Abstract
AIM: To compare the sensitivity and specificity of two imaging techniques, endoscopic ultrasound (EUS) and magnetic resonance imaging (MRI), in patients with rectal cancer after neoadjuvant chemoradiation therapy. And we compared EUS and MRI data with histological findings from surgical specimens.
METHODS: Thirty-nine consecutive patients (51.3% Male; mean age: 68.2 ± 8.9 years) with histologically confirmed distal rectal cancer were examined for staging. All patients underwent EUS and MRI imaging before and after neoadjuvant chemoradiation therapy.
RESULTS: After neoadjuvant chemoradiation, EUS and MRI correctly classified 46% (18/39) and 44% (17/39) of patients, respectively, in line with their histological T stage (P > 0.05). These proportions were higher for both techniques when nodal involvement was considered: 69% (27/39) and 62% (24/39). When patients were sorted into T and N subgroups, the diagnostic accuracy of EUS was better than MRI for patients with T0-T2 (44% vs 33%, P > 0.05) and N0 disease (87% vs 52%, P = 0.013). However, MRI was more accurate than EUS in T and N staging for patients with more advanced disease after radiotherapy, though these differences did not reach statistical significance.
CONCLUSION: EUS and MRI are accurate imaging techniques for staging rectal cancer. However, after neoadjuvant RT-CT, the role of both methods in the assessment of residual rectal tumors remains uncertain.
Keywords: Endoscopic ultrasound, Magnetic resonance imaging, Rectal cancer, Neoadjuvant chemoradiation therapy, Diagnostic accuracy
INTRODUCTION
Endoscopic ultrasound (EUS) is the most accurate imaging technique for evaluating local invasion of rectal cancer and perirectal lymph nodes. Its overall accuracy for T staging before radiation therapy (RT) ranges from 73% to 94%, and from 70% to 80% for N staging[1]. The findings of rectal EUS serve to decide the type of treatment: surgery or preoperative chemoradiation (RT-CT)[2]. The response to neoadjuvant therapy must also be assessed accurately in patients with rectal cancer.
The benefits of this treatment have been reported in different studies, reporting lower local recurrence rates in patients with locally advanced rectal tumors. Rectal EUS helps select a group with advanced locoregional disease (stage T3 or T4) in whom this preoperative treatment offers most benefit[3-5].
The accuracy of EUS in staging rectal cancer ranges from 80% to 95% compared to 65%-75% for computed tomography (CT) and 75%-85% for magnetic resonance imaging (MRI)[6-9]. There is still debate about the role of cross-sectional imaging to restage rectal cancer after RT-CT, and data are scanty on accuracy, sensitivity, and specificity rates, suggesting that these modalities are not efficient enough for restaging.
The aim of this prospective study was to evaluate the diagnostic accuracy of two imaging techniques, EUS and MRI, in patients with rectal cancer, before and after neoadjuvant RT-CT. We compared the EUS and MRI data with histological findings on surgical specimens.
MATERIALS AND METHODS
Over a 2-year period (January 2007 to January 2009), 50 patients with histologically confirmed distal rectal cancer were referred to our Unit for rectal endosonography to stage the disease. Out of these, thirty-nine consecutive cases (51.3% Male; mean age: 68.2 ± 8.9 years) were enrolled (drop-out from the study: 11 patients).
Both examinations were done, in random order, by two experienced endosonographers (> 300 rectal examinations/year) and an expert radiologist, before and after RT. The second examiner was blind to the first one’s conclusions. Patients were included in the study if they had rectal cancer shown by either EUS or MRI, and were scheduled for neoadjuvant RT.
Usually, 30-40 d after termination of the RT protocol, patients were re-assessed by either EUS or MRI, followed by surgical excision during the same week. All patients gave informed consent.
Subjects with a history of rectal surgery, recurrent rectal cancer, or severe systemic illness were excluded from the study. Ten patients had neoplastic sub-stenosis of the lumen; therefore, it was not possible to evaluate their iliac lymph nodes by EUS.
These two subgroups were formed to see whether the diagnostic accuracy of EUS or MRI was related to the tumor T stage after RT.
EUS protocol: Rectal EUS was performed using an oblique-forward viewing echo-endoscope (Pentax: FGG36UX, FG-38UX, EG-3630U). All examinations were done with the patient lying on the left side under conscious sedation (midazolam i.v.). Patients were prepared with colonic lavage before EUS. The echo-endoscope was inserted and advanced beyond the lesion, under direct vision, to the rectosigmoid junction, high enough to detect iliac vessels on the EUS picture. Tumors were targeted to determine the depth of infiltration into or through the rectal wall. Frequencies commonly employed ranged from 7.5 to 10 MHz (Figure 1).
Figure 1.
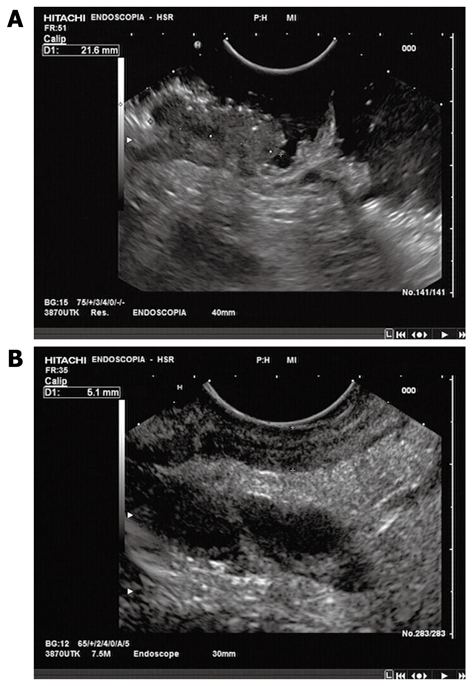
Rectal EUS. A: Before neoadjuvant chemoradiation treatment: evidence of perirectal fat tissue invasion, with fat speculation; B: After neoadjuvant chemoradiation treatment: reduction of tumor size and perirectal fat invasion.
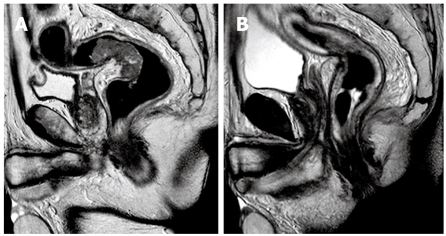
MRI protocol: All MRI examinations were performed on a 1.5T MRI scanner (Gyroscan Intera, Philips, The Nederlands). Iron contrast medium rectal distension and IM hypotonizing medication were performed. MRI examinations were performed before and after iv contrast media infusion (0.2 mL/kg). Multiplanar TSE T2 weighted sequences (TR: 4450, TE: 120, matrix: 512 × 344, NSA: 6, FOV: 240) were performed in order to local stage rectal cancer; axial TSE T2 weighted sequences (TR: 3950, TE: 80, matrix: 528 × 372, NSA: 3, FOV: 370) were performed to analyze the presence of pathological lymph nodes (N). Axial FFE T1 weighted sequences (TR: 196, TE: 4.6, matrix: 320 × 216, NSA: 4, FOV: 250) with and without Fat Saturation, before and after contrast medium and sagittal FFE T1 weighted sequences (TR: 196, TE: 4.6, matrix: 320 × 216, NSA: 4, FOV: 250) with Fat Saturation, after contrast medium, were also performed. The MRI protocol used in our hospital is very similar to that commonly reported in the literature. Our Institute can be considered a medium-high flow hospital for rectal cancer staging; in fact, we performed at least 50 rectal contrast enhanced MRI in 1 year (Figure 2).
Figure 2.
Rectal RMI. A: Before neoadjuvant chemoradiation treatment: evidence of perirectal fat tissue invasion, with fat spiculation, and enlarged mesorectal lymph nodes; B: After neoadjuvant chemoradiation treatment: reduction of tumor size, perirectal fat invasion, enlarged lymph nodes; non-homogeneous mesorectal fat tissue.
Neoadjuvant chemoradiation treatment: Chemoradiotherapy consisted of administration of Oxaliplatin 100 mg/m2 every 2 wk for three courses plus continuous infusion of 5-FU 200 mg/m2/die for six consecutive weeks. Concomitant hyperfractionated radiotherapy at a total dose of 45 Gy (1.25 Gy twice daily for 5 d every week with a four-field box technique, with 6-18 MeV photons) was started the same day as the second course of Oxaliplatin. EUS and pelvic MRI were repeated just before surgery to establish the clinical response.
Surgical treatment: Laparoscopic or laparotomic nerve sparing surgical resection (either low anterior or abdomino-perineal resection) with total mesorectal excision (TME) was scheduled 6-8 wk after completion of neoadjuvant treatment. Criteria for sphincter preservation were acceptable sphincter function, assessed with preoperative rectal manometry; and absence of direct invasion of the sphincter apparatus. These criteria remained unchanged throughout the treatment period. Creation of temporary loop ileostomy or colostomy was performed in selected cases as judged necessary by the surgeon.
Rectal cancer staging
Rectal tumors were staged using the tumor-node-metastasis system (TNM)[10].
Statistical analysis
Pre-operative EUS and MRI findings were compared to assess the concordance between the two methods. κ-statistics were used to check how well EUS and MRI classified subjects in the T and N stage groups. The degree of agreement was quantified by weighted κ, which assumes the categories (Tl, T2, etc.) are ordered and accounts for how far apart EUS and MRI are in classifying them.
EUS and MRI were repeated after RT, and the findings were compared with the T and N stages established on the basis of histological examination of surgical specimens. The proportions of concordant results between each method and histology were compared using the χ2 test (2 × 2 table). Patients were considered all together and sorted into two subgroups according to their histological T stage: the first group had T0-T2 stage cancer and the second T3-T4.
RESULTS
After neoadjuvant chemoradiation therapy, histological examination of surgical specimens was done for all patients, with the following results: 9 T0; 1 Tl; 8 T2; 21 T3. Nodal involvement was N0 in 23; N1 in 14; N2 in 2. There were 18 patients with T0-T2 disease, 21 T3 (no T4), 23 without nodal metastasis (N0 disease) and 16 with N1-N2 disease. EUS and MRI correctly classified patients in line with their histological T stage in 46% (18/39) and 44% (17/39) (NS) of cases, respectively. These proportions were higher for both techniques when nodal involvement was considered: staging was correct in 69% (27/39) and 62% (24/39). Interestingly, when patients were sorted into T and N subgroups, the diagnostic accuracy of EUS was better than MRI for patients with T0-T2 (44% vs 33%, NS) and N0 disease (87% vs 52%, P = 0.013). However, MRI was more accurate for patients with more advanced disease (both T and N) after RT, though these differences did not reach statistical significance (Tables 1 and 2).
Table 1.
Post-treatment stage: imaging diagnosis vs surgical specimens
| Stage | EUS | MRI |
| T0N0 | 6 | 4 |
| T0N1 | 0 | 0 |
| T0N2 | 0 | 0 |
| T1N0 | 1 | 0 |
| T1N1 | 0 | 0 |
| T1N2 | 0 | 0 |
| T2N0 | 0 | 0 |
| T2N1 | 0 | 1 |
| T2N2 | 0 | 0 |
| T3N0 | 2 | 2 |
| T3N1 | 4 | 6 |
| T3N2 | 0 | 0 |
| T4N0 | 0 | 0 |
| T4N1 | 0 | 0 |
| T4N2 | 0 | 0 |
Table 2.
Diagnostic accuracy (T and N-stage)
| All T | T0-T2 | T3-T4 | All N | N0 | N 1-2 | |
| n | 39 | 18 | 21 | 39 | 23 | 16 |
| MRI | 44% | 33% | 52% | 62% | 52% | 75% |
| EUS | 46% | 44% | 48% | 69% | 87% | 44% |
MRI: Magnetic resonance imaging; EUS: Endoscopic ultrasound.
DISCUSSION
The prognosis for rectal cancer strongly correlates with the histopathological stage at diagnosis. Many imaging techniques are available, but each one differs in accuracy and applicability[11-13]. Accurate staging is important for planning surgery and deciding on adjuvant treatment.
ERUS (endorectal ultrasound) and MRI are considered to be the most accurate modalities for determining local tumor stage. Two recent meta-analyses have compared EUS, CT and MRI for rectal cancer staging. Bipat et al[14] found that ERUS was the most accurate modality when compared with CT and MRI for the evaluation of the T stage in rectal cancer. For lymph node involvement, the results of ERUS, CT and MRI were comparable. However, the T-staging system does not discriminate between T3 tumors with close or involved circumferential resection clearance. In this meta-analysis, the distance of the tumor from the rectal fascia or the anticipated circumferential resection clearance was not evaluated. Lahaye et al[15] conducted another meta-analysis regarding the accuracy of preoperative imaging for predicting the two most important risk factors that they recognized for local recurrence in rectal cancer; the circumferential resection clearance and the lymph node status. Major progress has been made in the preoperative staging in rectal tumors by MRI and several authors have indicated that a tumor-free circumferential clearance of more than 1 mm can be predicted using this method. For nodal status, ERUS was slightly, but not significantly, better than MRI.
The introduction of trans-rectal EUS has made it easier to see the pattern of the layers of the rectal wall, improving treatment allocation by establishing the depth of tumor invasion more accurately[16-18]. EUS is the most accurate tool for evaluating local invasion of rectal cancer and perirectal lymph nodes. Its overall accuracy before radiation ranges from 73% to 94% in T staging, and from 70% to 80% in N staging. Comparative studies found EUS very accurate in staging rectal cancer from 80% to 95%, compared to 65% to 75% for CT and 75% to 85% for MRI[6-9].
CT and MRI have proved disappointing for detecting small neoplastic lesions. MRI is not significantly superior to CT because of the limited resolution of conventional MRI techniques. It has, however, been reported useful in determining the status of the circumferential resection margin (meso-rectum), which is important for assessing the risk of local recurrence[19].
Recent data suggest that EUS staging of rectal cancer after RT-CT is inaccurate[1], while MRI seems to be cost-effective in selecting appropriate patients for neoadjuvant therapy and its use is justified for local staging. After RT-CT, EUS and MRI might be useful to demonstrate tumor shrinkage and down-staging in responsive tumors, which might occasionally disappear completely[17,18,20-23]. Increasing reflectivity and signal changes indicate fibrosis, but unless significant tumor bulk remains, neither modality seems to be able to exclude the persistence of tumor cells within fibrosis[24-28].
Finally, EUS-FNA (endoscopic ultrasound-guided fine-needle aspiration) was proposed for N staging of rectal cancer following neoadjuvant chemoradiation[29].
This study compared rectal EUS and MRI for staging rectal cancer before and, in particular, after neoadjuvant RT-CT. As reported in other studies, at the first staging examination after the diagnosis of rectal cancer, EUS and MRI offer similar accuracies. However, if we consider the degree of agreement between the two procedures in rectal cancer, EUS (without lymph node biopsy) and MRI give concordant results in T and N staging only in 64% and 54% of patients. Moreover, they only give concordant results for both T and N stages in one third of patients. The strength of agreement between EUS and MRI in staging advanced rectal cancer was very poor, implying that each method has limitations.
After RT, both EUS and MRI offered poor diagnostic performance in the assessment of T and N stages compared to the “gold standard”, i.e. histological examination of surgical specimens. In the present study, compared to MRI, EUS offered significantly superior diagnostic accuracy in assessing nodal involvement after RT, giving much better results in patients with N0 disease. A higher proportion of false-positive results of MRI in patients without nodal metastasis are probably due to the effects of RT on perirectal tissues (edema, inflammation, and fibrosis), which renders MRI unreliable for excluding nodal disease. Both methods were weak in restaging the persistence and degree of tumor invasion of the rectal wall, and this might be mainly due to microscopic persistence (under-staging) in the wall or to inflammatory and scar changes in perirectal fat (over-staging). These might explain the lower sensitivity and accuracy of both methods in restaging after RT.
The recent development of elastosonography, a new real-time EUS modality that gives a fairly qualitative image of tissue elasticity, might improve the accuracy and sensitivity of EUS in this setting. In a preliminary report, we showed that adding elastosonography to real-time EUS boosted the accuracy in T staging of the disease[30]. The ability of elastosonography to distinguish tissues with different levels of elasticity means it can detect inflammatory (soft) tissues and tumor (hard) separately, particularly when the real-time modality does not exclude the suspicion of perirectal invasion (Figure 3).
Figure 3.
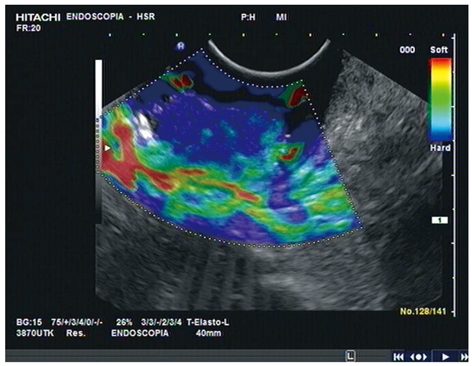
Rectal EUS and elastosonography before neoadjuvant chemoradiation treatment. Elastography showing hard tissue inside the lesion.
Therefore, in the modern era of GI-oncology it seems that patients benefit mostly from interdisciplinary approaches. In considering the value of a diagnostic technique, based on its ability to influence the therapeutic choices by allowing a better selection of patients, we believe that in this situation these two modalities are complementary.
In conclusion, EUS and MRI are both accurate for staging rectal cancer. In particular, in pre-therapy staging, EUS is a good modality for T staging while MRI obtains other information, including the clearance. However, after RT-CT, neither method is reliable for establishing the T stage. EUS seems significantly better than MRI for assessing the N stage, but until significant improvements in both methods are achieved, their use in this setting should be considered only in controlled trials.
COMMENTS
Background
Endoscopic ultrasound (EUS) and magnetic resonance imaging (MRI) are both accurate for staging rectal cancer. However, after radiation therapy-computed tomography (RT-CT), neither method is reliable for establishing the T stage. EUS seems significantly better than MRI for assessing the N stage but until significant improvements in both methods are achieved, their use in this setting should be considered only in controlled trials.
Innovations and breakthroughs
This study compared rectal EUS and MRI for staging rectal cancer after neoadjuvant RT-CT.
Peer review
This study compared rectal EUS and MRI for staging rectal cancer after neoadjuvant RT-CT and provides a good basis for selecting the best surgical or medical strategy in patients with rectal cancer.
Footnotes
Peer reviewer: Luis Bujanda, Professor, Donostia Hospital, San Sebastián, 20010, Spain
S- Editor Tian L L- Editor Stewart GJ E- Editor Ma WH
References
- 1.Vanagunas A, Lin DE, Stryker SJ. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am J Gastroenterol. 2004;99:109–112. doi: 10.1046/j.1572-0241.2003.04019.x. [DOI] [PubMed] [Google Scholar]
- 2.Savides TJ, Master SS. EUS in rectal cancer. Gastrointest Endosc. 2002;56:S12–S18. doi: 10.1016/s0016-5107(02)70079-5. [DOI] [PubMed] [Google Scholar]
- 3.Pahlman L, Glimelius B. Pre- or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann Surg. 1990;211:187–195. doi: 10.1097/00000658-199002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 5.Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99:623–627. doi: 10.1111/j.1572-0241.2004.04116.x. [DOI] [PubMed] [Google Scholar]
- 6.Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9–20. doi: 10.1007/s003840050002. [DOI] [PubMed] [Google Scholar]
- 7.Guinet C, Buy JN, Ghossain MA, Sezeur A, Mallet A, Bigot JM, Vadrot D, Ecoiffier J. Comparison of magnetic resonance imaging and computed tomography in the preoperative staging of rectal cancer. Arch Surg. 1990;125:385–388. doi: 10.1001/archsurg.1990.01410150107019. [DOI] [PubMed] [Google Scholar]
- 8.Rifkin MD, Ehrlich SM, Marks G. Staging of rectal carcinoma: prospective comparison of endorectal US and CT. Radiology. 1989;170:319–322. doi: 10.1148/radiology.170.2.2643135. [DOI] [PubMed] [Google Scholar]
- 9.Thaler W, Watzka S, Martin F, La Guardia G, Psenner K, Bonatti G, Fichtel G, Egarter-Vigl E, Marzoli GP. Preoperative staging of rectal cancer by endoluminal ultrasound vs magnetic resonance imaging. Preliminary results of a prospective, comparative study. Dis Colon Rectum. 1994;37:1189–1193. doi: 10.1007/BF02257780. [DOI] [PubMed] [Google Scholar]
- 10.American Joint Committee on Cancer. Colon and rectum. Manual for staging of cancer. 4th ed. Philadelphia: JB Lippincott; 1992. pp. 75–79. [Google Scholar]
- 11.Heriot AG, Grundy A, Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86:17–28. doi: 10.1046/j.1365-2168.1999.00996.x. [DOI] [PubMed] [Google Scholar]
- 12.Muthusamy VR, Chang KJ. Optimal methods for staging rectal cancer. Clin Cancer Res. 2007;13:6877s–6884s. doi: 10.1158/1078-0432.CCR-07-1137. [DOI] [PubMed] [Google Scholar]
- 13.Halefoglu AM, Yildirim S, Avlanmis O, Sakiz D, Baykan A. Endorectal ultrasonography versus phased-array magnetic resonance imaging for preoperative staging of rectal cancer. World J Gastroenterol. 2008;14:3504–3510. doi: 10.3748/wjg.14.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773–783. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 15.Lahaye MJ, Engelen SM, Nelemans PJ, Beets GL, van de Velde CJ, van Engelshoven JM, Beets-Tan RG. Imaging for predicting the risk factors--the circumferential resection margin and nodal disease--of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR. 2005;26:259–268. doi: 10.1053/j.sult.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Brown G, Davies S, Williams GT, Bourne MW, Newcombe RG, Radcliffe AG, Blethyn J, Dallimore NS, Rees BI, Phillips CJ, et al. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91:23–29. doi: 10.1038/sj.bjc.6601871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254–265. doi: 10.1245/s10434-008-0231-5. [DOI] [PubMed] [Google Scholar]
- 18.Puli SR, Reddy JB, Bechtold ML, Choudhary A, Antillon MR, Brugge WR. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:1255–1265. doi: 10.1245/s10434-009-0337-4. [DOI] [PubMed] [Google Scholar]
- 19.LeBlanc JK. Imaging and management of rectal cancer. Nat Clin Pract Gastroenterol Hepatol. 2007;4:665–676. doi: 10.1038/ncpgasthep0977. [DOI] [PubMed] [Google Scholar]
- 20.Bartram C, Brown G. Endorectal ultrasound and magnetic resonance imaging in rectal cancer staging. Gastroenterol Clin North Am. 2002;31:827–839. doi: 10.1016/s0889-8553(02)00027-4. [DOI] [PubMed] [Google Scholar]
- 21.Maor Y, Nadler M, Barshack I, Zmora O, Koller M, Kundel Y, Fidder H, Bar-Meir S, Avidan B. Endoscopic ultrasound staging of rectal cancer: diagnostic value before and following chemoradiation. J Gastroenterol Hepatol. 2006;21:454–458. doi: 10.1111/j.1440-1746.2005.03927.x. [DOI] [PubMed] [Google Scholar]
- 22.Dinter DJ, Hofheinz RD, Hartel M, Kaehler GF, Neff W, Diehl SJ. Preoperative staging of rectal tumors: comparison of endorectal ultrasound, hydro-CT, and high-resolution endorectal MRI. Onkologie. 2008;31:230–235. doi: 10.1159/000121359. [DOI] [PubMed] [Google Scholar]
- 23.Shihab OC, Moran BJ, Heald RJ, Quirke P, Brown G. MRI staging of low rectal cancer. Eur Radiol. 2009;19:643–650. doi: 10.1007/s00330-008-1184-6. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki C, Torkzad MR, Tanaka S, Palmer G, Lindholm J, Holm T, Blomqvist L. The importance of rectal cancer MRI protocols on interpretation accuracy. World J Surg Oncol. 2008;6:89. doi: 10.1186/1477-7819-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqui AA, Fayiga Y, Huerta S. The role of endoscopic ultrasound in the evaluation of rectal cancer. Int Semin Surg Oncol. 2006;3:36. doi: 10.1186/1477-7800-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torricelli P. Rectal cancer staging. Surg Oncol. 2007;16 Suppl 1:S49–S50. doi: 10.1016/j.suronc.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 27.Rovera F, Dionigi G, Boni L, Cutaia S, Diurni M, Dionigi R. The role of EUS and MRI in rectal cancer staging. Surg Oncol. 2007;16 Suppl 1:S51–S52. doi: 10.1016/j.suronc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 28.Lennon AM, Penman ID. Endoscopic ultrasound in cancer staging. Br Med Bull. 2007;84:81–98. doi: 10.1093/bmb/ldm033. [DOI] [PubMed] [Google Scholar]
- 29.Harewood GC. Utility of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am J Gastroenterol. 2004;99:953; author reply 954. doi: 10.1111/j.1572-0241.2004.40112.x. [DOI] [PubMed] [Google Scholar]
- 30.Mezzi G, Arcidiacono PG, Carrara S, Boemo C, Testoni PA. Elastosonography in malignant rectal disease: preliminary data. Endoscopy. 2007;39:375; author reply 375. doi: 10.1055/s-2007-966220. [DOI] [PubMed] [Google Scholar]