Abstract
Objective
Previous studies suggest possible modulatory effects of progesterone on nicotine addiction. The goal of this study was to determine the effects of progesterone, on acute physiological and subjective responses to intravenous (IV) nicotine in overnight abstinent male and female smokers.
Methods
Twelve smokers, 6 males and 6 females, participated in a double-blind, placebo-controlled, crossover study, which consisted of 2 experimental sessions. Before each session, subjects were treated orally with a single dose of either 200 mg progesterone or placebo. Starting 2 hours following the medication treatment, subjects received an IV saline injection, followed by 0.5 and 1.0 mg/70 kg IV nicotine.
Results
Progesterone treatment, compared to placebo, enhanced the ratings of “bad effects,” from IV nicotine and attenuated the rating of “drug liking.” Progesterone also enhanced suppression of smoking urges by nicotine as assessed by the Brief Questionnaire on Smoking Urges (BQSU).
Conclusions
These results suggest that progesterone may alter the subjective effects of nicotine as well as urges to smoke cigarettes. Further studies are warranted to examine the modulation of nicotine’s effects by gonadal hormones.
Keywords: nicotine, progesterone, IV nicotine, sex differences, gender differences, gonadal hormones
Introduction
Gonadal hormones, estradiol and progesterone have well-documented actions on dynamic brain functioning, including interactions with multiple neurotransmitters affecting the brain reward circuit (Jackson, Robinson et al. 2006). Changes in reward function during the menstrual cycle phases were examined in a recent functional MRI study (Dreher, Schmidt et al. 2007). Plasma estradiol levels in normally cycling women were positively correlated with the reward activation in hippocampal-amygdala region, indicating the modulation of the brain reward circuits by gonadal hormones (Dreher, Schmidt et al. 2007). With respect to tobacco addiction, in a recent study, Allen and coworkers reported than women who quit smoking during the follicular phase of the menstrual cycle, had shorter times to relapse than women who quit during the luteal phase (Allen, Bade et al. 2008). These findings, along with many others (Carpenter, Upadhyaya et al. 2006),support possible modulatory effects of gonadal hormones on nicotine addiction.
The goal of this study was to characterize progesterone’s effects on subjective and physiological responses to intravenous (IV) nicotine, and on nicotine withdrawal severity in overnight abstinent smokers. Previously, we reported that in overnight-abstinent female smokers, progesterone treatment, compared to placebo, attenuated the subjective effects of the first cigarette of the day and craving for cigarettes (Sofuoglu, Babb et al. 2001). To better characterize the interaction between progesterone and nicotine, we examined progesterone’s effects on IV nicotine, as a pure form of nicotine. The advantages of the IV route include rapid delivery, comparable to smoking, as well as accurate dosing. To our knowledge, this is the first study evaluating progesterone effects on IV nicotine responses in male and female smokers.
Methods
Participants
Six female and 6 male non-treatment seeking smokers were recruited from the New Haven area (7 African-Americans, 3 Caucasians, 1 Hispanic and 1 Native-American). Two additional subjects were enrolled but dropped out of the study and were not included in the analysis. The average age (SD) of the subjects were 35.7 (7.7). Subjects, on average, smoked 17.1 (6.5) cigarettes/day, and had a Fagerstrom Test for Nicotine Dependence (Heatherton, Kozlowski et al. 1991) score of 5.9 (1.6). Subjects had normal physical, laboratory and psychiatric examinations and were not dependent on alcohol or on any drugs other than nicotine. Experimental sessions were conducted in the Biostudies Unit located at the VA Connecticut Healthcare System (West Haven campus) and subjects were paid for participation. This study was approved by the VA Connecticut Healthcare System Human Subjects Subcommittee, and all subjects signed informed consent prior to their entry into the study.
Procedures
This outpatient, double-blind, placebo-controlled, crossover study had 2 experimental sessions, where subjects were randomly assigned to a sequence of two treatment conditions: placebo or progesterone. Each session started at 8 AM. On the experimental days, subjects were instructed not to smoke after midnight. Smoking abstinence was verified based on expired carbon monoxide levels (<10 ppm). Prior to each session, each subject had an indwelling intravenous catheter placed in an antecubital vein for nicotine infusion and blood drawing. After baseline measurements were obtained, subjects received the study medication followed by a light meal. Starting 2 hours after medication administration, when peak levels of progesterone were expected, subjects were given saline followed by 2 escalating doses of nicotine (0.5 and 1.0 mg/70 kg) intravenously. This cumulative dosing procedure within the same session has been successfully used in previous studies for other drugs of abuse (Chait, Corwin et al. 1988; Walsh, Preston et al. 1994; Walsh, Sullivan et al. 1996). The injections were given over 60 seconds, separated by 30 minute intervals. During the sessions, participants were sitting on a comfortable recliner chair. Cardiac rhythm was monitored continuously during sessions, and 12-lead ECGs were obtained before and at the end of the session.
To control for menstrual cycle phase, female smokers attended the experimental sessions during the early follicular phase of their menstrual cycle. During the early follicular phase, the first week from the beginning of the cycle, both the endogenous estradiol and progesterone levels are low and remain stable. Consequently, this timing minimizes the interaction between the endogenous sex hormones and progesterone treatment (Chabbert Buffet, Djakoure et al. 1998). Many previous studies have demonstrated the feasibility and safety of administering sex hormones to women during the early follicular phase of the menstrual cycle (Justice and de Wit 2000; Sofuoglu, Babb et al. 2001; Sofuoglu, Babb et al. 2002). Peak levels of progesterone are achieved during the luteal phase (Chabbert Buffet, Djakoure et al. 1998). In contrast, men have plasma levels of progesterone comparable to levels of women who are in the follicular phase (Zumoff, Miller et al. 1990). To complete the experimental sessions during the early follicular phase in female smokers and to minimize carryover effects from progesterone, the experimental sessions were 2-3 days apart.
Nicotine and Progesterone Administration
Nicotine bitartrate was obtained from Interchem Corporation (Paramus, NJ). Nicotine samples were prepared by the research pharmacy at the VA Connecticut Healthcare System in a 5 cc volume of saline. In order to examine the dose-response effects of nicotine, subjects were administered placebo (saline) followed by 2 ascending doses of nicotine (0.5 and 1.0 mg/70 kg). The nicotine doses selected were within the nicotine dose range that has been shown to produce robust and reproducible subjective and physiological effects (Henningfield, Miyasato et al. 1985; Jones, Garrett et al. 1999; Sofuoglu, Babb et al. 2003; Sofuoglu, Poling et al. 2006).
Micronized progesterone (Prometrium®) was obtained from Solvay Pharmaceuticals (Marietta, GA). After oral administration, micronized progesterone reaches its peak plasma levels in 2 to 3 hours and has an elimination half-life of 3 to 4 hours (Simon 1995; de Lignieres 1999). We used a single 200 mg progesterone capsule. This dose has been shown to elevate plasma progesterone levels in female smokers to levels comparable to those found during the luteal phase of the menstrual cycle (Sofuoglu, Babb et al. 2001).
Outcome measures
We obtained physiological and subjective measures during the course of this study. The physiological measures were systolic and diastolic blood pressure and heart rate. These measures were taken before medication treatment and every 20 min for 2 hours afterwards. Additional physiological measures were taken 5 minutes before and 1, 2, 3, 5, 8, 10, and 15 minutes after saline or nicotine injections.
The subjective effects of nicotine were assayed with four instruments. The Drug Effects Questionnaire (DEQ), used to assess the acute subjective effects of nicotine, consists of 5 items: “drug strength,” “good effects,” “bad effects,” “head rush”, and “like the drug.” Participants rated these items on a 100 mm scale, from 0 “not at all” to 100 “extremely.” The Brief Questionnaire on Smoking Urges (BQSU), is a 10-item scale was originally developed by Tiffany and Drobes (Tiffany and Drobes 1991; Cox, Tiffany et al. 2001). Smokers were asked how strongly they agree or disagree with items on a 7-point Likert scale. This scale has been found to be highly reliable and reflects levels of nicotine deprivation (Bell, Taylor et al. 1999; Morgan, Davies et al. 1999). The Nicotine Withdrawal Symptom Checklist (NWSC) measures withdrawal symptoms from tobacco and includes items of cigarette craving, irritability/anger, anxiety, difficulty concentrating, restlessness, increased appetite, depressed mood, and insomnia (Hughes and Hatsukami 1986; Hughes and Hatsukami 1997). We used a modified version of the NWSC in which participants were asked to rate these symptoms on a 100 mm scale, from “not at all” to “extremely “(e.g.,Buchhalter, Acosta et al. 2005). The Profile of Mood States (POMS), Bipolar Form, is a 72–item rating scale used to measure the effects of medication treatments on mood (McNair, Lorr et al. 1988). The POMS has 6 subscales: (1) composed-anxious; (2) agreeable-hostile; (3) elated-depressed; (4) confident-unsure; (5) energetic-tired; (6) clear headed-confused. The DEQ was given 5 minutes before and 1, 3, 5, 8, and 10 minutes after saline or nicotine injections. The NWSC and the BQSU were given at the beginning of each session, 5 minutes before saline and nicotine deliveries, and 30 minutes after the second nicotine administration. The POMS was given 3 times: at the beginning of the session, before saline administration and at the end of each experimental session.
Data Analysis
All analyses were conducted with the Statistical Analysis System Version 9.1.3. To examine treatment effects on outcome measures, we conducted repeated-measures, random-effects analysis of covariance. The model included a fixed main effect for treatment (placebo or progesterone), dose (saline, 0.5 or 1.0 mg nicotine),, and interaction between treatment and dose. We also included a random effect for subject and a blocking factor for treatment sequence. For blood pressure, heart rate, and DEQ measurements during IV saline or nicotine administration, multiple measurements were collected. In order to simplify the analysis and interpretation, a change score (maximum post dose score minus predose baseline) for each dose (saline, 0.5 mg or 1.0 mg nicotine/70 kg) was calculated as a summary index. For other outcomes, all data points were included in the model. Values of p<.05 were considered statistically significant, based on two-tailed tests. Type I error rate in post-hoc tests was maintained through Bonferroni adjustments.
Results
Physiological measures
Compared to placebo, progesterone treatment did not modify IV nicotine responses for heart rate [F(1,55) =1.5; p>0.05], systolic [F(1,55) =1.2; p>0.05] or diastolic [F(1,55) =0.2; p>0.05] blood pressure. There was a significant nicotine dose effect for the heart rate, systolic and diastolic blood pressure (p<0.001). Pairwise comparisons indicated that responses for all 3 outcomes were higher under the 0.5 mg or 1.0 mg nicotine than under the placebo condition (p<0.05).
Subjective Measures
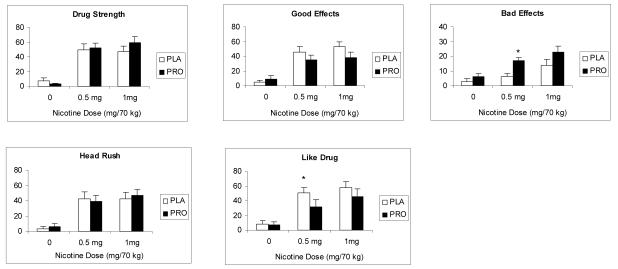
As shown in Figure 1, a significant treatment effect was observed for the rating of “drug liking” [F(1, 54) = 4.2; p<0.05] and “bad effects” [F(1, 54) = 4.3; p<0.05]. The rating of all items showed significant main effect of nicotine dose (p<0.001), indicating greater response to the 0.5 and 1.0 mg nicotine compared to saline.
Fig 1.
The average (with SEM) subjective responses to saline, 0.5 and 1.0 mg/ 70 kg intravenous nicotine under placebo and progesterone conditions. Bars represent the change (maximum post dose-baseline). Measurements were taken just before and 1, 3, 5, 8 and 10 minutes after each injection. Significant treatment differences for each nicotine dose are indicated by an asterisks (*).
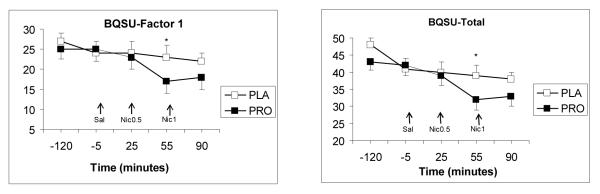
For the BQSU, a significant main effect for treatment was observed for the total score [F(1, 99) = 4.2, p <0.05], and for factor 1 (urge to smoke for stimulation) F(1, 99) = 4.5, p <0.05]. Pairwise comparisons indicated higher ratings under placebo condition (p<0.05). For the total NWSC score, no significant main effects for treatment or treatment-by-time were observed (p>0.05).
As sedation is one of the possible side effects of progesterone, the energetic-tired subscale of POMS was of particular interest. However, this subscale showed no treatment or treatment-by-time interactions (p>0.05). Similarly, other subscales of the POMS did not show a treatment or treatment-by-time interactions (p>0.05).
Plasma Progesterone and Estradiol Measurements
As expected, a significant treatment effect was observed for progesterone levels [F(1, 11) = 10.1, p <0.01], with higher values under progesterone treatment. In men, progesterone levels at baseline and 2 hours after progesterone treatment were 0.9 (0.2) and 50.0 (22.1), ng/ml, respectively. Under placebo treatment, the corresponding values were 1.3 (0.3) and 1.1(0.2) ng/ml. In women, plasma progesterone levels at baseline and 2 hours after progesterone administration were 1.7 (0.7) and 35.6 (14.3) ng/mL, respectively. Under placebo treatment, the corresponding values were 1.0 (0.3) and 1.4 (0.5) ng/mL. For women, baseline estradiol levels were 39.1 (5) and 38.5 (2.5) pg/mL under progesterone and placebo treatment, respectively.
Discussion
Progesterone treatment, compared to placebo, enhanced the ratings of “bad effects,” from IV nicotine and attenuated the rating of “drug liking” in male and female smokers. These effects of progesterone were unlikely due to non-specific mood-alteration, since measures of mood did not show changes in response to progesterone alone. We previously reported that progesterone treatment at 200 mg, attenuated the rating of “good effects” from smoking in female smokers who were in the follicular phase of their menstrual cycles (Sofuoglu, Babb et al. 2001). Consistent with these findings, in previous studies progesterone treatment attenuated some of the subjective responses to cocaine (Sofuoglu, Babb et al. 2002; Sofuoglu, Mitchell et al. 2004; Evans and Foltin 2006). This study further extends these findings by demonstrating that progesterone alters some of the subjective responses to pure nicotine in male and female smokers.
In this study, progesterone treatment, compared to placebo, enhanced nicotine’s effect in suppressing urges for smoking, as indexed by Factor 1 (urge to smoke for stimulation) of the BQSU and the total score of the BQSU. Consistent with these findings, in a previous study we reported progesterone treatment was associated with decreases in craving for cigarettes in female smokers (Sofuoglu, Babb et al. 2001). In that study, there was a trend for reduced smoking under progesterone treatment using a choice procedure. We are currently examining progesterone’s effects on tobacco withdrawal severity and smoking behavior in male and female smokers.
In our study, progesterone had opposite effects on the aversive and pleasurable effects of nicotine, simultaneously enhancing ratings of “bad effect” while attenuating “drug liking.” Both the aversive and rewarding nicotine effects seem to be require dopaminergic system activation, although more recent studies have demonstrated differences in the neurobiological mechanisms of nicotine reward and aversion (Becerra, Breiter et al. 2001; Jensen, McIntosh et al. 2003). Progesterone and its active metabolites, allopregnenolone and pregnenolone, have not been shown to directly affect the dopaminergic system, but they interact with other neurotransmitter receptors that modulate dopaminergic functioning, including positive modulatory effects on GABAA receptors, negative modulatory effects on NMDA receptors (Smith 1991; Cyr, Ghribi et al. 2000), as well as blockage of nicotinic receptors including the neuronal α4β2 subtype (Bullock, Clark et al. 1997; Dar and Zinder 1997; Paradiso, Sabey et al. 2000). Further studies are needed to clarify the neurobiological effects of progesterone on the reward system.
Plasma progesterone levels achieved following progesterone treatment were higher than those in our previous study with female smokers (43 vs. 14 pg/ml), although the same micronized progesterone formulation was used in both studies. These differences are possibly due to the effect of food on progesterone absorption. In our current study, to prevent nausea from intravenous nicotine, breakfast was provided early in the session, just after progesterone treatment. Food has been shown to increase the peak plasma concentrations of progesterone levels without affecting the time to reach the peak concentration (Simon, Robinson et al. 1993). Similar to previous studies, a significant variation was observed among subjects in plasma progesterone levels due to erratic absorption of micronized progesterone. Men had greater average plasma levels than women but this difference was not statistically significant.
This study also had other limitations. First, dose-dependent effects of progesterone were not examined. We selected a progesterone dose that would achieve plasma levels found during the luteal phase of the menstrual cycle. Second, the study had a single dose of treatment. It is possible that longer treatment duration might be associated with different treatment effects. Third, due to small sample size, sex differences in progesterone effects on nicotine response could not be addressed. Lastly, levels of active metabolites of progesterone, pregnenolone or allopregnenolone were not measured. These metabolites are likely play an important role in the CNS effects of progesterone (Baulieu 1998).
Conclusion
Progesterone treatment enhanced the ratings of “bad effects,” from IV nicotine and attenuated the rating of “drug liking.” Progesterone also enhanced suppression of smoking urges by intravenous nicotine. Further studies are warranted to examine the modulation of nicotine effects by gonadal hormones.
Fig 2.
The average (with SEM) total and Factor 1 scores of the BQSU. The measurements shown were taken at baseline, 5 minutes before saline and nicotine deliveries, and 30 minutes after the second nicotine administration. Arrows indicate the timing of saline and nicotine deliveries. Significant treatment differences at each time point are indicated by an asterisks (*).
Acknowledgments
This research was supported by the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC) and the National Institute on Drug Abuse grant R01-DA 14537. The authors were supported by career development awards, K02-DA021304 (MS) and K01-DA-019446 (MM).
References
- Allen SS, Bade T, Center B, et al. Menstrual phase effects on smoking relapse. Addiction. 2008;103(5):809–21. doi: 10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23(8):963–87. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, et al. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32(5):927–46. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, et al. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine Tob Res. 1999;1(1):45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, et al. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100(4):550–9. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Bullock AE, Clark AL, Grady SR, et al. Neurosteroids modulate nicotinic receptor function in mouse striatal and thalamic synaptosomes. Journal of Neurochemistry. 1997;68(6):2412–23. doi: 10.1046/j.1471-4159.1997.68062412.x. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, et al. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res. 2006;8(5):627–38. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Chabbert Buffet N, Djakoure C, Maitre SC, et al. Regulation of the human menstrual cycle. Front Neuroendocrinol. 1998;19(3):151–86. doi: 10.1006/frne.1998.0167. [DOI] [PubMed] [Google Scholar]
- Chait LD, Corwin RL, Johanson CE. A cumulative dosing procedure for administering marijuana smoke to humans. Pharmacol Biochem Behav. 1988;29(3):553–7. doi: 10.1016/0091-3057(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J Neuroendocrinol. 2000;12(5):445–52. doi: 10.1046/j.1365-2826.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- Dar DE, Zinder O. Short term effect of steroids on catecholamine secretion from bovine adrenal medulla chromaffin cells. Neuropharmacology. 1997;36(1112):1783–8. doi: 10.1016/s0028-3908(97)00150-0. [DOI] [PubMed] [Google Scholar]
- de Lignieres B. Oral micronized progesterone. Clin Ther. 1999;21(1):41–60. doi: 10.1016/S0149-2918(00)88267-3. discussion 1-2. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, et al. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104(7):2465–70. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous Progesterone Attenuates the Subjective Effects of Smoked Cocaine in Women, but not in Men. Neuropsychopharmacology. 2006;31(3):659–74. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, K Miyasato, Jasinski DR. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. J Pharmacol Exp Ther. 1985;234(1):1–12. [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Effects of three doses of transdermal nicotine on post-cessation eating, hunger and weight. J Subst Abuse. 1997;9:151–9. doi: 10.1016/s0899-3289(97)90013-4. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31(1):129–38. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, et al. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40(6):1251–7. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Jones HE, Garrett BE, Griffiths RR. Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. J Pharmacol Exp Ther. 1999;288(1):188–97. [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000;71(1):51–9. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Dropperman L. Profile of Mood States: Bipolar Form. Educational and Industrial Testing Service; San Diego: 1988. [Google Scholar]
- Morgan MJ, Davies GM, Willner P. The Questionnaire of Smoking Urges is sensitive to abstinence and exposure to smoking-related cues. Behav Pharmacol. 1999;10(67):619–26. doi: 10.1097/00008877-199911000-00008. [DOI] [PubMed] [Google Scholar]
- Paradiso K, Sabey K, Evers AS, et al. Steroid inhibition of rat neuronal nicotinic alpha4beta2 receptors expressed in HEK 293 cells. Mol Pharmacol. 2000;58(2):341–51. doi: 10.1124/mol.58.2.341. [DOI] [PubMed] [Google Scholar]
- Simon JA. Micronized progesterone: vaginal and oral uses. Clinical Obstetrics & Gynecology. 1995;38(4):902–14. [PubMed] [Google Scholar]
- Simon JA, Robinson DE, Andrews MC, et al. The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone. Fertil Steril. 1993;60(1):26–33. [PubMed] [Google Scholar]
- Smith SS. Progesterone administration attenuates excitatory amino acid responses of cerebellar Purkinje cells. Neuroscience. 1991;42(2):309–20. doi: 10.1016/0306-4522(91)90377-z. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb D, Hatsukami DK. Labetalol treatment enhances the attenuation of tobacco withdrawal symptoms by nicotine in abstinent smokers. Nicotine Tob Res. 2003;5(6):947–53. doi: 10.1080/14622200310001615312. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69(12):299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72(12):431–5. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78(4):699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Mouratidis M, et al. Effects of topiramate in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berl) 2006;184(34):645–51. doi: 10.1007/s00213-005-0296-9. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86(11):1467–76. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Sullivan JT, et al. Fluoxetine alters the effects of intravenous cocaine in humans. J Clin Psychopharmacol. 1994;14(6):396–407. [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, et al. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exp Ther. 1996;279(2):524–38. [PubMed] [Google Scholar]
- Zumoff B, Miller L, Levin J, et al. Follicular-phase serum progesterone levels of nonsmoking women do not differ from the levels of nonsmoking men. Steroids. 1990;55(12):557–9. doi: 10.1016/0039-128x(90)90052-d. [DOI] [PubMed] [Google Scholar]