Abstract
Background
Plasticity in the spinal dorsal horn is thought to underlie at least in part pain behavior following peripheral nerve injury. Homer1 proteins play an important role in synaptic plasticity through an activity-dependent remodeling of the post-synaptic density (PSD). In this study we examined the early consequences of the loose ligation of the sciatic nerve on the levels of Homer1a and Homer1b/c proteins in the PSD of spinal dorsal horn neurons.
Methods
Male rats were randomly assigned to control, sham-operated or ligated groups. Four hours after sciatic exposure or ligation the animals were anesthetized and euthanized. Dorsal horn ipsilateral and contralateral quadrants were homogenized and centrifuged to obtain a PSD-containing LP1 fraction. Homer1 isoforms were identified in Western immunoblots. In some animals, Homer1 small interfering RNA (siRNA), non-target siRNA, MK-801 or U-01026 were injected intrathecally before surgery to assess the effects of this treatment on the levels of Homer1 isoforms and on two signs of injury-associated pain behavior, a shift in weight-bearing distribution and thermal hyperalgesia.
Results
In ligated animals the protein levels of Homer1a increased and those of Homer1b/c decreased in the ipsilateral LP1 fraction of the spinal dorsal horn. In contrast, no changes were detected in the contralateral LP1 fraction of ligated animals, or either the ipsilateral or contralateral LP1 fraction of sham-operated animals. Intrathecal injections of Homer1 siRNA, but not non-target siRNA, 2h before the ligation prevented the accumulation of Homer1a and loss of Homer1b/c in the ipsilateral LP1 fraction. The same pre-treatment with Homer1 siRNA also alleviated both a shift in weight-bearing behavior and thermal hyperalgesia in the ligated animals. Intrathecal injections of MK-801 or U0126 15 min before the ligation similarly prevented the injury-associated changes in Homer1 protein levels and the behavioral signs of pain.
Conclusion
The ligation-associated changes in the protein levels of Homer1a and Homer 1b/c in the ipsilateral PSD of spinal dorsal horn neurons may be an important early reflection of the injury-associated plasticity that in time leads to the development of persistent pain.
Introduction
Excitatory synapses in the central nervous system are characterized by a prominent thickening of the post-synaptic membrane called the post-synaptic density (PSD). The PSD contains tight complexes of numerous proteins, including neurotransmitter receptors and associated regulatory and cytoskeletal proteins1,2. Recent evidence suggests that strong synaptic activity is accompanied by major changes in the PSD protein matrix, and it is clear now that dynamic regulation of the various constituents of the PSD plays an important role in the establishment of greater synaptic efficacy through activity-dependent changes in post-synaptic structure and function1,2.
The Homer protein family is a major constituent of the PSD3,4. All of the members of the Homer protein family contain an Ena/VASP Homology 1 (EVH1) domain at the N-terminal region, and all but Homer1a contain a coiled-coil (CC) domain at the C-terminal region3,4. The EVH1 domain can bind proline-rich motifs of various proteins including metabotropic glutamate receptors (mGluR). The forms of Homer that contain the CC domain (e.g., Homer1b, Homer1c) can form hetero or homomeric complexes, and this allows for a link between mGluR and other PSD proteins. Homer proteins may play an important role in the regulation of dendritic spine morphology in both synaptogenesis and synaptic plasticity through an activity-dependent remodeling of the PSD3,4.
Injury-associated plasticity in the spinal dorsal horn is thought to underlie at least in part pain behavior following peripheral nerve injury5, and we and others have previously reported an intriguing potential role for the Homer1 isoforms in pain6,7,8. In our most recent investigation7 we observed that an early but transient up-regulation of Homer1 gene expression is associated with loose ligation of the sciatic nerve. In the present study we focused on the early consequences of the nerve injury on the levels of Homer1 proteins in sub cellular fractions of spinal dorsal horn neurons.
We hypothesized that loose ligation of the sciatic nerve was associated with changes in Homer1 protein levels, and we surmised that these changes may be important early steps in the injury-associated plasticity that eventually leads to the development of persistent pain. We also hypothesized that inhibition of protein production by intrathecal pre-treatment with small interfering RNAs (siRNA) would prevent the changes in Homer1 levels and thus attenuate behavioral signs of pain. We further sought to examine whether the changes in Homer1 levels were dependent on the activation of NMDA receptors or extracellular signal-regulated kinase 1 & 2 (ERK1/2). There is a well-established association between peripheral nerve injury and the activation of NMDA receptors and ERK1/25, and we previously reported that the ligation-associated increases in Homer1a message7 were NMDA and ERK1/2-dependent.
Methods
Animals
Male Harlan-Sprague-Dawley rats (200–250g) were used. All experiments were conducted in accordance with guidelines accepted by the International Association for the Study of Pain9. The animal protocol was approved by the appropriate Animal Care and Use Committee at the University of Wisconsin-Madison.
Behavioral Tests
A dual channel scale (Incapacitance Meter™, Stoelting, Chicago, IL), which separately measures the weight borne by each hind limb, was used for the weight-bearing test. While normal rats distribute weight about equally, animals with a unilateral injury will shift their weight from an injured to a non-injured limb. This shift is taken as a measure of discomfort in the injured limb. A 1s weighing period was used to average 20 measurements, and the average ratio of the injured over uninjured weight distribution was then calculated for each animal.
Thermal hyperalgesia was assessed with the well-established hind paw withdrawal latency test10 using a plantar analgesia instrument (Stoelting, Chicago, IL). Animals were acclimated for 15–20 min. The ipsilateral, injured, paw was tested four times to obtain an average latency. Each of the four trials were separated by 5 min. Baseline withdrawal latencies were obtained for all animals before they were randomly assigned to control, sham-operated or ligated groups. Four hours after sciatic exposure or ligation the withdrawal latencies of all animals were obtained again (second latency test). The weight-bearing test preceded the second paw withdrawal latency test.
Intrathecal drug application
Homer1 (1μM) or non-target (control) siRNA (1μM) (Santa Cruz Biotechnology, Santa Cruz, CA or Dharmacon, Lafayette, CO) were injected intrathecally 2h before sciatic exposure or ligation in a volume of 10μl using the procedure of Mestre and colleagues11 as described in detail previously12. Homer1 siRNA targets Homer1 mRNA specifically while non-target (control) siRNA does not have any known gene target in human, rat or mouse. The siRNAs were mixed with diluted transfection reagent (Santa Cruz Biotechnology, Santa Cruz, CA) or oligofectamine (Invitrogen, Carlsbad, CA) and incubated for 20–30 min at room temperature before application.
MK-801 (20μg in 10μl saline), U01026 (5μg in 10μl of 10% DMSO) or vehicle (10μl) were injected 15 min before sciatic exposure or ligation. Both drugs were from Sigma-Aldrich, St. Louis, MO. For all intrathecal injections animals were briefly anesthetized as described below.
Anesthesia, sciatic ligation and tissue collection
Animals were anesthetized with isoflurane. Body temperature was kept at 37°C with a homeothermic blanket system. Anesthesia was sufficiently deep to prevent arousal but light enough to permit spontaneous respiration. Adequate anesthesia was assessed by monitoring blink or ear reflexes, withdrawal to toe pinches, respiratory rate, and absence of spontaneous movements.
Loose ligation of the sciatic nerve (chronic constriction injury) was performed using the Bennett and Xie13 procedure as described previously6,12. Briefly, the sciatic nerve was exposed and loosely ligated with 4 simple interrupted 4–0 chromic gut sutures placed about 1mm apart. In sham-operated animals the sciatic nerve was exposed but not ligated. Control animals were anesthetized but were not subject to surgery.
For tissue collection the animals were re-anesthetized and euthanized with an intracardiac injection of saturated potassium chloride. A laminectomy rapidly (<2 min) exposed the lumbar spinal cord at L5, and about 1cm of the cord was excised and cut into dorsal and ventral halves and the dorsal half further divided into ipsilateral and contralateral quadrants. All tissues were stored at −80°C until use.
Western immunoblots
Tissues were homogenized and sequentially centrifuged to yield the PSD-containing LP1 fraction as described previously6. After assaying for total protein content the fractions were processed using the Western immunoblot procedure as described previously6,7. Homer1a and Homer1b/c antibodies (both from Santa Cruz Biotechnology, Santa Cruz, CA) were each used at a dilution of 1:600. The Homer1a antibody was raised against a peptide mapping at the C-terminus of mouse origin. Homer1b/c antibody was raised against amino acids 181–354 of human Homer1b. Beta III tubulin served as the loading control (1:1000; Promega, Madison, WI). Protein levels were estimated from optical density measurements using the BioSpectrum 500 Image Analysis System (UVP, Upland, CA). The levels in sham-operated or ligated animals were normalized to those in control, uninjured animals, i.e., control values served as baseline reference values.
RT-PCR
Homer1a, lamin A/C and cyclophilin B mRNA levels in individual dorsal horn quadrants were determined by the real-time polymerase chain reaction (RT-PCR) as described previously7. Briefly, total RNA was isolated from homogenized tissues with Trizol (Invitrogen, Carlsbad, CA) and cDNA was synthesized from 1μg of the total RNA. Quantitative RT-PCR was then performed with the ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) by monitoring in real time the increase in fluorescence of SYBR-GREEN dye. Relative expression levels of Homer1a in each sample were determined using a standard curve of 3-fold serial dilutions. Average fold induction relative to control animals was determined after normalizing to the amount of 18S rRNA in each sample. A 2-fold or greater change was considered significant. Primer sequences are available upon request.
Statistical Analysis
ANOVA was used for the statistical data analysis. The main emphasis was on detecting differences in Homer1a or Homer1b/c protein or message levels between control, sham-operated and ligated animals, or vehicle and drug-treated animals. In the behavioral experiments the emphasis was on detecting differences in weight distribution or latencies between control, sham-operated and ligated or between the vehicle and drug-treated groups. Significant effects were further analyzed with Scheffe’s post-hoc test, and statistical difference was inferred at p ≤ 0.05. Each group consisted of 6 animals except for the initial siRNA study (Fig. 2) which was based on 4 animals per group. All data are expressed as mean±SEM.
Figure 2. Intrathecal pre-treatment with Homer1 siRNA prevented injury-associated increases in Homer1a message.
A All three doses of Homer1 siRNA prevented the injury-associated increases in Homer1a mRNA. Pre-treatment with non-target siRNA failed to prevent the increase. B. Homer1b/c, cyclophilin B or lamin A/C mRNA was not affected by the sciatic ligation and pre-treatment with Homer1 siRNA had no effect. #p<0.05, ##p<0.01.
Results
Increases in Homer1a and decreases in Homer 1b/c protein in the PSD of dorsal horn neurons were associated with the sciatic ligation
We focused on the 4h post-ligation period based on our previous finding that Homer1a gene expression was transiently up-regulated at this time7. In addition, this is the earliest time after surgery that the presence of pain behavior in ligated animals can be differentiated from the lack of pain behavior in sham-operated animals.
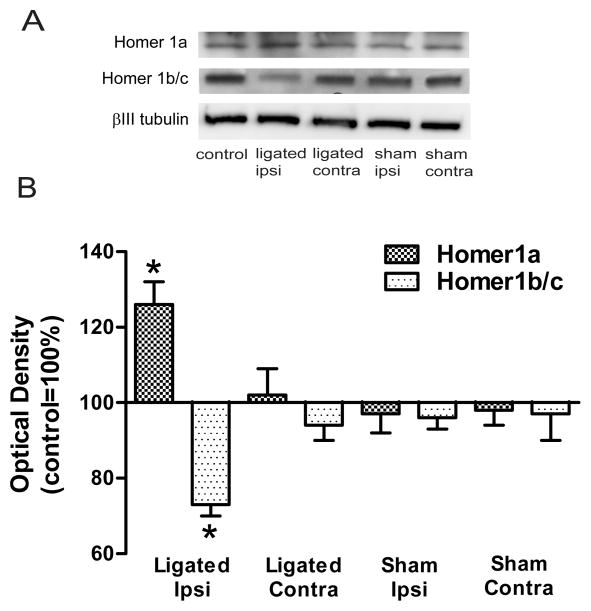
Homer1a protein levels were significantly higher in the ipsilateral PSD-containing LP1 fraction in ligated animals when compared to control animals, 126±6%, F(4,25)=6.3, p<0.001 (Fig. 1). Homer1a levels in the contralateral LP1 fraction of ligated animals (102±7%) or either the ipsilateral (97±5%) or contralateral (98±4%) LP1 fractions in sham-operated animals were not significantly different from controls.
Figure 1. Accumulation of Homer1a and loss of Homer1b/c in the PSD of spinal dorsal horn neurons were associated with loose ligation of the sciatic nerve.
A Immunoblots of Homer1a and Homer1b/c in representative LP1 fractions of the dorsal horn. Beta III-tubulin was the loading control. B: Summary plot of the estimated content of Homer1a and Homer1b/c. Note the divergent changes in levels in the ipsilateral PSD of ligated animals. In contrast, Homer1a and Homer1b/c levels in the contralateral PSD of ligated animals or either side in sham-operated animals were not different from controls. *p<0.001.
Homer1b and 1c have extensive overlapping sequences3,4 and we are not aware of a commercially available antibody that distinguishes between the two. So we used an antibody that detects both 1b and 1c but that does not cross-react with Homer2 or Homer3 isoforms.
The levels of Homer1b/c in the ipsilateral PSD-containing LP1 fraction of ligated animals were lower than controls, 73±3%, F(4,25)=7.2, p<0.001 (Fig. 1). On the other hand, Homer1b/c levels on the contralateral side in ligated animals (94±4%) or either the ipsilateral (96±3%) or contralateral (97±7%) side in sham-operated animals were not different from controls.
Intrathecal pre-treatment with Homer1 siRNA dose-dependently prevented the ligation-associated increases in Homer1a message
In an initial study we sought to determine both the optimal dose for Homer1a silencing and the specificity of the siRNA treatment.
Homer1a mRNA levels in the ipsilateral spinal dorsal horn at 4h post-ligation were significantly lower than those of vehicle-treated ligated animals (2.0±0.2) for all three Homer1 siRNA doses tested, 20μM: 1.3±0.3, p<0.05; 1μM: 0.9±0.3, p<0.01; and 0.1μM 1.3±0.1, p<0.01 (Fig. 2A). In contrast, in non-target siRNA-treated animals the levels of Homer1a mRNA in the ipsilateral spinal dorsal horn (2.1±0.2) were not different from those of vehicle-treated ligated animals (Fig. 2A). Given that we obtained the greatest effect with 1μM Homer1 siRNA we used this dose in the remainder of the study.
To further assess the specificity of our procedure we also processed the ipsilateral spinal dorsal horn from animals pre-treated with 1μM Homer1 siRNA for Homer1b/c, lamin A/C or cyclophilin B mRNA. Lamin A/C is an intermediate filament type protein that is a major component of nuclear lamina. Cyclophilin B is a protein located in the endoplasmic reticulum and associated with secretory pathways. Both of these proteins are expressed abundantly in mammalian cells.
As we reported previously, Homer1b/c message was not affected by the sciatic ligation7. Consequently as expected, 1μM siRNA pre-treatment did not modify the Homer1b/c mRNA levels (Fig. 2B). Similarly, neither lamin A/C nor cyclophilin B message was affected by the loose ligation of the sciatic nerve. Intrathecal pre-treatment with 1μM Homer1 siRNA also did not affect either lamin A/C or cyclophilin B message.
Intrathecal pre-treatment with Homer1 siRNA prevented the ligation-associated changes in Homer1a and Homer 1b/c protein levels in the PSD
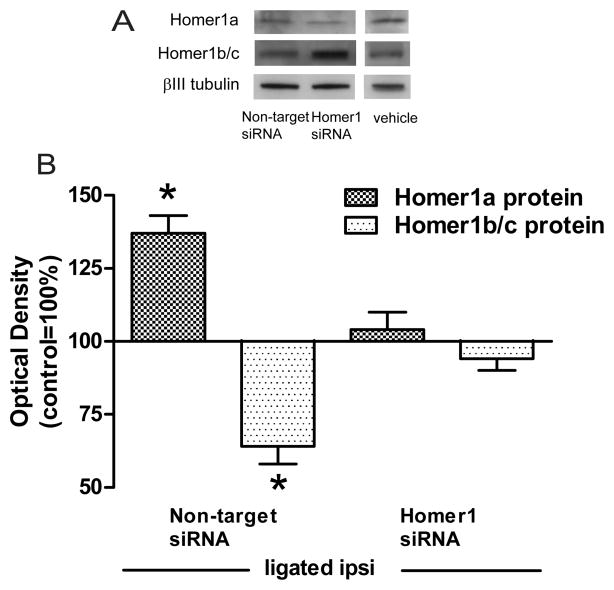
In ligated animals pre-treated with non-target siRNA there was a significant accumulation of Homer1a in the ipsilateral LP1 fraction of spinal dorsal horn neurons 4h post-ligation, 137±6%, F(2,15)=17.6, p<0.001 (Fig. 3). The increase in Homer1a protein in these ligated animals was comparable to that seen previously (Fig. 1). On the other hand, pre-treatment with Homer1 siRNA prevented this accumulation, 104±6%.
Figure 3. Ligation-associated changes in the protein levels of Homer1a or Homer1b/c in the ipsilateral PSD were prevented by intrathecal pre-treatment with Homer1 siRNA but not non-target siRNA.
A Homer1a and Homer 1b/c immunoblots in the ipsilateral LP1 fraction of ligated animals pre-treated intrathecally with either 1μM Homer1 siRNA or 1μM non-target siRNA. For comparison, immunoblots from vehicle-treated ligated animals shown in Fig. 5 are also included here. B: Summary plots of Homer1a and Homer1b/c levels. The ligation-associated divergent changes in Homer1 protein levels (Fig. 1) were prevented by pre-treatment with Homer1 siRNA but not with non-target siRNA. *p<0.001.
Homer1 siRNA pre-treatment also prevented the injury-associated reduction in Homer1b/c protein in the ipsilateral LP1 fraction of spinal dorsal horn neurons, 94±4% (Fig. 3). In contrast, Homer1b/c protein levels in non-target siRNA pre-treated animals were significantly lower than in controls, 64±6%, F(2,15)=26.3, p<0.001. These results suggested that silencing Homer1a message specifically prevented the injury-elicited accumulation of Homer1a and loss of Homer1b/c in the PSD.
A pre-emptive single intrathecal injection of Homer1 siRNA abolished the two behavioral signs of pain
Before the animals were euthanized and their tissues collected for the immunoblotting experiments, they were behaviorally tested to examine whether they exhibited signs of pain behavior.
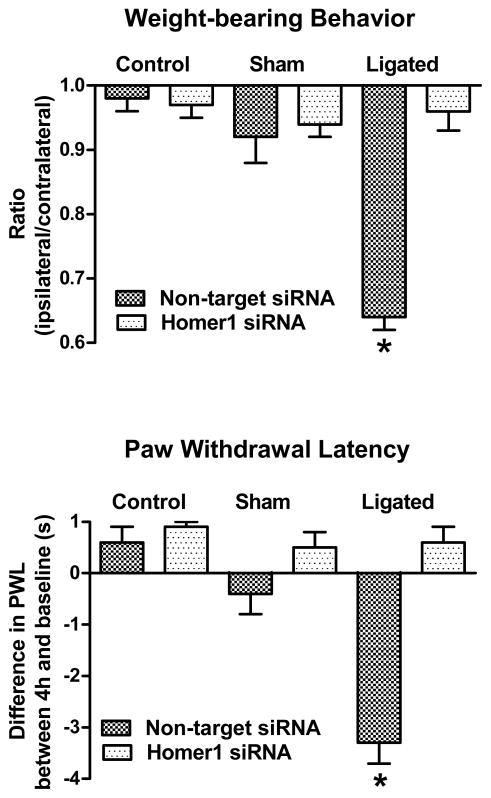
Non-target siRNA-treated ligated animals exhibited a significant shift in weight-bearing behavior 4h post-ligation as they placed less weight on their injured, ipsilateral limb, 0.64±0.02 (Fig. 4B). Pre-treatment with Homer1 siRNA effectively prevented this shift, 0.96±0.03. This difference between groups was significant, F(5,30)=26.0, p<0.001, and was due to the reduced ratio in vehicle-treated rats.
Figure 4. Pre-treatment with Homer1 siRNA abolished both behavioral signs of pain.
Intrathecal injection of Homer1 siRNA prevented an injury-associated shift in weight-bearing behavior suggesting pain relief. In contrast, pre-treatment with non-target siRNA did not modify the weight-bearing shift in ligated animals. Homer1 siRNA pre-treatment also abolished the injury-associated reduction in the second test withdrawal latency 4h after loose ligation of the sciatic nerve. Pre-treatment with non-target siRNA did not prevent this reduction. Pre-treatment with either non-target siRNA or Homer1 siRNA did not modify the weight distribution or thermal latency behavior of control, uninjured (no surgery) animals or of sham-operated animals.*p<0.001.
The same non-target siRNA-treated ligated animals also showed a significant reduction between the baseline (9.0±0.4s) and 4h post-ligation (5.8±0.2s) withdrawal latency of the injured, ipsilateral limb (Fig. 4A). Intrathecal pre-treatment with Homer1 siRNA effectively prevented this reduction (8.3±0.3s vs. 8.8±0.3s) suggesting the absence of thermal hyperalgesia (Fig. 4A). ANOVA indicated no difference among groups at baseline, F(5,30)=1.2, p<0.5, but a significant difference at 4h post-ligation, F(5,30)=35.4, p<0.001. This was due to the reduction in second test latency in Homer1a siRNA-treated animals. Neither non-target siRNA nor Homer1 siRNA significantly modified the weight-bearing behavior or the second test latency of control or sham-operated animals (Fig. 4).
These results suggested that application of Homer 1 siRNA at a dose sufficient to block the ligation-associated increases in Homer1a mRNA and the subsequent accumulation of Homer1a protein in the PSD of spinal dorsal horn neurons effectively abolished both of the early, injury-associated signs of pain behavior.
MK-801 and U0126 pre-treatment also prevented the ligation associated changes in Homer1a and Homer 1b/c protein levels in the PSD of spinal dorsal horn neurons
In these experiments we sought to determine whether blockade of NMDA receptors with MK-801 or ERK1/2 activity with the MEK inhibitor U0126 would modify the changes in Homer1 protein levels. We reported previously that the early, injury-elicited induction of Homer1a gene expression in the spinal dorsal horn was mediated at least in part by NMDA receptors as well as ERK1/27.
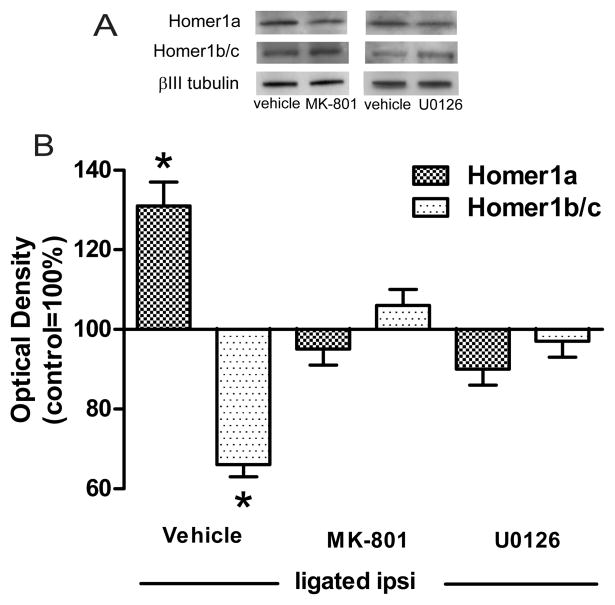
As expected, in vehicle-treated animals the sciatic ligation was associated with the accumulation of Homer1a in the ipsilateral LP1 fraction (131±6%). This injury-elicited increase in Homer1a protein levels was prevented by pre-treatment with either MK-801 (95±4%) or U0126 (90±4%), F(2,15)=21.9, p<0.001 (Fig. 5).
Figure 5. Ligation-associated changes in the levels of Homer1a or Homer1b/c were dependent on the activation of NMDA receptors and ERK1/2.
The ligation-associated divergent changes in Homer1 protein levels were prevented by intrathecal pre-treatment with either MK-801 or U0126. Representative immunoblots accompany the summary plot.*p<0.001.
The same pre-treatment also prevented the injury-associated reduction in Homer1b/c protein levels in the ipsilateral PSD of dorsal horn neurons. In other words, the levels of Homer1b/c in vehicle-treated ligated animals were significantly lower (66±3%) than in either MK-801 (106±4%) or U0126 (97±4) treated animals, F(2,15)=34.4, p<0.001.
MK-801 and U0126 similarly prevented both a shift in weight-bearing behavior and thermal hyperalgesia
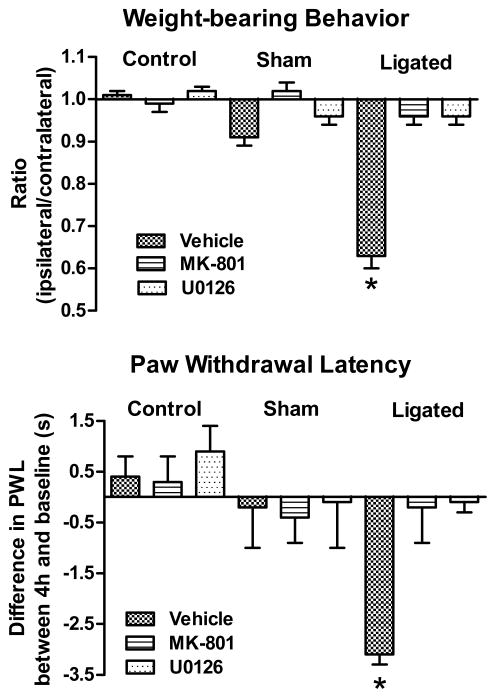
Vehicle-treated ligated animals exhibited a significant shift in weight-bearing behavior 4h post-ligation as their weight-bearing ratio was reduced to 0.63±0.03, F(8,45)=40.5, p<0.001 (Fig. 6). Intrathecal pre-treatment with either MK-801 or U0126 prevented this shift.
Figure 6. Early pain behavior was abolished by pre-treatment with either MK-801 or U0126.
Vehicle-treated ligated animals showed a significant shift in weight-bearing distribution. Pre-treatment with either MK-801 or U0126 prevented this shift suggesting pain relief. Vehicle-treated ligated animals also exhibited a significant reduction in their 4h post-ligation (second test) latency. Pre-treatment with either MK-801 or U0126 also prevented this reduction. Injections of vehicle, MK-801 or U0126 did not significantly modify the weight distribution or second test latency in either control or sham-operated animals. *p<0.001.
Vehicle-treated ligated animals similarly showed a reduction in the 4h post-ligation withdrawal latency of the injured, ipsilateral limb (4.8±0.3s compared to baseline 7.9±0.2s, Fig. 6). Intrathecal pre-treatment with MK-801 or U0126 prevented this reduction. ANOVA indicated no difference among groups at baseline, F (8,45)=0.4, p<1, but a significant difference at 4h post-ligation, F(8,45)=177.1, p<0.001. This was due to the significant reduction in the second test latency of vehicle-treated animals.
Control animals exhibited equal weight-bearing behavior and did not show a significant difference between baseline and second test latencies (Fig. 6). Vehicle-treated sham-operated animals similarly did not show a shift in weight-bearing behavior or significant difference between their baseline and second test latency 4h after sciatic nerve exposure (Fig. 6). Neither MK-801 nor U0126 modified the weight-bearing behavior or the second test latencies of control or sham-operated animals.
Discussion
In this study we examined the early consequences of the loose ligation of the sciatic nerve on the protein levels of Homer1 isoforms in the PSD of spinal dorsal horn neurons. Our data established that the sciatic ligation was associated with increases in Homer1a levels and decreases in Homer1b/c levels. These changes in the protein levels of both Homer1a and Homer1b/c were prevented specifically by pre-treatment with Homer1 siRNA. The same pre-treatment also prevented two ligation-associated early behavioral signs of pain. To our knowledge, this is the first demonstration of a change in content of Homer1 proteins in the PSD of spinal dorsal horn neurons which can be prevented by the same agents that abolish signs of pain behavior.
The injury-elicited increases in Homer1a protein levels followed closely the up-regulation of Homer1a gene expression reported in our previous study7. This confirmed the notion that the expression of this Homer isoform can be quickly induced by a variety of physiological stimuli14–18. These results also confirmed our previous finding that the early, injury-elicited induction of Homer1a gene expression in the spinal dorsal horn was accompanied by a rapid increase in protein synthesis, and was mediated at least in part by activation of both NMDA receptors and ERK1/27. Our present results further extended our previous findings by suggesting a direct relationship between injury-associated up-regulation of Homer1a and early behavioral signs of pain.
These results are in agreement with some aspects of a study of Homer1a in the mouse spinal cord which reported that chronic inflammation increased both gene and protein expression of this isoform, and that this increase was attenuated by a block of NMDA receptors or ERK1/2 activation8. This study also demonstrated that Homer1a as well as Homer1b/c were concentrated in the superficial layers of the dorsal horn, and that the inflammation elicited changes in both immunohistochemical and in situ hybridization staining patterns. However, this study concluded that Homer1a up-regulation was protective against the inflammatory pain because silencing Homer1a gene expression enhanced the pain while Homer1a over-expression in the spinal cord attenuated the hyperalgesia8. It is probable that differences in pain models and animal species contributed to the disparate outcome between this and our study. In addition, like synaptic plasticity19, the development of pain consists of early and late phases which are likely mediated by different mechanism5. Transient increases in Homer1a expression during one of these phases may affect synaptic plasticity differently, and this may have further contributed to the disparate outcomes.
We appreciate that while we have used a model of neuropathic pain in this study we only focused on the early consequences of the sciatic ligation. The pain behavior cannot thus be characterized as chronic but rather perhaps as ‘sub-chronic’20. Nevertheless we feel this is an important time point as it suggests that even as early as a few hours after the ligation there are changes in nociceptive processing occurring at the level of the spinal dorsal horn. This early spinal plasticity may then underlie at least to some extent the observable early pain behavior. It is of interest in this respect that in our hands at 4h post-surgery (but not before) the presence of pain behavior in ligated animals can be differentiated from the lack of pain behavior in sham-operated animals.
Low basal amounts of Homer1a protein in dorsal horn neurons were detected, but the sciatic ligation appeared to specifically elicit additional synthesis of the protein which then accumulated in the PSD. This accumulation of Homer1a and the observed loss of Homer1b/c imply a displacement of the latter by the former from the PSD. These data provide further support for the notion that Homer1a may participate in the early stages of synaptic plasticity. Activity-dependent up-regulation of Homer1a, its accumulation at postsynaptic sites, and its displacement of Homer1b/c is considered a critical step in the reorganization of the postsynaptic structure in synaptic plasticity3,4,14–18.
It is presently unclear how this increase in Homer1a protein at the PSD facilitates synaptic function in the short or the long-term. Potential Homer1a-mediated remodeling changes include increased insertion of AMPA receptors into the PSD membrane, enhancement of intracellular calcium release, increased activation of transient receptor potential canonical (TRPC) channels, and amplification of the link between metabotropic and ionotropic glutamate receptors21,22.
The injury-elicited accumulation of Homer1a in the PSD of spinal dorsal horn neurons may similarly engender synaptic remodeling and in this manner be a first step in a time-dependent process which contributes to the eventual development of neuropathic pain. The remodeled PSD may be a common reflection of the injury-elicited changes in several receptors, pathways, transcription factors and genes. As such, the PSD of spinal dorsal horn neurons may also be a suitable novel target for successful therapeutic intervention.
Acknowledgments
The authors wish to thank Anna M. Koos and DeAnna I. Mitchell for expert help with the animal surgeries and the behavioral testing.
Supported in part by National Institutes of Health grants NS034870 and NS055042.
Footnotes
Conflict of interest: None.
Implication Statement
Sciatic ligation-associated changes in the protein levels of the scaffolding proteins Homer1a and Homer 1b/c in the post-synaptic density of spinal dorsal horn neurons may be an important early reflection of nerve injury-associated plasticity that in time leads to persistent pain behavior.
References
- 1.Boeckers TM. The postsynaptic density. Cell Tissue Res. 2006;326:409–22. doi: 10.1007/s00441-006-0274-5. [DOI] [PubMed] [Google Scholar]
- 2.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: A more quantitative view. Annu Rev Biochem. 2007;76:823–47. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 3.de Bartolomeis A, Iasevoli F. The homer family and the signal transduction system at glutamatergic postsynaptic density: potential role in behavior and pharmacotherapy. Psychopharmacol Bull. 2003;37:51–83. [PubMed] [Google Scholar]
- 4.Worley PF, Zeng W, Huang G, Kim JY, Shin DM, Kim MS, Yuan JP, Kiselyov K, Muallem S. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium. 2007;42:363–71. doi: 10.1016/j.ceca.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Prog Brain Res. 2009;89:707–58. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 6.Miletic G, Miyabe T, Gebhardt GJ, Miletic V. Increased levels of Homer1b/c and Shank1a in the post-synaptic density of spinal dorsal horn neurons are associated with neuropathic pain in rats. Neurosci Lett. 2005;386:189–93. doi: 10.1016/j.neulet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Miyabe T, Miletic G, Miletic V. Loose ligation of the sciatic nerve in rats elicits transient up-regulation of Homer1a gene expression in the spinal dorsal horn. Neurosci Lett. 2006;398:296–99. doi: 10.1016/j.neulet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Tappe A, Klugmann M, Luo C, Hirlinger D, Agarwal N, Benrath J, Ehrengruber MU, During MJ, Kuner R. Synaptic scaffolding protein Homer1a protects against chronic inflammatory pain. Nat Med. 2006;12:677–81. doi: 10.1038/nm1406. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 10.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 11.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier AJ. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 12.Miletic G, Miletic V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain. 2008;137:532–39. doi: 10.1016/j.pain.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 14.Keuren-Jensen KV, Cline HT. Visual experience regulates metabotropic glutamate receptor-mediated plasticity of AMPA receptor synaptic transmission by homer1a induction. J Neurosci. 2006;26:7575–80. doi: 10.1523/JNEUROSCI.5083-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang GC, Mao LM, Liu XY, Parelkar NK, Arora A, Yang L, Hains M, Fibuch EE, Wang JQ. In vivo regulation of homer1a expression in the striatum by cocaine. Mol Pharmacol. 2007;71:1148–58. doi: 10.1124/mol.106.028399. [DOI] [PubMed] [Google Scholar]
- 16.Inoue Y, Udo H, Inokuchi K, Sugiyama H. Homer1a regulates the activity-induced remodeling of synaptic structures in cultured hippocampal neurons. Neuroscience. 2007;150:841–52. doi: 10.1016/j.neuroscience.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 17.Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: Implications for addiction. Biochem Pharmacol. 2008;75:112–13. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue Y, Honkura N, Kato A, Ogawa S, Udo H, Inokuchi K, Sugiyama H. Activity-inducible protein Homer1a/Vesl-1S promotes redistribution of postsynaptic protein Homer1c/Vesl-1L in cultured rat hippocampal neurons. Neurosci Lett. 2004;354:143–47. doi: 10.1016/j.neulet.2003.09.082. [DOI] [PubMed] [Google Scholar]
- 19.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 21.Ehrengruber MU, Kato A, Inokuchi K, Hennou S. Homer/Vesl proteins and their roles in CNS neurons. Mol Neurobiol. 2004;29:213–227. doi: 10.1385/MN:29:3:213. [DOI] [PubMed] [Google Scholar]
- 22.Worley PF, Zeng W, Huang G, Kim JY, Shin DM, Kim MS, Yuan JP, Kiselyov K, Muallem S. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium. 2007;42:363–371. doi: 10.1016/j.ceca.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]