Abstract
AIM: To investigate the possible use of the multiple cytokine production modulator, Y-40138, as a novel immunotherapy in the rat nonalcoholic steatohepatitis (NASH) model.
METHODS: We allocated 6-wk-old male F344 rats to choline-supplemented, L-amino acid-defined (CSAA) diet (control group), CSAA diet + Y-40138 (control + Y-40138 group), choline-deficient, L-amino acid-defined (CDAA) diet (NASH group), or CDAA diet + Y-40138 (NASH + Y-40138 group). In each group, we measured the plasma alanine aminotransferase (ALT) levels, and the plasma and liver levels of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin-10 (IL-10). Tissue specimens of phosphate buffered saline-perfused liver were subjected to hematoxylin and eosin staining, Azan staining, Sirius red staining, and immunohistochemical staining (for Kupffer cells and TNF-α). We then extracted Kupffer cells from the collagenase-perfused livers using the Percoll gradient centrifugation method, and measured the TNF-α levels in the supernatant (in vitro TNF-α production by Kupffer cells) using an enzyme-linked immunosorbent assay kit.
RESULTS: In comparison to the NASH group, serum ALT elevation was mild, production of serum and liver TNF-α and IFN-γ was inhibited, and IL-10 production was increased in the NASH + Y-40138 group. Amelioration of liver histology was also noted in the NASH + Y-40138 group. Kupffer cell immunohistochemical staining revealed no differences between groups, whereas TNF-α immunohistochemical staining showed fewer stained cells in the NASH + Y-40138 group than in the NASH group. The TNF-α levels in the in-vitro Kupffer cell culture supernatant were lower in the NASH + Y-40138 group than in the NASH group.
CONCLUSION: Administration of Y-40138 to NASH model rats reduced hepatic inflammation and suppressed fibrosis. These results indicate that the multiple cytokine production modulator, Y-40138, is promising as a novel treatment for NASH.
Keywords: Nonalcoholic steatohepatitis, Y-40138, Tumor necrosis factor α, Interferon γ, Interleukin-10, Kupffer cell, Innate immunity
INTRODUCTION
In recent years, nonalcoholic steatohepatitis (NASH) associated with obesity and diabetes mellitus has attracted a great deal of attention. Despite no history of alcohol intake, the histological findings of NASH are similar to those of alcoholic hepatitis, and it is a progressive condition. A 2-component theory has been proposed for the etiology of NASH, the underlying condition being simple steatosis, with a first component of fatty degeneration as a result of insulin resistance, followed by a second component of aggravating factors such as inflammatory cytokines, including tumour necrosis factor α (TNF-α), oxidative stress and endotoxins[1]. Kupffer cells, part of the innate immune system, produce various cytokines and are known to play a role in pathologies of the liver[2,3]. Abnormally functioning Kupffer cells are suspected to be involved in NASH[4]. Although the etiological mechanisms of NASH have not been fully elucidated, similar histopathological findings in NASH and alcoholic hepatitis suggest the existence of a common underlying mechanism. One possible mechanism is that increased endotoxin levels cause activation of Kupffer cells through surface Toll-like receptor-4 (TLR4), leading to increased expression of TNF-α and increased levels of reactive oxygen species (ROS)[5]. These induce inflammation and fibrosis, progressing to NASH.
It is known that the choline-deficient L-amino acid-defined (CDAA) fed rat model shows fatty accumulation and fibrosis, and after 16 wk the liver shows cirrhotic changes, and nodules appear as in hepatocellular carcinoma[6]. Although this model lacks obesity and insulin resistance, the 2 major characteristics of NASH in humans, the possible relation of Kupffer cells with inflammation, steatosis, and fibrosis may resemble human NASH[7].
In this study, we administered Y-40138, which is a reciprocal cytokine production modulator, inhibiting production of TNF-α and interferon γ (IFN-γ), and promoting production of interleukin-10 (IL-10)[8,9], to a CDAA-fed rat NASH model, to investigate its possible use as a novel immunotherapy for NASH.
MATERIALS AND METHODS
Animals and induction of NASH model
Male F344 rats (6-wk-old, weighing 180-200 g) were purchased from Japan SLC Inc. (Hamamatsu, Japan). The animals were housed in stainless steel mesh cages under controlled temperature (23 ± 3°C) and relative humidity (50% ± 20%) conditions with 10 to 15 air changes per hour, and light illumination for 12 h a day. They were allowed access to tap water ad libitum throughout the duration of the study. CDAA and choline-supplemented L-amino acid-defined (CSAA) feeds were purchased from CLEA Japan Inc. (Tokyo, Japan). The details of both diets are described elsewhere[10].
The study groups were fed a CDAA diet for 8 wk to produce NASH. After 8 wk, the control group rats (CSAA diet, n = 5), control + Y-40138 group rats (CSAA + Y-40138 diet, n = 5), NASH group rats (CDAA diet, n = 5), and NASH + Y-40138 group rats (CDAA diet + Y-40138, n = 5) were killed and bled from the portal vein. Plasma was obtained by centrifugation at 3000 g for 15 min, and stored at -20°C. Liver tissues were excised after perfusion with phosphate buffered saline via the portal vein. All procedures were approved by our Institutional Animal Care Committee, and conducted in accordance with the Nara Medical University Guidelines for the Care and Use of Laboratory Animals.
Compounds
Y-40138 was obtained from Tanabe Mitsubishi Pharma Co. (Osaka, Japan), and dissolved in 0.5% hydroxypropylmethylcellulose solution. Y-40138 was orally administered to rats (10 mg/kg per day) for 8 wk.
Plasma alanine aminotransferase (ALT)
The plasma ALT levels were measured in samples from rats of each group. The blood samples were centrifuged, and the plasma was collected and stored at -30˚C until used for ALT determination. The plasma ALT levels were measured using a 7170 Clinical Analyzer (Hitachi High-Technologies, Tokyo, Japan).
Plasma TNF-α, IFN-γ and IL-10
TNF-α, IFN-γ and IL-10 concentrations were measured in plasma samples (50 μL) from rats from each group using an enzyme-linked immunosorbent assay (ELISA) kit (BioSource International, Camarillo, CA, USA).
Liver TNF-α, IFN-γ and IL-10
Liver tissue samples were frozen in liquid nitrogen, powdered, and 30 mg samples were homogenized in 1 mL of lysis buffer. TNF-α, IFN-γ, IL-10 levels in the supernatant (50 μL) were measured using an ELISA kit (BioSource International).
Histological examination
Liver tissue samples were fixed in 10% formalin, embedded in paraffin, and 5 μm thick sections were cut from the paraffin blocks for staining with hematoxylin and eosin, Azan, and Sirius red. Histological grading and staging were performed using a modified scoring system based on the classifications of Matteoni et al[11] and Brunt et al[12].
Immunohistochemistry
Kupffer cell dynamics were analyzed by comparing cell counts in each group using immunohistochemical staining with anti-rat macrophage/dendritic cell monoclonal antibody (RM-4: Trans Genic Inc., Kobe, Japan), Vectastain ABC Elite Kit (Vector Laboratories, Burlingame, CA, USA), and DAB peroxidase substrate solution (Vector Laboratories), with counterstaining by Hematoxylin Mayer. The stained areas were analyzed and compared using NIH image software (Version 1.61, U. S. National Institute of Health, Bethesda, MD, USA).
TNF-α immunohistochemical staining was performed on the paraffin sections using an anti-rat TNF-α/TNFSF1A antibody (R&D Systems, Minneapolis, MN, USA). We then used Vectastain ABC Elite Kit, and DAB peroxidase substrate solution, with counterstaining by Hematoxylin Mayer. The stained areas were analyzed using NIH image software.
Isolation and culture of Kupffer cells
Kupffer cells were harvested from the liver using isolation buffers according to a previously described procedure[13,14]. The livers were perfused in situ with Ca2+-free minimum essential medium (Sigma, St. Louis, MI, USA), followed by 0.3% pronase (Roche Diagnostics Co., Indianapolis, IN, USA) and 0.05% collagenase type IV (Wako, Osaka, Japan) in Dulbecco’s modified Eagle’s medium/F-12 (Sigma) at a rate of 10 mL/min through the portal vein. The liver was carefully removed and minced using scissors. The minced livers were further incubated in a shaker water bath with 0.035% pronase and 62.5 U/mL DNase (Sigma) in Dulbecco’s modified Eagle’s medium/F-12 at 37°C for 20 min. The samples were then filtered through gauze mesh, and the parenchymal cells were removed using low-speed centrifugation. Percoll (GE Healthcare, Little Chalfont, UK) was used for density gradient centrifugation of the cells. Kupffer cells were collected from the layer between 1.04 and 1.06, and cultured in RPMI 1640 supplemented with 10% fetal bovine serum and SAG (50 μg/mL streptomycin, 50 μg/mL ampicillin, 0.3 g/L L-glutamine). The viability of the cultured Kupffer cells was determined using trypan blue exclusion. Kupffer cells were seeded at a density of 5×105 cells/mL on 12-well plastic plates with RPMI-1640 medium. For the lipopolysaccharide (LPS)-stimulated group, Kupffer cells were then exposed to 1 μg/mL of LPS (Sigma-Aldrich Corp.) and incubated for 4.5 h at 37°C in 50 mL/L CO2-humidified air. Cells not treated with LPS were used as a control. TNF-α levels in the supernatant were measured using an ELISA kit.
The purity of the isolated Kupffer cells was over 98%, as examined by the uptake of 1 μm latex beads[15], and the viability was over 97% using the trypan blue dye exclusion test.
Statistical analysis
All results are expressed as mean ± SD. Analyses were conducted using Statview software (version 5.0, SAS Institute, Cary, NC, USA). The statistical differences between groups were evaluated using the paired t-test. A P-value < 0.05 was considered statistically significant.
RESULTS
Plasma ALT levels
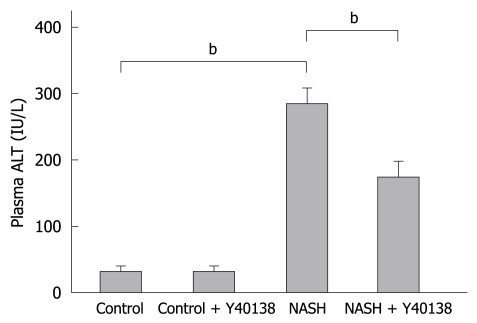
The mean plasma ALT level was 35.8 ± 4.7 IU/L in the control group, 38.3 ± 6.7 IU/L in the control + Y-40138 group, 279.0 ± 32.8 IU/L in the NASH group, and 171.3 ± 28.5 IU/L in the NASH + Y-40138 group. The NASH group showed a statistically significant elevation in the plasma ALT level in comparison to the control group (P < 0.01). The plasma ALT levels were significantly decreased in the NASH + Y-40138 group in comparison to the NASH group (P < 0.01) (Figure 1).
Figure 1.
The plasma alanine aminotransferase (ALT) levels in the nonalcoholic steatohepatitis (NASH) group were significantly elevated in comparison to the control group. The plasma ALT levels in the NASH + Y-40138 group were significantly decreased in comparison to the NASH group (each group n = 5). bP < 0.01.
Plasma TNF-α, IFN-γ and IL-10 levels
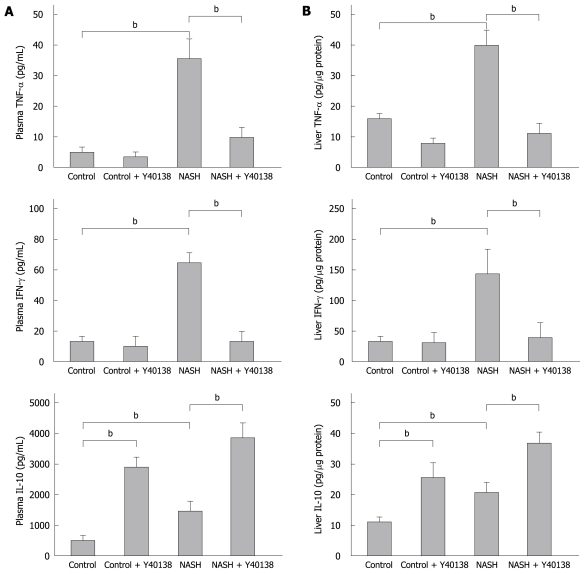
In comparison to the control group, the plasma TNF-α levels were significantly elevated in the NASH group (5.1 ± 1.4 pg/mL vs 35.2 ± 7.3 pg/mL, P < 0.01). The plasma TNF-α levels were significantly lower in the NASH + Y-40138 group (9.6 ± 3.6 pg/mL) than in the NASH group (P < 0.01). The plasma IFN-γ levels in the NASH group (62.4 ± 11.0 pg/mL, P < 0.01) were significantly elevated in comparison to the control group (12.3 ± 2.3 pg/mL). The plasma IFN-γ levels in the NASH + Y-40138 group (12.8 ± 6.3 pg/mL) were significantly decreased in comparison to the NASH group (P < 0.01). The plasma IL-10 levels in the control + Y-40138 group (2860 ± 350 pg/mL, P < 0.01) and the NASH group (1360 ± 380 pg/mL, P < 0.01) were significantly elevated in comparison to the control group (520 ± 96 pg/mL). The plasma IL-10 levels in the NASH + Y-40138 group (3820 ± 480 pg/mL) were significantly increased in comparison to the NASH group (P < 0.01) (Figure 2A).
Figure 2.
Tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin-10 (IL-10) levels in the plasma and liver (ELISA method) in each group (n = 5). A: The plasma TNF-α and IFN-γ levels were significantly elevated in the NASH group, and the increase was attenuated in the NASH + Y-40138 group. The IL-10 levels were increased in the NASH group and further increased in the NASH + Y-40138 group rats. bP < 0.01; B: The liver TNF-α, IFN-γ and IL-10 levels were significantly elevated in the livers of the NASH group rats. TNF-α and IFN-γ levels were attenuated in the NASH + Y-40138 group. The IL-10 levels further increased in NASH + Y-40138 group rats. bP < 0.01.
Liver TNF-α, IFN-γ and IL-10 levels
The liver TNF-α level in the NASH group was 39.1 ± 6.2 pg/μg protein, significantly higher than that in the control group (15.8 ± 3.6 pg/μg protein, P < 0.01). The liver TNF-α level in the NASH + Y-40138 group (12.4 ± 4.1 pg/μg protein) was significantly lower than that in the NASH group (P < 0.01). The liver IFN-γ level in the NASH group was 136 ± 39 pg/μg protein, significantly higher than that in the control group (36.3 ± 5.6 pg/μg protein, P < 0.01). The liver IFN-γ level in the NASH + Y-40138 group (45.6 ± 18.5 pg/μg protein) was significantly lower than that in the NASH group (P < 0.01). The liver IL-10 level in the control + Y-40138 group (24.7 ± 5.3 pg/μg protein) and the NASH group (19.3 ± 4.1 pg/μg protein) were significantly elevated in comparison to the control group (10.4 ± 2.5 pg/μg protein). The liver IL-10 level in the NASH + Y-40138 group (34.5 ± 4.3 pg/μg protein) was significantly higher than that in the NASH group (P < 0.01) (Figure 2B).
Histological findings
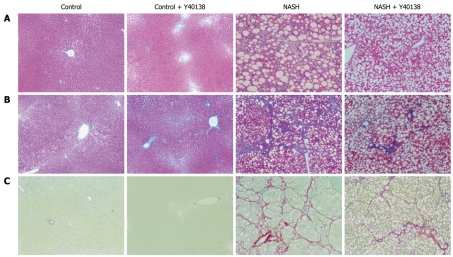
In contrast to the control group, marked inflammation, fat deposition, and fibrosis were seen in the NASH group, corresponding to Matteoni’s type 4 and grade 3/stage 3 of Brunt’s NASH classification. The NASH + Y-40138 group findings corresponded to Matteoni’s type 3 and Brunt’s grade 2/stage 2, and showed amelioration of inflammation and fibrosis in comparison to the NASH group (Figure 3).
Figure 3.
Histological analysis of liver sections. A: Hematoxylin and eosin stain (× 100); B: Azan stain (× 100); C: Sirius red stain (× 100). Histological findings of liver tissue from the NASH group rats corresponded to Matteoni’s type 4 and grade 3/stage 3 of Brunt’s NASH classification. Sirius red staining revealed abundant collagen. NASH + Y-40138 group findings corresponded to Matteoni’s type 3 and Brunt’s grade 2/stage 2, and showed amelioration of inflammation and fibrosis in comparison to the NASH group (n = 5).
Immunohistochemical findings
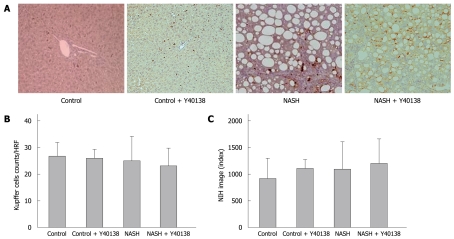
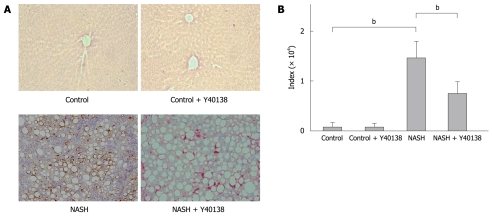
Kupffer cell immunohistochemical staining showed no significant difference in the number of stained cells per field between groups (Figure 4A and B). Moreover, quantitative analysis using NIH Image analysis software showed no significant difference between groups (Figure 4C).
Figure 4.
Kupffer cell immunohistochemical staining, Kupffer cell number and semiquantification in the liver. A: Kupffer cell immunohistochemical staining (× 200). Brown-stained cells are positive; B: No significant differences were seen between groups in the stained cell counts per field; C: Quantitative analysis revealed no significant differences between groups (n = 5). HRF: High rise filed.
The TNF-α immunoreactivity in the liver tissue samples from the NASH group rats was increased in comparison to the control group rats. Moreover, the immunopositive areas in those animals were localized either to the periphery of fatty spots or to the non-parenchymal cells (Figure 5A). Semiquantitative analysis of TNF-α staining revealed significantly increased immunoreactivity in the livers of the NASH group rats (3250 ± 390) in comparison to the control group rats (260 ± 80) (P < 0.01). Semiquantitative analysis of TNF-α staining revealed significantly decreased immunoreactivity in the livers of the NASH + Y-40138 group rats (820 ± 350) in comparison to the NASH group rats (P < 0.01) (Figure 5B).
Figure 5.
TNF-α immunohistochemical staining and semiquantification. A: TNF-α immunohistochemical staining (× 200). Brown-stained cells are positive; B: TNF-α immunoreactivities in the tissue samples from the NASH group were increased in comparison to the control group. The TNF-α immunoreactivities in the NASH + Y-40138 group were decreased in comparison to the NASH group (n = 5). bP < 0.01.
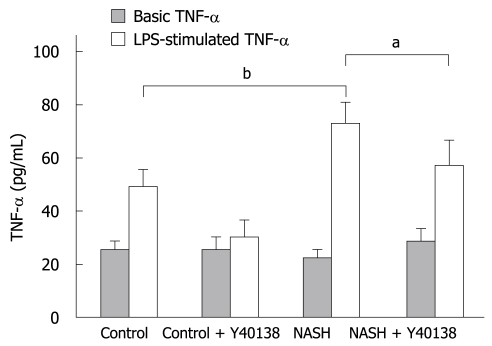
In-vitro TNF-α production by Kupffer cells
The viability of the cultured Kupffer cells was over 98% as determined by trypan blue exclusion. The basal TNF-α production by Kupffer cells isolated from the NASH group (26.6 ± 3.4 pg/mL) was equal to that from the control group (23.4 ± 4.8 pg/mL). After LPS stimulation, TNF-α production in the control group (48.9 ± 7.5 pg/mL) was significantly higher than the basal control level, and that in the NASH group (73.8 ± 8.4 pg/mL) was significantly higher than that in the control group (P < 0.01). TNF-α production in the NASH + Y-40138 group (57.2 ± 10.3 pg/mL) was significantly lower than that in the NASH group (P < 0.05) (Figure 6).
Figure 6.
In-vitro TNF-α production by Kupffer cells. The basal TNF-α production by Kupffer cells isolated from the NASH group (26.6 ± 3.4 pg/mL) was equal to that in the control group (23.4 ± 4.8 pg/mL). After lipopolysaccharide (LPS) stimulation, TNF-α production in the control group (48.9 ± 7.5 pg/mL) was significantly higher than the basal control level, and was significantly higher in the NASH group (73.8 ± 8.4 pg/mL) than in the control group (n = 5). bP < 0.01. TNF-α production in the NASH + Y-40138 group (57.2 ± 10.3 pg/mL) was significantly decreased in comparison to the NASH group (n = 5). aP < 0.05.
DISCUSSION
Kupffer cells located in the hepatic sinusoids are important hepatic cells that possess phagocytic activity, antigen presenting activity, and produce inflammatory cytokines, making them responsible for the hepatic innate immunity. Inflammatory cytokines, in particular TNF-α, are reportedly involved in the etiology and progression of alcoholic hepatitis[16]. The histopathological findings in NASH are similar to those detected in alcoholic hepatitis, suggesting the existence of a common underlying mechanism. A possible mechanism is that increased endotoxin levels cause activation of Kupffer cells through TLR4, leading to increased expression of TNF-α and increased levels of ROS. These induce inflammation and fibrosis, progressing to NASH. The elevation in the hepatic tissue and plasma levels of TNF-α noticed in NASH[2] has been attributed to a combination of secretion by fat cells associated with obesity, and secretion by Kupffer cells activated by endotoxins[17].
Once the trigger of TLR4 signal transmission has been initiated, Kupffer cells produce inflammatory cytokines including TNF-α, IL-1β, and IL-6, as well as the anti-inflammatory cytokine IL-10[18]. Kupffer cells also induce the inflammatory response through production of transforming growth factor-1, matrix metalloproteinase, platelet-derived growth factor, and ROS[19]. NASH is characterized by fatty deposition in the hepatocytes, inflammatory cell infiltration, with consequent liver fibrosis[20].
Methionine/choline deficient (MCD) and CDAA diets are widely used to produce NASH animal models[4]. Although these animals models lack obesity and insulin resistance, 2 of the major characteristics of NASH in humans, the possible relation of Kupffer cells with inflammation, steatosis, and fibrosis may resemble the human NASH[7]. We also demonstrated decreased Kupffer cell phagocytic activity in this model[4]. Increased sensitivity to LPS, the TLR4 ligand, occurs in MCD and CDAA-induced NASH, triggering the production of inflammatory cytokines[3].
TNF-α is a cytokine widely involved in biodefence and immune mechanisms based on inflammation, and continued overproduction is harmful to the organisms, with potentially serious toxicity, resulting in various morbidities[21]. Anti-cytokine antibodies such as infliximab are now widely used clinically as pharmacotherapy for inflammatory diseases[19].
In contrast, IL-10 is produced by macrophages and T-cells and has anti-inflammatory properties, inhibiting the production of inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8[22]. Therefore, IL-10 has shown efficacy in models of sepsis and rheumatoid arthritis[23] and clinically useful therapeutic effects have been reported for IL-10 in the treatment of steroid-resistant Crohn’s disease[24].
The chemical name of the new synthetic compound Y-40138 is N-[1-(4-[4-(pyrimidin-2-yl) piperazin-1-yl] methylphenyl) cyclopropyl] acetamide/hydrochloride. Its molecular formula is C20H25N5O/HCl, its molecular weight is 387.91 (free base: 351.45). Although the site of action of Y-40138 has not yet been elucidated, it is known to have reciprocal actions in inhibiting production of TNF-α and IFN-γ, and increasing production of IL-10, as well as inhibiting production of chemokines and inflammatory cell chemotaxis, mechanisms not seen in any other agents[8,9]. Previous studies on Y-40138 have demonstrated improved survival rates in a D-galactosamine/LPS-induced mouse fulminant hepatic failure model[25,26] and a LPS-stimulated mouse sepsis model[27], and reduced ALT levels in a concanavalin A-induced mouse hepatitis model[28].
In this study using the rat NASH model, we detected increased plasma levels of ALT and TNF-α, and confirmed increased TNF-α levels in the liver using ELISA and immunohistochemical staining. These results indicate that TNF-α is involved in this NASH model, as in alcoholic hepatitis. Administration of Y-40138 to these same model rats reduced the plasma levels of ALT and TNF-α, and also decreased the TNF-α levels in the liver. We detected increased levels of IL-10 in the peripheral blood and liver. The TNF-α production by the isolated Kupffer cells was similar in the control and NASH rats. Kupffer cells from the control rats were sensitive to LPS stimulation. Furthermore, those from NASH were more sensitive to LPS stimulation, suggesting that continuous inflammation leads to up-regulation of the LPS-induced TNF-α production by the activated Kupffer cells. Administration of Y-40138 to the same model rats decreased LPS-induced TNF-α production in vitro. We presume that IL-10 restrains the production of TNF-α and IFN-γ via inhibition of the activation of Kupffer cells through STAT3 (signal transducer and activator of transcription 3).
In conclusion, the multiple cytokine production modulator Y-40138 ameliorated hepatic inflammation and inhibited fibrotic changes in the liver. These results indicate that Y-40138 is promising as a novel treatment for NASH. Y-40138 may have a role in suppressing the progress of hepatitis and prolonging the lives of patients with liver damage such as NASH in which cytokines and/or chemokines are involved.
COMMENTS
Background
Kupffer cells, part of the innate immune system, produce various cytokines and are known to play a role in pathologies of the liver. Abnormally functioning Kupffer cells are thought to be involved in nonalcoholic steatohepatitis (NASH). Although the etiological mechanisms of NASH have not been fully elucidated yet, observing similar histopathological findings in NASH and alcoholic hepatitis suggests the existence of a common underlying mechanism. In this study, the authors administered the multiple cytokine production modulator, Y-40138, to NASH model rats, to investigate its possible use as a novel immunotherapy for NASH.
Research frontiers
Administration of Y-40138 to NASH model rats reduced hepatic inflammation and suppressed fibrosis. These results indicate that the multiple cytokine production modulator, Y-40138, is promising as a novel treatment for NASH. Therefore, Y-40138 may contribute to suppressing the progress of hepatitis and prolonging the life of patients with liver damage such as NASH.
Applications
The present study is an experimental research using the rat NASH model, which was made by feeding a choline-deficient L-amino-acid-defined diet to rats, to examine their innate immunity. The authors believe that Y-40138 is a useful immunotherapy for NASH.
Terminology
The chemical name of the new synthetic compound Y-40138 is N-[1-(4-[4-(pyrimidin-2-yl) piperazin-1-yl] methylphenyl) cyclopropyl] acetamide/hydrochloride. Although the mechanism of action of Y-40138 has not been fully elucidated yet, it is known to have reciprocal actions in inhibiting the production of tumour necrosis factor-α and interferon-γ, and increasing the production of IL-10, as well as inhibiting the production of chemokines and inflammatory cell chemotaxis, and no other agents are known to have such mechanisms.
Peer review
This is an interesting study on the use of a cytokine modulator in the treatment of an experimental NASH model. The paper is well written and the research is well designed and executed.
Acknowledgments
We gratefully acknowledge Tanabe Mitsubishi Pharma Co. for generously providing us with Y-40138 for our study.
Footnotes
Supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, No. 19590784
Peer reviewers: Bhupinder S Anand, Professor, Digestive Diseases Section (111D), VA Medical Center, 2002 Holcombe Blvd., Houston, TX 77030, United States; Dr. Satoshi Yamagiwa, Division of Gastroenterology and Hepatology, Niigata University Graduate School of Medical and Dental Sciences, 757 Asahimachi-dori, Chuo-ku, Niigata, 951-8510, Japan; Maurizio Parola, Professor, Department of Experimental Medicine and Oncology, University of Torino, Corso Raffaello 30, 10125 Torino, Italy
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
References
- 1.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 2.Tsujimoto T, Kuriyama S, Yamazaki M, Nakatani Y, Okuda H, Yoshiji H, Fukui H. Augmented hepatocellular carcinoma progression and depressed Kupffer cell activity in rat cirrhotic livers. Int J Oncol. 2001;18:41–47. doi: 10.3892/ijo.18.1.41. [DOI] [PubMed] [Google Scholar]
- 3.Kawaratani H, Tsujimoto T, Kitazawa T, Kitade M, Yoshiji H, Uemura M, Fukui H. Innate immune reactivity of the liver in rats fed a choline-deficient L-amino-acid-defined diet. World J Gastroenterol. 2008;14:6655–6661. doi: 10.3748/wjg.14.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsujimoto T, Kawaratani H, Kitazawa T, Hirai T, Ohishi H, Kitade M, Yoshiji H, Uemura M, Fukui H. Decreased phagocytic activity of Kupffer cells in a rat nonalcoholic steatohepatitis model. World J Gastroenterol. 2008;14:6036–6043. doi: 10.3748/wjg.14.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaida I, Kubota M, Kayano K, Takenaka K, Mori K, Okita K. Prevention of fibrosis reduces enzyme-altered lesions in the rat liver. Carcinogenesis. 1994;15:2201–2206. doi: 10.1093/carcin/15.10.2201. [DOI] [PubMed] [Google Scholar]
- 7.Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21:89–104. doi: 10.1055/s-2001-12932. [DOI] [PubMed] [Google Scholar]
- 8.Haddad JJ, Safieh-Garabedian B, Saadé NE, Land SC. The biphasic immunoregulation of pyrimidylpiperazine (Y-40138) is IL-10 sensitive and requires NF-kappa B targeting in the alveolar epithelium. Br J Pharmacol. 2001;133:49–60. doi: 10.1038/sj.bjp.0704041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisadome M, Fukuda T, Adachi K, Komatsu H. Combination benefit of a pyrimidylpiperazine derivative (Y-40138) and methotrexate in arthritic rats. Eur J Pharmacol. 2004;497:351–359. doi: 10.1016/j.ejphar.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 10.Nakae D, Mizumoto Y, Yoshiji H, Andoh N, Horiguchi K, Shiraiwa K, Kobayashi E, Endoh T, Shimoji N, Tamura K. Different roles of 8-hydroxyguanine formation and 2-thiobarbituric acid-reacting substance generation in the early phase of liver carcinogenesis induced by a choline-deficient, L-amino acid-defined diet in rats. Jpn J Cancer Res. 1994;85:499–505. doi: 10.1111/j.1349-7006.1994.tb02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 12.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 13.Seglen PO. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973;82:391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- 14.Olynyk JK, Clarke SL. Isolation and primary culture of rat Kupffer cells. J Gastroenterol Hepatol. 1998;13:842–845. doi: 10.1111/j.1440-1746.1998.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 15.Kubo S, Rodriguez T Jr, Roh MS, Oyedeji C, Romsdahl MM, Nishioka K. Stimulation of phagocytic activity of murine Kupffer cells by tuftsin. Hepatology. 1994;19:1044–1049. [PubMed] [Google Scholar]
- 16.Hansen J, Cherwitz DL, Allen JI. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994;20:461–474. [PubMed] [Google Scholar]
- 17.Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886–1900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Seki E, Tsutsui H, Nakano H, Tsuji N, Hoshino K, Adachi O, Adachi K, Futatsugi S, Kuida K, Takeuchi O, et al. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol. 2001;166:2651–2657. doi: 10.4049/jimmunol.166.4.2651. [DOI] [PubMed] [Google Scholar]
- 19.Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182–1197. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 21.Kitazawa T, Tsujimoto T, Kawaratani H, Fujimoto M, Fukui H. Expression of Toll-like receptor 4 in various organs in rats with D-galactosamine-induced acute hepatic failure. J Gastroenterol Hepatol. 2008;23:e494–e498. doi: 10.1111/j.1440-1746.2007.05246.x. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimura A, Mori H, Ohishi M, Aki D, Hanada T. Negative regulation of cytokine signaling influences inflammation. Curr Opin Immunol. 2003;15:704–708. doi: 10.1016/j.coi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Walmsley M, Katsikis PD, Abney E, Parry S, Williams RO, Maini RN, Feldmann M. Interleukin-10 inhibition of the progression of established collagen-induced arthritis. Arthritis Rheum. 1996;39:495–503. doi: 10.1002/art.1780390318. [DOI] [PubMed] [Google Scholar]
- 24.van Deventer SJ, Elson CO, Fedorak RN. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Crohn's Disease Study Group. Gastroenterology. 1997;113:383–389. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda T, Hisadome M, Komatsu H. Treatment with Y-40138, a multiple cytokine production modulator, inhibits lipopolysaccharide- or tumour necrosis factor-alpha-induced production of pro-inflammatory cytokines and augments interleukin-10. J Pharm Pharmacol. 2005;57:1461–1466. doi: 10.1211/jpp.57.11.0012. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda T, Mogami A, Tanaka H, Yoshikawa T, Hisadome M, Komatsu H. Y-40138, a multiple cytokine production modulator, protects against D-galactosamine and lipopolysaccharide-induced hepatitis. Life Sci. 2006;79:822–827. doi: 10.1016/j.lfs.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda T, Sumichika H, Murata M, Hanano T, Adachi K, Hisadome M. A novel dual regulator of tumor necrosis factor-alpha and interleukin-10 protects mice from endotoxin-induced shock. Eur J Pharmacol. 2000;391:317–320. doi: 10.1016/s0014-2999(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda T, Mogami A, Hisadome M, Komatsu H. Therapeutic administration of Y-40138, a multiple cytokine modulator, inhibits concanavalin A-induced hepatitis in mice. Eur J Pharmacol. 2005;523:137–142. doi: 10.1016/j.ejphar.2005.08.060. [DOI] [PubMed] [Google Scholar]