SUMMARY
The myeloperoxidase (MPO)-derived oxidant hypochlorous acid (HOCl/OCl−) is implicated in the pathogenesis of atherosclerosis and other inflammatory states. We have synthesized a novel imaging probe, sulfonaphthoaminophenyl fluorescein (SNAPF), that selectively reacts to HOCl. SNAPF detects HOCl produced by stimulated MPO-expressing cells cultured from human whole blood, as well as HOCl from bone marrow (BM)-derived macrophages isolated from transgenic mice that express human MPO. Two lines of evidence indicate that SNAPF permits the in vivo imaging of HOCl production. First, we used this approach to demonstrate HOCl production by neutrophils in experimental murine peritonitis. Second, we detected HOCl production by MPO expressing cells in human atherosclerotic arteries. Thus, fluorescence reflectance imaging by SNAPF may provide a valuable non-invasive molecular imaging tool for implicating HOCl and MPO in the damage of inflamed tissues.
INTRODUCTION
Oxidative stress likely participates in the pathogenesis of diseases as diverse as cancer [1], those that affect the lungs [2] and skin disease [3]. Oxidative processes in tissues can involve Fenton reactions, in which free metal ions (most commonly Fe2+) interact with peroxide to yield oxidizing products such as the hydroxyl radical and superoxide [4,5,6]. An alternative pathway for the production of oxidizing agents, independent of Fenton chemistry, involves the action of myeloperoxidase (MPO). MPO is a heme enzyme secreted in large amounts by activated neutrophils along with superoxide. Superoxide dismutates spontaneously or enzymatically to hydrogen peroxide, a relatively unreactive oxidant, which is then used as a substrate by MPO to produce hypochlorous acid (HOCl) [7]. With a pKa of 7.463 at 35°[8], under physiological conditions, approximately half of the HOCl dissociates to the hypochlorite anion (OCl−), one of the most powerful natural oxidants. Hypochlorite likely functions to injure microorganisms oxidatively, however it can also have detrimental effects on host tissue. Neutrophil derived HOCl has been implicated in lung injury [9,10,11], rheumatoid arthritis [12,13], hepatic ischemia-reperfusion injury [14] and renal disease [15,16] amongst others.
Oxidative stress within the vasculature plays multiple roles in the development of atherosclerosis, particularly with regard to oxidative modifications of lipoproteins [17]. Monocytes and neutrophils, cells which do not accumulate appreciably in atherosclerotic plaques, typically contain high levels of MPO. Yet, a subset of monocyte-derived macrophages in human atheromatous lesions retain MPO [18]. These MPO+ macrophages colocalize with hypochlorite-modified proteins [19]. Furthermore, human atherosclerotic plaques harbor 3-chlorotyrosine, a product of MPO-mediated reactions [20]. Conversely, murine macrophages from sites of atherosclerotic plaques do not contain immunoreactive MPO or 3-chlorotyrosine [21], making mice an inappropriate model for investigating the role of macrophage-derived MPO in the pathogenesis of disease. This limitation has recently been circumvented by the creation of transgenic mice that express human MPO, h-MPOTg [22]. Irradiated LDL receptor deficient (LDLR−/−) animals receiving bone marrow from transgenic animals develop accelerated atherosclerosis during hypercholesterolemia when compared to those with wild type donors. MPO resides within plaques from the transgenic, but not wild type, BM recipients. Moreover, elevated levels of plasma MPO independently predict risk for coronary events and myocardial infarction in clinical studies [23, 24, 25].
Within the context of cardiovascular disease, the effects of HOCl on tissue are numerous. For example, hypochlorite can cause endothelial cell (EC) apoptosis, a process during which the dying ECs release tissue factors that may contribute to thrombosis [26]. Hypochlorite can derivatize both low- and high- density lipoproteins. Along with numerous other oxidants, it oxidizes LDL to OxLDL, a pro-atherogenic form that readily enters macrophages, thus promoting foam cell formation in early lesion development [27, 28, 29]. Oxidized LDL has chemotactic activity [30, 31, 32] and is immunogenic [33, 34]. Hypochlorite oxidizes tyrosine 192 on apo-A1, the major protein of HDL [35]. This oxidation impairs cholesterol transport via the cell membrane transporter ATP binding cassette transporter A1 (ABCA-1) [36, 37].
To study the contribution of oxidative species to disease processes, a number of fluorescent probes have been developed. Most detect multiple reactive oxygen species (ROS), and can be prone to auto-oxidation. It is likely that different ROS play distinct roles in specific pathological processes, hence methods for selective detection of specific ROS are desirable. To this end, Setsukinai et al [38] developed two novel auto-oxidation resistant fluoroscein-based probes, 2-[6-(4′-hydroxy)phenoxy-3H-xanthen-3-on-9-yl]benzoic acid (APF) and 2-[6-(4′-amino)phenoxy-3H-xanthen-3-on-9-yl]benzoic acid (HPF), which detect only the highly reactive oxygen species (hROS) hydroxyl radical (OH.), peroxynitrite (ONOO−), and HOCl. Furthermore, because APF but not HPF detects HOCl, use of the two probes in tandem might distinguish HOCl presence from other hROS.
Modification of the APF probe to render it detectable with longer emission wavelengths would increase its suitability for bioimaging, since lipids and hemoglobin within normal tissue absorb light within the visible light range (which includes the emission spectrum of fluorescein, ~520 nm). In particular, elastin produces strong green autofluorescence in arteries, rendering probes that emit in the green range unsuitable for atherosclerosis research. The absorption coefficient of tissue components is lowest in the far red/near infra red (NIR) range from approximately 650–900 nm [39]. In this work development of a new water-soluble probe for hypochlorite, SNAPF, which has maximal excitation and emission wavelengths of 614 and 676 nm, respectively, is reported.
The current study investigated the utility of SNAPF as a sensor for the detection of HOCl-generating MPO positive cells, and thus probable oxidative stress-related damage, in inflammatory states. We demonstrate that SNAPF detects HOCl formation after stimulation of human neutrophils, MPO-containing human macrophages, and transgenic mouse macrophages that express human MPO. In addition, we demonstrate the ability of SNAPF to detect HOCl generation in vivo, and consequently to potentially directly implicate HOCl-mediated tissue injury in inflammation in a non-invasive manner, using fluorescence reflectance imaging (FRI).
RESULTS
SNAPF Probe Synthesis and Characterization
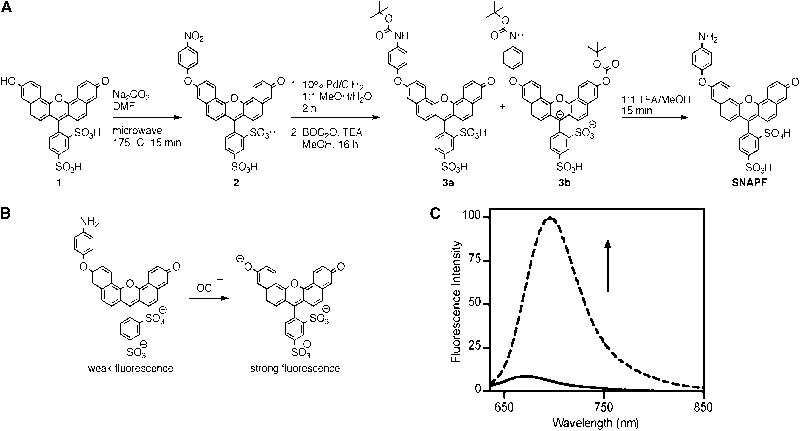
In an effort to prepare a water-soluble far-red or NIR probe for hypochlorite, a xanthene based dye scaffold was chosen. This allowed for the preparation of red-shifted hypochlorite sensors based on an activation strategy similar to that reported for APF, where oxidative cleavage of a 4-aminophenylether modified fluorescein, which has quenched fluorescence, results in generation of free fluorescein with strong fluorescence emission. SNAPF is prepared in a three-step process from sulfonaphthofluorescein, 1, as detailed in Fig. 1a. The 4-nitrophenyl modified intermediate 2 was prepared in moderate yield by nucleophilic attack of dye 1 on 1-fluoro-4-nitrobenzene in the presence of sodium carbonate. The nitro group of 2 is then reduced with hydrogen gas in the presence of palladium on carbon catalyst to yield crude SNAPF. During the course of this reduction, a significant amount of free dye 1 is generated. Dye 1 and SNAPF have nearly identical retention times by HPLC and cannot be resolved easily. To facilitate isolation of pure SNAPF, the crude reduction product was protected with BOC anhydride. This enabled easy separation of the mono- and di-BOC functionalized SNAPF derivatives, 3a and 3b, respectively, by preparative HPLC. Purified SNAPF is then isolated by treatment of 3a and 3b with a methanolic solution of TFA.
Figure 1.
Synthesis of SNAPF. a) Synthesis of SNAPF. b) Activation of SNAPF after exposure to excess HOCl at room temperature for 5 min. c) Fluorescence emission traces, exciting with 625 nm light, of SNAPF before (solid line) and after (dashed line) exposure to excess sodium hypochlorite in PBS, pH 7.4.
As with APF, the reaction of SNAPF with strong oxidants results in oxidative cleavage of the 4-aminophenyl moiety, generating highly fluorescent free dye 1 (Fig. 1b). Treatment of SNAPF with excess HOCl in PBS results in a greater than 8-fold increase in fluorescence emission (Fig. 1c), which corresponds to generation of 1. Once generated, deprotonated dye 1 has an absorption maximum at 614 nm and emission at 676 nm [40].
SNAPF Detects Chemically and Enzymatically Generated HOCl in vitro
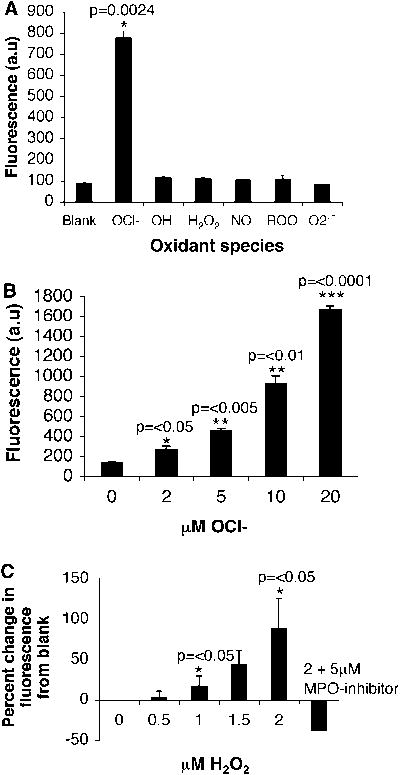
To establish which oxidative species SNAPF reports, we exposed 10 μM SNAPF solutions to a range of chemically-generated oxidants (Fig. 2a). Of the oxidants tested, a significant increase in fluorescence occurs only with HOCl, indicating the selectivity of SNAPF for hypochlorite. In addition, the fluorescence emission of activated SNAPF after exposure to HOCl increases in a concentration-dependent manner (Fig. 2b).
Figure 2.
Fluorescence response of SNAPF after activation. a) SNAPF selectively detects HOCl when reacted against a panel of biological oxidants. All oxidants are 100 μM except HOCl, which is 10 μM and were generated as described previously (38) (n=3). Briefly, b) Increasing concentrations of native HOCl result in an increase in SNAPF generated fluorescence (n=3). c) Purified MPO, 11.2 nM, generates increasing amounts of SNAPF-detectable HOCl when higher amounts of H2O2 are added as substrate. Fluorescence generated by addition of 2 μM H2O2 is ablated by co-incubation of MPO with a selective MPO inhibitor at a final concentration of 5 μM (n=3).
SNAPF can also detect HOCl generated by purified MPO, after incubation with the enzyme at 37°C for 30 minutes. As expected, the probe (at 10 μM) does not detect generation of HOCl in this system in the absence of the required MPO substrate, H2O2 (Fig. 2c). Pre-incubation of the enzyme with 5μM MPO inhibitor (4-aminobenzoic acid hydrazide) before the addition of 2 μM H2O2, ablated signal generation, although the inhibitor is not effective when added after the peroxide, presumably due to the high rate of HOCl generation. The MPO inhibitor similarly affected detection of HOCl by APF (data not shown).
SNAPF Detects HOCl Generated By Stimulated Human Neutrophils
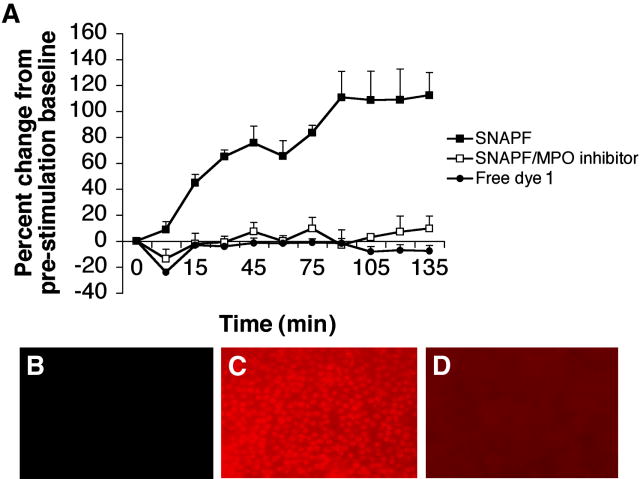
Since MPO is expressed at high levels by neutrophils, we wished to establish first whether SNAPF could detect HOCl generation by these cells following activation with PMA. Freshly isolated neutrophils incubated with 10 μM SNAPF (or SNAPF + 2 μM MPO inhibitor) were stimulated with PMA at a concentration of 400 nM in the buffer and monitored by fluorescence spectrophotometry. The fluorescent signal from the activated probe rapidly increased following cell stimulation, and reached a peak around 90 minutes post-stimulation after which it appeared to remain stable (Fig. 3a). The fluorescence increase quantified with the plate reader translated well to fluorescence microscopy; neutrophils incubated with SNAPF were visibly brighter following PMA stimulation (Fig. 3b, 3c). In both quantitative and qualitative data sets, co-incubation of the neutrophils with MPO inhibitor ablated the fluorescence of SNAPF following cell stimulation (Fig. 3a, 3d). Co-incubation with MPO inhibitor at a final concentration of 2 μM was found to be sufficient to diminish the fluorescent signal.
Figure 3.
SNAPF detects HOCl generated by stimulated human neutrophils. SNAPF, co-incubated with neutrophils isolated from fresh venous blood, fluoresces after stimulation of the neutrophils with 400 nM PMA. The fluorescence emission is quantifiable on a plate reader (a) and the increase in fluorescence is sufficient to permit imaging using fluorescence microscopy (b–d). Neutrophils coincubated with SNAPF pre (b) and post (c) stimulation with PMA. The increase in fluorescence post stimulation is attenuated by coincubation with 2 μM MPO inhibitor (a & d, n=4).
SNAPF Detects HOCl Generated From Stimulated Murine Macrophages Transgenic for Human MPO
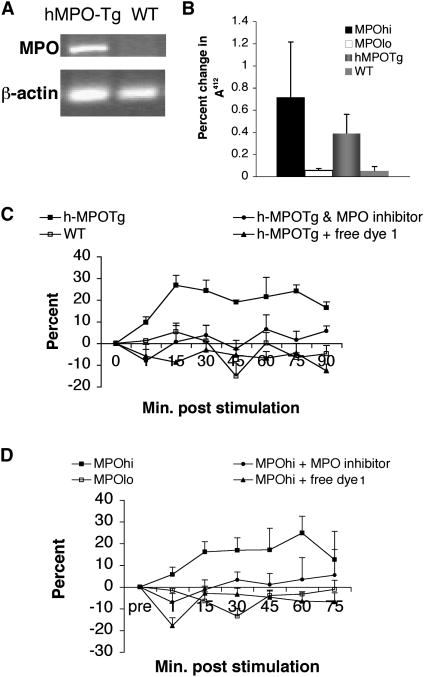
As noted above, mouse macrophages do not express MPO. Therefore, to examine whether SNAPF can detect HOCl generated by stimulation of MPO-containing macrophages, we first isolated and cultured bone marrow-derived macrophages, using standard techniques, from mice transgenic for human MPO with age-matched wild type mice as controls. Reverse transcriptase amplification of cDNA reveals transcription of the hMPO gene in h-MPOTg, but not WT, mice (Fig. 4a). The translated protein is enzymatically active (Fig. 4b). Fluorescence readings of stimulated h-MPOTg and WT BM-derived macrophages co-incubated with probe taken over a 90-minute time period show a ~25% increase in h-MPOTg cell fluorescence which peaks around 15 minutes post stimulation, and reaches a statistically significant difference from WT cells by 75 minutes post stimulation (Fig. 4c). h-MPOTg or WT macrophages cultured in 96 well plates were also used to demonstrate visually the post-stimulation increase in fluorescence observed with the plate readers via fluorescence microscopy. h-MPOTg, but not WT, macrophages show an increase in fluorescence following stimulation abrogated by co-incubation with 2 μM MPO inhibitor (Fig. 5a–f).
Figure 4.
MPO+ macrophages contain active MPO that generates HOCl detectable by SNAPF, following stimulation. a) The gene for hMPO is transcribed in bone marrow derived macrophages from hMPO-Tg, but not WT, mice as demonstrated by RT-PCR. This is translated into functionally active MPO, established by a dTNB assay (b). Results are expressed as a percentage decrease in absorbance at 412 nm per μg protein (n=3). Enzymatically active MPO is also present in human MPOhi cells (b, n=4). HOCl generated by hMPO-Tg BM macrophages (c, n=3) or human MPOhi macrophages (d, n=5) is quantifiably detected by SNAPF using a fluorescent plate reader, following cell stimulation with 400 nM PMA. Results are expressed as a percentage change in fluorescence from baseline readings (pre-stimulation) over time.
Figure 5.
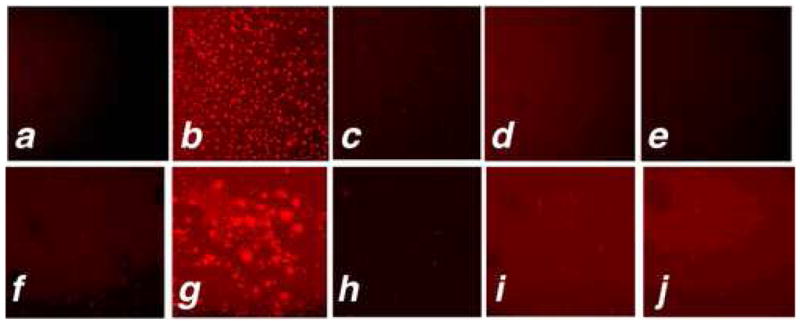
SNAPF is activated upon incubation with MPO+ macrophages stimulated with PMA, detectable by fluorescence microscopy. BM-derived macrophages from h-MPOTg and WT mice, or human MPOhi or MPOlo macrophages were incubated with 10 μM SNAPF for ten minutes prior to stimulation with 400 nM PMA. Images were acquired 30 minutes post-stimulation, using an APC filter set. a) h-MPOTg cells pre-stimulation. b) h-MPOTg cells post-stimulation. c) h-MPOTg cells co-incubated with 2 μM MPO inhibitor, post-stimulation. d) and e), WT cells pre- and post-stimulation. f) and g), MPOhi cells pre-and post stimulation, h), MPOhi cells post stimulation co-incubated with MPO inhibitor. i) and j), MPOlo cells pre-and post-stimulation. Results are representative of at least three separate experiments.
SNAPF Identifies HOCl Generated From Stimulated Human MPO-containing Macrophages
To study whether SNAPF can detect HOCl generated by stimulation of MPO+ human macrophages, human monocytes were isolated from whole blood then cultured to either the MPO-expressing phenotype (MPOhi, cultured in serum free media with addition of GM-CSF [26]), or as classical macrophages (MPOlo). MPOlo cells do not contain any MPO protein as previously shown by Western blotting [26]. MPOhi cells contain enzymatically active MPO that produce HOCl after stimulation of the cells with PMA as measured by the oxidation of TNB to DTNB in a taurine-containing buffer (Fig. 4b). Unstimulated MPOhi cells do not demonstrate HOCl release (data not shown).
We performed a time course analysis of cells stimulated with PMA at a final concentration of 400 nM using a fluorescent plate reader and achieved results similar to those for h-MPOTg macrophages (Fig. 4d). In this case fluorescence emission from the activated probe in MPOhi cells peaked at around 60 minutes post stimulation, although the fluorescence signal was statistically different from WT fluorescence earlier in the time course, at 15–30 minutes post stimulation. The increase in fluorescence was also imaged after stimulation of human MPOhi and MPOlo cells using fluorescence microscopy, and again results comparable to those from the mouse experiments were found (Fig. 5g-l).
SNAPF Detects HOCl Generation in vivo
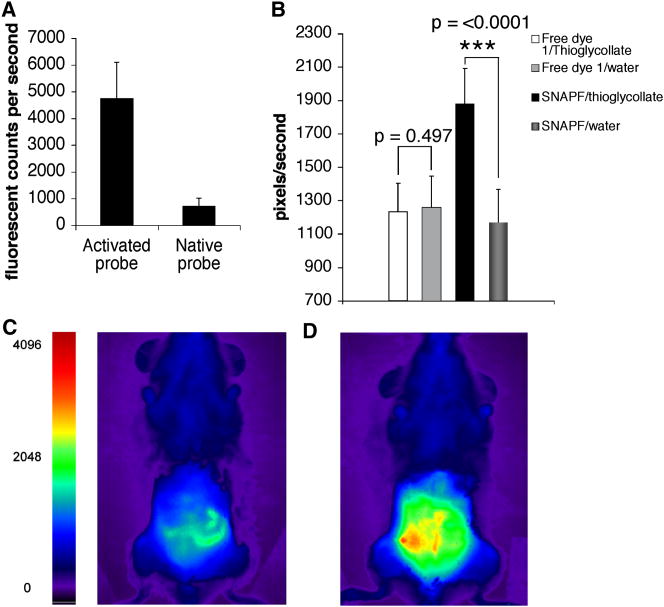
We first determined whether the increase in fluorescence of activated SNAPF could be distinguished by FRI in vitro. Incubation of 10 μM SNAPF with 20 μM HOCl at 37°C for 30 minutes results in a seven-fold increase in fluorescence of the probe as detected by FRI (Fig. 6a). Given this result, we subsequently injected the probe (50 nmoles) into h-MPOTg mice that had peritonitis induced by thioglycollate. Thioglycollate was injected 24 hours before probe administration, providing an expected mix of about 60% neutrophils and 40% monocytes/macrophages in the peritoneal inflammatory milieu2. When SNAPF was injected i.p., a 1.4-fold increase in fluorescence of the peritoneum in vivo is observed after one hour (Fig. 6b–d). Fluorescence emission did not increase when SNAPF was injected into control mice, nor did fluorescence increase in animals with peritonitis and control mice when dye 1 is used as a control in place of SNAPF (Fig. 6b).
Figure 6.
FRI imaging reveals SNAPF activation by HOCl in vivo. Comparison of FRI images of SNAPF alone or incubated with 20 μM HOCl shows an approximate 7-fold increase in SNAPF fluorescence in vitro using this imaging system (a). We then injected 50 nanomoles of SNAPF i.p into hMPO-Tg mice with thioglycollate-induced peritonitis and imaged the animals after one hour. There was a ~1.4-fold increase in peritnoneal fluorescence (b), presumably due to contact of SNAPF with HOCl generated by activated neutrophils and macrophages present. Fluorescence was ~1.6-fold higher post-SNAPF injection in mice injected with thioglycollate as opposed to water. c), FRI image of h-MPOtg mouse pre- SNAPF injection, d), the same animal post-injection of SNAPF. There was no visible fluorescence when SNAPF was injected into mice that had been injected with saline solution as opposed to thioglycollate, nor was there any increase in fluorescence of a wavelength-matched control dye, 1, when injected into mice with peritonitis compared to saline-injected animals (b) (n=4 per group).
SNAPF Detects HOCl in Human Atherosclerotic Plaque
Having established that the probe can detect MPO activity in isolated cells and in whole animals in vivo, we determined whether we could use SNAPF to provide direct evidence of HOCl in human atherosclerotic plaque. Frozen sections of atherosclerotic human carotid arteries yielded SNAPF-derived fluorescence. This fluorescence is distinct from autofluorescence visible through the FITC filter set (Fig. 7a–c). Subsequent immunohistochemical staining of parallel sections co-localized both macrophages and MPO with the fluorescent areas (Fig. 7d–e). SNAPF was also incubated with thinly sliced (~1mm thick) sections of freshly excised atheromatous carotid arteries and again showed highly fluorescent regions on fluorescence microscopy, suggesting probe activation (Fig. 7e–g). Following imaging, the sections were frozen in OCT and sectioned to 6 μm thick slices. Immunohistochemistry demonstrated the presence of MPO+ cells and macrophages within the samples (Fig. 7h–i).
Figure 7.
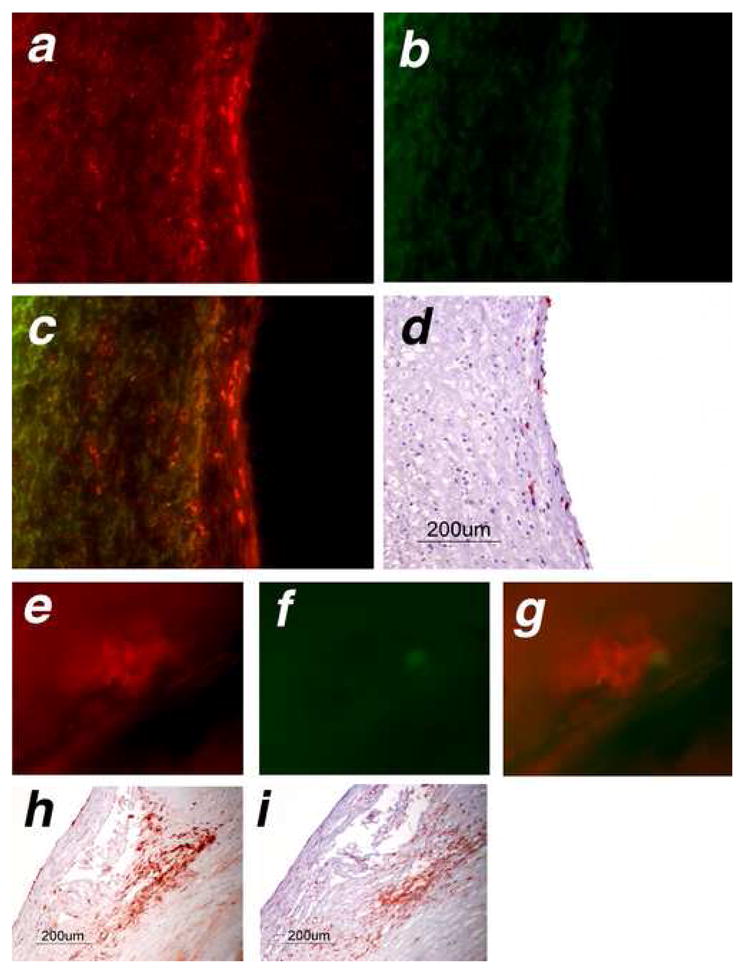
SNAPF detects areas of HOCl generation in frozen sections of human atherosclerotic plaque, and is activated after incubation with fresh atheromatous carotid endarterectomies.. Frozen serial sections cut from atheromatous carotid endarterectomies (n=4) were incubated at 37°C for 1 hour with 10 μM SNAPF, or with buffer alone as a control, then viewed by fluorescence microscopy. Sections incubated with SNAPF were observed using an APC (a, colored in red) and a FITC (b, colored in green) filter set. Merged images (c) show that fluorescent areas using the APC filter set, which detects SNAPF, are distinct from areas that fluoresce using the FITC filter set (autofluorescence). The areas which are fluorescent using the APC filter set correspond to the location of MPO+ cells in the section (d).
Freshly excised human endarterectomies (n=4) were obtained from the operating theaters of the Brigham and Women’s Hospital, cut into thin slices and incubated with 10 μM SNAPF at 37°C for one hour. The slices were then viewed under a fluorescent microscope. Areas which were fluorescent when viewed using the APC filter set (e, pseudocolored in red) were not fluorescent with the FITC filter set (f, green) when exposed for the same amount of time, indicating that this was likely not autofluorescence. Merged images show distinct red colored areas (g). The slices contained MPO+ cells, established by immunohistochemical staining of adjacent tissue (h). MPO+ cells colocalised with cells, which stained positively for macrophages in adjacent sections (i).
DISCUSSION
Oxidative stress has far reaching effects within living tissue. We now understand that not all biological oxidants behave in the same manner in, or have equivalent effects on, cells and tissues. Tools that report the distinct roles of different oxidant species in disease processes would help unravel pathophysiologic mechanisms and might ultimately furnish insight enabling the development and evaluation of more targeted therapies. MPO produces a number of oxidants, one of which is HOCl. To distinguish the action of HOCl from other oxidants, as we become increasingly aware of the multiplicity of its effects on tissue components, would provide valuable insights into oxidative mechanisms in diverse diseases. The SNAPF probe described in this work can selectively detect HOCl including that generated by purified MPO. No other fluorescent probe thus far has shown such high specificity for HOCl over other biologically relevant oxidants, although the prior sensors, HPF and APF, might be used in tandem to distinguish HOCl from hydroxyl radical and peroxynitrite. SNAPF obviates the need for dual probes in the detection of HOCl. In addition SNAPF has absorption and fluorescence emission maxima in the farred, which maximizes tissue penetration and minimizes the potential of interference from tissue autofluorescence, a particular concern in the study of elastin-rich arterial tissues. We demonstrated that SNAPF can detect HOCl generated from stimulated human neutrophils, the classic source of MPO. Thus this probe could serve as a valuable tool for investigation of multiple disease states characterized by acute inflammation involving infiltration of polymorphonuclear monocytes.
In our experiments, human and murine MPO+ macrophages appear to generate sufficient HOCl after stimulation with PMA to be detected by SNAPF, both quantitatively and qualitatively. Although PMA is not a physiologically relevant stimulus in the context of atheroma, HOCl production in MPOhi macrophages is expected following cell activation by stimulants present within an atherosclerotic lesion, such as CD40L and cholesterol crystals [41]. This study aimed to determine whether SNAPF could detect HOCl generated from living cells; for this purpose stimulation with PMA was appropriate as our previous studies showed that MPOhi cells stimulated with PMA generate substantial HOCl. A study using an alternative stimulus, N-formyl-methionylleucylphenylalanine (FMLP), demonstrated that stimulation of human neutrophils pre-incubated with tumor necrosis factor-α resulted in rapid accumulation of HOCl over the first 30 minutes of stimulation, reaching a maximum concentration approximately 60 minutes post stimulation [42]. Fluorescence time course data using SNAPF to detect HOCl also suggests a rapid generation of HOCl following cell stimulation, with comparable times for maximum concentration reached (60–75 minutes for macrophages, 90 minutes for neutrophils).
Since degranulation of certain leukocytes following stimulation releases pre-formed MPO, we may expect the extracellular milieu to become highly fluorescent when cells are incubated with SNAPF. Instead we see enhanced fluorescence of cells themselves, in agreement with the previous data from Setsukinai et al, who demonstrated increased fluorescence of porcine neutrophils incubated with APF following stimulation [38]. The reasons for this may be twofold: the probe may be taken up into the cells by pinocytosis (as suggested for APF) and activated intracellularly by HOCl generation within phagocytic vesicles. Also, it is believed that extracellularly released HOCl is short-lived and acts very locally, which may give the appearance of the macrophages or neutrophils becoming fluorescent as SNAPF is activated in close proximity to the cells.
The in vitro experiments demonstrate that SNAPF reports HOCl at concentrations as low as 2 μM. Due to the short-lived nature of HOCl, however, it is difficult to measure accurately HOCl levels in vivo. In vitro experiments have shown that 1 × 106 neutrophils/mL will generate ~43 μM HOCl 60 minutes after stimulation with PMA [43]. The sensitivity of SNAPF should therefore suffice to detect levels of HOCl expected even in a low-grade hypochlorite-induced oxidative state.
Results from the concurrent use of the MPO inhibitor with SNAPF in these studies suggest that the observed increases in fluorescence likely result from the specific reaction of SNAPF with MPO-derived HOCl. As an additional control for both in vivo whole animal experiments, and in vitro cell culture work, we used sulfonaphthofluorescein, 1, in place of SNAPF. Fluorescence emission did not increase in any of the experiments with the free dye 1, thereby excluding activation of the naphthofluorescein itself by HOCl.
As well as cell culture-derived HOCl, SNAPF detects HOCl generation in both fresh and frozen samples of atherosclerotic human arteries, and in vivo in mice with thioglycollate induced peritonitis. We believe this is the first described direct imaging evidence of HOCl within atherosclerotic plaque; previous reports have by necessity focused on the presence of HOCl-modified proteins within lesions and atheromatous vessel walls [19, 20, 36]. In its current form, SNAPF does not lend itself to in vivo imaging by intravenous injection due to the small size of the molecule. We find that in experimental peritonitis the rapid washout time of the probe makes it impractical for this purpose. Conjugation of the sensor moiety to a non-immunogenic pegylated co-polymer, a technique used in our laboratory, might evade this problem, and should be a goal of future research.
The excitation and emission wavelengths employed by SNAPF render the probe suitable for in vivo imaging, up to a tissue depth of around 1 cm. Use of these longer wavelengths circumvents difficulties found with the majority of currently available fluorescent sensors for oxidative species, which utilize shorter wavelengths [44]. Although these probes certainly have value for in vitro studies, imaging the molecular processes that occur in vivo requires imaging modalities that use fluorescent sensors less affected by normal tissue components. SNAPF could provide such a sensor.
SIGNIFICANCE
Traditional methods of visualizing vascular disease, such as angiography or ultrasound, furnish valuable tools in the evaluation of atherosclerosis. These modalities, however, have considerable limitations as they reflect anatomy or structure rather than functions or molecular processes. Too often, atherosclerosis is only diagnosed after the onset of symptoms, when the disease is already quite advanced. Pre-clinical diagnosis of atherosclerosis is thus a major unmet medical need. Detection of structural changes in the vasculature such as arterial calcification or stenosis may not distinguish functional aspects of plaques that correlate with their propensity to provoke thromboses, and hence clinical events such as stroke or myocardial infarction. Novel methods utilizing molecular imaging techniques can complement the more established approaches by offering a window into the dynamic biological processes such as inflammation by which a plaque may develop or become susceptible to rupture, invisible to traditional imaging techniques. Imaging techniques that report on specific disease-related molecular events, reaching beyond anatomy, may ultimately allow for the identification of vulnerable plaques before they precipitate disastrous clinical events. Such approaches could also provide much-needed tools for proof-of-concept studies and initial clinical evaluation of novel therapeutics. To this end, we have developed a novel fluorescent probe, SNAPF, which is active in the far red range of light and selectively detects HOCl, an oxidant that is implicated in many disease processes including atherosclerosis. Indeed, we have provided direct evidence of the presence of HOCl within human atherosclerotic plaque, an accomplishment previously hindered by the short half-life of the oxidant. The present results, including the use of SNAPF in a non invasive in vivo imaging study, indicate that SNAPF could provide a valuable tool to monitor selectively the impact of HOCl on the atherogenic process, as well as other diseases, in vivo.
EXPERIMENTAL PROCEDURES
General Considerations
APF and HPF were purchased from Alexis Biochemicals, ferrous perchlorate was obtained from GFS Chemicals (Columbus, Ohio), and recombinant human GM-CSF and recombinant mouse G-CSF from Cell Sciences (Canton, MA). Bovine liver catalase, Krebs-Henseleit buffer, taurine, 5,5′-dithio-bis(2-nitrobenzoic acid), 3-(aminopropyl)-1-hydroxy-3-isopropyl-2-oxo-1-triazene, 10% palladium on carbon, hydrogen gas, and 1-fluoro-4-nitrobenzene were all purchased from Sigma-Aldrich (St Louis, MO). Hydrogen peroxide was purchased from EMD Chemicals Inc. (Gibbstown, NJ), MPO and MPO inhibitor (4-aminobenzoic acid hydrazide) from EMD Biosciences Inc. (San Diego, CA), rabbit anti-myeloperoxidase from Biodesign (Saco, ME), and 2,2′-azobis (2-amidinopropane) dihydrochloride from Wako Chemicals USA Inc, (Richmond VA). Compound 1 was prepared as reported [40]. All microwave reactions were performed using a CEM (Matthews, NC) Discover LabMate microwave reactor. Preparative high performance liquid chromatography (HPLC) was performed on Varian (Palo Alto, Ca) ProStar instrument with a ProStar UV/Vis detector model 320 set at 500 nm. All HPLC data were collected with a Grace-Vydac (Columbia, MD) column, part number 218TP5210, with a flow rate of 6 mL/min, unless noted otherwise. Buffer A consisted of water and 0.1% TFA, whereas buffer B was composed of 90% acetonitrile, 9.9% water and 0.1% TFA. Nuclear magnetic resonance spectra were acquired on a Bruker Biospin (Billerica, MA) DPX-400 spectrometer at ambient temperature and referenced to tetramethylsilane (TMS) as an internal standard. High-resolution electrospray ionization (ESI) mass spectra were collected on a Bruker Daltonics (Billerica, MA) APEXII 3 T Fourier transform mass spectrometer in the Department of Chemistry Instrumentation Facility (DCIF) at the Massachusetts Institute of Technology. Low-resolution ESI mass spectra were obtained on a ZQ mass detector from Waters Corp. (Milford, MA). Fluorescence data were collected on a Horiba Jobin Yvon (Edison, NJ) Fluorolog-3 spectrofluorometer and were corrected for the detector sensitivity.
Mice
C57BL/6J mice transgenic for human MPO (h-MPOTg) were engineered as described previously [22]. WT C57BL/6 mice were purchased from Taconic. All mice were housed in a specified pathogen free environment with 12 hour light/dark cycles, and were given free access to standard chow and water. Before in vivo imaging, mice were transferred to a non-fluorescent diet (AIN-76 purified diet, Harlan) for at least five days.
Probe Synthesis
Intermediate 2. To a solution of 1 (592 mg, 1.1 mmol) in 25 mL anhydrous dimethylformamide (DMF) was added sodium carbonate (424 mg, 4 mmol). The mixture was heated in an oil bath until a blue suspension was obtained. To this suspension was added 1-fluoro-4-nitrobenzene (564 mg, 4 mmol) and the reaction was heated to 175 °C with a microwave reactor in a sealed reaction vessel for 15 min. Upon cooling, the excess sodium carbonate was filtered off and the filtrate was concentrated to 5 mL by rotary evaporation. The crude product was then precipitated by addition of tert-butyl methyl ether (40 mL). The precipitate was isolated by centrifugation, and washed with an additional portion of tert-butyl methyl ether (40 mL). This crude material was purified by reverse phase column chromatography using a 70 g RPC18 Isolute cartridge (Biotage, Charlottesville, VA) (eluting with: water, 0.1% TFA, and a 0 to 50% MeOH gradient in 10% increments) to afford pure 2 (285 mg, 39%) as an brown-orange solid. 1H NMR (400 MHz, d6-DMSO): δ 9.07 (1H, d, J = 8.0 Hz), 8.91 (1H, d, J = 8.0 Hz), 8.41 (1H, s), 8.36 (2H, d, J = 8.9 Hz), 8.00 (1H, d, J = 9.2 Hz), 7.91 (1 H, d, J = 7.8 Hz), 7.87 (1H, d, J = 9.4 Hz), 7.73 (1H, s), 7.61 (1H, d, J = 7.8 Hz), 7.46 (2H, d, J = 8.9 Hz), 7.41 (1H, d, J = 9.2 Hz), 7.22–7.29 (4 H, m). HRMS (ESI) [M+H]+ calcd. for C33H20NO11S2+: 670.0472, found: 670.0455.
SNAPF
To a solution of 2 (36 mg, 0.054 mmol) in MeOH (7.6 mL) and water (0.4 mL) was added 10% palladium on carbon (10 mg). This suspension was allowed to stir under 1 atm of H2, during which the crude product precipitated out of solution. After stirring for 2 h, the reaction mixture was filtered through a 0.45 μ nylon filter and the filtrate was discarded. The remaining solid was washed with 1:1 water/DMF (3 × 5 mL) giving a red solution. This solution was dried by rotary evaporation, giving crude SNAPF (8 mg) as a burgundy red solid. For purification, the crude material was BOC protected by treatment with di-tert-butyl dicarbonate (BOC2O) (22 mg, 0.1 mmol) and triethylamine (TEA) (13 μL, 0.1 mmol) with stirring in MeOH (2 mL) for 16 h. This treatment results in formation of a mixture of mono- and di-BOC protected probe, 3a and 3b, respectively. Following solvent removal, the protected intermediates were isolated by preparative RPC18 HPLC (eluting with a gradient from 0 to 75% buffer B with a flow rate of 6 mL/min over 50 min) giving 3a (3.6 mg, 9%, LRMS (ESI) [M+H]+ calcd. for C38H30NO11S2+: 740.13, found: 740.12) and 3b (2.0 mg, 4%, LRMS (ESI) [M+H]+ calcd. for C43H38NO13S2+: 840.18, found: 840.35) as orange-red solids. Intermediates 3a and 3b were then combined and treated with 4 mL of a 1:1 MeOH/TFA solution for 15 min. After solvent removal by rotary evaporation and drying in vacuo, pure SNAPF (3.5 mg, 10%) was obtained as a red-pink solid. Prepared SNAPF was solubilised in phosphate buffer pH 7.4 with 0.1% DMF as a co-solvent, and stored at −20°C in stock solutions of 1mM for experimental use. 1H NMR (400 MHz, d6-DMSO): δ 9.29 (1H, d, J = 7.9 Hz), 9.21 (1H, d, J = 8.4 Hz), 8.34 (1H, s), 8.01 (1H, d, J = 8.6 Hz), 7.93 (1H, d, J = 9.2 Hz), 7.87 (1 H, d, J = 7.8 Hz), 7.63 (1H, d, J = 9.0 Hz), 7.49-7.44 (3H, m), 7.34-7.26 (3H, m), 7.14 (2H, d, J = 6.2 Hz), 6.98-6.92 (2H, m). HRMS (ESI) [M+H]+ calcd. for C33H22NO9S2+: 640.0731, found: 640.0722.
Neutrophil Isolation
Neutrophils were isolated by drawing venous blood from human volunteers and immediately centrifuging the blood through a density gradient at room temperature (Polymorphprep, Axis-Shield). This method separates distinct bands of mononuclear cells and polymorphonuclear leucocytes from erythrocytes. The band containing polymorphonuclear leucocytes was collected and washed twice in Hanks’ balanced salt solution (HBSS), then the cells were resuspended in phosphate buffer pH 7.4 containing 150 mM sodium chloride and the appropriate fluorescent probes. Isolated cells were used in experiments without delay.
Macrophage Isolation
Human monocytes were isolated from donated whole blood using a Ficoll density gradient, washed several times in HBSS, and matured into macrophages either with, for MPOhi cells, or without (for MPOlo) 500 U/mL GM-CSF as described previously [41]. MPOhi cells were cultured in serum-free medium. Murine macrophages were isolated by flushing bone marrow from femurs and tibias of donor mice and cell suspensions centrifuged through a Ficoll density gradient. Adherent bone marrow derived cells were then cultured to macrophages by adding a final concentration of 50 ng/mL M-CSF/5% fetal bovine serum to the culture medium.
Reverse Transcriptase Polymerase Chain Reaction
RNA was isolated from bone marrow derived macrophages using standard procedures (RNEasy mini kit, Qiagen). cDNA was then generated from the RNA using a reverse transcriptase reaction (SuperscriptII RNAse reverse transcriptase, Invitrogen). cDNA samples were amplified by PCR using primers specific for human MPO (forward 5′ ACCCCTGCCAAGCTGA 3′, reverse 5′ GGGGATATGAGTTGGACA, annealing at 53°C) and mouse β-actin (forward 5′ CCTGAGCGCAAGTACTCTGTGT 3′, reverse 5′ GCTGATCCACATCTGCTGGAA 3′, annealing at 60°C). RT-PCR products were separated with agarose gel electrophoresis.
MPO activity
Generation of HOCl by MPO was assessed by chlorination of taurine in Krebs-Henseleit (KH) buffer. Cultured cells were scraped into KH buffer containing 20 mM taurine and stimulated with a final concentration of 400 nM phorbol myristate acetate (PMA) at 37°C for two hours with periodic shaking. The reaction was stopped by the addition of catalase at 20 μg/mL of buffer followed by a ten minute incubation on melting ice. Cell suspensions were then centrifuged at 13,000rpm for 5 minutes at 4°C, and a final concentration of 0.225 mM 5-thio-2-nitrobenzoic acid (TNB) added to the supernatant. The reaction was allowed to proceed for five minutes in the dark. Absorbance readings were measured at 412 nm for each sample, using buffer alone with TNB as a blank. Meanwhile, cell pellets were resuspended in triton homogenizing buffer, lysed by sonication for 1 minute and protein measurements were taken for each sample. Results were expressed as the percent decrease in absorbance at 412 nm from the blank sample per μg of protein.
Fluorescence microscopy
Cells were cultured in black, clear bottomed 96 well plates (Costar). On the day of the experiment, cells were washed twice in HBSS and incubated in 0.1 M sodium phosphate buffer pH 7.4, containing 150 mM sodium chloride and appropriate probes, with or without MPO inhibitor. Cells were incubated at 37°C in the dark for ten minutes, then pre-stimulation images were acquired. Cells were then stimulated with PMA at a concentration of 400 nM in the buffer and incubated, protected from light, at 37°C for a further 60 minutes, after which post-stimulation images were obtained. Images were acquired using either a FITC (excitation 480/30 nm, emission 535/40 nm) or APC (excitation 620/60 nm, emission 700/75 nm) filter set on a Nikon Eclipse TE2000U fluorescence microscope, utilizing Spot software.
Quantification of fluorescence
Experimental cells were cultured in black, clear bottomed 96 well plates (Costar), washed twice with HBSS then incubated at 37 °C in the dark in sodium phosphate buffer containing 10 μM of the indicated probes, with or without MPO inhibitor, as above. Chemical reactions to generate the ROS were performed as described previously [38] in 0.5 mL tubes, and were transferred into wells of the 96-well plates. Briefly, OH. was generated by 100 μM ferrous perchlorate/1 μM H2O2, HOCl by addition of 10 μM NaOCl, superoxide (O2.−) by the addition of 100 μM KO2, H2O2 by addition of 100 μM H2O2, nitric oxide (NO) by addition of 100 μM 3-(aminopropyl)-1-hydroxy-3-isopropyl-2-oxo-1-triazene and alkylperoxyl radical (ROO.) by addition of 100 μM 2,2′-Azobis(2-amidinopropane)dihydrochloride. Each reaction was incubated with 10 μM SNAPF. Fluorescence readings from the plates were measured on a Safire2 plate reader (Tecan) using Magellan software. For APF, excitation and emission filters were 495 nm and 520 nm respectively, for SNAPF excitation and emission filters of 600 nm and 700 nm were used.
Human Atherosclerotic Tissue
Freshly excised atherosclerotic human carotid arteries were obtained from the operating theaters at Brigham and Women’s Hospital, Boston according to protocols approved by the Institutional Review Board. Carotids were either sliced thinly, then incubated with the probes at 37 °C in phosphate buffer for fluorescence imaging, or were frozen in OCT and sectioned into 6 micrometer thick slices. Frozen sections were warmed to room temperature, then incubated with 100 μL of phosphate buffer containing 10 μM probe for 1 h, after which they were examined by fluorescence microscopy. Serial sections were stained immunohistochemically for macrophages (primary antibody anti-CD68, 1:700, DAKO) or myeloperoxidase (rabbit anti-MPO, 1:400, DAKO), using 3-amino, 9-ethyl-carbazole (AEC) as a substrate.
In vivo imaging
Sterile peritonitis was induced in hMPO-Tg mice by intraperitoneal injection of 1 mL of a 3% thioglycollate solution in water. After 24 hours, live mice were imaged by FRI on a Siemens BonSAI system with filters for far-red imaging (excitation 660 nm, emission 735 nm). The fluorescent probes were injected into the mice intraperitoneally, and the animals were imaged again after one hour. The changes in fluorescence signal intensity over baseline were quantified by region of interest (ROI) analysis using OsiriX version 2.5.1.
Statistical analysis
Statistical significance was calculated by determining p-values using the paired T-test.
Acknowledgments
This work was funded by the NHLBI Program for Excellence in Nanotechnology, funding number UO1 HL080731 (RW).
We thank Dr. Galina Sukhova for samples of human atherosclerotic plaque, and we also thank Elissa Simon-Morrissey, Eugenia Shvartz and Lindsey MacFarlane for technical assistance.
Footnotes
JS and JWH, personal communication
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 2.Bowler RP, Crapo JD. Oxidative stress in allergic respiratory disease. J Allergy Clin Immunol. 2002;110:349–356. doi: 10.1067/mai.2002.126780. [DOI] [PubMed] [Google Scholar]
- 3.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 4.McCord JM. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974;185:529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- 5.McCord JM, Day ED. Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS letters. 1978;86:139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- 6.Repine JE, Fox RB, Berger EM. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981;256:7094–7096. [PubMed] [Google Scholar]
- 7.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 8.Morris JC. The acid ionization constant of HOCl from 5 to 35. J Phys Chem. 1966;70:3798–3805. [Google Scholar]
- 9.Hammerschmidt S, Buchler N, Wahn N. Tissue lipid peroxidation and reduced glutathione depletion in hypochlorite-induced lung injury. Chest. 2002;121:573–581. doi: 10.1378/chest.121.2.573. [DOI] [PubMed] [Google Scholar]
- 10.Venglarik CJ, Giron-Calle J, Wigley AF, Malle E, Watanabe N, Forman HJ. Hypochlorous acid alters bronchial epithelial cell membrane properties and prevention by extracellular glutathione. J Appl Physiol. 2003;95:2444–2452. doi: 10.1152/japplphysiol.00002.2003. [DOI] [PubMed] [Google Scholar]
- 11.Hammerschmidt S, Vogel T, Jockel S, Gessner C, Seyfarth HJ, Gillissen A, Wirtz H. Protein kinase C inhibition attenuates hypochlorite-induced acute lung injury. Respir Med. 2007 doi: 10.1016/j.rmed.2006.11.003. (Epub) [DOI] [PubMed] [Google Scholar]
- 12.Wu SM, Pizzo SV. 2-Macroglobulin from rheumatid arthritis synovial fluid: Functional analysis defines a role for oxidation in inflammation. Arch Biochem Biophys. 2001;391:119–126. doi: 10.1006/abbi.2001.2408. [DOI] [PubMed] [Google Scholar]
- 13.Jasin HE. Oxidative modification of inflammatory synovial fluid immunoglobulin G. Inflammation. 1993;17:167–181. doi: 10.1007/BF00916103. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischaemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289:G760–G767. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- 15.Malle E, Buch T, Grone HJ. Myeloperoxidase in kidney disease. Kidney International. 2003;64:1956–1967. doi: 10.1046/j.1523-1755.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama Y, Lindholm B, Stenvinkel P. Inflammation and oxidative stress in ESRD – the role of myeloperoxidase. J Nephrol. 2004;17:72–76. [PubMed] [Google Scholar]
- 17.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherosclerosis. Free Radic Biol Med. 1996;20:707–728. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 18.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97:1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, Lim LL, Shi W, Hazen SL, Jacob JS, Crowley JR, Heinecke JW, Lusis AJ. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107:419–430. doi: 10.1172/JCI8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111:2798–2804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 24.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. NEJM. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 25.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression. Arterioscler Thromb Vasc Biol. 2004;24:1309–1314. doi: 10.1161/01.ATV.0000131784.50633.4f. [DOI] [PubMed] [Google Scholar]
- 27.Hazell LJ, Stocker R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of lipoprotein into a high-uptake form for macrophages. Biochem J. 1993;290:165–172. doi: 10.1042/bj2900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katsura M, Fortser LA, Ferns GA, Anggard EE. Oxidative modification of low-density lipoprotein by human polymorphonuclear leucocytes to a form recognized by the lipoprotein scavenger pathway. Biochim Biophys Acta. 1994;1213:231–237. doi: 10.1016/0005-2760(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 30.McMurray HF, Parthasarathy S, Steinberg D. Oxidative modification of low-density lipoprotein by human polymorphonuclear leucocytes to a form recognized by the lipoprotein scavenger pathway. J Clin Invest. 1993;92:1004–1008. doi: 10.1172/JCI116605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terkeltaub R, Banka CL, Solan J, Santoro D, Brand K, Curtiss LK. Oxidized LDL induces monocytic cell expression of interleukin-8, a chemokine with T-lymphocyte chemotactic activity. Arterioscler Thromb. 1994;14:47–53. doi: 10.1161/01.atv.14.1.47. [DOI] [PubMed] [Google Scholar]
- 32.Wang GP, Deng ZD, Ni J, Qu ZL. Oxidized low density lipoprotein and very low density lipoprotein enhance expression of monocyte chemoattractant protein-1 in rabbit peritoneal exudate macrophages. Atherosclerosis. 1997;133:31–36. doi: 10.1016/s0021-9150(97)00109-3. [DOI] [PubMed] [Google Scholar]
- 33.Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 34.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergt C, Fu X, Huq NP, Kao J, Heinecke JW. Lysine residues direct the chlorination of tyrosines in YXXK motifs of apolipoprotein A-1 when hypochlorous acid oxidizes high density lipoprotein. J Biol Chem. 2003;279:7856–7866. doi: 10.1074/jbc.M309046200. [DOI] [PubMed] [Google Scholar]
- 36.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao B, Oda MN, Bergt C, Fu X, Green PS, Brot N, Oram JF, Heinecke JW. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-1. J Biol Chem. 2006;281:9001–9004. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 38.Setsukinai KI, Urano Y, Kakinuma K, Majima HJ, Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 39.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nature Medicine. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 40.Hilderbrand SH, Weissleder R. One-Pot synthesis of new symmetric and asymmetric xanthene dyes. Tet Lett. 2007;48:4383–4385. doi: 10.1016/j.tetlet.2007.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugiyama S, Okada Y, Sukhova G, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Test ST. Effect of tumor necrosis factor on the generation of chlorinated oxidants by adherent human neutrophils. J Leukocyte Biol. 1991;50:131–139. doi: 10.1002/jlb.50.2.131. [DOI] [PubMed] [Google Scholar]
- 43.Bergt C, Marsche G, Panzenboeck U, Heinecke JW, Malle E, Sattler W. Human neutrophils employ the myeloperoxidase/hydrogen peroxide/chloride system to oxidatively damage apolipoprotein A-1. Eur J Biochem. 2001;268:3523–3531. doi: 10.1046/j.1432-1327.2001.02253.x. [DOI] [PubMed] [Google Scholar]
- 44.Gomes A, Fernandes E, Lima JL. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]