECs yielded equivalent results: siRNA-mediated knock-down of E2F2 reduced cytoplasmic ECE-1 (ECE-1b) levels and increased plasma membrane ECE-1 (ECE-1a, -1c, -1d) levels (Supplemental Figure S8). Importantly, the increase in plasma-membrane ECE-1 in the E2F2−/− lung ECs was associated with an enhanced conversion of exogenous BigET-1 into ET-1 (Figure 4D). These observations are consistent with a study by Muller et al, who showed that ECE-1b decreases the expression and activity of ECE-1a, -1c, and -1d13. Collectively, the results presented here and by Muller et al. suggest that the enhanced contractility of E2F2−/− aortic vessels in response to BigET-1 (Figure 1B and 1C) may evolve indirectly through the downregulation of late endosomal ECE-1b levels and a subsequent increase in ECE-1a, -1c, and -1d activity.
Figure 4. Loss of E2F2 increases levels of the plasma-membrane ECE-1 isoforms and reduces levels of the intracellular ECE-1 isoforms.
(A) Representative Western blotting for ECE-1 (Upper) and quantification of relative ECE-1 levels (Lower) in the whole-cell lysates from the lung tissues of E2F2−/− mice and their WT littermates. The levels of ECE-1 were determined densitometrically, normalized to the levels of α-actin, and expressed as the fold-difference from the levels measured in WT lung tissues. Control assessments evaluated ECE-1 levels in HUVECs and HeLa cell lysates and recombinant human ECE-1 (rhECE-1) protein (n=6 per group; **P<0.01, N.S., not significant vs. WT lung tissue; N/A, not applicable for normalization to α-actin). (B) Western blotting analyses of ECE-1 protein levels at the plasma membrane and in the cytosol of the primary lung ECs isolated from E2F2−/− and WT mice. Cells were fractionated with a Sulfo-NHS-SS-Biotin kit. The levels of ECE-1 in the membrane and cytosol were normalized to the levels of Tie-2 and Calnexin, respectively, and expressed as the fold-difference from the levels measured in WT ECs. (n=5 per group; **P<0.01 vs. WT). (C) Confocal microscopy of mouse lung ECs stained with an anti-ECE-1 antibody (red) and counter-stained with DAPI (blue) (original magnification 400 X); representative results from 4 separate experiments are shown. The anti-ECE-1 antibody recognizes an epitope shared by all four ECE-1 isoforms (ECE-1a, -1b, -1c, -1d). (D) ET-1 levels in the culture media of WT and E2F2−/− lung ECs after incubation with 10−7 M BigET-1 for 60 min (n=6 per group. ***P<0.001). The ET-1 levels were measured with ELISA and normalized to the quantity of cells.
Discussion
Hypertension is a multifactorial disease in which genetic traits play an important role33, 34. Recent studies indicate that transcriptional regulators in both vascular ECs and SMCs can critically impact BP control27, 35. Our investigations provide the first evidence that E2F2, a classic cell-cycle regulator, influences BP. Data from our studies suggest that endogenous levels of E2F2 transcriptional activity in non-proliferating endothelium regulate vessel tone through the ECE-1/ET-1 system: E2F2 acts as a transactivator of ECE-1b transcription, and Sam68 functions as a cofactor that represses E2F2-induced ECE-1b transcription in ECs. Thus, the physiological state could regulate BP, at least in part, by balancing E2F2 and Sam68 activity to fine-tune ECE-1b expression. If so, deregulated E2F2 transcriptional activity (e.g., in E2F2−/− mice or in women carrying the C-338A ECE-1b promoter polymorphism) may contribute to the pathogenesis of hypertension.
The ECE-1/ET-1 system has been recognized to regulate BP through multiple mechanisms including maintenance of the system vessel resistance and renal functions6, 36–38. Increasing evidence emphasizes aberrant vessel tone in the pathogenesis of hypertension39, 40. We observed a striking abnormality of vascular reactivity in E2F2−/− mice. However, increased ECE-1/ET-1 activity has also been shown to increase oxidative stress41 and to mediate Angiotensin II-induced hypertension42. Importantly, the kidney has been shown to be both a source of ET-1 generation and an important target organ of this peptide, where it mediates natriuretic and diuretic effects through the ETB receptor subtype43, 44. Therefore we cannot rule out a possible contribution of these factors to the hypertension phenotype of E2F2−/− mice.
The (C-338A) ECE-1b promoter polymorphism has been linked to hypertension in women but not in men16, 17. This gender difference may be partially due to the fact that the higher levels of estrogen in women suppress ET-1 synthesis to a greater extent, so women are more sensitive to the increase of ECE-1 activity45. However, this effect was not seen in a related study of human Alzheimer’s disease for which ECE-1 activity also plays an important role28 (see below). In our experimental settings, the elevated BP existed in both female and male E2F2−/− mice.
Higher levels of basal promoter activity were observed for the polymorphic ECE-1b/A promoter than for the ECE-1b/C promoter, and E2F2 siRNA strongly inhibited both promoters, yielding comparable levels of residual activity. Nevertheless, the ECE-1b/A promoter was only marginally activated by E2F2 overexpression. This apparent discrepancy could evolve from intrinsic competition between E2F2 and other cofactors that bind to the ECE-1b promoter. Overexpression of one factor (e.g., E2F2) could induce conformational changes in other cofactors, and interactions between the cofactor complex and their cognate cis-elements in the ECE-1b/A and ECE-1b/C promoters could differ. However, this explanation has yet to be evaluated experimentally.
Funalot et al. have recently reported that the E2F2 binding site polymorphism in the ECE-1b promoter is associated with a lower risk of late-onset Alzheimer’s disease28. Since the deposition of β-amyloid in the brain is a pathological hallmark of Alzheimer’s disease and the ECE-1 activity degrades β-amyloid46, this data corroborates our findings for the regulation of ECE-1 activity by this E2F2-binding site in the ECE-1b promoter. In addition, E2F2 may have a role in other pathological conditions that have been linked to ECE-1 activity, including pulmonary hypertension, myocardial infarction, and renal failure47. Thus, the implications of our findings presented here may extend to a broad range of pathological conditions and normal developmental processes.
Supplementary Material
Acknowledgments
We thank Dr. Gustavo Leone (The Ohio State University) for providing E2F2 knockout mice, Dr. Funalot (INSERM, France) for ECE-1b/A and ECE-1b/C promoter-alkaline phosphatase reporter plasmids, Dr. Farbio Martelli (IRCSS, Italy) for E2F2 expression plasmid, Dr. Chi Wai Eric So (The Institute of Cancer Research, Sutton, UK) for myc-Sam68 plasmid, Dr. Yves Goldberg (INSERM, Grenoble, France) for pEGFP-Sam68 plasmid, and Drs. Lizhao Wu (The Ohio State University), Ping Lu (CSEMC, Boston), Hiromichi Hamada (CSEMC, Boston), T Ouimet (INSERM, France), Jianing Zhang (Northwestern University), Ms. Andrea Wecker (CSEMC, Boston) and Shirley Yang (Northwestern University) for technical help. We thank Dr. W. Kevin Meisner for editorial assistance.
Sources of Funding
This work was supported by American Heart Association Grant 0430135N (to G.Q.), National Institutes of Health Grants HL-077378 (to M.E. Mendelsohn), HL-53354, HL-57516, HL-80137, HL-63414, HL-77428, and HL-66957 (to D.W.L.).
Footnotes
CLINICAL PERSPECTIVE
Hypertension is largely attributed to abnormal renal sodium handling; however, a growing body of evidence now suggests that primary abnormalities in vessels can also cause aberrations in blood pressure. Very often, the source of the abnormality resides in the endothelial cells that regulate the functional state of the entire vessel, and this knowledge has directed our search for new diagnostic and therapeutic targets. To date, the role of transcriptional mechanisms in blood-pressure regulation is poorly characterized. In this study, we found that E2F2, a transcription factor involved in cell-cycle control, regulates blood pressure by modulating vessel contractility. This previously unknown function of E2F2 evolves from the molecule’s unique role in endothelial cells: suppression of endothelin-converting enzyme 1 (ECE-1). ECE-1 converts the inactive precursor molecule big endothelin-1 (BigET-1) into the potent vasoconstrictor endothelin-1 (ET-1), and genetic deletion of E2F2 in mice was associated with both exaggerated vessel contractility in response to BigET-1 stimulation and high blood pressure. E2F2 suppresses ECE-1 activity indirectly by promoting transcription of ECE-1b, the negative isoform of ECE-1, and our results also indicate that Sam68 functions as a cofactor that represses E2F2-mediated ECE-1b transcription. These findings are especially provocative, because clinical studies have identified an E2F binding-site polymorphism on the ECE-1b promoter that is strongly associated with hypertension in women. Collectively, our results and observations from other labs may have identified a previously unknown mechanism of blood-pressure maintenance that operates through the E2F2/Sam68-ECE-1 pathway, and deregulation of this pathway may contribute to blood-pressure disorders, including hypertension in humans.
Disclosures
None.
References
- 1.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.D’Orleans-Juste P, Plante M, Honore JC, Carrier E, Labonte J. Synthesis and degradation of endothelin-1. Can J Physiol Pharmacol. 2003;81:503–510. doi: 10.1139/y03-032. [DOI] [PubMed] [Google Scholar]
- 3.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 5.Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao WH, Kamada N. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- 6.Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 8.Yanagisawa H, Yanagisawa M, Kapur RP, Richardson JA, Williams SC, Clouthier DE, de Wit D, Emoto N, Hammer RE. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125:825–836. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]
- 9.Schweizer A, Valdenaire O, Nelbock P, Deuschle U, Dumas Milne Edwards JB, Stumpf JG, Loffler BM. Human endothelin-converting enzyme (ECE-1): three isoforms with distinct subcellular localizations. The Biochemical journal. 1997;328 (Pt 3):871–877. doi: 10.1042/bj3280871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdenaire O, Lepailleur-Enouf D, Egidy G, Thouard A, Barret A, Vranckx R, Tougard C, Michel JB. A fourth isoform of endothelin-converting enzyme (ECE-1) is generated from an additional promoter molecular cloning and characterization. Eur J Biochem. 1999;264:341–349. doi: 10.1046/j.1432-1327.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- 11.Azarani A, Boileau G, Crine P. Recombinant human endothelin-converting enzyme ECE-1b is located in an intracellular compartment when expressed in polarized Madin-Darby canine kidney cells. The Biochemical journal. 1998;333 (Pt 2):439–448. doi: 10.1042/bj3330439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi M, Fukuda K, Shimada K, Barnes K, Turner AJ, Ikeda M, Koike H, Yamamoto Y, Tanzawa K. Localization of rat endothelin-converting enzyme to vascular endothelial cells and some secretory cells. The Biochemical journal. 1995;311 (Pt 2):657–665. doi: 10.1042/bj3110657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller L, Barret A, Etienne E, Meidan R, Valdenaire O, Corvol P, Tougard C. Heterodimerization of endothelin-converting enzyme-1 isoforms regulates the subcellular distribution of this metalloprotease. J Biol Chem. 2003;278:545–555. doi: 10.1074/jbc.M208949200. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Kroger B, Jacob E, Seulberger H, Subkowski T, Otter R, Meyer T, Schmalzing G, Hillen H. Molecular characterization of human and bovine endothelin converting enzyme (ECE-1) FEBS Lett. 1994;356:238–243. doi: 10.1016/0014-5793(94)01277-6. [DOI] [PubMed] [Google Scholar]
- 15.Shimada K, Takahashi M, Turner AJ, Tanzawa K. Rat endothelin-converting enzyme-1 forms a dimer through Cys412 with a similar catalytic mechanism and a distinct substrate binding mechanism compared with neutral endopeptidase-24.11. The Biochemical journal. 1996;315 (Pt 3):863–867. doi: 10.1042/bj3150863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funalot B, Courbon D, Brousseau T, Poirier O, Berr C, Cambien F, Amouyel P, Schwartz JC, Ducimetiere P. Genes encoding endothelin-converting enzyme-1 and endothelin-1 interact to influence blood pressure in women: the EVA study. J Hypertens. 2004;22:739–743. doi: 10.1097/00004872-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Funke-Kaiser H, Reichenberger F, Kopke K, Herrmann SM, Pfeifer J, Orzechowski HD, Zidek W, Paul M, Brand E. Differential binding of transcription factor E2F-2 to the endothelin-converting enzyme-1b promoter affects blood pressure regulation. Hum Mol Genet. 2003;12:423–433. doi: 10.1093/hmg/ddg040. [DOI] [PubMed] [Google Scholar]
- 18.Tiret L, Poirier O, Hallet V, McDonagh TA, Morrison C, McMurray JJ, Dargie HJ, Arveiler D, Ruidavets JB, Luc G, Evans A, Cambien F. The Lys198Asn polymorphism in the endothelin-1 gene is associated with blood pressure in overweight people. Hypertension. 1999;33:1169–1174. doi: 10.1161/01.hyp.33.5.1169. [DOI] [PubMed] [Google Scholar]
- 19.Attwooll C, Lazzerini Denchi E, Helin K. The E2F family: specific functions and overlapping interests. Embo J. 2004;23:4709–4716. doi: 10.1038/sj.emboj.7600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD, Helin K. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 2001;15:267–285. doi: 10.1101/gad.864201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells J, Graveel CR, Bartley SM, Madore SJ, Farnham PJ. The identification of E2F1-specific target genes. Proc Natl Acad Sci U S A. 2002;99:3890–3895. doi: 10.1073/pnas.062047499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeGregori J. The genetics of the E2F family of transcription factors: shared functions and unique roles. Biochim Biophys Acta. 2002;1602:131–150. doi: 10.1016/s0304-419x(02)00051-3. [DOI] [PubMed] [Google Scholar]
- 23.Murga M, Fernandez-Capetillo O, Field SJ, Moreno B, Borlado LR, Fujiwara Y, Balomenos D, Vicario A, Carrera AC, Orkin SH, Greenberg ME, Zubiaga AM. Mutation of E2F2 in mice causes enhanced T lymphocyte proliferation, leading to the development of autoimmunity. Immunity. 2001;15:959–970. doi: 10.1016/s1074-7613(01)00254-0. [DOI] [PubMed] [Google Scholar]
- 24.Qin G, Kishore R, Dolan CM, Silver M, Wecker A, Luedemann CN, Thorne T, Hanley A, Curry C, Heyd L, Dinesh D, Kearney M, Martelli F, Murayama T, Goukassian DA, Zhu Y, Losordo DW. Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent transcriptional control of VEGF. Proc Natl Acad Sci U S A. 2006;103:11015–11020. doi: 10.1073/pnas.0509533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, Greenberg ME, Orkin S, Nevins JR, Robinson ML, Leone G. The E2F1-3 transcription factors are essential for cellular proliferation. Nature. 2001;414:457–462. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- 26.Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 28.Funalot B, Ouimet T, Claperon A, Fallet C, Delacourte A, Epelbaum J, Subkowski T, Leonard N, Codron V, David JP, Amouyel P, Schwartz JC, Helbecque N. Endothelin-converting enzyme-1 is expressed in human cerebral cortex and protects against Alzheimer’s disease. Mol Psychiatry. 2004;9:1122–1128. 1059. doi: 10.1038/sj.mp.4001584. [DOI] [PubMed] [Google Scholar]
- 29.van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW. Isolation of endothelial cells from fresh tissues. Nat Protoc. 2008;3:1085–1091. doi: 10.1038/nprot.2008.71. [DOI] [PubMed] [Google Scholar]
- 30.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 31.Qin G, Ii M, Silver M, Wecker A, Bord E, Ma H, Gavin M, Goukassian DA, Yoon YS, Papayannopoulou T, Asahara T, Kearney M, Thorne T, Curry C, Eaton L, Heyd L, Dinesh D, Kishore R, Zhu Y, Losordo DW. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203:153–163. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukong KE, Richard S. Sam68, the KH domain-containing superSTAR. Biochim Biophys Acta. 2003;1653:73–86. doi: 10.1016/j.bbcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi N, Smithies O. Human genetics, animal models and computer simulations for studying hypertension. Trends Genet. 2004;20:136–145. doi: 10.1016/j.tig.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Kleinert H, Wallerath T, Euchenhofer C, Ihrig-Biedert I, Li H, Forstermann U. Estrogens increase transcription of the human endothelial NO synthase gene: analysis of the transcription factors involved. Hypertension. 1998;31:582–588. doi: 10.1161/01.hyp.31.2.582. [DOI] [PubMed] [Google Scholar]
- 36.Haynes WG, Ferro CJ, O’Kane KP, Somerville D, Lomax CC, Webb DJ. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation. 1996;93:1860–1870. doi: 10.1161/01.cir.93.10.1860. [DOI] [PubMed] [Google Scholar]
- 37.Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan Hypertension Investigators. N Engl J Med. 1998;338:784–790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Rivero G, Ruiz-Torres MP, Rivas-Elena JV, Jerkic M, Diez-Marques ML, Lopez-Novoa JM, Blasco MA, Rodriguez-Puyol D. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation. 2006;114:309–317. doi: 10.1161/CIRCULATIONAHA.105.611111. [DOI] [PubMed] [Google Scholar]
- 39.Mendelsohn ME. In hypertension, the kidney is not always the heart of the matter. J Clin Invest. 2005;115:840–844. doi: 10.1172/JCI24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell. 2006;124:929–942. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 41.Laplante MA, de Champlain J. The interrelation of the angiotensin and endothelin systems on the modulation of NAD(P)H oxidase. Can J Physiol Pharmacol. 2006;84:21–28. doi: 10.1139/Y05-146. [DOI] [PubMed] [Google Scholar]
- 42.Laplante MA, Wu R, Moreau P, de Champlain J. Endothelin mediates superoxide production in angiotensin II-induced hypertension in rats. Free Radic Biol Med. 2005;38:589–596. doi: 10.1016/j.freeradbiomed.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman A, Abassi ZA, Brodsky S, Ramadan R, Winaver J. Mechanisms of big endothelin-1-induced diuresis and natriuresis : role of ET(B) receptors. Hypertension. 2000;35:732–739. doi: 10.1161/01.hyp.35.3.732. [DOI] [PubMed] [Google Scholar]
- 44.Rothermund L, Luckert S, Kossmehl P, Paul M, Kreutz R. Renal endothelin ET(A)/ET(B) receptor imbalance differentiates salt-sensitive from salt-resistant spontaneous hypertension. Hypertension. 2001;37:275–280. doi: 10.1161/01.hyp.37.2.275. [DOI] [PubMed] [Google Scholar]
- 45.Webb CM, Ghatei MA, McNeill JG, Collins P. 17beta-estradiol decreases endothelin-1 levels in the coronary circulation of postmenopausal women with coronary artery disease. Circulation. 2000;102:1617–1622. doi: 10.1161/01.cir.102.14.1617. [DOI] [PubMed] [Google Scholar]
- 46.Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB. Alzheimer’s disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem. 2003;278:2081–2084. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- 47.Attina T, Camidge R, Newby DE, Webb DJ. Endothelin antagonism in pulmonary hypertension, heart failure, and beyond. Heart. 2005;91:825–831. doi: 10.1136/hrt.2004.053991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.