Abstract
Objective
Smooth muscle cell (SMC) differentiation is a dynamic process that must be tightly regulated for proper vascular development and to control the onset of vascular disease. Our lab previously reported that a specific focal adhesion kinase (FAK) inhibitor termed FRNK (FAK Related Non-Kinase) is selectively expressed in large arterioles when SMC are transitioning from a synthetic to contractile phenotype and that FRNK inhibits FAK-dependent SMC proliferation and migration. Herein, we sought to determine whether FRNK expression modulates SMC phenotypes in vivo.
Methods and Results
We present evidence that FRNK−/− mice exhibit attenuated SM marker gene expression during post-natal vessel growth and following vascular injury. We also show that FRNK expression is regulated by TGF-β and that forced expression of FRNK in cultured cells induces serum- and TGF-β-stimulated SM marker gene expression, while FRNK deletion or expression of a constitutively activated FAK variant attenuated SM gene transcription.
Conclusions
These data highlight the possibility that extrinsic signals regulate the SMC gene profile, at least in part, by modulating the expression of FRNK and that tight regulation of FAK activity by FRNK is important for proper SMC differentiation during development and following vascular injury.
Keywords: integrins, smooth muscle, differentiation, vascular remodeling
While medial smooth muscle cells (SMC) found in the mature vessel are fully differentiated and express high levels of SM contractile proteins, these cells do not terminally differentiate and can transition to a synthetic phenotype characterized by low levels of SM contractile gene expression and responsiveness to pro-growth and migratory signals. This unique plasticity is critical for proper vessel development, blood pressure homeostasis, and injury repair processes.1,2
A number of secreted growth factors (i.e. platelet derived growth factor (PDGF-BB), sphingosine 1-phosphate (S1P), transforming growth factor-β (TGF-β) and contractile agonists (angiotensin II, endothelin-1 and thrombin) have been shown to regulate SMC phenotype in vitro and in vivo.3 In addition to levels of circulating factors, studies have also shown that the extracellular matrix (ECM) that surrounds SMC in the vessel wall can impart control over SM phenotypes.4 Indeed, genetic ablation of either fibronectin or the α5 integrin fibronectin receptor results in embryonic lethality associated with impaired SMC investment of both embryonic and extraembryonic vessels.5, 6 Although the precise signaling mechanisms by which these diverse agonists and ECM regulate SMC transcription have not been completely delineated, several studies indicate that many (but not all) SM-specific genes (particularly contractile genes) depend on the presence of an serum response factor (SRF) DNA binding element termed a CArG box (CC(A/T)6GG) and many of these aforementioned intrinsic factors alter SRF or SRF-cofactor activity.3, 7–10
One of the major proteins involved in the integrin intracellular signaling cascade is the non-receptor protein tyrosine kinase, focal adhesion kinase (FAK), which is strongly and rapidly activated by various aforementioned growth factors and by ligation of all β1, β3 or β5 containing integrins.11 Although a direct role for FAK in vascular growth and development has yet to be examined, germline deletion of FAK phenocopies the lethal defects observed in fibronectin−/− and α5 integrin−/− embryos. Interestingly, our lab recently showed that FAK activity is regulated in a unique fashion in SMC, whereby a separate protein comprising the carboxyterminus of FAK, termed FRNK (FAK Related Non Kinase) that acts as a dominant-interfering mutant for FAK is selectively expressed in SMC with very high levels found in the large arterioles. FRNK transcription results from the utilization of an alternative start site within the FAK gene and FRNK expression is independently regulated by a distinct promoter embedded within FAK intronic sequences.12, 13 Whereas FAK protein levels remain relatively constant during vascular development, FRNK protein levels are dynamically increased in neonatal vessels and in adult vessels two weeks following injury, when SMC are transitioning from a synthetic to contractile phenotype.
The aim of this study was to determine whether FRNK expression plays a direct role in the phenotypic modulation of SMC in vivo. Herein we present evidence that FRNK−/− mice exhibit repressed SM marker gene expression during post-natal vessel growth and following vascular injury. These data highlight the possibility that extrinsic signals regulate the SMC gene profile by modulating the dynamic expression of FRNK.
METHODS
Mice were housed in an AALAC accredited University Animal Care Facility and all experimental procedures were approved by the University of North Carolina animal care and use committee (IACUC). All quantitative data represent at least three separate experiments presented as mean +/− SEM. Means were compared by 2-tailed Students’s t test and p<0.05 was considered statistically significant as indicated by an asterisk. All other data including Western analysis are representative of at least three individual experiments. Please see Online Supplemental Data section for a complete description of the animal models, reagents, DNA constructs, and general methods used for these studies.
RESULTS
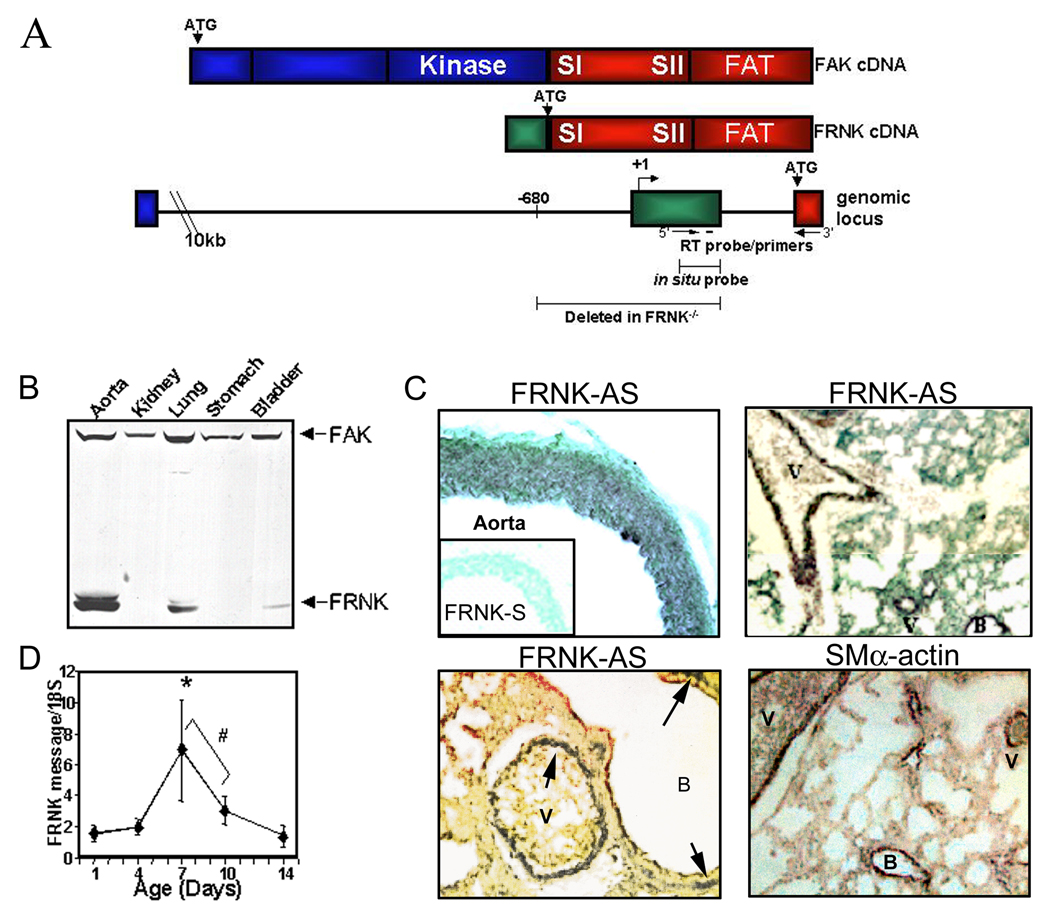
The 41/43 kDa FRNK protein is selectively expressed in the vasculature from approximately E12.5 onward with highest levels observed in large arterioles and lung of neonatal rats (post-natal day 4–14) (Fig 1B).12,14 FRNK protein was also detected in cells derived from human aorta and coronary arteries and coronary SMC cultures derived from explanted pro-epicardial organs, but not in cultured endothelial cells (please see www.ahajournals.org, online Fig I). As previously reported (and depicted in Fig 1A), FRNK expression is regulated by a promoter embedded within the fak gene and frnk transcription initiates from a non-coding exon located approximately 300 bp upstream of the FRNK translational start site in the mouse gene. 12, 13 Since FRNK shares the same amino acid sequence as the C-terminus of FAK, we have been unable to develop a specific antibody that recognizes FRNK (but not FAK) for immunohistochemical analysis of FRNK expression patterns. However, since the frnk non-coding exon is selectively protected in RNA isolated from SMC and SMC-containing tissues,12 we developed an in situ probe directed against this unique sequence to examine FRNK expression patterns in vivo. Our in situ analysis of tissues harvested from post-natal day 7 mice revealed that FRNK is expressed throughout the media of large arterial vessels, but is not expressed in either the endothelium or adventitial layer (Fig 1C, top). Some visceral SMC staining was apparent as well, with high levels observed in the lung (Fig 1C) and bladder (not shown). FRNK expression in the lung was localized to the smooth muscle lining of the bronchi in addition to vessels, similar to the staining pattern observed for SMα-actin. 16 Further analysis of FRNK expression in the developing mouse vasculature using quantitative RT-PCR revealed a dynamic up-regulation of FRNK mRNA in aorta between 7 and 10 days post-natal, corroborating our previous Western analysis (Fig 1D).
Figure 1. FRNK is expressed selectively in smooth muscle containing-tissues.
A. Schematic of FAK and FRNK cDNA and genomic locus. Shaded areas represent FAK-specific (blue), FRNK-specific (green), or common (red) coding regions. B. Western analysis of 14d postnatal rat tissues. C. In situ hybridization for FRNK and SMα-actin in postnatal day 7 mouse aorta and lung. D. Quantitative RT-PCR for FRNK in thoracic aorta. (Mean +/− SEM n >/= 4, * p < 0.01, # p < 0.05).
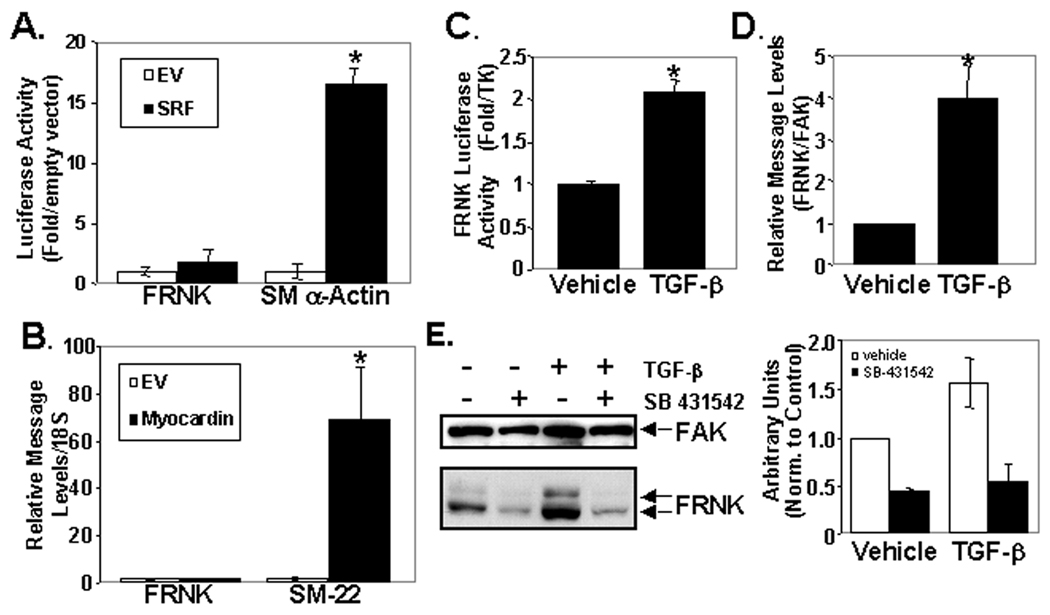
We reasoned that up-regulation of FRNK in the neonatal vessels may serve to buffer FAK-dependent signals in this environment that is particularly rich in growth factors and matrices known to be up-stream activators of FAK. Thus we sought to identify some of the mechanisms involved in the transient and dynamic regulation of FRNK expression in post-natal vessels. To determine the relative stability of FRNK protein, we treated SMC with cyclohexamide for various times and analyzed FRNK and FAK levels. In support of the idea that FRNK expression can be tightly regulated, we found that FRNK protein turnover is relatively rapid, with an apparent half-life of approximately 4.5 hr (please see www.ahajournals.org, online Fig IIA). The level of FAK protein was not significantly changed during the 8 hr time-course examined, consistent with previous reports that FAK protein is extremely stable with a half-life exceeding 20 hr26. We next sought to identify factors that can modulate dynamic FRNK expression during vascular morphogenesis. We first utilized a FRNK promoter reporter construct to define factors that regulate FRNK transcription. A fragment comprising approximately 6kb of sequence upstream of the FRNK ATG (−5388 to +876) was previously shown to drive SM-specific expression of LacZ in vivo in a pattern reminiscent of FRNK expression.14 Importantly, we found that the corresponding FRNK promoter attached to a luciferase reporter (FRNK-Luc) exhibited high activity in cultured SMC in comparison to 10T1/2 cells (consistent with the levels of FRNK expressed in these two cell types, please see www.ahajournals.org, online Fig IIB). Although FRNK is expressed in a SM-restricted fashion, careful analysis of the FRNK promoter region from chicken, mouse and human sequence did not reveal any conserved CArG elements, known to direct SRF-dependent transcription. Indeed, co-expression of SRF and FRNK-Luc in SRF−/− ES cells did not alter basal FRNK-Luc promoter activity in these cells, while reconstitution of SRF induced expression of a SMα-actin-Luc construct approximately 15-fold (Fig 2A). Moreover, when expressed in SMC, the FRNK-Luc construct was un-responsive to over-expression of the myocardin family of SRF co-factors, which stimulated strong activation of the SMα-actin-Luc reporter (please see www.ahajournals.org, online Fig IIC). Furthermore, ectopic expression of myocardin did not alter FRNK message levels but induced a striking 60-fold increase in SM22 message (Fig 2B). Collectively, these data provide strong support for FRNK being expressed in a non-SRF/CARG dependent fashion as previously suggested.22
Figure 2. FRNK expression is regulated by TGF-β but is SRF/CArG independent.
A. SRF−/− ES cells were co-transfected with indicated constructs and processed for luciferase activity. B. qRT-PCR in 10T1/2 cells transfected with Flag or Flag-Myocardin. C,D. FRNK luciferase assay in 10T1/2 cells (C) or qRT-PCR in rat aortic SMC dosed with vehicle or TGF-β (1 ng/mL) for 24 hr in media containing 0.2% serum. E. Western analysis of rat SMC pretreated with SB 431542 for 15 min and then dosed with TGF-β (1 ng/mL) or vehicle for 48 hours. N>/=3.
We next screened a number of cytokines/growth factors known to be released following vessel injury for their ability to increase FRNK promoter and protein levels in cultured rat aortic SMC. We found that TGF-β induced a marked increase in FRNK-Luc activity, FRNK mRNA (as assessed by quantitative RT-PCR) and protein levels compared to vehicle treated cells (Fig 2C–E), while other agonists including the potent SMC mitogens PDGF-BB, S1P, angiotensin II, thrombin, and basic-fibroblast growth factor had no effect (data not shown). The ability of SB 431542 (a selective inhibitor of the TGF-β1 activin receptor-like kinase; ALK-5) to dramatically reduce expression of FRNK in cultured SMC under serum-starved conditions or following TGF-β treatment strongly supports the idea that TGF-β is a major regulator of FRNK expression (Fig 2E).
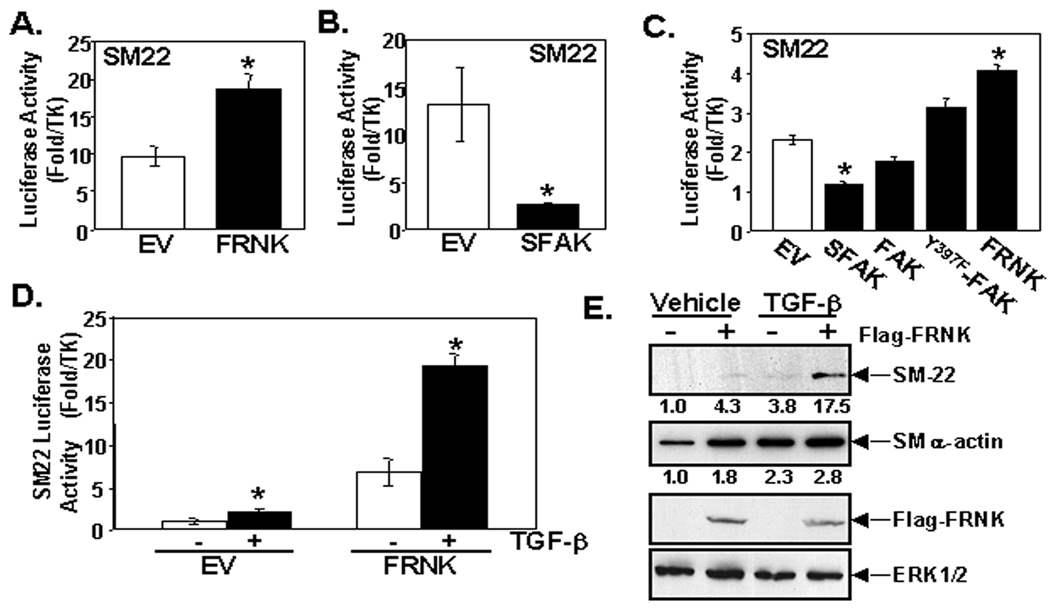
Since TGF-β induces FRNK expression and SM differentiation, we theorized that FRNK might promote SMC phenotypic switching from a synthetic to contractile state. To directly explore this possibility, we performed SMC promoter-reporter assays in 10T1/2 cells, a multi-potential SMC precursor line that has been shown to markedly up-regulate SMC-specific gene expression upon stimulation with serum, S1P, or TGF-β.19, 20 While FRNK protein is not expressed in detectible levels in 10T1/2, FRNK message is detectible by quantitative RT-PQR (data not shown) and forced expression of FRNK in these cells inhibited FAK activity and resulted in a two to four-fold increase in SM22, SMα-actin and SM-MHC promoter activity, without effecting myocardin levels (Fig 3A, please see www.ahajournals.org, online Fig IIIA,B). We reasoned that FRNK expression likely promotes SMC differentiation by relieving FAK-dependent repressive signals. To determine whether FAK activation limits SM marker gene expression we ectopically expressed a constitutively active FAK variant (termed SuperFAK21) that leads to enhanced FAK activity as assessed by phosphorylated Y397FAK levels. As expected, SuperFAK expression in 10T1/2 cells significantly reduced SM22 (Fig 3B) and SMα-actin reporter gene expression (please see www.ahajournals.org, online Fig IIIC). Similarly, ectopic expression of FRNK and FAK variants in primary rat aortic SMC revealed an inverse relationship between FAK activity and SM gene expression (i.e. low FAK activity correlates with high levels of SM markers, Fig 3C, please see www.ahajournals.org, online Fig IIID). Taken together, our findings provide compelling evidence that FAK activity limits SMC differentiation, and up-regulation of FRNK promotes differentiation by attenuating FAK activity.
Figure 3. SM marker gene expression is induced by FRNK expression.
A–D. SM-22 luciferase assay in 10T1/2 cells (A, B, D) or rat aortic SMC of cells (C) performed in 10% serum-containing media (A–C) or media containing 0.2% serum and treated with vehicle or TGF-β (1 ng/mL) for 24 hrs (D). Mean +/− SEM; n>/=3. E. Western analysis of 10T1/2 cells transfected with Flag or Flag-FRNK and treated with TGF-β (1 ng/mL) for 48 hrs in media containing 0.2% serum. Densitometry of 4 separate experiments is indicated numerically.
We next examined TGF-β stimulated SM gene expression in our cell culture models. We found that TGF-β and FRNK acted in concert to induce SM22 reporter activity in 10T1/2 cells (Fig 3D). Importantly, similar results were observed when we analyzed endogenous SM gene expression by Western analysis. SM22 protein was un-detectible in 10T1/2 cells under basal conditions, but either expression of FRNK or treatment of cells with TGF-β for 48 hours induced a modest increase in SM-22, and when combined, these agents markedly increased SM22 expression (Fig 3E). FRNK and TGF-β each individually led to a similar marked induction of SMα-actin protein levels in 10T1/2 cells, however, the combined treatment had a more modest effect, most likely due to the already high levels of SMα-actin induced by each factor alone. Collectively, these data provide support for a model whereby TGF-β induces FRNK expression and that FRNK, in turn, contributes to TGF-β induced SMC differentiation by dampening FAK-dependent signals.
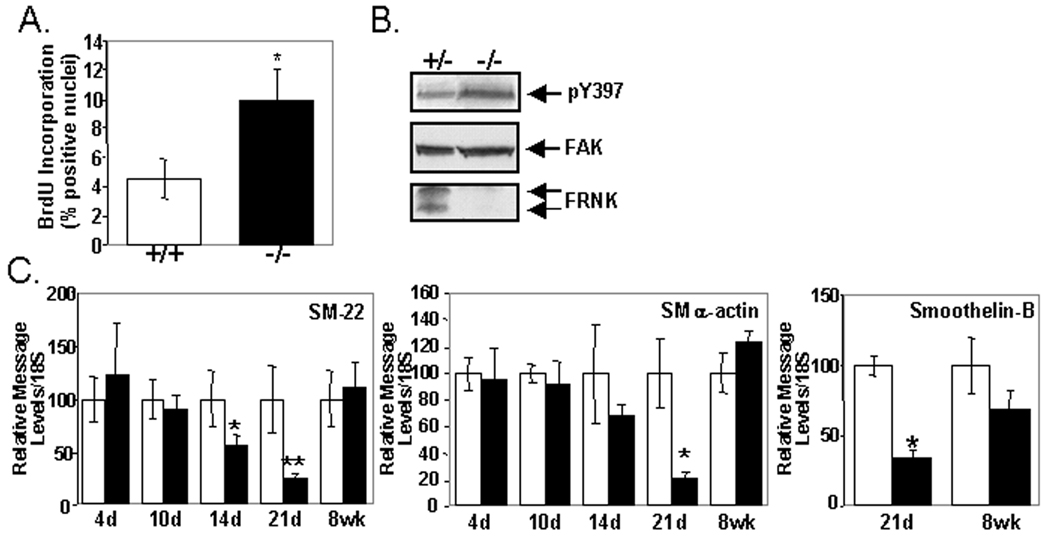
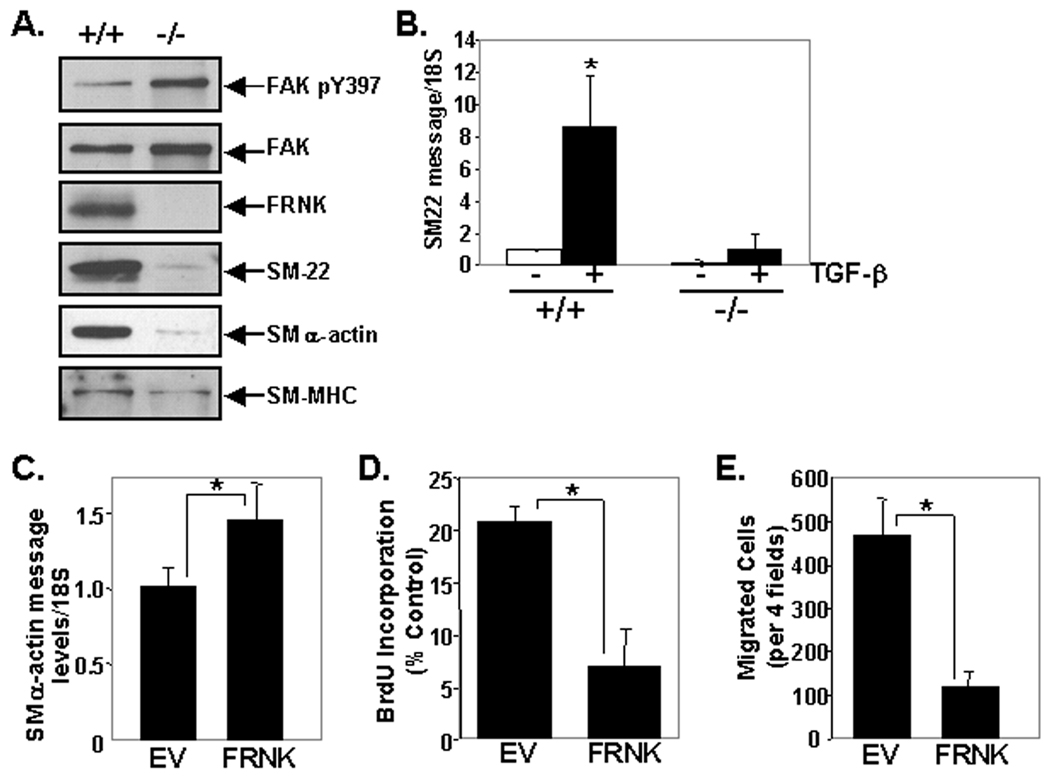
We next strove to evaluate FRNK's function to regulate SMC phenotype in post-natal vessels. FRNK−/− mice were recently generated by a strategy that resulted in deletion of a 1kb fragment that included the non-coding exon and 700 bp of upstream fak intronic sequence (see schematic in Fig 1A). Although these mice were born in the expected Mendelian frequency and showed no gross phenotype, they were not specifically examined for defects in SMC differentiation marker gene expression or growth. 14 Since the timing of FRNK expression during development and following vascular injury correlates with the conversion of SMC from a synthetic to contractile phenotype, we first examined the proliferation index of medial SMC in post-natal vessels from wild type or FRNK−/− mice. As shown in Figure 4A, medial SMC in postnatal FRNK−/− vessels exhibit a significantly higher index of BrdU incorporation in comparison to littermate control vessels. Importantly, SMC tissue from postnatal FRNK−/− pups reveal lack of FRNK protein and higher levels of active pY397FAK, in comparison to wild type littermate controls, while total FAK protein levels are unaltered (Fig 4B).
Figure 4. FRNK−/− mice exhibit increased SM proliferation and attenuated expression of SM marker genes during development.
A. BrdU incorporation in post-natal day 4 thoracic aorta collected from wild type (+/+) or FRNK−/− (−/−) mice (Mean +/− SEM (n=5, p<0.03). B. Western analysis in post-natal day 4 bladders from heterozygous (+/−) or FRNK−/− (−/−) pups. C. Quantitative RT-PCR for indicated SM differentiation markers in +/+ and −/− thoracic aorta. Data is normalized to expression of 18S and is expressed as the mean percent of wild type expression at each time point +/− SEM (n > 4 for each time point, ** p < 0.01, * p < 0.05).
We next analyzed the dynamic expression of the contractile genes, SMα-actin, SM-22, and smoothelin B in the aorta of post-natal control and FRNK−/− mice by quantitative RT-PCR. Expression of these contractile genes is enhanced approximately 6–10 fold from 4 day post-natal to adult vessels in wild type mice (data not shown). Interestingly, we found that FRNK−/− vessels contain significantly fewer transcripts of these genes in the 2–3 week period of vessel development when compared to age and littermate controls, when FRNK protein is most highly abundant in wild type vessels (Fig 4C).12 However, no significant differences in SM marker gene expression was observed in adult aorta (see data for 8 week animals in Fig 4C) or carotid vessels isolated from wild type or FRNK−/− mice (Fig 5A), indicating that the lag in SM differentiation observed in the neonatal vessels was normalized during vessel maturation. In spite of these transient significant differences in SMC phenotype, we did not detect any significant difference in medial thickness of FRNK−/− adult arterioles (aorta or carotids) in comparison to littermate control vessels analyzed from 7 days post-natal to adult (please see www.ahajournals.org, online Fig IV). Also, we found that adult FRNK−/− mice did not exhibit significant changes in mean systolic blood pressure (as assessed by tail cuff measurements) or pressor responses (as assessed by intra-aortic catheterization) following phenylephrine treatment in comparison to wild type littermate controls (please see www.ahajournals.org, online Fig IV), indicating that FRNK expression does not function to regulate vascular homeostasis in adult mice (see online Supplemental Data section for further discussion).
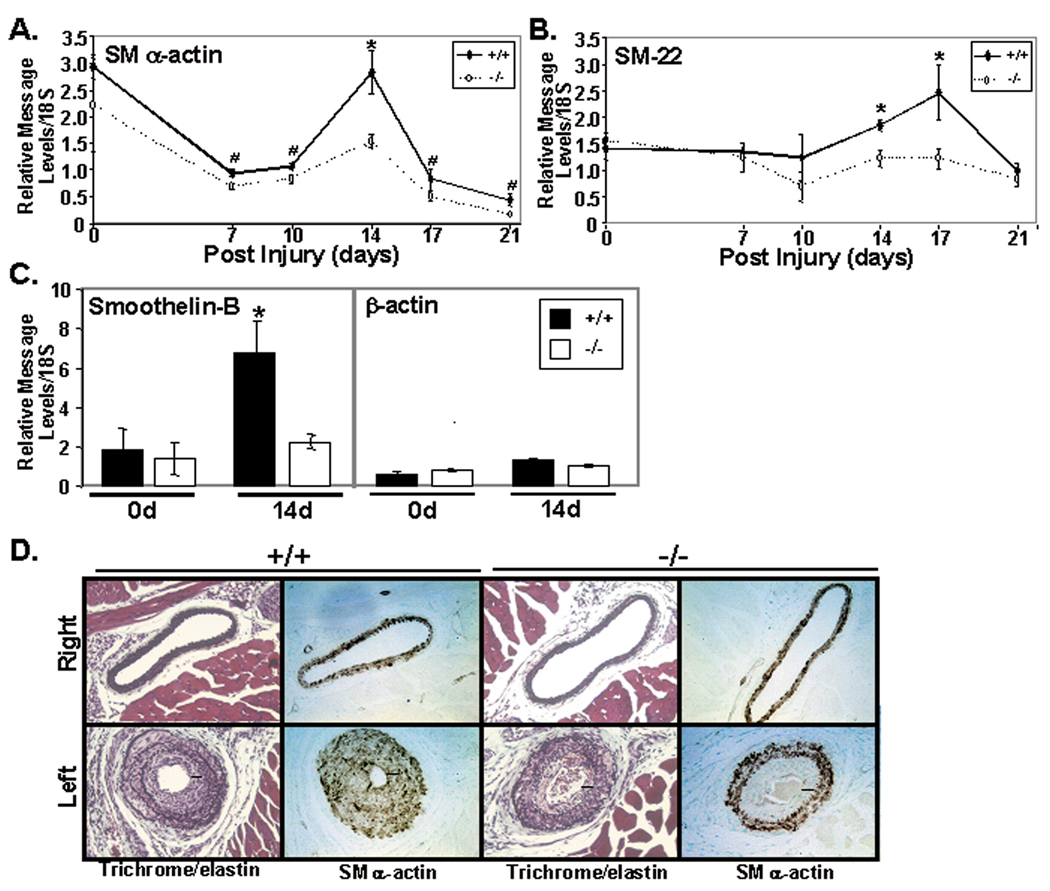
Figure 5. FRNK−/− mice show decreased expression of SM marker genes following carotid artery ligation.
Wild type (+/+) or FRNK−/− (−/−) mice were subjected to left carotid blood flow. A-C. qRT-PCR for the indicated SM markers and β-actin (control). Data is presented as the left carotid normalized to expression in the wild type right (control) carotid artery at each timepoint +/− SEM (n >4, * p<0.01, # p<0.05). D. Verhoeff’s elastin and immunohistochemistry for SM α-actin in carotids from wild type (+/+) or FRNK−/− (−/−) mice 14 days following ligation. Black bars indicate thickness of neointima. Data are representative of at least three separate animals from each genotype.
Since we previously reported that FRNK expression is relatively low in adult vessels, but is dramatically up-regulated by 14 days following catheter-induced vessel injury, we postulated that FRNK might also regulate SMC phenotypic switching during restenosis. Numerous studies have revealed that contractile gene expression is dynamically regulated following vascular injury induced by either vessel ligation or surgical endothelial denudation. Typically, reduced SMC marker gene expression is apparent within 3–7 days following injury followed by a burst of re-expression after approximately 2–3 weeks.17, 18 To examine a possible involvement of FRNK in the regulation of SMC re-differentiation following vascular injury, we performed quantitative RT-PCR for SM marker genes in wild type and FRNK−/− carotid arteries 7–21 days following carotid ligation. As shown in figure 5, significant decreases in re-expression of SMα-actin, SM-22, and smoothelin B (but not β-actin) were observed in FRNK−/− vessels in comparison to littermate controls, indicating that the recovery of SM differentiation was defective in injured FRNK−/− arteries. Notably, no significant differences in SM gene expression were observed in the control un-ligated right carotid artery at the time points examined (data not shown). Concomitantly, immunohistochemical analysis for SMα-actin revealed a striking difference between wild type and FRNK−/− vessels 14 days after ligation. While, robust SMα-actin staining was observed throughout the media and neo-intima of wild type vessels, SMα-actin expression in the neoinitma of FRNK−/− vessels was markedly reduced (Fig 5D). While we anticipated that this phenotype might lead to a more exacerbated injury response, morphometric analysis at this time point revealed no significant differences in the extent of remodeling induced by ligation of wild type and FRNK−/− vessels, likely due to the fact that the ligation induces a very strong proliferative response in the wild type mice (please see www.ahajournals.org, online Fig V). Indeed, by 21 days after ligation full occlusion of injured vessels was observed in both wild type and FRNK−/− mice. Collectively, these data provide strong support for the hypothesis that up-regulation of FRNK expression following vessel injury is required for appropriate SMC maturation that occurs subsequent to injury repair.
Finally, we utilized cultures derived from FRNK−/− mice to confirm our previous in vitro and in vivo findings. We found that aortic SMC isolates from FRNK−/− mice consistently exhibited enhanced pY397FAK, and lower levels of the contractile proteins SM22, SMα-actin, and SM-MHC than SMC isolates from aged matched (and passage-matched) control mice (Fig 6A), while these cells maintained similar morphology (please see www.ahajournals.org, online Fig VI). The low levels of SMC proteins in FRNK−/− SMC may reflect the inability of these cells to maintain a differentiated phenotype in these pro-growth culture conditions (see online Supplemental Data section for further discussion). In support of the notion that FRNK acts to enhance TGF-β dependent gene expression, we found that TGF-β-induced expression of endogenous SM22 was significantly decreased in FRNK−/− SMC in comparison to matched wild type SMC isolates (Fig 6B). We also noticed that the FRNK−/− cells exhibited high growth rates and were more motile than control SMC isolates (data not shown). To directly test that these differences were due to lack of FRNK expression, we next re-constituted FRNK in FRNK−/− cells. As shown in figure 6 C–E, re-expression of FRNK in these cultures significantly increased SMα-actin expression, but significantly decreased both BrdU incorporation and PDGF-stimulated motility.
Figure 6. Cultured SMC isolated from FRNK−/− mice exhibit a synthetic phenotype.
A. Western analysis of primary cell isolates harvested from 6 week post-natal wild type (+/+) or FRNK−/− (−/−) mice (data are representative of three separate cell isolates per genotype). B. qRT-PCR for SM-22 message in wild type (+/+) or FRNK−/− (−/−) cells serum starved and treated with TGF-β for 24 hr. C–E. FRNK−/− cells infected with GFP or GFP-FRNK adenovirus were subjected to qRT-PCR (C), BrdU incorporation (D) or chemotaxis (E) assays. Data are presented as mean +/− SEM (n=3).
DISCUSSION
FRNK, a dominant interfering mutant that attenuates FAK activity, exhibits selective and dynamic expression in SMC. We previously reported that FRNK attenuates SMC proliferation and migration by regulating FAK/Rac1-dependent signaling.23 Herein we have found an additional function for FAK/FRNK signaling in SMC. We present evidence that FRNK deletion (by homologous recombination) represses SM gene expression during post-natal vessel growth and following vascular injury. We also show that FRNK expression is regulated by TGF-β and that forced expression of FRNK induces SM marker gene expression in cultured cells grown in serum and enhances TGF-β-stimulated SM marker gene expression. Conversely, FRNK deletion or expression of a constitutively activated FAK variant attenuated SM gene transcription. These data suggest that enhanced FAK activity is permissive for SMC growth and migration, but limits SM differentiation, and that tight regulation of FAK activity is likely important for proper SMC phenotypic modulation during development and following vascular injury.
FAK is activated by a process that involves dimerization and intermolecular phosphorylation of tyrosine 397 in trans.24 Phosphorylation of Y397 results in subsequent recruitment of the tyrosine kinase Src (and/or Fyn), which phosphorylates and further activates FAK (and phosphorylates certain FAK binding partners).11 FRNK likely attenuates FAK activity by inducing the formation of FRNK/FAK heterodimers that are incapable of Y397 phosphorylation and Src binding. In support of this notion, expression of wild type FAK (and Src) can rescue FRNK-dependent inhibition of FAK (and paxillin) phosphorylation, while expression of a phospho-deficient Y397FFAK cannot.25 Our findings indicating that FAK activity is enhanced in tissues and cells derived from FRNK−/− mice strongly supports the hypothesis that the dynamic regulation of FRNK expression can impart specific spatial and temporal control of FAK activity in vivo. In this regard, it is interesting to note that FAK protein is extremely stable with a reported half-life exceeding 20 hr,26 while we found that FRNK protein turnover is rapid. We speculate that transient FRNK expression is particularly important in the vasculature, where direct apposition of SMC with extracellular matrix could lead to high levels of FAK activation and un-controlled SM growth. It is feasible, however, that FRNK has additional FAK-independent functions, and future studies will examine this possibility.
In support of the idea that FRNK/FAK signaling plays an active role in regulating smooth muscle cell phenotypes, FAK−/− mouse embryonic fibroblasts were recently reported to exhibit a myo-fibroblast appearance as assessed by high levels of SM α-actin containing stress fibers relative to control fibroblasts.27 Interestingly, we recently found that FAK-deficient SMC (like FRNK over-expressing SMC) exhibit enhanced TGF-β stimulated SM marker gene expression (un-published observations; see Supplemental Data section for further discussion). In addition, recent studies have provided evidence for a role of FAK in promoting striated muscle cell differentiation as the dynamic regulation of FAK activity was found to be essential for differentiation of C2C12 myoblasts into myotubes.28 Also, a role for FAK in the promotion of cardiogenesis was suggested by studies in which stable expression of FRNK in ES cells was shown to induce cardiac α-myosin heavy chain and sarcomeric myosin expression.29 Since SRF plays a critical role in the regulation of contractile gene expression in each of these muscle types, it will be of future interest to determine whether limiting FAK activity regulates SRF activity and/or co-factor recruitment.
Since FRNK exhibited a striking SMC-restricted expression pattern, we sought to explore the mechanisms underlying its transcriptional regulation. Interestingly, we found that the FRNK promoter does not contain a canonical CArG box, is not affected by SRF deletion, and is un-responsive to over-expression of the myocardin family of potent SRF co-factors. Thus, FRNK belongs to a sub-class of smooth muscle specific genes that are regulated in a CArG-independent fashion including aortic carboxypeptidase-like protein (ACLP), cysteine-rich protein 2 (CRP2), and histidine-rich calcium-binding protein (HRCBP) (see Supplemental Data for further discussion)30–32 Interestingly, TGF-β, a strong activator of SMC differentiation, led to significant up-regulation of FRNK expression. Thus, future exploration of the TGF-β-dependent mechanisms that control FRNK expression may lead to identification of additional transcription factors that regulate SMC phenotypes.
Although reported to affect a wide variety of cellular processes a principal function for FAK in numerous cell types is its ability to modulate integrin and growth factor receptor-stimulated cellular migration.11 Direct evidence for the role of FAK in modulating fibronectin-dependent motility was previously shown using FAK−/− fibroblasts, endothelial cells, neurons, and keratinocytes.33–36 We previously showed that FRNK expression in SMC attenuated FAK-dependent PDGF-stimulated chemotaxis12 and our recent studies reveal that FAK−/− SMC exhibit a similar defect (un-published observations). Since FRNK appears to play a dual role in SM function; aiding to block SMC growth and migration and to promote SMC differentiation, FRNK may function as a toggle in the regulation of SMC phenotypes.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by grants from NIH-NHLBI (HL-081844 and HL-071054 to JMT; HL070953 to CPM) and the American Heart Association (0355776U to JMT; 0555476U to C.P.M). RLS was supported by an NIH Training Grant GM008581-06A2.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Hungerford JE, Owens GK, Argraves WS, Little CD. Development of the aortic vessel wall as defined by vascular smooth muscle and extracellular matrix markers. Dev Biol. 1996;178:375–392. doi: 10.1006/dbio.1996.0225. [DOI] [PubMed] [Google Scholar]
- 3.Sinha S, Hoofnagle MH, Kingston PA, McCanna ME, Owens GK. Transforming Growth Factor-{beta}1 Signaling Contributes to Development of Smooth Muscle Cells from Embryonic Stem Cells. Am J Physiol Cell Physiol. 2004 doi: 10.1152/ajpcell.00221.2004. [DOI] [PubMed] [Google Scholar]
- 4.Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 2000;81:173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouvard D, Brakebusch C, Gustafsson E, Aszodi A, Bengtsson T, Berna A, Fassler R. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89:211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- 6.George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- 7.Kato Y, Kuwabara T, Warashina M, Toda H, Taira K. Relationships between the activities in vitro and in vivo of various kinds of ribozyme and their intracellular localization in mammalian cells. J Biol Chem. 2001;276:15378–15385. doi: 10.1074/jbc.M010570200. [DOI] [PubMed] [Google Scholar]
- 8.Qiu P, Feng XH, Li L. Interaction of Smad3 and SRF-associated complex mediates TGF-beta1 signals to regulate SM22 transcription during myofibroblast differentiation. J Mol Cell Cardiol. 2003;35:1407–1420. doi: 10.1016/j.yjmcc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Qiu P, Ritchie RP, Fu Z, Cao D, Cumming J, Miano JM, Wang DZ, Li HJ, Li L. Myocardin enhances Smad3-mediated transforming growth factor-beta1 signaling in a CArG box-independent manner: Smad-binding element is an important cis element for SM22alpha transcription in vivo. Circ Res. 2005;97:983–991. doi: 10.1161/01.RES.0000190604.90049.71. [DOI] [PubMed] [Google Scholar]
- 10.Qiu P, Ritchie RP, Gong XQ, Hamamori Y, Li L. Dynamic changes in chromatin acetylation and the expression of histone acetyltransferases and histone deacetylases regulate the SM22alpha transcription in response to Smad3-mediated TGFbeta1 signaling. Biochem Biophys Res Commun. 2006;348:351–358. doi: 10.1016/j.bbrc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 12.Taylor JM, Mack CP, Nolan K, Regan CP, Owens GK, Parsons JT. Selective expression of an endogenous inhibitor of FAK regulates proliferation and migration of vascular smooth muscle cells. Mol Cell Biol. 2001;21:1565–1572. doi: 10.1128/MCB.21.5.1565-1572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan K, Lacoste J, Parsons JT. Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol Cell Biol. 1999;19:6120–6129. doi: 10.1128/mcb.19.9.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayasaka H, Simon K, Hershey ED, Masumoto KH, Parsons JT. FRNK, the autonomously expressed C-terminal region of focal adhesion kinase, is uniquely regulated in vascular smooth muscle: analysis of expression in transgenic mice. J Cell Biochem. 2005;95:1248–1263. doi: 10.1002/jcb.20501. [DOI] [PubMed] [Google Scholar]
- 15.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 16.Chang DF, Belaguli NS, Iyer D, Roberts WB, Wu SP, Dong XR, Marx JG, Moore MS, Beckerle MC, Majesky MW, Schwartz RJ. Cysteine-rich LIM-only proteins CRP1 and CRP2 are potent smooth muscle differentiation cofactors. Dev Cell. 2003;4:107–118. doi: 10.1016/s1534-5807(02)00396-9. [DOI] [PubMed] [Google Scholar]
- 17.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 19.Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 21.Gabarra-Niecko V, Keely PJ, Schaller MD. Characterization of an activated mutant of focal adhesion kinase: 'SuperFAK'. Biochem J. 2002;365:591–603. doi: 10.1042/BJ20020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 23.Sundberg LJ, Galante LM, Bill HM, Mack CP, Taylor JM. An endogenous inhibitor of focal adhesion kinase blocks Rac1/JNK but not Ras/ERK-dependent signaling in vascular smooth muscle cells. J Biol Chem. 2003;278:29783–29791. doi: 10.1074/jbc.M303771200. [DOI] [PubMed] [Google Scholar]
- 24.Katz BZ, Miyamoto S, Teramoto H, Zohar M, Krylov D, Vinson C, Gutkind JS, Yamada KM. Direct transmembrane clustering and cytoplasmic dimerization of focal adhesion kinase initiates its tyrosine phosphorylation. Biochim Biophys Acta. 2002;1592:141–152. doi: 10.1016/s0167-4889(02)00308-7. [DOI] [PubMed] [Google Scholar]
- 25.Richardson A, Malik RK, Hildebrand JD, Parsons JT. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochel HJ, Schulte TW, Nguyen P, Trepel J, Neckers L. The benzoquinone ansamycin geldanamycin stimulates proteolytic degradation of focal adhesion kinase. Mol Genet Metab. 1999;66:24–30. doi: 10.1006/mgme.1998.2774. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg RS, Bernstein AM, Benezra M, Gelman IH, Taliana L, Masur SK. FAK-dependent regulation of myofibroblast differentiation. Faseb J. 2006;20:1006–1008. doi: 10.1096/fj.05-4838fje. [DOI] [PubMed] [Google Scholar]
- 28.Clemente CF, Corat MA, Saad ST, Franchini KG. Differentiation of C2C12 myoblasts is critically regulated by FAK signaling. Am J Physiol Regul Integr Comp Physiol. 2005;289:R862–R870. doi: 10.1152/ajpregu.00348.2004. [DOI] [PubMed] [Google Scholar]
- 29.Hakuno D, Takahashi T, Lammerding J, Lee RT. Focal adhesion kinase signaling regulates cardiogenesis of embryonic stem cells. J Biol Chem. 2005;280:39534–39544. doi: 10.1074/jbc.M505575200. [DOI] [PubMed] [Google Scholar]
- 30.Layne MD, Yet SF, Maemura K, Hsieh CM, Liu X, Ith B, Lee ME, Perrella MA. Characterization of the mouse aortic carboxypeptidase-like protein promoter reveals activity in differentiated and dedifferentiated vascular smooth muscle cells. Circ Res. 2002;90:728–736. doi: 10.1161/01.res.0000013289.97650.c8. [DOI] [PubMed] [Google Scholar]
- 31.Chang YF, Wei J, Liu X, Chen YH, Layne MD, Yet SF. Identification of a CArG-independent region of the cysteine-rich protein 2 promoter that directs expression in the developing vasculature. Am J Physiol Heart Circ Physiol. 2003;285:H1675–H1683. doi: 10.1152/ajpheart.00165.2003. [DOI] [PubMed] [Google Scholar]
- 32.Anderson JP, Dodou E, Heidt AB, De Val SJ, Jaehnig EJ, Greene SB, Olson EN, Black BL. HRC is a direct transcriptional target of MEF2 during cardiac, skeletal, and arterial smooth muscle development in vivo. Mol Cell Biol. 2004;24:3757–3768. doi: 10.1128/MCB.24.9.3757-3768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 34.Ilic D, Kovacic B, Johkura K, Schlaepfer DD, Tomasevic N, Han Q, Kim JB, Howerton K, Baumbusch C, Ogiwara N, Streblow DN, Nelson JA, Dazin P, Shino Y, Sasaki K, Damsky CH. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci. 2004;117:177–187. doi: 10.1242/jcs.00845. [DOI] [PubMed] [Google Scholar]
- 35.Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- 36.Gates RE, King LE, Jr, Hanks SK, Nanney LB. Potential role for focal adhesion kinase in migrating and proliferating keratinocytes near epidermal wounds and in culture. Cell Growth Differ. 1994;5:891–899. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.