Abstract
Wnts play pivotal roles during development and in the mature nervous system. However, the mechanism by which Wnts traffic between cells has remained elusive. Here we demonstrate a novel mechanism of Wnt transmission through release of exosome-like vesicles containing the Wnt-binding protein Evenness Interrupted/Wntless/Sprinter (Evi/Wls/Srt). We show that at the Drosophila larval neuromuscular junction (NMJ), presynaptic vesicular release of Evi is required for the secretion of the Wnt, Wingless (Wg). We also show that Evi acts cell-autonomously in the postsynaptic Wnt-receiving cell to target dGRIP, a Wg-receptor-interacting protein, to postsynaptic sites. Upon Evi loss of function, dGRIP is not properly targeted to synaptic sites, interfering with postsynaptic Wnt signal transduction. These findings uncover a previously unknown cellular mechanism by which a secreted Wnt is transported across synapses by Evi-containing vesicles, and reveal novel trafficking functions of Evi in both the Wnt-producing and the Wnt-receiving cell.
INTRODUCTION
Members of the Wnt family of morphogens orchestrate a myriad of developmental processes in all metazoan organisms studied to date (Siegfried and Perrimon, 1994). These include the establishment of cell identity during pattern formation, control of cell proliferation and migration, and cytoskeletal remodeling. Wnts are also known to coordinate major aspects of the nervous system from early development to adult function, in which they regulate neural stem cell proliferation, axon pathfinding, synapse differentiation and plasticity, as well as learning (Ataman et al., 2008; Salinas and Zou, 2008; Speese and Budnik, 2007; Zhao et al., 2005). Not surprisingly, alterations in Wnt signaling in humans have been linked to a number of cognitive disorders, such as schizophrenia and Alzheimer’s disease (De Ferrari and Moon, 2006).
Wnts activate a variety of intracellular signal transduction pathways that regulate gene expression and cytoskeletal organization events (Gordon and Nusse, 2006; Salinas and Zou, 2008). The best understood signaling pathway is the canonical Wnt pathway, in which Wnt ligands bind to the Frizzled (Fz) family of serpentine receptors. Receptor activation in turn stabilizes cytoplasmic β-catenin, which enters the nucleus and regulates gene expression. In a divergent canonical pathway, GSK3-β operates through a non-genomic mechanism, by phosphorylating microtubule-associated proteins, thereby regulating microtubule stability. Alternative signal transduction mechanisms activated by Wnt ligands include the planar cell polarity (PCP) pathway, and the Wnt/Ca++ pathway. Recent studies at the Drosophila neuromuscular junction (NMJ) and in the developing mammalian nervous system have uncovered a novel transduction mechanism, in which Wnt receptors themselves are cleaved and translocated into the nucleus (Lyu et al., 2008; Mathew et al., 2005). These non-exclusive transduction cascades provide alternative mechanisms for cells to regulate diverse processes in different spatio-temporal contexts.
While considerable progress has been made in elucidating the signaling pathways activated by Wnts, much less is known about how Wnts are secreted and transported to distant locales. At the Drosophila imaginal wing disc, the Wnt-1 homolog Wingless (Wg) is secreted by a discrete row of Wg-producing cells. Secreted Wg forms a long-range gradient expanding many cell diameters away from the source of Wg secretion (Neumann and Cohen, 1997). The mechanisms by which Wg is transported from its site of secretion to distant target cells have remained poorly understood. Wnt proteins are highly hydrophobic and tightly associated to cell membranes owing to palmitoyl modifications essential for biological activity (Willert et al., 2003). Thus, unescorted Wnt molecules are not easily diffusible in the extracellular milieu. Several mechanisms have been proposed to explain the movement of Wnt molecules from their site of secretion, including their association with glycosaminoglycan-modified proteins at the extracellular matrix (Baeg et al., 2001), the formation of exosome-like vesicles called argosomes (Greco et al., 2001), extracellular lipoprotein particles (Panakova et al., 2005), transcytosis (Coudreuse et al., 2006), or a combination of the above. However, the exact mechanism employed during intercellular Wnt transport has remained elusive.
Recent studies have identified a type II multi-pass transmembrane protein called Evenness Interrupted/Wntless/Sprinter (Evi/Wls/Srt), which appears to be specifically required in vivo for Wnt secretion in epithelial cells of flies and human cultured cells (Banziger et al., 2006; Bartscherer et al., 2006; Goodman et al., 2006). In the wing epithelium of Drosophila, Wg cannot be secreted from the evi mutant cells and this leads to the accumulation of Wg within these cells. In contrast, the secretion of other morphogens, such as Hedgehog (Hh), remains unaffected, suggesting that Evi is dedicated to the secretion of Wnt proteins. Further analysis has suggested that Evi functions as a Wnt cargo receptor during trafficking from the Golgi to the plasma membrane, and recycled back to the Golgi through the retromer complex (Belenkaya et al., 2008; Franch-Marro et al., 2008; Pan et al., 2008; Port et al., 2008; Yang et al., 2008).
In the nervous system, Wnts are released by pre- or postsynaptic cells and function in either a retrograde or anterograde manner (Salinas and Zou, 2008; Speese and Budnik, 2007). Similar to other cell types, the mechanisms by which Wnts are transported between synaptic compartments are principally unexplored. Considering that Wnt-1 is released from synapses in an activity-dependent manner (Ataman et al., 2008), and the substantial short and long-term effects of Wnt signaling on neurons, elucidating the mechanisms by which Wnt secretion/transport is regulated in the nervous system remains an important problem.
Here we have addressed this key question by using the glutamatergic synapses of the Drosophila larval NMJ, where Wnt-1/ Wingless (Wg) is secreted from motorneurons. We report that Evi is localized at these synapses and its function is indispensable for proper Wg secretion and signaling. We also demonstrate a novel mechanism for transport of the Wg signal across the synapse through the release of Evi-containing exosome-like vesicles. Further, we show that Evi is required for the proper trafficking of the Wg receptor DFrizzled-2 (DFz2), through actions that involve the DFz2-interacting protein dGRIP, a PDZ protein required for the transport of internalized DFz2 vesicles towards the nucleus (Ataman et al., 2006; Mathew et al., 2005).
RESULTS
Evi is required for Wg secretion at the NMJ
Previous studies have suggested that Evi is required for Wg secretion in non-neuronal cells (Banziger et al., 2006; Bartscherer et al., 2006). Since Wg is secreted from motorneurons at the fly NMJ (Ataman et al., 2008; Packard et al., 2002) we first examined the distribution of Wg at the NMJ of evi null mutants, which survive to the third instar larval stage (Bartscherer et al., 2006). We found that secreted Wg levels were substantially reduced at postsynaptic muscles in evi mutants (Fig. 1A, C, E). However, this reduction was not limited to the postsynaptic compartment, but was also observed in the presynaptic boutons as determined by volumetric quantifications of the Wg signal inside the presynaptic bouton demarcated by anti-HRP staining (Fig. 1A, C, E). A similar decrease was observed when Evi was downregulated using Evi-RNAi expressed in neurons using the elav-Gal4 driver (Suppl. Fig. 1A, B). These results could indicate that Evi might be required for the stability or synthesis of Wg in motorneurons. However, we did not observe any changes in Wg levels in the nervous system (Fig. 1F). Thus, at the NMJ, Evi is required for the transport and/or secretion of Wg in presynaptic terminals. A prediction of this hypothesis is that Wg should accumulate in the cell bodies or axons of motorneurons. We found that there was a substantial increase in Wg immunoreactivity levels in motorneuron cell bodies and longitudinal axons within the neuropil (Suppl. Fig. 1C–E).
Figure 1. Wg localization at the NMJ is regulated by presynaptic Evi.
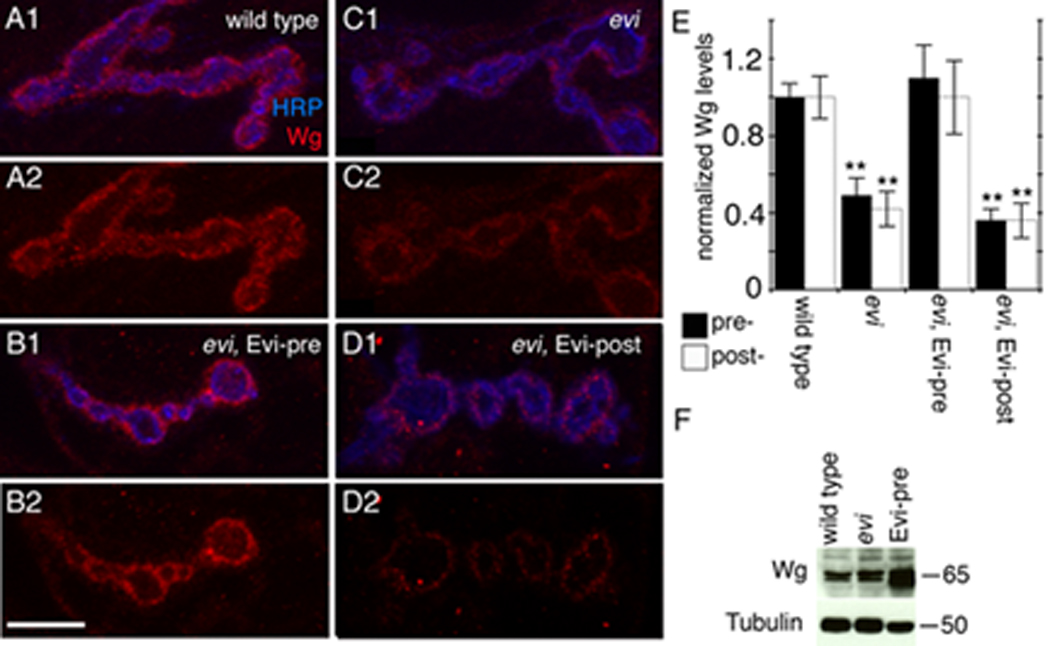
(A–D) Single confocal slices of NMJs labeled with anti-HRP and anti-Wg in (A) wild type, (B) an evi mutant expressing transgenic Evi in motorneurons (evi, Evi-pre), (C) an evi mutant, and (D) an evi mutant expressing transgenic Evi in muscles (evi, Evi-post). (E) Normalized Wg levels inside synaptic boutons (pre-) and at the postsynaptic region (post-). (F) Western blot of larval brain extracts. Numbers at the right of the blot represent molecular weigh in KDa. Calibration bar is 7 µm.
The above model was further tested by rescue experiments. Expressing an Evi-GFP transgene in the motorneurons of evi mutants, by using the Gal4 driver C380, completely rescued the low levels of Wg in both the pre- and postsynaptic compartments (Fig. 1B, E). Notably, however, expression of the Evi-GFP transgene in postsynaptic muscles, by using the Gal4 strain BG487, did not (Fig. 1D, E). Thus, Evi is required in motorneurons for normal Wg transport and/or secretion.
We also observed that mutations in evi mimicked synaptic phenotypes previously observed in mutations affecting Wg signaling (Ataman et al., 2006; Mathew et al., 2005; Packard et al., 2002). As muscle fibers grow in size, the Drosophila larval NMJ continuously expands by adding new synaptic boutons. This expansion is critically dependent on Wg signaling (Packard et al., 2002). Wg appears to be secreted by motorneurons, and suppressing Wg secretion substantially reduces synaptic bouton proliferation. Further, in wg mutants many boutons are misshapen, and some remain in an undifferentiated state (ghost boutons), lacking active zones and postsynaptic apparatus. Conversely, increasing Wg secretion by overexpressing Wg in motorneurons enhances formation of synaptic boutons. In the presynaptic cell Wg activates a divergent canonical pathway that regulates microtubules (Ataman et al., 2008; Franco et al., 2004; Miech et al., 2008). In the postsynaptic muscle cell Wg initiates an atypical pathway in which the DFz2 receptor itself is cleaved and a fragment imported to the nucleus (Ataman et al., 2006; Ataman et al., 2008; Mathew et al., 2005). In evi mutants the total number of synaptic boutons was decreased by over 50%, without any change in muscle size, and the boutons had an aberrant morphology being large and deformed (Fig. 2A–E; Suppl. Fig. 2). In addition, evi NMJs had a significantly higher number of ghost boutons (Fig. 2D arrows, F). The decrease in bouton number was only partially rescued by expressing Evi in either the pre- or postsynaptic cell (Fig. 2E). However, it was completely rescued by simultaneously expressing Evi in both cells (Fig. 2E). In the case of ghost boutons, expressing the Evi transgene in motorneurons or in both motorneurons and muscles completely rescued the abnormal increase in ghost boutons in evi mutants (Fig. 2F). Expressing Evi in muscles using the weaker Gal4 driver BG487-Gal4 did not rescue the increase in ghost boutons, but this phenotype was completely rescued by using the stronger muscle Gal4 driver C57-Gal4 (Fig. 2F). Thus, although Evi is required only in motorneurons for proper Wg transport and/or secretion, Evi is needed both in neurons and muscles for normal synaptic growth.
Figure 2. Mutations in evi mimic abnormal synaptic phenotypes observed in wg mutants.
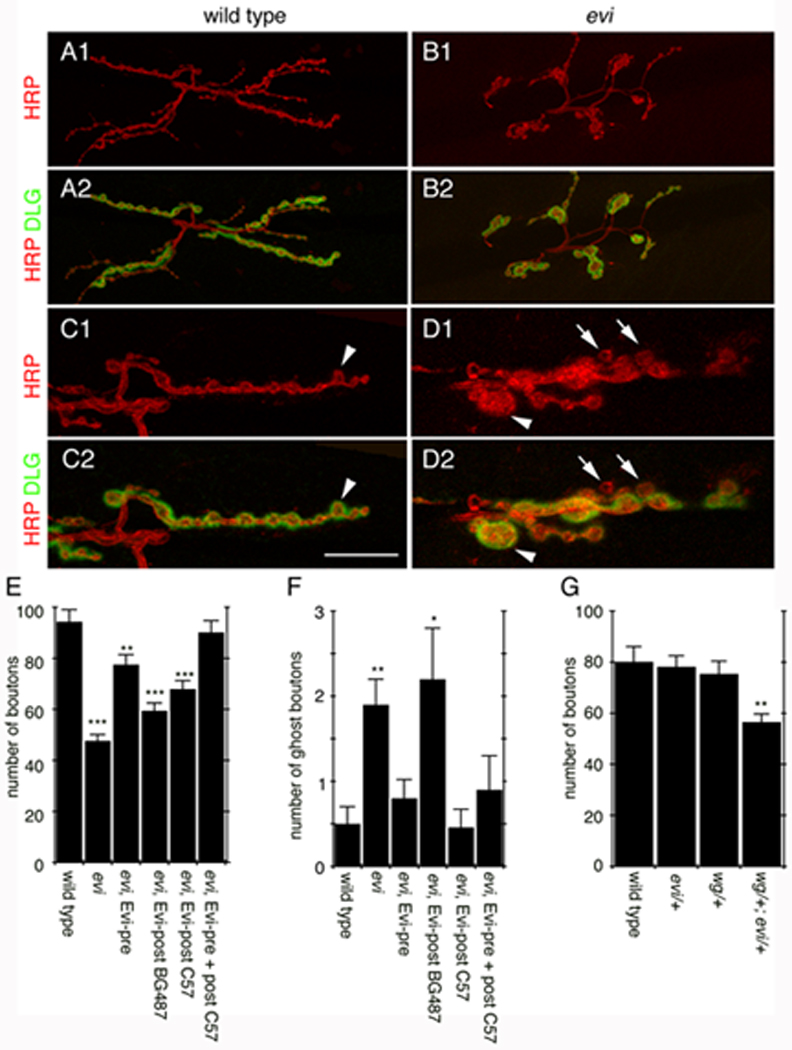
(A–D) Confocal images of NMJs labeled with anti-HRP and anti-DLG in (A, C) wild type, and (B, D) evi. (A, B) Projections of entire NMJs. (C, D) Single confocal slices of NMJ branches (arrowheads in C, D= abnormal boutons; arrows= ghost boutons). (E–G) Number of (E, G) boutons and (F) ghost boutons. Calibration bar is 30 µm for A, B and 13 µm for C, D.
The similarity in the synaptic phenotypes between evi and wg mutants at the NMJ, together with previous evidence suggesting that both proteins establish biochemical interactions (Banziger et al., 2006), raised the question of whether there were genetic interactions between evi and wg during NMJ growth. This was addressed by analysis of transheterozygotes. The number of boutons was normal in heterozygotes, but there was a supra-additive reduction in the number of boutons in the transheterozygote (expected decrease in bouton number by simple additivity in wg/+; evi/+ is 14.2% vs. 27% observed in wg/+; evi/+ transheterozygotes; Fig. 2G), suggesting that evi and wg genetically interact during synaptic bouton proliferation.
Evi is localized both pre- and postsynaptically and is transferred trans-synaptically from the pre- to the postsynaptic compartment
To examine the synaptic localization of Evi, we generated two antibodies directed either to a predicted extracellular (Evi-Nex) or intracellular (Evi-Cin) region of the Evi protein (Fig. 3A; Suppl. Fig. 3A, B). Both antibodies strongly labeled the NMJ in similar patterns (Fig. 3B, C). This immunoreactivity was specific, as it was severely decreased in evi mutants (Fig. 3D, E). Immunoreactive Evi label was observed both in pre- and postsynaptic compartments at the NMJ, as determined by double labeling with anti-HRP, which defines the boundary of the presynaptic compartment. However, Evi was particularly enriched at the postsynaptic junctional region (Fig. 3B, C), the same region occupied by secreted Wg, and its receptor DFz2 at the NMJ (Packard et al., 2002). In this region Evi immunoreactivity was present in a punctate pattern presumably reflecting vesicular structures (Fig. 3B, C).
Figure 3. Evi is localized pre- and postsynaptically at the NMJ and it is transported trans-synaptically as an intact protein.
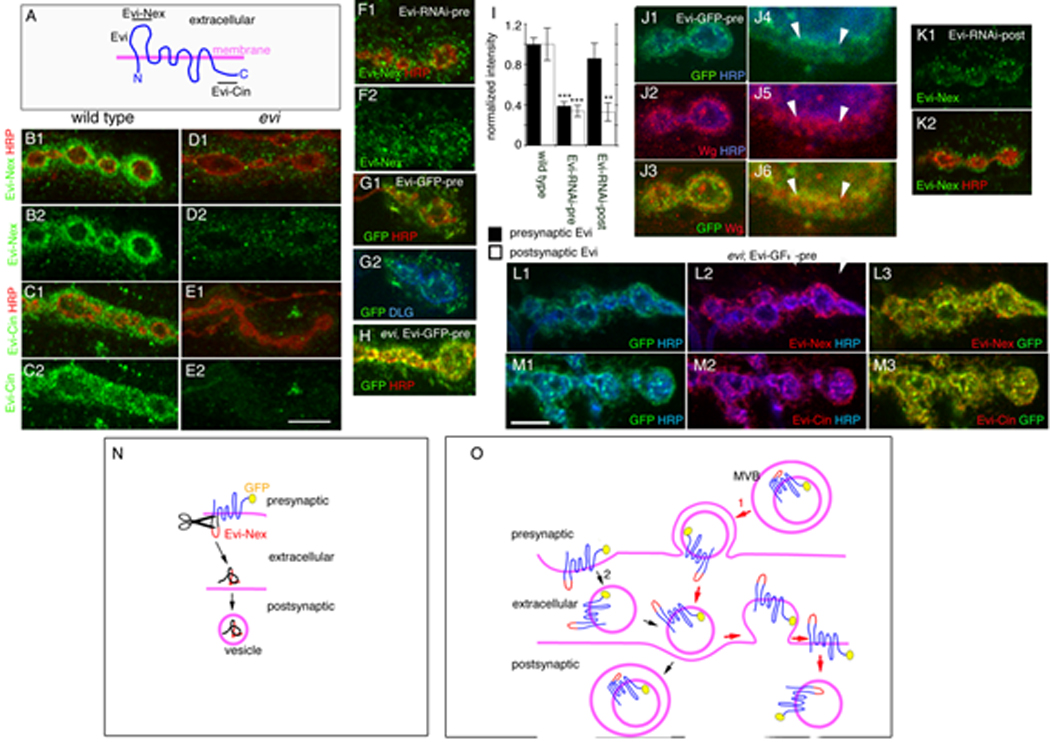
(A) Predicted structure of Evi and the regions (underlined) used for generation of the Evi-Nex and Evi-Cin antibodies. (B–E)Single confocal slices of NMJs double stained with anti-HRP and antibodies to (B, D) Evi-Nex and (C, E) Evi-Cin, in (B, C) wild type, (D, E) evi. Single confocal slices of (F) Evi-RNAi-pre stained with Evi-Nex and HRP, (G) Evi-GFP-pre triple stained with anti-GFP, anti-HRP and anti-Dlg or (H) evi;Evi-GFP-pre stained with anti-GFP, anti-HRP. (I) Normalized pre- and postsynaptic Evi levels. (J) Single confocal slices of a bouton at (J1–J3) low and (J4–J6) high magnification in Evi-GFP-pre stained with anti-GFP, anti-Wg and anti-HRP. (L,M) Images of NMJs from evi;Evi-GFP-pre triple stained with GFP, anti-HRP and antibodies to (L2,L3) Evi-Nex or (M2,M3) Evi-Cin. (N, O) Models on the potential mode of Evi trans-synaptic transfer. In (N) an extracellular region of Evi is cleaved and transported to the postsynaptic compartment. In (O) Evi is transferred as an intact protein through the use of vesicular compartments. Calibration bar is 2 µm for Fig. 3E 5–8 and 6 µm for the rest of the panels.
The rescue experiments suggested that Evi functions both pre- and postsynaptically during synaptic bouton proliferation, but that it is required solely presynaptically for Wg transport and/or secretion. To further determine the requirement of Evi in the pre- and postsynaptic side, we expressed Evi-RNAi with the cell-specific Gal4 drivers. Surprisingly, expressing Evi-RNAi in the motorneurons (Evi-RNAi-pre) led not only to a reduction in Evi immunoreactivity inside presynaptic terminals, but also substantially reduced the label at the postsynaptic region (Fig. 3F, I). The observation that presynaptic knockdown of Evi has a trans-synaptic effect on Evi levels in muscle was quite unexpected. Such a phenomenon is not observed when knocking down other well-known synaptic proteins such as dGRIP (Ataman et al., 2006) or spectrin (Pielage et al., 2005). This observation was also not due to a leaky Gal4 driver, as C380-Gal4 expresses Gal4 in motorneurons and not in muscles (Budnik et al., 1996; Sanyal et al., 2003). Further, expressing a nuclear LacZ (UAS-LacZ-NLS) with C380-Gal4 resulted in strong labeling of neuronal but not muscle nuclei (Suppl. Fig 3D, E), and expressing myristylated-mRFP (myr-mRFP) using C380-Gal4 did not result in postsynaptic myr-mRFP signal (Suppl. Fig. 3C). These observations suggest that postsynaptic Evi is at least partly derived from the presynaptic motorneurons. The possibility that Evi could be transferred from the pre- to the postsynaptic compartment was tested by expressing Evi-GFP in motorneurons (Evi-GFP-pre). Notably, GFP was observed both in presynaptic boutons as well as at the postsynaptic junctional region (Fig. 3G), supporting the notion that Evi could be transferred from pre- to postsynaptic compartments. This transfer was unlikely to result from Evi overexpression, as when the Evi-GFP transgene was expressed presynaptically in an evi mutant background, at levels similar to endogenous levels (Suppl. Fig. 3F), a similar distribution of the GFP label in the postsynaptic side was observed (Fig. 3H) (Suppl. Fig. 3F). This transfer of presynaptic Evi was also clearly observed in vivo in samples expressing myr-mRFP and Evi-GFP in motorneurons and imaged live (Suppl. Fig. 3C).
Given that Wg is secreted by presynaptic boutons and that Evi is required for normal Wg secretion, we next examined if presynaptically derived Evi colocalized with endogenous secreted Wg observed at postsynaptic sites. For these experiments we expressed Evi-GFP in motorneurons and examined both the Wg and Evi-GFP labels at the postsynaptic compartment. We found that there was substantial colocalization between Wg and Evi-GFP distal to the bouton rim, right outside the HRP label (Fig. 3J), consistent with the idea that secreted Evi vesicles contain Wg.
In contrast to the expression of Evi-RNAi in motorneurons, expressing Evi-RNAi in the muscles (Evi-RNAi-post), although significantly reducing the levels of Evi protein in the postsynaptic compartment, did not change the levels of Evi in presynaptic boutons (Fig. 3K, I). These results demonstrate that Evi is expressed by both motorneurons and muscles, but that there is a unidirectional transfer of Evi from presynaptic boutons to the postsynaptic region.
Considering that Evi is a multipass transmembrane protein, two possible scenarios might account for the above transfer of Evi from the pre- to the postsynaptic region. One possibility is that an extracellular region of Evi is cleaved, as is the case for other membrane receptors (Selkoe et al., 1996) (Fig. 3N). However, this possibility is highly unlikely, as in the Evi-GFP transgene the GFP tag is fused to the intracellular C-terminal region of Evi, and thus the transfer must include the intracellular domain. An alternative possibility is that the entire Evi protein could be transported in the form of a vesicle from the pre- to the postsynaptic compartment (Fig. 3O), as has been previously suggested with argosomes, vesicular structures that can transport Wg from cell to cell (Greco et al., 2001). To address this possibility, we took advantage of the Evi-Nex antibody, which recognizes an epitope localized at the first extracellular loop of Evi (Fig. 3A; red region in Fig. 3N, O), and which is separated from the C-terminal GFP tag by 7 transmembrane domains. For these experiments, we expressed Evi-GFP in motorneurons in an evi null mutant background and determined whether the postsynaptic GFP signal colocalized with the Evi-Nex and Evi-Cin immunoreactivity. We found that anti-Evi-Nex and anti-Evi-Cin immunoreactivities were exactly colocalized with GFP at the postsynaptic region (Fig. 3L, M; Suppl. Fig. 3G, H). Thus, these results support the notion that intact Evi is transferred across the synapse likely in a vesicle.
We also examined Drosophila Schneider-2 (S2) cells transfected with the Evi-GFP construct. We found that untransfected S2 cells in contact with Evi-GFP-transfected cells often contained Evi-GFP positive puncta within their cytoplasm (Fig. 4A, arrowheads). To verify that this was due to transfer of Evi-GFP from transfected to non-transfected cells, Evi-untransfected cells were separately transfected with mCherry and mixed with the Evi-GFP transfected cells. Again, we found that mCherry-positive (Evi-untransfected) cells had GFP puncta within their cytoplasm (Fig. 4B), suggesting that Evi-transfected cells transferred Evi to nearby cells.
Figure 4. Evi is transferred from cell to cell and to the medium.
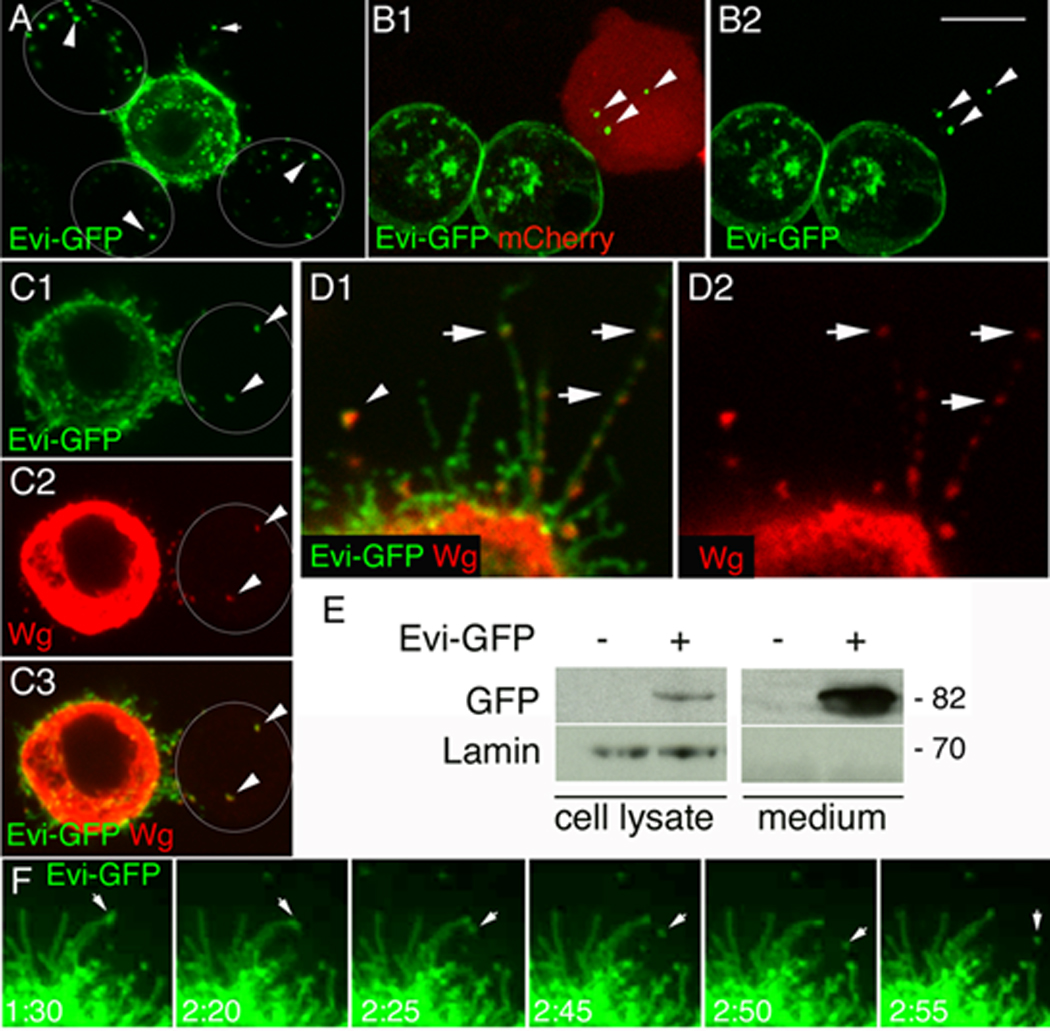
(A, B) Single confocal slices of S2 cells (A) either untransfected (outlined by white circles) or transfected with Evi-GFP and (B) either transfected with mCherry or Evi-GFP (arrowheads= Evi-negative cells; arrows= Evi in the media). (C) Evi-GFP and Wg are transferred together into an untransfected cell (arrowheads) (D) Wg localizes with Evi into punctuate structures within filopodia (arrows), as well as in the medium (arrowhead) (E) Western blot of lysates and media from Evi-GFP transfected S2 cells. (F) Time-lapse imaging of an S2 cell transfected with Evi-GFP showing the shedding of an Evi-GFP vesicle to the medium (arrows). Calibration bar is 3µm for panel 4D and 8 µm for the rest of the panels. Time points in 4F are in min.
We also found that Evi-GFP puncta were observed in the medium, suggesting the secretion of Evi vesicles into the medium (Fig. 4A, arrow). To determine if the Evi vesicles that were transferred to adjacent cells contained Wg, we cotransfected S2 cells with Evi-GFP and Wg. We found that the Evi vesicles transferred to adjacent cells or to the medium contained Wg (Fig. 4C, D, arrowhead). Interestingly, in these double transfected cells Wg localized to varicosities within filopodia (arrows in Fig. 4D). These filopodia were also present in untransfected cells as seen with phalloidin staining to label endogenous F-actin (Suppl. Fig. 4C). Two other membrane proteins, DFz2 and rCD2-mRFP, which also become localized to filopodia were not observed to be secreted (Suppl. Fig. 4A, B). We also carried out a Western blot analysis of the S2 cells and the culture medium. We found that indeed the culture medium contained full-length Evi protein, suggesting that Evi was secreted to the medium (Fig. 4E). The above observation was directly visualized by time-lapse imaging the Evi-GFP fluorescence. We found that Evi-GFP puncta trafficked within highly dynamic filopodia-like structures in the S2 cells and that some of these puncta were secreted to the media in a time-frame of several minutes (Fig. 4F; Suppl. Movie 1, Suppl. Movie 2, arrows). Thus, release and trans-cellular transfer of Evi vesicles to adjacent cells is a common biological mechanism utilized by both neuronal and non-neuronal cells.
Evi is present in multiple compartments at the NMJ
To determine the subcellular localization of Evi within pre- and postsynaptic compartments we next carried out immunoelectron microscopy studies with the Evi antibodies. For these experiments 1.4 nm gold conjugated secondary antibodies followed by silver intensification were used to mark sites of Evi-antibody binding using the pre-embedding technique. Consistent with our immunofluorescence studies, Evi was found to be localized in several pre- and postsynaptic structures.
At the postsynaptic junctional region, Evi was found within the subsynaptic reticulum (SSR), a system of muscle-derived membrane folds that completely surrounds synaptic boutons (Fig. 5A; the presynaptic bouton highlighted in pink overlay). Within the SSR, silver-intensified gold particles were observed in close association with the membrane folds (Fig. 5E, arrows). Notably, gold particles were also found inside approximately 200 nm in diameter membranous vesicles within the SSR (Fig. 5A, B; arrows and insets). In summary, at the postsynaptic region, Evi is present in association with SSR membranes and with novel postsynaptic vesicles.
Figure 5. Evi is localized to pre- and postsynaptic vesicular structures as well as pre- and post-perisynaptic membranes.
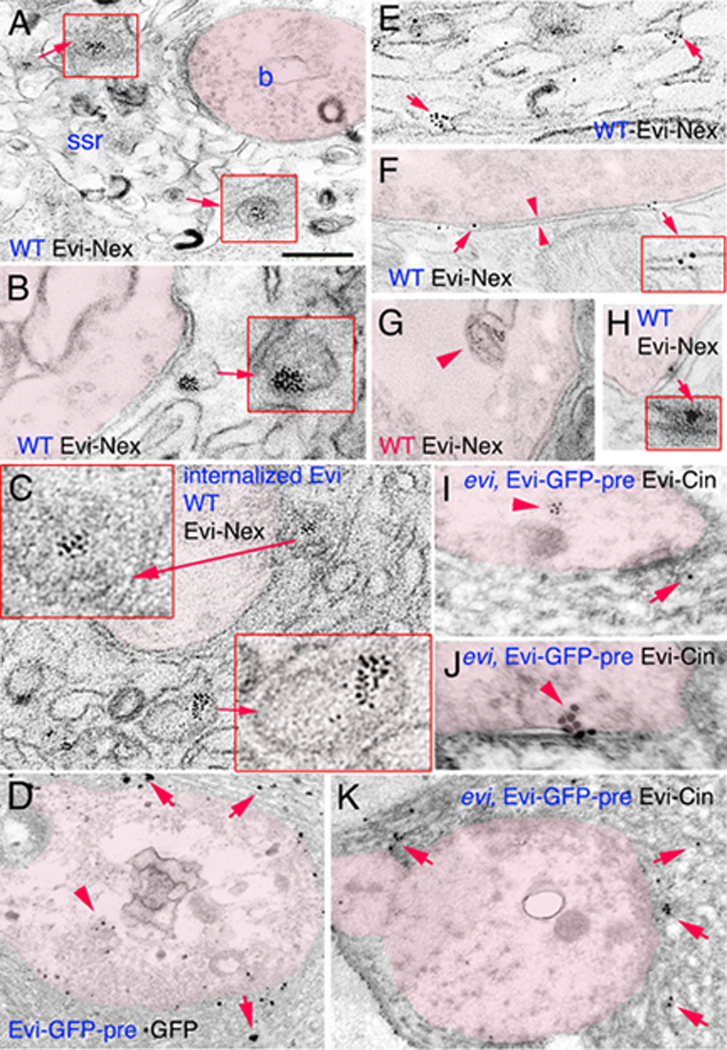
(A–K) Electron micrographs of synaptic bouton regions in preparations stained with antibodies to Evi-Nex or GFP, labeled with silver intensified 1.4nm gold secondaries, or antibodies to Evi-Cin labeled with 18 nm gold secondaries. The presynaptic compartment has been overlayed in pink, and insets are high magnification views of the structures indicated by the arrows. In (A, B, D–H) samples were stained pre-embedding with anti-Evi-Nex or anti-GFP. In (C) samples were processed for an internalization assay. (I–K) Samples were stained post-embedding with anti-Evi-Cin. (A, B) Immunoreactive vesicles found at the SSR region. (C) Internalized Evi is found in postsynaptic SSR vesicles. (D, K) Localization of label at SSR membranes. (E–H) Evi label at the perisynaptic region of pre- and postsynaptic membranes. Arrowheads in (F) mark the active zone. (I) Evi localization at a presynaptic multimembrane body. (J) Evi-immunoreactive gold particles at the presynaptic region and the synaptic cleft. Calibration bar is 0.6µm in A, C, D, K; 0.3µm in B, E–H; 0.2µm in the insets of A; 0.15µm in the inset of B, and 0.1 µm in the inset of C.
Evi was also associated with the pre- and postsynaptic membrane (Fig. 5F, arrows) and sometimes the signal was observed at the synaptic bouton cleft (Fig. 5H, arrow and inset). Within the presynaptic bouton, Evi was observed in large multi-membrane structures (Fig. 5G, arrowhead). Thus, Evi is present in multiple structures at synapses, including pre-and postsynaptic vesicular structures, the SSR, and synaptic membranes.
To determine if these vesicles were endocytosed from the muscle surface, we next conducted an internalization assay. These experiments were facilitated by the finding that the Evi-Nex antibody can bind to surface Evi in vivo (Suppl. Fig. 3A). For these studies, unfixed and unpermeabilized body wall muscles were incubated with the Evi-Nex antibodies in the cold, washed, and brought to room temperature for 30 min prior to fixation. Then, samples were permeabilized and incubated with the gold-conjugated secondary antibody, followed by preparation for EM. Interestingly we found that the Evi label was found at SSR membranes as well as inside the large SSR vesicles (Fig. 5C, arrows and insets), suggesting that at least a subset of these postsynaptic vesicles are derived from the endocytosis of postsynaptic surface Evi.
To verify that Evi was transferred from presynaptic boutons to the postsynaptic SSR at the ultrastructural level, GFP-tagged Evi was expressed in motorneurons using the C380 Gal4 driver, and the NMJ was examined by immunoelectron microscopy using an anti-GFP antibody. We found that the GFP label was found not only within synaptic boutons (Fig. 5D, arrowhead), but throughout the postsynaptic SSR membrane (Fig. 5D). We also expressed Evi-GFP in the motorneurons of evi mutants and immunolabeled Evi with the Evi-Cin antibody using the postembedding technique. Again, we found the label both in the presynaptic compartment (Fig. 5 I, arrowhead), at the synaptic bouton cleft (Fig. 5J, arrowhead) and the postsynaptic SSR region (Fig. 5I, K, arrows). Thus, Evi is transferred trans-synaptically as expected from the observations at the light level.
Postsynaptic Evi is required for the trafficking of DFz2 through the DFz2-interacting protein dGRIP
Given that presynaptic Evi alone is not sufficient for normal NMJ development we predicted that Evi was also endogenously expressed in muscle. To test this prediction we carried out real time PCR experiments from body wall muscle mRNA. We found that there were significant levels of Evi mRNA in muscles and that these levels were substantially decreased upon expressing Evi-RNAi (Suppl. Fig. 5). What is the cell-autonomous role of Evi in the postsynaptic target cell? To address this issue we examined postsynaptic Wg signaling while downregulating Evi selectively in the muscle using Evi-RNAi. Previous studies suggest that Wg is secreted by presynaptic boutons (Packard et al., 2002), and unraveled a novel postsynaptic Wg signal transduction pathway in the postsynaptic muscles, the Frizzled Nuclear Import (FNI) pathway (Speese and Budnik, 2007), which is also shared by other Wnt receptors (Lyu et al., 2008). In this pathway, the Wg receptor, DFz2, is internalized from the postsynaptic muscle membrane and back-transported from the synapse to the nucleus through a mechanism that requires an interaction between the PDZ-binding C-terminal tail of DFz2 and the PDZ4-5 domain of the 7-PDZ protein dGRIP (Ataman et al., 2006). The entire cytoplasmic domain of DFz2 (DFz2-C) is then cleaved and imported into the nucleus (Mathew et al., 2005).
In muscles expressing Evi-RNAi we found that DFz2 was localized normally at the postsynaptic region of the NMJ. However, the postsynaptic levels of DFz2 were substantially increased (Fig. 6A, B, E). In contrast, no such increase in DFz2 levels was observed in the presynaptic cell upon expression of Evi-RNAi in motorneurons (normalized presynaptic DFz2 intensity in wild type is 1.0 ± 0.07 vs 0.93±0.07 in Evi-RNAi-pre). The same phenotype has been previously observed when the transport of DFz2 from the synapse to the nucleus is prevented by interfering with dGRIP function in muscles (Ataman et al., 2006). Interestingly, a similar accumulation of Wg at the postsynaptic region was observed upon downregulating Evi in muscle (Fig. 6C, D, E), consistent with the notion that Wg is trafficked with its receptor (Gagliardi et al., 2008). To determine if the increase in DFz2 at synapses of Evi-RNAi-post larvae was due to a defect in the internalization and/or trafficking of DFz2, we carried out DFz2 internalization assays. In these experiments we used an anti-DFz2-N antibody that binds to the extracellular domain of the receptor in vivo, allowing us to follow the fate of internalized DFz2. Dissected third instar body wall muscles were incubated with the DFz2-N antibody at 4°C in vivo, and after washing the excess antibody, samples were brought to room temperature and fixed at 5 and 60 minutes after the antibody-binding step. To determine the fraction of DFz2 that remained at the surface, samples were then incubated with Alexa 647-conjugated secondary antibody in the absence of detergent permeabilization as previously reported (Ataman et al., 2006; Mathew et al., 2005). To determine the amount of internalized DFz2, the above procedure was followed by permeabilization and incubation with an FITC-conjugated secondary antibody.
Figure 6. Evi downregulation in muscle results in postsynaptic Wg and DFz2 accumulation and alterations in the Frizzled Nuclear Import Wg pathway.
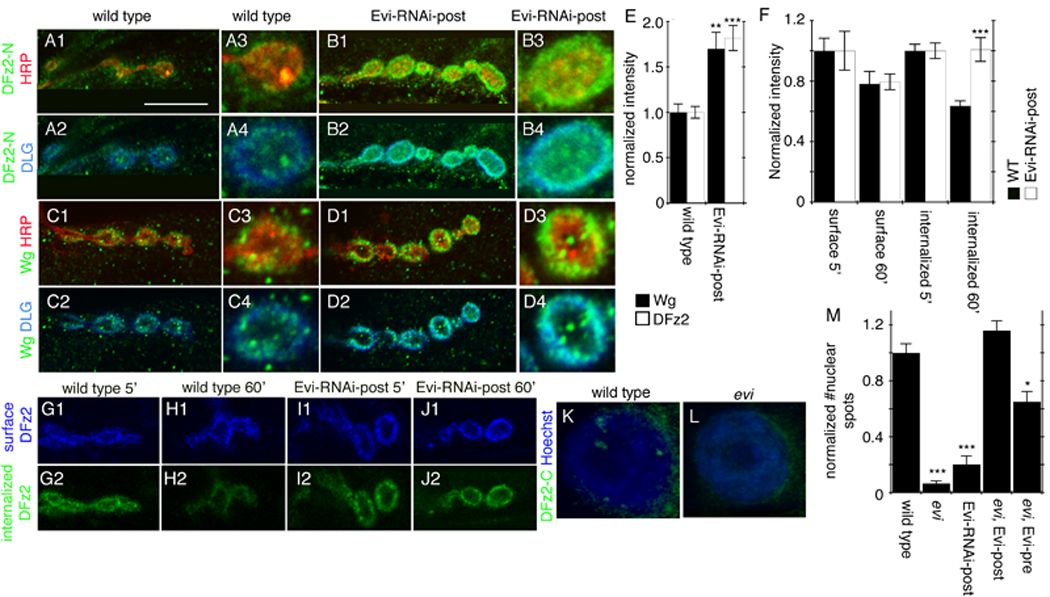
(A–D) Single confocal slices of NMJs triple stained with antibodies to HRP, DLG and (A, B) DFz2 or (C, D) Wg in (A, C) wild type and (B, D) Evi-RNAi-Post. (E) Wg and DFz2 immunoreactivity levels at the postsynaptic region. (F) Intensity of surface and internalized DFz2 at 5 and 60 min after the antibody-binding step in wild type and Evi-RNAi-post. (G–J) Single confocal slices of NMJs subjected to the internalization assay, showing (G1–J1) surface DFz2 and (G2–J2) internalized DFz2 (G, I) at 5 min and (H, J) 60 min after the antibody binding step, in (G, H) wild type, and (I, J) Evi-RNAi-post. (K, L) Confocal slices of muscle nuclei in preparations stained with anti-DFz2-C and Hoechst in (K) wild type and (L) evi mutants. (M) Normalized number of DFz2-C nuclear spots. Calibration bar is 10 µm for panels A-H1-2, I-L; 5 µm for panels A-D3-4.
As in previous studies (Ataman et al., 2006; Mathew et al., 2005), in wild type samples, surface DFz2 was internalized and observed near synaptic boutons at 5 min after the antibody-binding step (Fig. 6G2, F). However, at 60 min after the antibody-binding step, internalized DFz2 was significantly reduced at the NMJ as a result of its trafficking away from the synapse (Fig. 6H2, F; (Ataman et al., 2006; Mathew et al., 2005)). In contrast, upon expressing Evi-RNAi in muscles, no decrease in internalized synaptic DFz2 was observed at 60 min (Fig. 6I2, J2, F). No significant changes were observed in surface DFz2 in both genotypes (Fig. 6G1–J1, F), suggesting that only a small pool of the DFz2-antibody complexes become internalized. Thus, similar to alterations in dGRIP, a decrease in Evi function in muscles appears to interfere with the trafficking of DFz2 away from the synapse. This conclusion was further supported by examination of the levels of DFz2-C imported into the muscle nuclei. Previous studies show that the C-terminal region of DFz2 is cleaved and imported into the nucleus, where it is observed in the form of discrete immunofluorescent puncta (Fig. 6K; (Mathew et al., 2005)). In evi mutants and upon expressing Evi-RNAi in muscles alone, nuclear DFz2-C puncta were almost completely abolished (Fig. 6K, L, M), in agreement with the model that in the absence of Evi function, DFz2 is not properly transported to the muscle nucleus. Furthermore, a complete rescue of the DFz2-C nuclear spots to wild type levels was observed in the evi mutant by expressing the Evi transgene in the muscle alone. In contrast, expressing Evi in motorneurons provided only a partial rescue of the nuclear DFz2-C foci (Fig 6M). Therefore, we conclude that muscle Evi is involved in the trafficking of DFz2 to the nucleus.
Given the substantial similarities between the phenotypes observed upon knocking down evi and dgrip in postsynaptic DFz2 trafficking as well as in the synaptic morphology and NMJ growth (Ataman et al., 2006), we next examined whether interfering with Evi function could be disrupting the postsynaptic function of dGRIP. For these studies we examined the localization of dGRIP in larvae expressing Evi-RNAi in muscles. In wild type, dGRIP is present in small trafficking vesicles highly concentrated at postsynaptic sites (Fig. 7A, arrows; (Ataman et al., 2006)), as well as in Golgi bodies in juxtaposition to the cis-Golgi marker Lavalamp (Lva; Fig. 7A; arrowheads (Ataman et al., 2006)). Notably, we found that upon knocking-down Evi specifically in muscles, dGRIP was substantially reduced from postsynaptic sites as well as from Golgi bodies in muscle (Fig. 7B, C). In addition, dGRIP was localized throughout the muscle submembrane region in a diffuse manner (Fig. 7B, C). Thus, Evi controls dGRIP localization at the postsynaptic muscle region and in the absence of Evi function dGRIP is not normally localized to the Golgi and synapses, likely disrupting postsynaptic DFz2 trafficking. A prediction of this model is that overexpressing dGRIP in muscles should overcome some of the defects arising from the lack of Evi in muscles. To test this model we overexpressed dGRIP in muscles while downregulating Evi in these cells. We found that the DFz2 accumulation at the postsynaptic region of Evi-RNAi-post was completely rescued, and indeed the postsynaptic levels of DFz2 became significantly lower than wild type (Fig. 7D). In addition, both the number of synaptic boutons and nuclear DFz2C spots were partially rescued by overexpressing dGRIP in evi mutants (Fig. 7E, F).
Figure 7. Downregulating Evi in postsynaptic muscles alters the localization of dGRIP, and proposed function of Evi in the pre- and postsynaptic compartment.
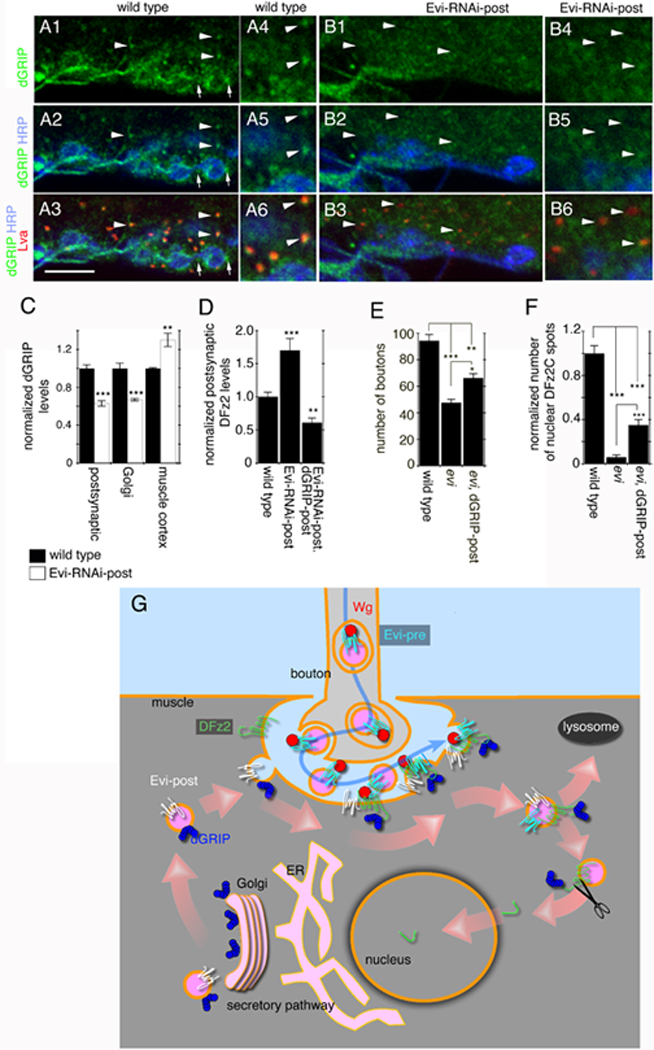
(A, B) Single confocal slices of NMJs triple labeled with antibodies to HRP, dGRIP and Lva in (A) wild type, and (B) Evi-RNAi-post (arrows in A= synaptic dGRIP; arrowheads in A, B= Golgi bodies. (C) dGRIP levels at the postsynaptic junctional region, Golgi bodies, and muscle cortex, in wild type and Evi-RNAi-post. (D) Postsynaptic DFz2 levels in wild type, Evi-RNAi-post and Evi-RNAi-post, dGRIP-post. (E) Bouton number in wild type, evi mutants, and evi mutants expressing dGRIP-post. (F) DFz2C spots in wild type, evi mutants, and evi mutants expressing dGRIP-post. (G) Proposed model for Evi function in the pre- and postsynaptic compartment (see text). Calibration bar is 6 µm for A-B1-3, and 3 µm for A-B4-6.
An additional prediction is that a population of Evi vesicles should traffic with dGRIP vesicles. To determine if this was the case we performed time lapse imaging of muscles expressing both Evi-GFP and dGRIP-mRFP. We found that in many instances Evi and dGRIP vesicles colocalized and followed the same trajectory (Suppl. Movie 3, Suppl. Movie 4). These results demonstrate that Evi, in addition to its important role in the Wg-secreting cell, has a critical function in Wg-target cells, as it mediates the transport of the downstream Wg signaling component, dGRIP.
DISCUSSION
Here we show that the multipass transmembrane protein Evi has a critical role in trans-synaptic Wnt-1/Wg transport through vesicular structures. To our knowledge, this is the first report to identify trans-synaptic communication through a vesicular structure. Further, our studies highlight a novel mechanism by which secreted factors can be transmitted from cell to cell. We propose that presynaptic Evi is required for trafficking Wg from the cell body to the presynaptic terminals, and across the synaptic cleft, to present Wg to postsynaptic DFz2 receptors (Fig. 7G). On the other hand, postsynaptic Evi is required to transport dGRIP to postsynaptic sites. At the postsynaptic region dGRIP interacts with postsynaptic DFz2 receptors and participates in the trafficking of DFz2 to the nucleus, where its C-terminal tail is cleaved, and imported to the nucleus (Fig. 7G).
Previous studies had implicated Evi only in the secretion of Wnts in Wnt-expressing cells (e.g., (Banziger et al., 2006)). However, endogenous Evi was also found in Wnt-target cells (Port et al., 2008), where it plays an as yet unidentified role. Our studies here identify an unprecedented role for Evi in Wg receiving cells in trafficking the Wg receptor DFz2 through the regulation of the synaptic targeting of the DFz2-interacting protein dGRIP, which was previously shown to function in transporting DFz2 receptors from the postsynaptic membrane to the muscle nucleus (Ataman et al., 2006). These studies unravel new processes and cellular mechanisms by which Evi functions as an essential component of synaptic Wnt signaling.
Trans-synaptic signaling in the nervous system: role of Wnts
Intercellular communication in the brain is primarily accomplished through the exocytosis of neurotransmitter-laden vesicles or by direct current conduction through gap junctions. Pre- and postsynaptic partners also release factors important for cell survival, synapse development, synapse maintenance, and synaptic plasticity (reviewed in Lu and Figurov 1997; reviewed in Marques 2005). Among these are neurotrophins such as Bone Derived Neurotrophic Factor (BDNF) and Nerve Growth Factor (NGF), members of the Bone Morphogenetic Protein (BMP) family and Wnts. These molecules are released from pre- or postsynaptic terminals and they function in retrograde or anterograde manners to influence synaptic growth, function and plasticity. At the Drosophila larval NMJ, continuous coordination of synaptic growth in relationship to muscle size requires the release of a retrograde signal of the BMP family which acts on BMP receptors in the presynaptic cell (Marques, 2005). This correlated synaptic growth is also controlled by the release of Wg, which is thought to act on DFz2 receptors in both the pre- and postsynaptic cells where it initiates alternative transduction pathways (Ataman et al., 2008; Franco et al., 2004; Miech et al., 2008).
A major gap in our understanding of how Wnts function is the mechanism by which they reach their destination once released. Despite the presence of charged amino acid residues in the primary sequence of Wnts, Wnts are hydrophobic molecules tightly bound to cell membranes due to the addition of palmitate moieties during maturation (Willert et al., 2003; Zhai et al., 2004). This hydrophobic nature of Wnts argues against the simple model of passive diffusion in the extracellular milieu. The studies presented here suggest that one mechanism for this transport is the association of Wg with Evi-containing vesicles, which are released from presynaptic boutons and become localized to postsynaptic sites. This model is supported by several lines of evidence. (1) Downregulating Evi in the presynaptic motorneurons (Evi-RNAi-pre) led not only to a reduction in Evi immunoreactivity inside presynaptic terminals, but also substantially reduced the label at the postsynaptic region. (2) Expressing Evi-GFP in motorneurons (Evi-GFP-pre) led to the localization of the GFP label in the postsynaptic junctional region in the form of puncta which colocalized with both the Evi-Cin and Evi-Nex antibodies, and which showed substantial colocalization with secreted Wg at the postsynapse. (3) Evi-GFP could be transferred from transfected to untransfected S2 cells, and S2 cell culture medium contained full-length Evi protein, suggesting that Evi was also secreted in cultured cells. In addition, the secreted and transferred Evi vesicles contained Wg. This trans-synaptic transfer of a synaptogenic signal through specialized vesicles containing a dedicated membrane protein is a novel signaling mechanism in the nervous system that might be used for a number of secreted signaling factors.
The release of endosomal vesicles, called exosomes, has been reported in a variety of tissues, including cultured neurons (Faure et al., 2006; Fevrier and Raposo, 2004; van Niel et al., 2006). These exosomes are released by the fusion of MVBs with the plasma membrane and are thought to be involved both in the removal of cellular debris as well as in intercellular communication. For example, in the immune system integrin- and MHC-containing exosomes are used for antigen presentation, and they are able to prime T lymphocytes in vivo (van Niel et al., 2006). In cultured cortical neurons the release of exosomes containing the cell adhesion molecule L1, the GPI-anchored prion protein and the GluR2/3 subunit of glutamate receptors has been reported, in a process that is regulated by membrane depolarization (Faure et al., 2006). Our finding that exosome-like vesicles containing a synaptogenic factor are released at synapses provides a novel mechanism for trans-synaptic communication.
The mechanism by which Evi-containing vesicles are released from the presynaptic cell is not known, but a few potential possibilities are depicted in Fig. 3O. For example Evi might be transported within the presynaptic cell in multivesicular bodies (MVB) that fuse with the plasma membrane thus releasing the Evi vesicle. In turn, after presentation of Wg to DFz2 receptors, the vesicle might fuse with the postsynaptic membrane. Interestingly, we found that Evi in presynaptic terminals was present in multimembrane compartments. Similarly, in Wg-secreting wing disc epithelial cells, Evi (Franch-Marro et al., 2008) and Wg (van den Heuvel et al., 1989) have been shown to be localized within MVBs. We also found that Evi label was found in association with postsynaptic SSR membranes and in the form of approximately 200 nm vesicles in the SSR. Our internalization assays suggest that these vesicles are endocytosed from the postsynaptic membrane.
Cell-autonomous role of Evi in Wg-target muscles
Besides its involvement in transporting the Wg signal across the synapse, we also found that Evi had a cell-autonomous function in the postsynaptic target cell, as revealed by specifically downregulating Evi in muscle. In this case both Wg and DFz2 accumulated at the postsynaptic region. In addition, DFz2 did not traffic normally from the NMJ and the nuclear import of DFz2-C was largely abolished. These findings suggest that Evi, beyond the regulation of Wnt secretion, organizes further downstream signaling events in the Wg-target cell.
The phenotypes observed upon downregulating Evi in muscle cells were highly reminiscent of those observed upon loss of dGRIP function. Further, decreasing Evi levels led to the virtual elimination of synaptic and Golgi dGRIP. The evidence relating Evi to dGRIP function is further supported by the findings that Evi and dGRIP are often observed trafficking in the same vesicles, and that overexpressing dGRIP in either evi mutants or Evi-RNAi-post elicited partial rescue of phenotypes resulting from evi loss of function. Thus, Evi appears to be required for the trafficking of dGRIP to synaptic sites where dGRIP binds DFz2 receptors and functions to traffic them towards the nucleus. The elimination of dGRIP from the Golgi complex might arise from a defect in its recycling to the Golgi due to its abnormal targeting to synapses. In the absence of postsynaptic dGRIP, DFz2 is not trafficked towards the nucleus leading to its accumulation at postsynaptic sites. Interestingly, Evi has been demonstrated to be involved in trafficking Wg from the Golgi to the plasma membrane in Wg-secreting cells (Belenkaya et al., 2008; Franch-Marro et al., 2008; Pan et al., 2008; Port et al., 2008; Yang et al., 2008). Our studies showing that Evi is required for the trafficking of dGRIP to postsynaptic sites suggest that Evi might have a role not solely in transporting Wnts, but also in trafficking components associated with Wnt pathways.
Although this study identifies a pre- and postsynaptic role for Evi, it is clear that both roles are not completely independent. For example, we found that restoring Evi levels only in the motorneurons of evi mutants was sufficient for a complete rescue of the ghost bouton phenotype and resulted in a partial rescue in the number of nuclear DFz2C spots. These results were surprising given that in the absence of postsynaptic Evi, dGRIP does not traffic normally, and thus interferes with postsynaptic Wnt signaling. A potential explanation is that the transferred presynaptic Evi can partially compensate for the lack of Evi in the postsynaptic cell.
In conclusion, our studies identify a novel mechanism by which the Wnt-1/Wg signal is transmitted across the synapse, through the use of an Evi vesicle, and find an additional cell-autonomous role of Evi in Wnt-receiving cell, the synaptic recruitment of dGRIP, which functions in transporting the signal to the muscle nucleus.
MATERIALS AND METHODS
Fly Strains
Flies were reared in standard Drosophila media at 25°C unless otherwise stated. (See Suppl. Materials for fly strains.) RNAi crosses and controls were performed at 29°C. The wgts flies were tested at the restrictive temperature (25°C).
Cytochemistry
Third instar larvae were dissected in Ca2++-free saline and fixed in either 4% Paraformaldehyde or non-alcoholic Bouin’s fixative (see Suppl. Methods for antibodies and Hoechst conditions).
Image Quantification
Confocal images were aquired using a Zeiss Pascal Confocal Microscope. Preparations from different genotypes were processed simultaneously and imaged using identical confocal acquisition parameters. Fluorescence signal intensity was quantified by volumetric measurements of confocal stacks using Volocity 4.0 Software (see Suppl. Materials). Measurements were taken from muscles 6 and 7, abdominal segment 3. A Student’s t test was performed for pair-wise comparisons between each genotype and controls. Error bars in the histograms represent mean±SEM, where ***= p<0.0001; **= p<0.001; *=p<0.05.
Schneider-2 (S2) cell cultures
S2 cells were transfected as described in Suppl Materials. For Evi-GFP transfer experiments, pAc-Evi-EGFP (Bartscherer et al., 2006) transfected S2 cells were washed 24 hours after transfection and mixed with pAc-mCherry transfected S2 cells. For co-transfection experiments we used, pAc-Wg (Bartscherer et al., 2006), pAc-Evi-EGFP, pAc-rCD2-RFP and pAc-DFz2-myc (Mathew et al., 2005). Cells were then grown for 24–48 hours and processed for immunocytochemistry.
Live Imaging
Live imaging of transfected S2-cells and body wall muscles was performed using an Improvision Spinning Disk confocal microscope as described in Suppl. Materials.
Western blots
Western blots were performed as in (Mendoza-Topaz et al., 2008). For examination of Evi in S2 cells and the culture medium, transfected cells were washed with fresh medium 24 hours after transfection, and 24 to 48 hours later cells and medium were harvested for immunoblotting (Suppl. Materials).
Immunoelectron microscopy
For the pre-embedding technique, third instar body wall muscles were fixed and incubated with anti-Evi-Nex (1:100) or anti-GFP (1:300) followed by anti-rabbit IgG-1.4 nm-nanogold (1:50; Nanoprobes, NY), and intensification using HQ silver reagents (Nanoprobes, NY). The EM Internalization assay was performed as above, except that 1.4 nm nanogold secondary antibody was used after permeabilization. For the post-embedding technique, samples were fixed and then embedded in LR White resin followed by antibody staining on grids with secondaries conjugated to 18nm gold (1:75; Jackson). Transmission electron microscopy analysis was performed as described by (Torroja et al., 1999). See Suppl. Materials for further details.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Marc Freeman, Michael Francis, and Motojiro Yoshihara as well as members of the Budnik lab for helpful comments and critical discussion on the manuscript. We especially thank John Nunnari for great help with immunoelectron microscopy, Yuly Fuentes for providing the images with UAS-LacZ-NLS, and Dr. Sean Speese for help with the qPCR. We also thank Dr. Michael Boutros for providing the evi2 and Evi-EGFP fly lines as well as the Evi-EGFP and Wg plasmids for studies in S2 cells. This work was supported by NIH grant RO1 MH070000 to VB. Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ataman B, Ashley J, Gorczyca D, Gorczyca M, Mathew D, Wichmann C, Sigrist SJ, Budnik V. Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proc Natl Acad Sci U S A. 2006;103:7841–7846. doi: 10.1073/pnas.0600387103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312:921–924. doi: 10.1126/science.1124856. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco B, Bogdanik L, Bobinnec Y, Debec A, Bockaert J, Parmentier ML, Grau Y. Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila. J Neurosci. 2004;24:6573–6577. doi: 10.1523/JNEUROSCI.1580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi M, Piddini E, Vincent JP. Endocytosis: a positive or a negative influence on Wnt signalling? Traffic. 2008;9:1–9. doi: 10.1111/j.1600-0854.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, Spana EP, Selva EM. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Greco V, Hannus M, Eaton S. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106:633–645. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- Lyu J, Yamamoto V, Lu W. Cleavage of the Wnt receptor Ryk regulates neuronal differentiation during cortical neurogenesis. Dev Cell. 2008;15:773–780. doi: 10.1016/j.devcel.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Marques G. Morphogens and synaptogenesis in Drosophila. J Neurobiol. 2005;64:417–434. doi: 10.1002/neu.20165. [DOI] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Topaz C, Urra F, Barria R, Albornoz V, Ugalde D, Thomas U, Gundelfinger ED, Delgado R, Kukuljan M, Sanxaridis PD, et al. DLGS97/SAP97 is developmentally upregulated and is required for complex adult behaviors and synapse morphology and function. J Neurosci. 2008;28:304–314. doi: 10.1523/JNEUROSCI.4395-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech C, Pauer HU, He X, Schwarz TL. Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction. J Neurosci. 2008;28:10875–10884. doi: 10.1523/JNEUROSCI.0164-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development. 1997;124:871–880. doi: 10.1242/dev.124.4.871. [DOI] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell. 2008;14:132–139. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. Presynaptic spectrin is essential for synapse stabilization. Curr Biol. 2005;15:918–928. doi: 10.1016/j.cub.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Narayanan R, Consoulas C, Ramaswami M. Evidence for cell autonomous AP1 function in regulation of Drosophila motor-neuron plasticity. BMC Neurosci. 2003;4:20. doi: 10.1186/1471-2202-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Yamazaki T, Citron M, Podlisny MB, Koo EH, Teplow DB, Haass C. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann N Y Acad Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Perrimon N. Drosophila wingless: a paradigm for the function and mechanism of Wnt signaling. Bioessays. 1994;16:395–404. doi: 10.1002/bies.950160607. [DOI] [PubMed] [Google Scholar]
- Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroja L, Chu H, Kotovsky I, White K. Neuronal overexpression of APPL, the Drosophila homologue of the amyloid precursor protein (APP), disrupts axonal transport. Curr Biol. 1999;9:489–492. doi: 10.1016/s0960-9822(99)80215-2. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Nusse R, Johnston P, Lawrence PA. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell. 1989;59:739–749. doi: 10.1016/0092-8674(89)90020-2. [DOI] [PubMed] [Google Scholar]
- van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem. 2004;279:33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- Zhao C, Aviles C, Abel RA, Almli CR, McQuillen P, Pleasure SJ. Hippocampal and visuospatial learning defects in mice with a deletion of frizzled 9, a gene in the Williams syndrome deletion interval. Development. 2005;132:2917–2927. doi: 10.1242/dev.01871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


