Abstract
4-hydroxynonenal (4-HNE) is a mutagenic α,β-unsaturated aldehyde produced during oxidative injury that is conjugated by several glutathione S-transferase (GST) isoforms. The alpha class human GSTA4-4 enzyme (hGSTA4-4) has a particularly high catalytic efficiency toward 4-HNE conjugation. However, hGST4-4 expression is low in most human cells and there are other aldehyde metabolizing enzymes that detoxify 4HNE. In the current study, we determined the effect of over-expression of hGSTA4 mRNA on the sensitivity of HepG2 cells to 4-HNE injury. HepG2 cells transfected with an hGSTA4 vector construct exhibited high steady-state hGSTA4 mRNA, high GST-4HNE catalytic activities, but lower basal glutathione (GSH) concentrations relative to insert-free vector (control) cells. Exposure to 4-HNE elicited an increase in GSH concentrations in the control and hGSTA4 cells, although the dose-response of GSH induction differed among the two cell types. Specifically, hGSTA4 cells had significantly higher GSH concentrations when exposed to 5–15 μM 4-HNE, but not at 20 μM 4-HNE, suggesting extensive GSH utilization at high concentrations of 4-HNE. The hGSTA4 cells exhibited a significant growth advantage relative to control cells in the absence of 4-HNE, and a trend towards increased growth at low dose exposures to 4-HNE. However, the hGSTA4 cells did not exhibit a growth advantage relative to control cells at higher 4-HNE exposures associated with increased GSH utilization. As expected, the hGSTA4 cells showed resistance to 4-HNE-stimulated lipid peroxidation at all 4-HNE doses. In summary, our data indicates that over-expression of hGSTA4 at levels conferring high GST-4-HNE conjugating activity confers a partial growth advantage to HepG2 cells and protects against 4-HNE oxidative injury. However, the loss of proliferative capacity of hGSTA4 cells challenged with levels of 4-HNE associated with severe oxidative stress indicates a role of other aldehyde metabolizing enzymes, and/or GSH-electrophile transporter proteins, in providing full cellular protection against 4-HNE toxicity.
Keywords: glutathione S-transferase, hGSTA4, 4-hydroxynonenal, lipid peroxidation
1. Introduction
A number of breakdown products are released under conditions of cellular oxidative stress that decompose to produce high concentrations of highly reactive α,β-unsaturated aldehydes. In particular, 4-hydroxynonenal (4-HNE) is a mutagenic aldehyde released during lipid peroxidation which readily forms covalent adducts with DNA and proteins (Esterbauer et al., 1991). Physiological conditions that elevate cellular 4-HNE can result in a loss of mitochondrial function and increased cellular necrosis leading to a loss of overall cell viability (Ramachandran et al., 2001). Coincidentally, elevated tissue 4-HNE concentrations have been associated with several human diseases including cancer, Parkinson’s disease, Alzheimer’s disease, atherosclerosis, pulmonary inflammation, rheumatoid arthritis and ophthalmologic disorders (reviewed in Esterbauer et al., 1991). In addition to its cellular toxicity, 4-HNE plays an important role in cell signaling pathways and can mediate cell cycle arrest (Yang et al., 2003), apoptosis (Raza and John, 2006), and stimulate cell proliferation (Moneypenny and Gallagher, 2005).
Given the high reactivity and physiological relevance of 4HNE, a number of enzyme systems have evolved to regulate 4-HNE levels in different cells and tissues. The primary enzymatic pathways of 4-HNE detoxification in liver include aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), aldehyde reductase (ALRD), and GST (Canuto et al., 1993; Luckey and Petersen, 2001, Gallagher and Gardner, 2002; Gardner et al., 2003). Of these enzyme systems, GST is a predominate mechanism for protection against 4-HNE toxicity in mammalian liver (Hartley and Petersen, 1997; Gardner et al., 2003). Interestingly, the major human liver GST isoform, hGSTA1-1, is relatively inefficient at conjugating 4-HNE (Km= 50 mM, kcat/Km= 0.058 s−1mM−1 (Hubatsch et al., 1998; Coles and Kadlubar, 2005) and other alkenal substrates. However, another alpha class isoform, hGSTA4-4, appears to be the most efficient human GST isoform that mediates 4-HNE conjugation with a Km of 49 μM and a rapid kcat/Km of 2.7 s−1mM−1 (Cheng et al., 2001). Despite its high catalytic efficiency toward 4-HNE, the contribution of this GST isoform toward 4-HNE removal among competing enzymatic pathways is unknown due to its relatively low expression in human tissues (Gallagher and Gardner, 2002).
Over-expression of an alpha class GST in mice with high 4-HNE conjugating activity (mGSTA4-4) provides cellular protection against the cytotoxic effects of oxidative stress mediated by hydrogen peroxide and organic hydroperoxides (Zimniak et al., 1997) and protects against 4-HNE induced apoptosis by inhibition of JNK-mediated cell signaling (Cheng et al., 2001). In addition, incorporation of hGSTA4-4 into adherent cell lines can cause immortalization from changes in 4-HNE mediated signaling events (Sharma et al., 2004). However, what is not known is if cells expressing high levels of hGSTA4-4 are resistant to 4-HNE-mediated oxidative damage relative to cells expressing low, but normal physiological hGSTA4-4 levels. In the current study, we tested the hypothesis that stable transfection of hGSTA4 mRNA would result in an increased cellular capacity to detoxify 4-HNE and confer protection against 4HNE-mediated oxidative damage. Human HepG2 cells were selected for study due to their low constitutive expression of the hGSTA4-4 protein (Zimniak et al., 1997).
2. MATERIALS AND METHODS
2.1 Chemicals and Reagents
All custom designed primers, platinum Taq polymerase, T4 DNA ligase, penicillin, streptomycin, fetal bovine serum (FBS), Trizol reagent and the pcDNA3.1 mammalian expression vector were purchased from Invitrogen Inc. (Carlsbad, CA). BamHI and HindIII were purchased from New England Biolabs (Ipswich, MA) and the human HepG2 cells were purchased from American Type Culture Collection (Manassas, VA). Agarose was obtained from Bio-Rad Laboratories (Hercules, CA), and the pGEM-TZ cloning vector was purchased from Promega Corp (Madison, WI). Earle’s MEM media was purchased from Gibco-BRL (Gaithersburg, MD). Trypan blue, dimethyl sulfoxide (DMSO), and other biochemicals were purchased from Sigma Chemical Company (St. Louis, MO). Racemic 4-HNE was purchased from Cayman Chemical (Ann Arbor, MI) and Alamar blue was purchased from Bio-Source International (Camarillo, CA).
2.2 Cell culture and transfection
Human hepatoma HepG2 cells were grown in Earle’s MEM medium containing 10% heat inactivated fetal calf serum, 100 μg streptomycin/ml, 100 units penicillin/ml in the presence of 5% CO2 (Zimniak et al., 1997). The hGSTA4 cDNA (Gardner and Gallagher, 2001) was excised from pGEM-TZ plasmid and the cDNA insert was blunt-end ligated into the pcDNA3.1 mammalian expression vector. Initially, the hGSTA4 construct lacked a functional Kozak initiator sequence and contained splice sites that caused mRNA splicing in vitro. Furthermore, a point mutation that resulted in an unwanted proline→leucine mutation was found in the recombinant cDNA. The native 5′ untranslated region which contains a Kozak sequence was added and a silent point mutation to eliminate the donor splice site, and the proline→leucine mutation was removed. HepG2 cells were then transfected with the modified pcDNA3.1/hGSTA4 plasmid by calcium phosphate co-precipitation (Watanabe et al., 1997) and several cell lines were selected in geneticin. Other HepG2 cells were transfected with the insert-free pcDNA3.1 vector (hereby termed “control cells”).
2.3 Biochemical and molecular characterization of transfected cells
HepG2 cell lysates were sonicated in 0.1M KH2PO4 (pH 7.4) and centrifuged at 10,000 × g and immediately stored at −80°C. Total cellular RNA was prepared separately using the Trizol reagent (Doi et al., 2004). GST activity in cell supernatants was determined using 1-chloro–2,4-dinitrobenzene (CDNB) and 4-HNE as substrates (Gallagher and Gardner, 2002). GSH peroxidase activity in cell lysates was determined using cumene hydroperoxide (CumOOH) and hydrogen peroxide as substrates (Hughes and Gallagher, 2004). Protein concentrations in cell lysates were determined by the bicinochoninic method with bovine serum albumin as the standard. Total GSH concentrations were determined in acidified 10,000 × g supernatants of HepG2 cells by the method of Baker et al. (Baker et al., 1990) using a 96-well microplate reader (Spectramax 250, Molecular Devices, Sunnyvale CA). Standard curve values were obtained from serial dilutions of GSH standards.
Analysis of steady state hGSTA4 mRNA expression was performed using semi-quantitative multiplexed RT-PCR and hGSTA4-specific PCR primers (forward primer 5′-AGTTGCAGGATGGTAACCACC-3′, reverse primer-5′-ATGGCCTAAAGATGTTGTAGACGG 3′) and including the housekeeping gene β-globin (forward primer 5′-GAAGAGCCAGGACAGGTACGG-3′, reverse primer 5′-ACCAACTTCATCCACGTTCACC-3′). Each PCR reaction included total first strand cDNA from HepG2 cells, 20 mM Tris-HCl, pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM of each dNTP, and 100 ng of β-globin and GST primers. After a 5 min incubation at room temperature, Taq DNA polymerase was added to the reactions. PCR was carried for 30 cycles of 30 s at 94°C, 30 s at 56°C, 30 s at 72°C; and 5 min at 72°C. PCR products were separated on a 2% agarose gel and quantitated using a Bio-Rad Fluor-S imaging system. The conditions were chosen so that none of the RNAs analyzed reached a plateau at the end of the amplification protocol (i.e. in the exponential phase of amplification) and also so that the two sets of primers used in each reaction were not competitive. Each set of reactions included a no-sample negative control. In addition, negative controls containing RNA in lieu of cDNA were included to exclude genomic DNA contamination in the reactions.
2.4 Effect of over-expression of hGSTA4 on cell growth, GSH concentrations and lipid peroxidation by 4HNE
For initial 4-HNE dose-response experiments, HepG2 cells were plated in 96 well plates and cultured according to Zimniak et al (Zimniak et al., 1997). Cell culture media was supplemented with ethanol vehicle control (0.05% v/v), 5, 10, 15 or 20 μM 4-HNE without a media change prior to addition of 10% Alamar blue. At 1, 2, 4. 8 and 24 hr, the cells were analyzed for the effects of 4-HNE on cell growth by Alamar blue reduction at 554 nM excitation/590 nM emission (Ahmed, 1994) using a 96-well fluorescence microplate reader. To analyze treatment-related effects on relative cell growth, the initial Alamar blue fluorescence intensity measurements taken at 1 hr post-addition of test agent (or vehicle) were subtracted from values obtained at 2, 4, 8 and 24 hrs post-dosing. Three replicates were carried out and analyzed statistically as described below in section 2.5.
After establishing a dose-response and time course of 4-HNE effects on cell growth, subsequent oxidative damage experiments involved incubation of HepG2 cells to 0–20 μM 4-HNE for 2 hr, a time frame consistent with the ability of 4-HNE to elicit sublethal cellular oxidative stress (Cheng et al., 2001, Raza and John, 2006). 4-HNE is fully metabolized or has reacted with cellular constituents by 2 hr of exposure (Hartley and Petersen, 1997) and thus later time points were not analyzed. The cells were trypsinized and analyzed for cellular GSH concentrations. The extent of lipid peroxidation was determined by measuring thiobarbituric acid-reactive substances (TBARS) in 10,000 × g cell supernatants prepared in 0.1 mM KCl containing 0.5% butylated hydroxytoluene (BHT) as a reductant to reduce the generation of aldehydes during tissue processing (Buege and Aust, 1978).
2.5 Statistical Analysis
Values reported reflect either the mean (S.E.M.) or mean (S.D.) of triplicate incubations (for biochemical characterization of transfected cell lines) or three replicate cell culture experiments. The effect of 4-HNE on the rates of cell growth in control and hGSTA4 cells was analyzed using two-way analysis of variance (ANOVA) and Bonferoni’s post-hoc test. 4-HNE-treatment effects on cellular GSH concentrations and lipid peroxidation in control and hGSTA4 cells were analyzed using one-way ANOVA and Dunnett’s t-test. All treatment-related effects were considered to be statistically significant at p ≤ 0.05. Data was analyzed using SigmaStat 2.0 software (Jandel Scientific, San Rafael, CA) or GraphPad Prism version 4 (Hearne Scientific Software, Chicago, IL) for personal computer.
3. Results
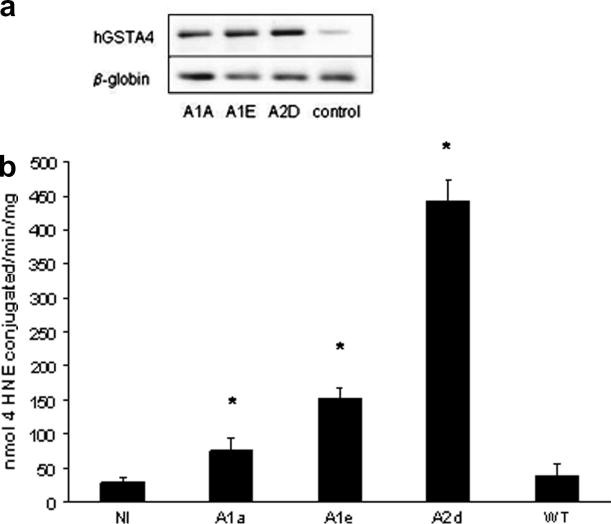
A number of hGSTA4 transfected cell lines were selected in the presence of geneticin and analyzed for expression levels of hGSTA4 mRNA and GST-4HNE activity. After an initial screening, we identified three clonal lines that over-expressed hGSTA4 mRNA relative to wild type or control cells. These lines were identified as lines A1A, A1D, and A2E. Of the three hGSTA4 transfected cell lines, A2D exhibited particularly strong expression of hGSTA4 mRNA relative to the other cell lines and had the highest GST-4-HNE activities (Figure 1). Specifically, the GST-4HNE conjugation activities in the control (insert-free vector transfected), A1A, A1E and A2D cell lines were 29(7), 76(18), 152(15) and 444(32) nmol 4-HNE conjugated/min/mg protein, respectively (Figure 1B). Cell line A2D was selected for further study and are subsequently referred to as “hGSTA4 cells.” The hGSTA4 mRNA levels from non-transfected (wild type) and insert free vector-transfected (control) HepG2 cells did not differ (Figure 1). Similarly, the GSH concentrations in non-transfected cells did not differ from the insert free vector-transfected (control) cells were similar, suggesting that the transfection process did not directly alter the GSH pathway (data not shown). In addition to the high GST-4-HNE conjugating capacity, the hGSTA4 cells also exhibited significantly higher GST catalytic activities toward CDNB (1.6-fold above controls, table 1), but did not statistically differ from the control cells with regard to GSH peroxidase activities toward CumOOH or H2O2 (table 1). The relatively high GST catalytic activities in the hGSTA4 cells were associated with 38% lower basal GSH concentrations than observed in the control cells (table 1).
Figure 1.
The effect of transfecting HepG2 cells with hGSTA4 on (A) hGSTA4 mRNA expression by semi-quantitative RT-PCR. β-globin mRNA is presented as a housekeeping gene in the reactions. (B) GST-4-HNE conjugating activity in cell lysates. In panel B, asterisks indicate significant differences in GST-4-HNE activities from no- insert controls (control cells) at p≤0.05. Baseline GST-4-HNE activities in wild-type HepG2 cells (WT) are included for comparative purposes.
Table 1.
Comparative GSH-dependent catalytic activities and GSH concentrations in control (no-insert vector transfected) and hGSTA4-transfected cells*.
| Cell line | GSH nmol/g | GST-4HNE nmol/min/mg | GST-CDNB nmol/min/mg | GSH-CumOOH nmol/min/mg | GSH-H2O2 nmol/min/mg |
|---|---|---|---|---|---|
| control | 1300(92)* | 29(7) | 17(1) | 39(5) | 14(1) |
| hGSTA4 | 801(64) | 444(31)* | 28(1)* | 53(8) | 13(1) |
data represents the mean (SEM) of three cellular preparations with significant differences noted at p<0.05.
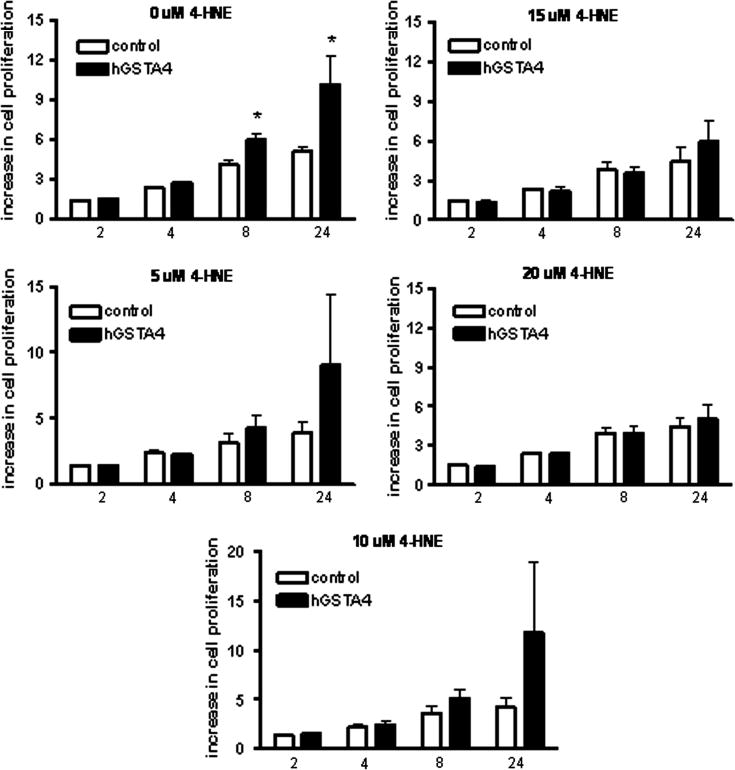
As observed in figure 2, the hGSTA4 cells exhibited a significant growth advantage relative to control cells in the absence of 4-HNE. The growth advantage in hGSTA4 cells was statistically significant at 8 hr (43% increase relative to control cells) and at 24 hr time points (79% increase relative to control cells, Figure 2). A trend toward increased cell proliferation was observed in hGSTA4 cells exposed to 5–10 μM 4HNE relative to control cells at similar time points, however, these data were not statistically significant (Figure 2). When challenged with higher concentrations of 4-HNE (15–20 μM), there was no observable growth advantage of the hGSTA4-transfected cells relative to control cells at any of the time points measured.
Figure 2.
Effect of 4-HNE on cell proliferation in control and hGSTA4-transfected cells. Cells were incubated in 96 well plates in the presence of 5–20 μM 4-HNE (or 0.05% ethanol control, 0 μM) and10% Alamar blue. Alamar blue fluorescence was measured at 1, 2, 4, 8, and 24 hr after initial incubation as described in methods. Values represent the % increase in Alamar blue fluorescence from initial 1 hr post-exposure measurements, and data reflect the mean (SEM) of three experiments. Asterisks indicate significantly greater rate of cell proliferation rates in hGSTA4 cells relative to control (no-insert vector transfected) cells at p≤0.05 using two-way ANOVA and Bonferoni’s post-hoc comparisons test.
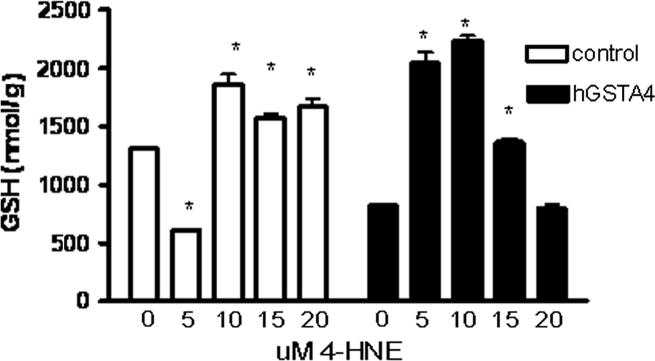
To evaluate the functional consequences of hGSTA4 transfection on GSH levels and susceptibility to oxidative damage, hGSTA4 and control cells were exposed to levels of 4-HNE associated with cellular oxidative stress for a period of 2 hr. As observed in Figure 3, exposure to a relatively low dose of 5 μM 4-HNE for two hours caused a significant decrease in GSH concentrations in control cells, whereas higher doses of 4-HNE stimulated an increase in cellular GSH levels. In contrast, exposure of hGSTA4 cells to 5–15 μM 4-HNE stimulated an increase in GSH concentrations, with the greatest increase in GSH levels occurring in cells exposed to 10 μM 4-HNE (3-fold higher GSH levels than controls, Figure 3). Under conditions of high 4-HNE exposure (20 μM), the GSH levels in hGSTA4 cells did not differ from unexposed hGSTA4 cells (Figure 3).
Figure 3.
Effect of 4-HNE on GSH concentrations in control and hGSTA4-transfected cells. HepG2 cells were incubated in the presence of 5–20 μM 4-HNE (or 0.05% ethanol control) and assayed at 2 hr for GSH concentrations. Data represent mean ± standard deviation of three replicate experiments and asterisks indicate significant treatment differences in GSH concentrations from 0 μM control for each cell type at p≤0.05 using one-way ANOVA and the Dunnett’s t-test.
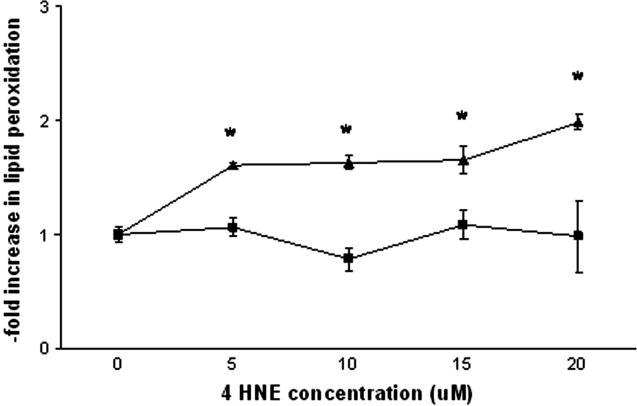
As observed in Figure 4, the hGSTA4 cells were protected against lipid peroxidation in the presence of all concentrations of 4-HNE. In contrast, exposure to 4-HNE increased the extent of lipid peroxidation in control cells at all doses. Specifically, exposure to 5 μM, 10 μM, 15 μM and 20 μM 4-HNE resulted in 1.60-, 1.63-, 1.65- and 1.98-fold increases, respectively, in lipid peroxidation when compared to control cells receiving vehicle (Figure 4).
Figure 4.
Effect of 4-HNE on the extent of lipid peroxidation in control (triangles) and hGSTA4-transfected cells (squares). Cells were incubated in the presence of 0–20 μM 4-HNE (or 0.05% ethanol control) and assayed at 2 hr for the extent of lipid peroxidation. Data represents mean ± SEM of three replicate experiments and asterisks indicate significant differences in lipid peroxidation among control and hGSTA4 cells using one-way ANOVA at p≤0.05.
4. Discussion
As discussed, 4-HNE is a cytotoxic endogenous product produced in relatively high amounts during the peroxidation of cell lipids, a common occurrence on exposure to pro-oxidant drugs and xenobiotics. 4-HNE accumulation during the initial stages of oxidative injury can exacerbate the cellular oxidative damage cascade by eliciting further protein and oxidative injury via altering mitochondrial function and redox status which decreases reactive oxygen species clearance (Raza and John, 2005), and also by electrophilic attack on nucleophilic sites of proteins (Esterbauer, 1991). Accordingly, the biochemical pathways that maintain cellular 4-HNE levels are of importance in maintaining cellular function under oxidative stress. The fact that hGSTA4-4 is a polymorphic enzyme (Coppede et al., 2005) that is present at low levels in human tissues raises the intriguing hypothesis that elevation of hGSTA4-4 expression may potentially protect against 4-HNE cellular injury. Others have reported that over-expression of mGSTA4-4, an alpha class GST in mice that exhibits high 4-HNE conjugating activity, protects against cellular oxidative damage in vitro (Zimniak et al., 1997). However, human hGSTA4-4 shares only 59% sequence with the mouse A4-4 form at the protein level (Bruns et al., 1999). In addition, mGSTA4-4 and the rat orthologue (rGSTA4-4) are expressed at relatively high levels in tissues relative to the human GSTA4-4 form (Gallagher and Gardner, 2002; Morel et al., 2002). Thus, it was important to target the hGSTA4-4 subclass with regard to potential chemotherapeutic implications for humans.
In the present study, we observed that hGSTA4-transfected cells expressed high levels of hGSTA4 mRNA and an increased capacity to conjugate 4-HNE relative to control cells. While we did not quantitate the levels of hGSTA4-4 protein in the transfected cells, the high hGSTA4 mRNA levels and GST-4-HNE activities in the transfected cells relative to controls indicates translation of the mRNA into functional protein. The fact that the transfected cells had significantly lower basal GSH concentrations than the control cells implies an increased utilization of GSH due to high expression and activity of the hGSTA4-4 enzyme and associated demand for GSH. The somewhat higher GSH peroxidase activity toward CumOOH in the hGSTA4 cells may also have reflected the higher expression of the hGSTA4-4 protein, which has catalytic activity towards this substrate (Hubatsch et al., 1998) and which likely placed further demands on constitutive cellular GSH levels. In contrast, GSTs do not catalyze the GSH-mediated reduction of H2O2, which is primarily catalyzed by cytosolic selenium-dependent GSH peroxidase (Hayes and Pulford, 1996), and whose activities did not differ among hGSTA4 and control cells.
Our human HepG2 cells over-expressing hGSTA4-4 exhibited a growth advantage relative to control cells by analysis of cell proliferation using the Alamar Blue assay. The growth advantage was marked in the absence of 4-HNE, but declined under increasing concentrations of 4-HNE. These data suggest that other factors such as removal of GSH-4-HNE conjugates by GSH conjugate efflux transporter proteins may play an important role in cell protection against 4HNE injury (Morrow et. al, 1998, 2000). It also must be pointed out that although the Alamar Blue assay is widely employed in toxicological studies to assess cell viability and cell proliferation rates, the assay provides a relative measure of cell proliferation, but not an absolute one. In particular, others have noted that the fluorescent product of Alamar Blue in cell media can sometimes lead to an overestimation of cell population (O’ Brien et al., 2000).
Interestingly, exposure to 4-HNE elicited an increase in GSH concentrations in both the control and the hGSTA4 cells, although the dose-response of GSH induction in the two cell lines was very different. Others have shown that 4-HNE exposure can increase cellular GSH through induction of glutamylcysteine ligase (GCL) activity by transcriptional activation of the GCL catalytic (GCLc) and modulator (GCLm) subunit mRNAs (Dickinson et al., 2002). The fact that cellular GSH concentrations in hGSTA4 cells exposed to 15 μM 4-HNE did not differ from vehicle-exposed hGSTA4 cells, and were similar to unexposed hGSTA4 cells when exposed to 20 μM 4-HNE, indicates that the increased 4-HNE-GSH conjugation in the presence of high concentrations of 4-HNE may have surpassed the ability of the hGSTA4 cells to synthesize GSH. The observed loss of GSH at a low level 4-HNE exposure (5 μM) in the control cells is consistent with a report by Malone and Hernandez (2006) who observed a significant depletion of GSH in astrocytes exposed to 4-HNE for 1–3 hrs. Collectively, our data suggests that GST expression may be an important co-regulator of GSH levels in cells exposed to 4-HNE.
Although we did not measure 4-HNE concentrations in unexposed or wild type HepG2 cells, others have reported cellular basal 4-HNE levels of approximately 1–5 μM (Esterbauer et al., 1991). When challenged with exogenous 4-HNE, the cellular antioxidant defenses in the control HepG2 cells could not protect against lipid peroxidation. In contrast, even at the highest dose of 4-HNE tested in our study (20 μM), the hGSTA4 cells were still protected against lipid peroxidation, despite exhibiting relatively lower levels of GSH compared to control cells. It is highly possible that under conditions of chronic or higher 4-HNE exposure that the GSH biosynthetic capacity in the hGSTA4 transfected cells may not be able to sustain protection against 4-HNE-mediated lipid peroxidation. We did not expose cells to 4-HNE at concentrations exceeding 20 μM due to a lack of physiological relevance, as higher levels of 4-HNE are typically generated only under conditions of severe oxidative stress (Esterbauer et al., 1991).
It must be pointed out that the GST-4-HNE activity levels in our hGSTA4 transfected cells was relatively high, and as discussed, hGSTA4-4 is not the only biochemical pathway for protection against 4-HNE-oxidative injury in human cells. Accordingly, of interest would be assessing the contribution of other 4-HNE metabolizing enzymes in protecting against 4-HNE oxidative damage our cell system. In particular, the oxidative production of 4-hydroxy-2-nonenoic acid (HNA, an ALDH metabolic product) along with HNE-GSH adducts in rat Kuppfer cells indicates a combined response of oxidative metabolism via the ALDH enzyme system and GST conjugation in mediating 4-HNE oxidative injury in liver cells (Luckey and Petersen, 2001). Other factors such as removal of GSH-4-HNE conjugates by GSH conjugate efflux transporter proteins can play an important role in cell protection against 4-HNE injury (Morrow et al., 2000). In this regard, both the multidrug resistance-associated proteins MRP1 and MRP2 (Morrow et al., 2000; Ji et al., 2004) and Ral-binding GTPase-activating protein RLIP76 (Sharma et al., 2001) are important cellular transporters for the removal of GSH conjugates. Of these transporters, RLIP76 may be the predominant pathway for removing GSH-HNE and other GSH-electrophile conjugates (Sharma et al., 2001). It would thus be of interest to observe if co-transfection of cells with both hGSTA4 and RLIP76 further enhances cellular resistance against 4-HNE-mediated oxidative injury, especially at high 4-HNE concentrations (>20 μM).
In conclusion, we have shown that over-expression of hGSTA4 confers high 4-HNE conjugation activity and protects against 4-HNE mediated lipid peroxidation. It should be noted that our laboratory, as well as others, typically use a racemic 4-HNE mixture of (R)- and (S)-enantiomers in cell studies, as this mixture is formed in biological systems (Gueraud et al., 2005). However, the (R)- and (S)-enantiomers can differ with respect to their biological properties and there may be differences in the reduction and retro-Michael conversion steps of the metabolism between the conjugates originating from the two enantiomers (Gueraud et al., 2005). Additional studies regarding the specific activities of hGSTA4-4 and other human GST isoforms towards these two enantiomers, and the contributions of competing aldehyde metabolizing enzymes and GST-electrophile transporters will help shed further light on the chemoprotective utility of GST over-expression in human cells.
Acknowledgments
This project was supported in part by a grant from the National Institutes of Health (R01-ES09427 and P42-ES004696). The technical assistance of Ms Karen Sheehy was greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SA, Gogal RM, Waksh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods. 1994;170:211–24. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem. 1990;190:360–365. doi: 10.1016/0003-2697(90)90208-q. [DOI] [PubMed] [Google Scholar]
- Bruns CM, Hubatsch I, Ridderstrom M, Mannervik B, Tainer JA. Human glutathione transferase 4-4 crystal structures and mutagenesis reveal the basis of high catalytic efficiency with toxic lipid peroxidation products. J Mol Biol. 1999;288:427–439. doi: 10.1006/jmbi.1999.2697. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Canuto RA, Muzio G, Maggiora M, Biocca ME, Dianzani MU. Glutathione-S-transferase, alcohol dehydrogenase and aldehyde reductase activities during diethylnitrosamine-carcinogenesis in rat liver. Cancer Lett. 1993;68:177–183. doi: 10.1016/0304-3835(93)90144-x. [DOI] [PubMed] [Google Scholar]
- Cheng JZ, Singhal SS, Sharma A, Saini M, Yang Y, Awasthi S, Zimniak P, Awasthi YC. Transfection of mGSTA4 in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis by inhibiting JNK-mediated signaling. Arch Biochem Biophys. 2001;392:197–207. doi: 10.1006/abbi.2001.2452. [DOI] [PubMed] [Google Scholar]
- Coles BF, Kadlubar FF. Human alpha class glutathione S-transferases: genetic polymorphism, expression, and susceptibility to disease. Methods Enzymol. 2005;401:9–42. doi: 10.1016/S0076-6879(05)01002-5. [DOI] [PubMed] [Google Scholar]
- Coppede F, Armani C, Bidia DD, Petrozzi L, Bonuccelli U, Migliore L. Molecular implications of the human glutathione transferase A-4 gene (hGSTA4) polymorphisms in neurodegenerative diseases. Mutat Res. 2005;579:107–114. doi: 10.1016/j.mrfmmm.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic Biol Med. 2002;33:974. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- Doi AM, Pham RT, Hughes EM, Barber DS, Gallagher EP. Molecular cloning and characterization of a glutathione S-transferase from largemouth bass (Micropterus salmoides) liver that is involved in the detoxification of 4-hydroxynonenal. Biochem Pharmacol. 2004;67:2129–2139. doi: 10.1016/j.bcp.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Esterbauer HRJS, Zollner H. Chemistry and biology of 4-hydroxynonenal. malondialdehyde and related aldehydes. Free Rad Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Gallagher EP, Gardner JL. Comparative expression of two alpha class glutathione S-transferases in human adult and prenatal liver tissues. Biochem Pharmacol. 2002;63:2025–2036. doi: 10.1016/s0006-2952(02)01017-1. [DOI] [PubMed] [Google Scholar]
- Gardner JL, Doi AM, Pham RT, Huisden CM, Gallagher EP. Ontogenic differences in human liver 4-hydroxynonenal detoxification are associated with in vitro injury to fetal hematopoietic stem cells. Toxicol Appl Pharmacol. 2003;191:95–106. doi: 10.1016/s0041-008x(03)00220-5. [DOI] [PubMed] [Google Scholar]
- Gardner JL, Gallagher EP. Development of a peptide antibody against human glutathione S-transferase A4 (hGSTA4-4) reveals preferential localization in hepatic mitochondria. Arch Biochem Biophys. 2001;390:19–27. doi: 10.1006/abbi.2001.2352. [DOI] [PubMed] [Google Scholar]
- Gueraud F, Crouzet F, Alary J, Rao D, Debrauwer L, Laurent F, Cravedi JP. Enantioselective metabolism of (R)- and (S)-4-hydroxy-2-nonenal in rat. Biofactors. 2005;24:97–104. doi: 10.1002/biof.5520240111. [DOI] [PubMed] [Google Scholar]
- Hartley DP, Petersen DR. Co-metabolism of ethanol, ethanol-derived acetaldehyde, and 4-hydroxynonenal in isolated rat hepatocytes. Alcohol Clin Exp Res. 1997;21:298–304. [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. CRC Crit Rev Biochem Mol Biol. 1996;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Hubatsch I, Ridderstrom M, Mannervik B. Human glutathione transferase A4-4: an alpha class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem J. 1998;330(1):175–179. doi: 10.1042/bj3300175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EM, Gallagher EP. Effects of 17-beta estradiol and 4-nonylphenol on phase II electrophilic detoxification pathways in largemouth bass (Micropterus salmoides) liver. Comp Biochem Physiol C Toxicol Pharmacol. 2004;137:237–247. doi: 10.1016/j.cca.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Ji B, Ito K, Horie T. Multidrug resistance-associated protein 2 (MRP2) enhances 4-hydroxynonenal-induced toxicity in Madin-Darby canine kidney II cells. Chem Res Toxicol. 2004;17:158–164. doi: 10.1021/tx034067m. [DOI] [PubMed] [Google Scholar]
- Luckey SW, Petersen DR. Metabolism of 4-hydroxynonenal by rat Kupffer cells. Archives Biochem Biophs. 2001;389:77–83. doi: 10.1006/abbi.2001.2307. [DOI] [PubMed] [Google Scholar]
- Malone PE, Hernandez ER. 4-Hydroxynonenal, a product of oxidative stress, leads to an antioxidant response in optic nerve head astrocytes. Exp Eye Research. 2006 doi: 10.1016/j.exer.2006.10.020. e-pub in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneypenny CG, Gallagher EP. 4-Hydroxynonenal inhibits cell proliferation and alters differentiation pathways in human fetal liver hematopoietic stem cells. Biochem Pharmacol. 2005;69:105–112. doi: 10.1016/j.bcp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Morel F, Rauch C, Coles B, Le Ferrec E, Guillouzo A. The human glutathione transferase alpha locus: genomic organization of the gene cluster and functional characterization of the genetic polymorphism in the hGSTA1 promoter. Pharmacogenetics. 2002;12:277–286. doi: 10.1097/00008571-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Morrow CS, Smitherman PK, Townsend AJ. Role of multidrug-resistance protein 2 in glutathione S-transferase P1-1-mediated resistance to 4-nitroquinoline 1-oxide toxicities in HepG2 cells. Mol Carcinog. 2000;29:170–178. doi: 10.1002/1098-2744(200011)29:3<170::aid-mc6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- O’ Brien J, Wilson I, Orton T, Pognon F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Perez A, Chen J, Senthil D, Schenker S, Henderson GI. In utero ethanol exposure causes mitochondrial dysfunction, which can result in apoptotic cell death in fetal brain: a potential role for 4- hydroxynonenal. Alcohol Clin Exp Res. 2001;25:862–871. [PubMed] [Google Scholar]
- Raza H, John A. 4-Hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4-4 and cytochrome P450 2E1 in PC12 cells. Toxicol Appl Pharmacol. 2006;216:309–318. doi: 10.1016/j.taap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Sharma R, Brown D, Awasthi S, Yang Y, Sharma A, Patrick B, Saini MK, Singh SP, Zimniak P, Singh SV, Awasthi YC. Transfection with 4-hydroxynonenal-metabolizing glutathione S-transferase isozymes leads to phenotypic transformation and immortalization of adherent cells. Eur J Biochem. 2004;271:1690–1701. doi: 10.1111/j.1432-1033.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- Sharma R, Singhal SS, Cheng J, Yang Y, Sharma A, Zimniak P, Awasthi S, Awasthi YC. RLIP76 is the major ATP-dependent transporter of glutathione-conjugates and doxorubicin in human erythrocytes. Arch Biochem Biophys. 2001;391:171–179. doi: 10.1006/abbi.2001.2395. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Shirayoshi Y, Koshimizu U, Hashimoto S, Yonehara S, Eguchi Y, Tsujimoto Y, Nakatsuji N. Gene transfection of mouse primordial germ cells in vitro and analysis of their survival and growth control. Exp Cell Res. 1997;230:76–83. doi: 10.1006/excr.1996.3366. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol. 2003;50:319–336. [PubMed] [Google Scholar]
- Zimniak L, Awasthi S, Srivastava SK, Zimniak P. Increased resistance to oxidative stress in transfected cultured cells overexpressing glutathione S-transferase mGSTA4-4. Toxicol Appl Pharmacol. 1997;143:221–229. doi: 10.1006/taap.1996.8070. [DOI] [PubMed] [Google Scholar]