Abstract
Background
African Americans (AA) have a greater post-glucose-challenge insulin response than European Americans (EA). Factors underlying this response are unknown.
Objective
To determine the insulin, C-peptide, and incretin responses to a mixed macronutrient meal in AA and EA children. We hypothesized that: 1) AA would have greater postprandial insulin and C-peptide responses; 2) AA would have higher incretin responses; 3) the greater β-cell response among AA would be explained by greater incretin responses.
Design
Subjects were 34 AA and 18 EA children. Glucose, insulin, C-peptide, glucagon-like peptide-1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP) were measured after consumption of a liquid mixed meal. Insulin, C-peptide, and incretin responses were derived from the area-under-the curve (AUC) for minutes 0-30 (“early response”) and minutes 30-180 (“late response”) following meal ingestion
Results
The early insulin response was higher in AA (14,565 ±6,840 pmol/L × 30min) vs. EA (7,450 ±4,077 pmol/L × 30min, P<0.001). Early C-peptide AUC did not differ by ethnicity (34.8 ±12.5 vs. 28.6 ±12.5nmol/L ×30mins, for AA and EA, respectively; P=0.10). Early and late GLP-1 responses were lower in AA vs EA (108.1 ±56.4 vs. 160.5 ±90.8pmol/L ×30mins) and (509.4 ±286.9 vs. 781.9 ±483.4pmol/L ×150mins), respectively (P<0.05 for both). The GIP response did not differ between groups.
Conclusion
Greater early insulin response in AA vs. EA is not due to differences in circulating GLP-1 or GIP, and may be due to lesser insulin clearance. Further research is needed to determine the physiologic implications of lower GLP-1 among AA.
INTRODUCTION
African American (AA) children and adults have significantly greater risk for Type 2 diabetes (T2D) compared to their European American (EA) counterparts (1;2). The cause of this risk disparity is incompletely understood. Recent findings indicate that these risk differences are not completely accounted for by differences in obesity, diet, physical activity, and other environmental risk factors (3). Hence, biologic factors may make an important contribution.
Numerous well-controlled studies have documented differences in glucose metabolism between AA and EA. Most prominent of these differences is the higher insulin response of AA (4). The higher insulin responses of AA (5-7) have been found to be independent of differences in insulin sensitivity, body fat, diet, and other lifestyle variables (7, 8). It has been suggested that this response may predispose AA to greater T2D risk relative to EA (8, 9). Hence, it will be important to fully characterize and determine the factors underlying this phenomenon.
To date, the majority of studies designed to investigate and characterize higher postchallenge insulin among AA vs. EA have utilized only intravenous or oral glucose tolerance tests (9-12). Although protocols involving intravenous administration of glucose provide robust models for assessing insulin dynamics, such tests do not reflect the normal physiology of food intake and subsequent metabolic responses (13). In particular, the contribution of the entero-insular axis to insulin dynamics is not reflected in the response to an intravenous glucose challenge. In addition, the role of nutrients other than glucose, are not assessed when an oral glucose tolerance test is administered. Hence, characterization of the postchallenge insulin response under physiologic conditions is warranted.
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are important determinants of postprandial insulin secretion. Combined they are considered the principal components of the endocrine portion of the entero-insular axis (14;15). To date, few investigations have quantified incretin responses in AA and EA. Obese AA adults were found to have significantly higher fasting and postchallenge GLP-1 concentrations than EA adults (16), however, recent findings in obese adolescents seem to contradict these findings (17).
The purpose of this study was to characterize the insulin, C-peptide, and incretin responses to a mixed macronutrient test meal in a group of AA and EA children. As C-peptide and insulin are released in equimolar concentrations and, unlike insulin, hepatic C-peptide clearance is negligible– peripheral vein C-peptide concentrations reflect prehepatic insulin secretion, i.e. β-cell response (13). In addition, insulin and C-peptide concentrations, when interpreted simultaneously, are indicative of hepatic insulin clearance. Thus, we tested the hypotheses that: 1) AA would have greater postprandial insulin and C-peptide responses; 2) AA would have higher incretin responses; 3) the greater β-cell response among AA would be explained by greater incretin responses.
RESEARCH DESIGN AND METHODS
Subjects
Fifty-two children were recruited from the Birmingham, Alabama region through flyers, local media advertisement, public health fairs, and word-of mouth. Children were between 7 and 12 years of age, were free of major illness, were not taking any prescription medications known to affect glucose metabolism or body composition, and had normal glucose tolerance (2-hr oral glucose tolerance test) on entry into the study. The Institutional Review Board of the University of Alabama at Birmingham (UAB) approved all procedures. Parents and children provided written informed consent and assent, respectively, before entry to the study. Parental and child ethnicity was assigned according to parental report. Each child classified as AA or EA had both parents classified as AA or EA, respectively. Based on adult data (16), a sample size of n=50 provided 80% power to detect an ethnic difference in GLP-1 at a two-tailed α-level of 0.05.
Study Design
Study design was cross-sectional and observational. Data were collected during an overnight in-patient visit to the General Clinical Research Center (GCRC) at UAB. All children arrived between 1600h and 1800h. Each child underwent a physical examination, including pubertal assessment by an experienced pediatrician according to the criteria of Marshall and Tanner (18, 19). Children received a standard meal (hamburger and fried potatoes), which they consumed by 2000h. Each child and parent was assigned a room and instructed to minimize physical activity. Children did not receive or consume any caloric or caffeinated beverages or snacks after 2000h. In the morning each child underwent a mixed meal tolerance test (MMTT) which was administered at approximately 0700. After completion of the MMTT, children and parents were escorted to the Webb Nutrition Sciences building for body composition analysis. Physical activity and dietary questionnaires were administered during this visit.
Mixed meal tolerance test
A flexible intravenous catheter was placed in the antecubital space of the left arm. Two blood samples were taken over a 15-min period for determination of basal glucose and insulin (the average of the values is used for basal “fasting” concentrations). At time “0”, a liquid meal was administered (Ensure, (Ross Laboratories, IL, USA; 250 kcal; 6g fat, 40g carbohydrate, and 9g of protein). Children were instructed to consume the meal within 5 minutes of administration. Blood was drawn at baseline and at 5, 10, 15, 20, 25, 30, 45, 60, 90, 120, 150, and 180 minutes after the start of meal ingestion. Dipeptidyl peptidase IV (DPPIV) inhibitor (Linco Research, St. Charles, MO; 10μL/ml) was added to all blood samples at collection to inhibit incretin degradation. Plasma samples were stored at -85°C until assay. For data analysis, postprandial responses were determined as the area under the curve (AUC) using the trapezoidal method (20). Early responses were defined AUC for the first 30 minutes, and late phase responses as the AUC for the last 150 minutes post challenge (21).
Body Composition
Total body composition (fat and lean mass) was determined using a Lunar Prodigy densitometer (GE/Lunar Radiation Corp., Madison, WI). Subjects were scanned in the supine position with hands placed at their sides. Height was measured to the nearest centimeter using a wall-mounted stadiometer and body weight was measured on an electronic scale while children wore light clothing.
Assays
Glucose was assayed using the glucose oxidase method on a Sirrus analyzer (Stanbio, Boerne, TX). Insulin was assayed in duplicate 100μl aliquots by radioimmunoassay (Linco Research, St. Charles, MO). In our laboratory, this assay has a sensitivity of 3.35 μIU/ml, a mean intra-assay CV of 3.49%, and a mean interassay CV of 5.57%. C-peptide was assayed in duplicate 25μl aliquots with a double antibody radioimmunoassay (Diagnostic Products Corp., Los Angeles. CA). In our laboratory this assay has a sensitivity of 0.318ng/mL, a mean intraassay CV of 3.57%, and a mean interassay CV of 5.59%. Intact GLP-1 was analyzed in duplicate in 100μl aliquots using ELISA (Linco Research, St. Charles, MO). This ELISA is highly specific for the immunologic measurement of the active forms of GLP-1 (7-36 amide and 7-37). In our laboratory, this assay has a sensitivity of 2 pmol/L, a mean intraassay CV of 7.4%, and a mean interassay CV of 10.7%. Total GIP was analyzed in duplicate 20μl aliquots by ELISA (Linco Research, St. Charles, MO). In our laboratory, this assay has a sensitivity of 8.2 pg/mL, a mean intraassay CV of 4.5%, and a mean interassay CV of 7.2%. The antibody has 100% cross reactivity with both forms of GIP (1-42 and 3-42).
Dietary intake and physical activity assessment
24-hour dietary recalls were administered by trained technicians according to the multiple pass method, as previously described (22), on the evening of the overnight visit. Recall data were entered into the Nutrition System for Research (v5, University of Minnesota) for determination of daily macronutrient intake. The physical activity questionnaire for children (PAQ-C) was developed by the Centers for Disease Control, and is designed to assess physical activity in the week prior to interview (23, 24). This questionnaire was administered on the evening of the overnight visit. The scoring range is 1 to 5, 5 being highest physical activity level.
Statistical Analyses
Two-way ANOVA was used to assess potential effects of ethnicity and gender on the continuously distributed variables analyzed. The Mann-Whitney U-test was used to determine differences in the categorical variables “Tanner stage.” Independent samples t-tests were used to compare postprandial responses between AA and EA. Relationships between insulin and C-peptide and incretin responses were assessed using Pearson correlation analysis. Multiple linear regression analyses were used to determine whether ethnicity was independently related to insulin and C-peptide responses after adjusting for glucose and incretin responses. Variable distributions that deviated from normal were log transformed prior to analysis. Statistical significance was set at P<0.05. Data were analyzed using SPSS version 10.0 (SPSS Inc., Chicago, IL). Power calculations were performed using PowPal5 software (Gorman, Primavera, & Allison, 1993).
RESULTS
Descriptive data
Descriptive data are presented in Table 1. AA and EA did not differ with respect to age, body composition, dietary intake, or physical activity. AA children were more mature. Fasting concentrations of glucose, C-peptide, insulin, and incretin peptides did not differ between groups. Similarly, groups did not differ in their mean intake of total energy or macronutrients, or in their levels of physical activity (Table 1). There were no differences in gender distribution between groups (P=0.30). Neither gender nor the gender-by-ethnicity interaction was significantly related to any of the variables described in Table 1.
Table 1.
Descriptive data.
| AA (n=34) | EA (n=18) | P | |
|---|---|---|---|
| Sex (male / female)* | 22 / 12 | 11 / 7 | 0.74 |
| Tanner stage (I / II / III)* | 20 / 9 / 5 | 14 / 4 / 0 | 0.03 |
| Age (yrs.) | 9.9 ±1.3 | 9.9 ±1.9 | 0.86 |
| Weight (kg) | 38.5 ±9.9 | 40.9 ±13.0 | 0.47 |
| Body mass index (kg/m2) | 19.0 ±3.7 | 19.7 ±4.3 | 0.57 |
| Fat mass (kg) | 9.4 ±7.1 | 12.7 ±7.9 | 0.14 |
| Lean mass (kg) | 27.3 ±5.0 | 25.8 ±6.0 | 0.34 |
| Fasting glucose (mmol/L) | 5.1 ±0.3 | 5.1 ±0.4 | 0.78 |
| Fasting C-peptide (nmol/L) | 0.4 ±0.2 | 0.5 ±0.2 | 0.77 |
| Fasting insulin (pmol/L) | 86.2 ±37.2 | 68.3 ±32.7 | 0.09 |
| Fasting GLP-1 (pmol/L) | 2.9 ±1.9 | 3.9 ±2.9 | 0.08 |
| Fasting GIP (pg/mL) | 46.5 ±27.4 | 49.1 ±31.3 | 0.98 |
| Energy intake (Kcal/day) | 1729 ±551 | 1878 ±483 | 0.29 |
| Total carbohydate intake (g/day) | 218 ±80 | 242 ±68 | 0.23 |
| Total sugar intake (g/day) | 107 ±57 | 114 ±36 | 0.57 |
| Total fat intake (g/day) | 70 ±29 | 73 ±23 | 0.63 |
| Total protein intake (g/day) | 63 ±19 | 69 ±22 | 0.28 |
| Physical activity score† | 2.7 ±0.9 | 2.8±0.6 | 0.43 |
P-value is for ethnic difference by two-way ANOVA. Gender and gender × ethnicity effects were not detected.
Ethnic differences in gender and tanner stage distributions were determined by Mann Whitney U-test.
Postprandial responses
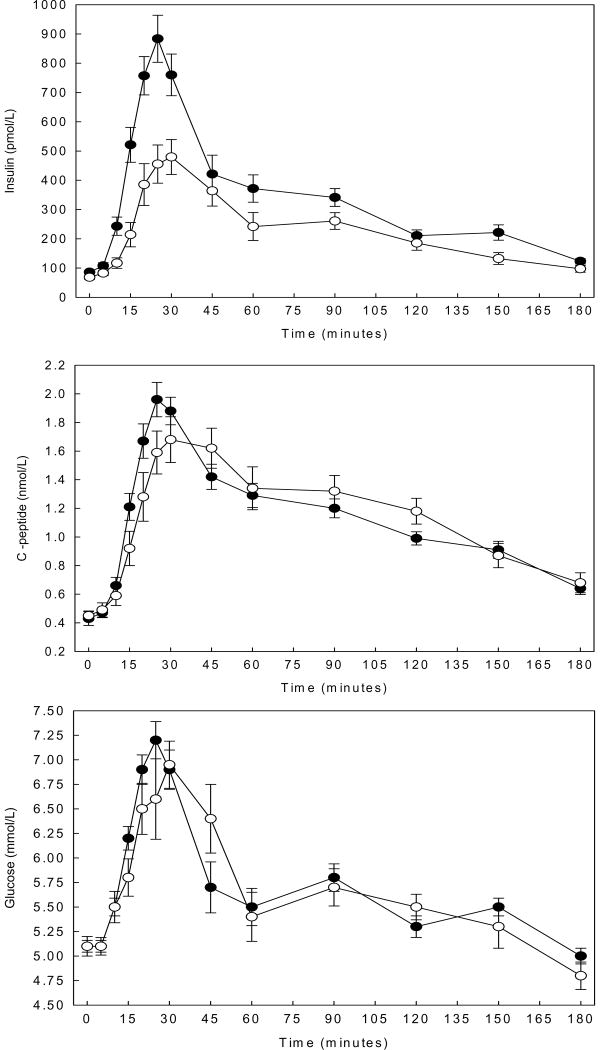
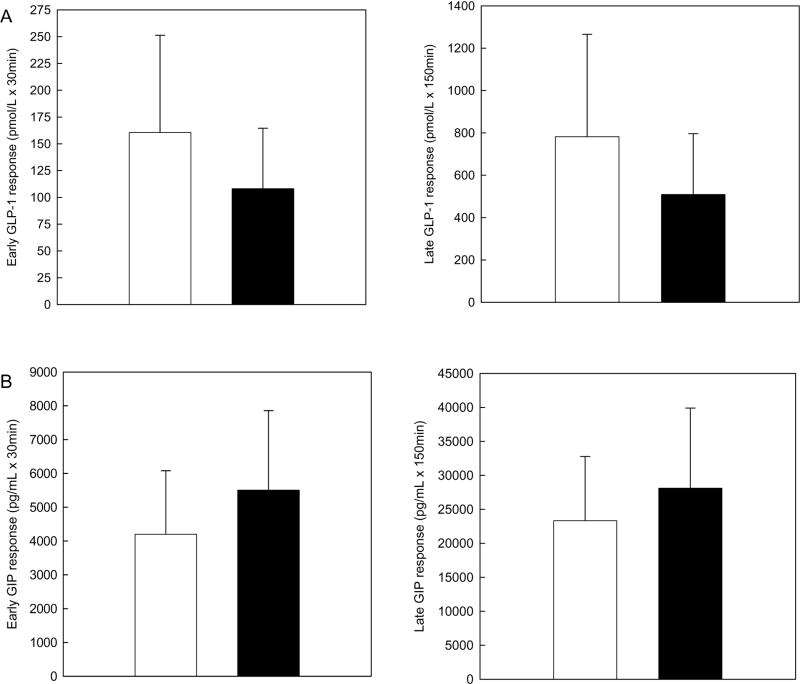
Postprandial insulin, C-peptide, and glucose responses are depicted in Figure 1. Mean early insulin response was approximately two-fold higher in AA compared to EA (14565 ±6840 vs. 7450 ±4077pmol/L ×30mins, P<0.01). The early insulin response remained higher in AA children after adjustment for the early glucose response (P<0.01). The early C-peptide response did not differ between AA and EA prior to (34.8 ±12.5 vs. 28.6 ±12.5nmol/L ×30mins, P=0.10) and following adjustment for the early glucose response (P=0.28). Late insulin (41140 ±18000 vs. 38947 ±20180pmol/L x150mins, P=0.69), late C-peptide (166.6 ±38.9 vs. 179.7 ±58.4nmol/L ×150mins, P=0.34), and early (172.3 ±17.4 vs. 164.4 ±23.1mmol/L ×30mins, P=0.17) and late (744.2 ±89.1 vs. 757.4 ±74.2mmol/L ×150mins, P=0.59) glucose responses did not differ by ethnicity. Early and late postprandial incretin responses are depicted in Figure 2. Mean early (108.1±56.4 vs. 160.5±90.8pmol/L ×30mins, P=0.03) and late (509.4 ±286.9 vs. 781.9±483.4pmol/L ×150mins, P=0.04) GLP-1 responses were significantly lower in the AA vs. EA children. Early (5505.9±2350.3 vs. 4203.5±1876pg/mL × 30mins, P=0.12) and late (28107 ±11796 vs. 23334 ±9447pg/mL × 150mins, P=0.17) GIP responses did not differ between groups.
Figure 1.
Insulin, C-peptide, and glucose responses in African American (AA) and European American (EA) children following ingestion of a mixed meal. Data are means ±SE, ●AA n=34 ○EA n=18. “Early response” is defined as the area-under-the curve (AUC) for minutes 0-30 and “late response” as the AUC for minutes 30-180 post challenge. Means were compared by independent samples t-tests.
Panel A. Insulin response. AA had a higher mean early insulin response (P<0.001). AA and EA children did not differ in late insulin response.
Panel B. C-peptide response. Mean early and late C-peptide response did not differ in AA and EA children.
Panel C. Glucose response. Mean early and late glucose response did not differ in AA and EA children.
Figure 2.
Incretin responses in African American (AA) and European American (EA) children following ingestion of a mixed meal. Data are means ±SD, ■AA n=34 □EA n=18. “Early response” is defined as the area-under-the curve (AUC) for minutes 0-30 and “late response” as the AUC for minutes 30-180 post challenge. Means were compared by independent samples t-tests.
Panel A. *AA had lower early (P=0.03) and lower late (P=0.04) glucagon-like peptide-1 (GLP-1) responses compared to EA.
Panel B. Mean early (P=0.12) and late (P=0.17) glucose-dependent insulinotropic polypeptide (GIP) responses did not differ in AA and EA children.
Associations among postprandial incretin, C-peptide, and insulin responses
The early GLP-1 response was not correlated with the early insulin (r = -0.16, P=0.33) or early C-peptide (r = -0.13, P=0.36) responses. In contrast, the early GIP response was significantly related to the early insulin response (r=0.54, P<0.001) and the early C-peptide response (r=0.56, P<0.001). The late GLP-1 and GIP responses were not associated with the late insulin or C-peptide responses (data not shown). These relationships were similar when the data were analyzed within each ethnic group. In multiple regression analyses, ethnicity remained a significant predictor of EIR after adjustment for early glucose and early GIP responses (Table 2). Only the early GIP and glucose responses were found to be significant predictors of the early C-peptide response (Table 2).
Table 2.
Results from multiple linear regression analyses for the dependent variables early insulin response (a) and early C-peptide response (b), n=52.
| a. Dependent variable: early insulin response. R2=0.48. | |||
|---|---|---|---|
| B | SE | P-value | |
| Model | -5.09 | 2.03 | 0.016 |
| Ethnicity | 0.19 | 0.06 | 0.004 |
| Early glucose response | 2.03 | 0.61 | 0.002 |
| Early GIP response | 0.46 | 0.14 | 0.002 |
| b. Dependent variable: early C-peptide response. R2=0.46. | |||
| Model | -5.02 | 1.4 | 0.001 |
| Ethnicity | 0.003 | 0.05 | 0.95 |
| Early glucose response | 1.63 | 0.43 | <0.001 |
| Early GIP response | 0.36 | 0.10 | 0.001 |
All variables were log transformed. Ethnicity was entered as a categorical variable: 1 = European American; 2 = African American. Early response is defined as the area under the curve for minutes 0-30 post challenge. Model a: R2 excluding ethnicity = 0.39. Model b: R2 excluding ethnicity = 0.48.
DISCUSSION
The purpose of this study was to determine the insulin, C-peptide, and incretin responses to a mixed macronutrient test meal in a group of AA and EA children. We tested the specific hypotheses that: 1) AA would have greater postprandial insulin and C-peptide responses; 2) AA would have higher incretin responses; 3) the greater β-cell response among AA would be explained by greater incretin responses. As expected, we found that AA children had a significantly higher early insulin response; however, early and late C-peptide responses did not differ statistically by ethnicity. In contrast to our hypothesis, the postprandial GLP-1 response was lower in AA, and was not associated with the early postprandial insulin response. Notably, GIP was significantly positively associated with the early insulin response but did not differ significantly by ethnicity. Taken together, these results suggest that incretins do not explain greater postchallenge insulin among AA vs. EA, and suggest that greater postchallenge insulin among AA is due to lesser insulin clearance.
Our data demonstrated that AA had greater postprandial insulin responses than EA of similar age, body composition, dietary intake, and physical activity. These results agree with those of previous studies that have documented greater first phase or acute insulin responses to intravenous glucose in AA and EA (6, 7, 9-11). Insulin responses quantified from the peripheral circulation reflect both the β-cell response and the extent of hepatic insulin clearance. Hence, it is important to also consider C-peptide concentrations; insulin and C-peptide are secreted in equimolar concentrations but hepatic C-peptide clearance is negligible relative to that of insulin (13). In contrast to findings from intravenous glucose challenge tests, with the meal test we did not find greater post-challenge C-peptide concentrations in AA children. Hence, our data suggest that, following a mixed macronutrient test meal, a greater β-cell response is not the primary cause of higher insulin responses in AA. Therefore, the greater insulin response of AA vs. EA to a meal stimulus is likely due to lesser hepatic insulin clearance.
Differences among studies in whether an ethnic-specific C-peptide response is detected may be due to the stimulus employed; i.e. oral meal test vs. intravenous glucose test (13). Specifically, the rate at which the β-cells are exposed to nutrients and the dynamics of the secretory response differ with test type (13). Thus, intravenous and oral tests are likely to capture unique aspects of the insulin response. From a cellular perspective, the immediate release of previously docked insulin secretary granules is probably responsible for the higher β-cell response observed in AA in the first few minutes following an intravenous glucose challenge (10, 13). However, rapid depletion of these immediately available granules and subsequent reliance upon granule movement and other processes during the extended early phase following the meal test, may impair the ability to detect the acute C-peptide response. Thus, we propose that the higher C-peptide or β-cell response of AA vs. EA is apparent only during the acute response to a challenge, which is most easily observed during an intravenous glucose tolerance test. Importantly, our meal test data are compatible with previous data showing that C-peptide concentrations following oral glucose did not differ among adult and adolescent AA and EA, despite significantly higher post-challenge insulin concentrations in AA (12, 25). Understanding test-related differences in the assessment of insulin dynamics is of critical importance to future studies designed to address β-cell function, particularly in heterogeneous populations.
Regardless of the physiology underlying the response, compared to EA, AA exhibit greater circulating insulin in the postprandial period. In a recent longitudinal analysis, greater insulin responses in AA children were shown to account for their lower adiponectin concentrations compared to EA children (26), indicating that excessive circulating insulin may indirectly compromise insulin sensitivity. In addition, hyperinsulinemia has been shown to be an independent risk factor for T2D and coronary heart disease (27, 28), and chronic hyperinsulinemia has been shown to directly induce insulin resistance in vitro (29). The potential long-term consequences of postprandial hyperinsulinemia among AA deserve further study.
This is the first study to characterize the major components of the endocrine portion of the entero-insular axis and their potential role in insulin response in healthy AA and EA children. We did not find significant ethnic differences in GIP responses. Unexpectedly, we found that early and late phase GLP-1 responses were lower in AA compared to EA children. Hence, our findings indicate that circulating incretins are unlikely to be responsible for higher postchallenge insulin among AA vs. EA.
Our GLP-1 findings agree those of Velasquez-Mieyer et al. who found that among severely obese adolescents, AA had lower GLP-1 responses to oral glucose (17). Importantly, our results extend these findings to demonstrate for the first time that ethnic differences in GLP-1 are also present in healthy children with normal body weight and normal glucose tolerance. Hence, lower GLP-1 in AA vs. EA children is not likely to be due to obesity or glucose intolerance and may be an inherent metabolic characteristic of this population. Given the many extra-pancreatic effects of GLP-1, including improvements in insulin-mediated glucose uptake, reductions in gastric emptying, and inhibition of food intake (30), lower GLP-1 in AA may predispose to T2D or other metabolic diseases. Clearly, it will be important to determine the contribution, if any, of lower GLP-1 to metabolic disease risk in AA in future studies.
We did not find an association between the postprandial GLP-1 response and the insulin response. This finding appears to conflict with previous findings in which GLP-1 infusion was shown to elicit an increase in insulin response under steady state glycemia (31-36). However, in these infusion studies, the concentrations of GLP-1 achieved were higher than those measured after the meal in the present study. Indeed, our findings are in agreement with data from experiments in humans (37) and in dogs (38) that demonstrated that physiologic concentrations of circulating GLP-1 do not seem to have an effect on insulin secretion. Moreover, a recent study in adults also failed to demonstrate an association between postprandial insulin and GLP-1 responses (39). These findings do not, however, preclude a role for GLP-1 in postprandial insulin secretion. Given the short circulating half-life of GLP-1, it is likely that peripheral venous concentrations of intact GLP-1 do not adequately reflect that which is secreted from the intestinal L-cell. Hence, these concentrations do not reflect local or portal vein peptide concentrations that may indirectly promote insulin secretion (30). In contrast to GLP-1, the early postprandial GIP response was strongly correlated with the early insulin response. Previous studies undertaken in rodents (40) support the notion that GIP is the dominant circulating incretin peptide. Although the AA children in our study had higher GIP responses, these differences were not statistically significant and suggest that GIP is unlikely to contribute to the higher insulin response of AA. Considering both the GIP and GLP-1 data presented, results from this study do not support a major role for circulating incretins in the higher insulin response of AA vs. EA children.
In conclusion, AA children had a higher postprandial early insulin response relative to their EA counterparts. This difference was not explained by differences in circulating concentrations of the incretin peptides, but may have been due in part to lower hepatic insulin clearance. Importantly, we observed that AA had lower GLP-1 than EA. This finding, if confirmed, could have important implications for T2D and/or other metabolic disease prevention and treatment in AA.
Acknowledgments
We acknowledge the support and efforts of Amanda Willig, Alexandra McPherson, Fernando Gomez, Paul Zuckerman, Buddy Sirikul Ph.D., David Bryan, Betty Darnell, Mia Amaya MD, John Paul Clancy M.D., Jamy Ard M.D., and Frank Franklin M.D. We also thank the Nursing, Bionutrition and Metabolic Kitchen staff (Yolanda Guyton, Cluster Boyd, Dena Jackson, Cassandra Thomas, and Karen Thomas; for additional recruitment efforts) and other staff of General Clinical Research Center for their efforts in support of this study. Portions of this study have been published on-line at Proquest/UMI dissertation publishing. PBH was supported in part by a predoctoral fellowship from the American Heart Association and a partial scholarship from the Diabetes Trust Fund of Alabama.
Grant Support: National Institutes of Health grants: R01-DK067426, P30-DK56336, and M01-RR-00032. American Heart Association: 0515149B.
Footnotes
Author contributions: PBH & BAG conceived and designed the study and wrote the manuscript. JRF, WMG, & WTG aided in statistical analyses, data interprtetation, and edited the manuscript.
Conflict of interest statement: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is an uncopyedited author manuscript that has been accepted for publication in The American Journal of Clinical Nutrition, copyright American Society for Nutrition (ASN). This manuscript may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, US Code) without permission of the copyright owner, the ASN. The final copyedited article, which is the version of record, can be found at http://www.ajcn.org/. The ASN disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Pettitt DJ, Jones KL, Arslanian SA. Type 2 diabetes mellitus in minority children and adolescents. An emerging problem. Endocrinol Metab Clin North Am. 1999;28:709–29. viii. doi: 10.1016/s0889-8529(05)70098-0. [DOI] [PubMed] [Google Scholar]
- 3.Shai I, Jiang R, Manson JE, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29:1585–1590. doi: 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- 4.Abate N, Chandalia M. The impact of ethnicity on type 2 diabetes. J Diabetes Complications. 2003;17:39–58. doi: 10.1016/s1056-8727(02)00190-3. [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM, D’Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 6.Arslanian S, Suprasongsin C, Janosky JE. Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab. 1997;82:1923–1927. doi: 10.1210/jcem.82.6.4002. [DOI] [PubMed] [Google Scholar]
- 7.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 8.Ku CY, Gower BA, Hunter GR, Goran MI. Racial differences in insulin secretion and sensitivity in prepubertal children: role of physical fitness and physical activity. Obes Res. 2000;8:506–515. doi: 10.1038/oby.2000.63. [DOI] [PubMed] [Google Scholar]
- 9.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25:2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 10.Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in african-american and caucasian children. J Clin Endocrinol Metab. 2002;87:2218–2224. doi: 10.1210/jcem.87.5.8498. [DOI] [PubMed] [Google Scholar]
- 11.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51:3014–3019. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 12.Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49:571–9. doi: 10.1007/s00125-005-0109-z. [DOI] [PubMed] [Google Scholar]
- 13.Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, Butler P, Rizza R. Assessment of μcell function in humans, simultaneously with insulin sensitivity and hepatic insulin extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293:E1–15. doi: 10.1152/ajpendo.00421.2006. [DOI] [PubMed] [Google Scholar]
- 14.Vilsboll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia. 2004;47:357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 15.Vilsboll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003;114:115–121. doi: 10.1016/s0167-0115(03)00111-3. [DOI] [PubMed] [Google Scholar]
- 16.Velasquez-Mieyer PA, Cowan PA, Umpierrez GE, Lustig RH, Cashion AK, Burghen GA. Racial differences in glucagon-like peptide-1 (GLP-1) concentrations and insulin dynamics during oral glucose tolerance test in obese subjects. Int J Obes Relat Metab Disord. 2003;27:1359–1364. doi: 10.1038/sj.ijo.0802415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velasquez-Mieyer PA, Cowan PA. Perez-Faustinelli S, Nieto-Martinez R, Villegas-Barreto C, Tolley EA, Alpert BS. Racial disparity in glucagon-like peptide 1 and inflammation markers among severely obese adolescents. Diabetes Care. 2008;31:770–5. doi: 10.2337/dc07-1525. [DOI] [PubMed] [Google Scholar]
- 18.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caumo A, Luzi L. First-phase insulin secretion: does it exist in real life? Considerations on shape and function. Am J Physiol Endocrinol Metab. 2004;287:E371–E385. doi: 10.1152/ajpendo.00139.2003. [DOI] [PubMed] [Google Scholar]
- 22.Lindquist CH, Gower BA, Goran MI. Role of dietary factors in ethnic differences in early risk of cardiovascular disease and type 2 diabetes. Am J Clin Nutr. 2000;71:725–732. doi: 10.1093/ajcn/71.3.725. [DOI] [PubMed] [Google Scholar]
- 23.Kowalski KC, Crocker PRE. Validation of the physical activity questionnaire for older children. Pediatr Exerc Sci. 1997;9:174–186. doi: 10.1123/pes.19.1.6. [DOI] [PubMed] [Google Scholar]
- 24.Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exer. 1997;29:1344–1349. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Osei K, Schuster DP, Owusu SK, Amoah AG. Race and ethnicity determine serum insulin and C-peptide concentrations and hepatic insulin extraction and insulin clearance: comparative studies of three populations of West African ancestry and white Americans. Metabolism. 1997;46:53–58. doi: 10.1016/s0026-0495(97)90167-0. [DOI] [PubMed] [Google Scholar]
- 26.Bush NC, Darnell BE, Oster RA, Goran MI, Gower BA. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes. 2005;54:2772–2778. doi: 10.2337/diabetes.54.9.2772. [DOI] [PubMed] [Google Scholar]
- 27.Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49:2094–2101. doi: 10.2337/diabetes.49.12.2094. [DOI] [PubMed] [Google Scholar]
- 28.Despres JP, Lamarche B, Mauriege P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 29.Yuan L, Ziegler R, Hamann A. Chronic hyperinsulinism induced down-regulation of insulin post-receptor signaling transduction in Hep G2 cells. J Huazhong Univ Sci Technolog Med Sci. 2002;22:313–316. doi: 10.1007/BF02896773. [DOI] [PubMed] [Google Scholar]
- 30.Drucker DJ. The biology of incretin hormones. Cell Metabolism. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 32.Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Insulinotropic action of glucagonlike peptide-I-(7-37) in diabetic and nondiabetic subjects. Diabetes Care. 1992;15:270–276. doi: 10.2337/diacare.15.2.270. [DOI] [PubMed] [Google Scholar]
- 33.Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 34.Schirra J, Leicht P, Hildebrand P, et al. Mechanisms of the antidiabetic action of subcutaneous glucagon-like peptide-1(7-36)amide in non-insulin dependent diabetes mellitus. J Endocrinol. 1998;156:177–186. doi: 10.1677/joe.0.1560177. [DOI] [PubMed] [Google Scholar]
- 35.Elahi D, McAloon-Dyke M, Fukagawa NK, et al. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul Pept. 1994;51:63–74. doi: 10.1016/0167-0115(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 36.Vahl TP, Paty BW, Fuller BD, Prigeon RL, D’Alessio DA. Effects of GLP-1-(7-36)NH2, GLP-1-(7-37), and GLP-1- (9-36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab. 2003;88:1772–1779. doi: 10.1210/jc.2002-021479. [DOI] [PubMed] [Google Scholar]
- 37.Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 38.Ionut V, Liberty IF, Hucking K, et al. Exogenously imposed postprandial-like rises in systemic glucose and GLP-1 do not produce an incretin effect, suggesting an indirect mechanism of GLP-1 action. Am J Physiol Endocrinol Metab. 2006;291:E779–E785. doi: 10.1152/ajpendo.00106.2005. [DOI] [PubMed] [Google Scholar]
- 39.Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose intolerance. Diabetes. 2008;57:678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 40.Gault VA, O’Harte FP, Harriott P, Mooney MH, Green BD, Flatt PR. Effects of the novel (Pro3)GIP antagonist and exendin(9-39)amide on GIP- and GLP-1-induced cyclic AMP generation, insulin secretion and postprandial insulin release in obese diabetic (ob/ob) mice: evidence that GIP is the major physiological incretin. Diabetologia. 2003;46:222–30. doi: 10.1007/s00125-002-1028-x. [DOI] [PubMed] [Google Scholar]