Abstract
To determine the impact of a low Mg2+/pH defined growth medium (MgM) on the proteome of Salmonella enterica serotype Typhimurium, we cultured S. Typhimurium cells in the medium under two different conditions termed MgM Shock and MgM Dilution and then comparatively analyzed the bacterial cells harvested from these conditions by a global proteomic approach. Proteomic results showed that MgM Shock and MgM Dilution differentially affected the S. Typhimurium proteome. MgM Shock induced a group of proteins whose induction usually occurred at low O2 level, while MgM Dilution induced those related to the type III secretion system (T3SS) of Salmonella Pathogenicity Island 2 (SPI2) and those involved in thiamine or biotin biosynthesis. The metabolic state of the S. Typhimurium cells grown under MgM Shock condition also differed significantly from that under MgM Dilution condition. Western blot analysis not only confirmed the proteomic results, but also showed that the abundances of SPI2-T3SS proteins SsaQ and SseE and biotin biosynthesis proteins BioB and BioD increased after S. Typhimurium infection of RAW 264.7 macrophages. Deletion of the gene encoding BioB reduced the bacterial ability to replicate inside the macrophages, suggesting a biotin-limited environment encountered by S. Typhimurium within RAW 264.7 macrophages.
Keywords: Salmonella enterica serotype Typhimurium, Low Mg2+/pH defined growth medium, Salmonella-containing vacuole, Salmonella Pathogenicity Island 2, Type III secretion system, Biotin biosynthesis
Introduction
To establish systemic infection in susceptible mice, the facultative intracellular pathogen Salmonella enterica serotype Typhimurium must survive and replicate inside host macrophages (Fields et al., 1986). Once it is taken up by macrophages, S. Typhimurium resides in a membrane-bound structure called the Salmonella-containing vacuole (SCV). Many bacterial proteins, such as those related to the type III secretion system (T3SS) of Salmonella Pathogenicity Island 2 (SPI2), are involved in helping S. Typhimurium replicate inside the SCV (Hensel, 2000). Identification and characterization of the proteins involved in S. Typhimurium intramacrophage survival have contributed significantly to the elucidation of the molecular mechanisms underlying the ability of S. Typhimurium to evade the host macrophage defense mechanisms and adapt to its local environment.
By using a bottom-up global proteomic approach, we previously analyzed the proteome of S. Typhimurium strain 14028 after the bacterial cells were isolated from murine macrophages RAW 264.7. A total of 315 S. Typhimurium proteins were identified. While most identified proteins were housekeeping-related, and their abundances remained relatively constant during the time course of infection, the abundances of 39 S. Typhimurium proteins increased significantly after the infection, which included STM3117-3119. Western blot analysis confirmed the increased abundances of STM3117-3119 proteins. Deletion of the gene encoding for STM3117 resulted in the failure of S. Typhimurium to replicate inside RAW 264.7 macrophages (Shi et al., 2006). Although the exact functions of STM3117-3119 proteins have yet to be determined, these results prove that a global discovery-based proteomic approach is a powerful tool to identify and characterize S. Typhimurium proteins involved in intramacrophage survival.
Despite our successful application of the proteomic method in discovering a novel S. Typhimurium protein involved in intramacrophage survival, the 315 identified S. Typhimurium proteins were <7% of the ~4800 S. Typhimurium proteins annotated at that time. Furthermore, only one SPI2-T3SS-related protein was identified. This low coverage of S. Typhimurium protein identification is most likely attributed to the limited bacterial cells isolated from the macrophages and the interfering background of residual host proteins, which render the abundances of most S. Typhimurium proteins below the detection level of the proteomic method used. To circumvent the limitations associated with low numbers of bacterial cells isolated from the host cells, one alternative approach was to analyze the S. Typhimurium proteome after bacterial cells were cultured under the conditions that mimicked certain conditions found inside the SCV, such as in a low Mg2+/pH defined growth medium (MgM) (Beuzon et al., 1999, Eriksson et al., 2003). Compared to Luria-Bertani (LB) medium, MgM selectively induced a group of S. Typhimurium and S. enterica serotype Typhi proteins. Some of these MgM-induced proteins, such as PduB and SrfH of S. Typhimurium (Adkins et al., 2006) and T1108, T1476 and HlyE of S. Typhi (Ansong et al., 2008), also increased in their abundances after Salmonella infection of macrophages, confirming some similarity among Salmonella cells colonizing macrophages.
To further characterize the impact of MgM on the S. Typhimurium proteome, we cultured S. Typhimurium cells in the medium under two contrast conditions (i.e., MgM Shock, the conditions used previously, and MgM Dilution) and then performed a comparative analysis of the bacterial cells grown under these two different conditions by using a liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based proteomic approach.
Materials and Methods
Reagents and Standard Procedures
All cell culture reagents were purchased from Invitrogen (Carlsbad, CA). Bacterial DnaK antibody was purchased from StressGen (Victoria, BC, Canada). OctA-Probe was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All chemicals used for tryptic digestion and biotin were purchased from Sigma (St. Louis, MO). Protein concentrations were measured with a bicinchoninic acid (BCA) protein assay kit from Pierce (Rockford, IL). SDS-PAGE and Western blot analyses were conducted according to the instructions from Invitrogen.
Bacterial Strains and Culture Conditions
Bacterial strains and plasmids used in this study are listed in Table S1. All S. Typhimurium strains were normally grown in LB medium. Kanamycin and ampicillin were used at 50 μg/ml. The MgM was described previously (Beuzon et al., 1999). Two different culture conditions were used with MgM during this study. First, after bacterial cells were grown in LB at 37 °C with agitation (200 rpm) until their OD600 were ~ 2 [i.e., stationary (Stat) phase], they were washed with MgM once, resuspended with the same volume of MgM and were then grown at 37 °C with agitation for 4 hr (i.e., MgM Shock) (Adkins et al., 2006, Ansong et al., 2008, Manes et al., 2007). Second, the overnight cultures were diluted at 1: 200 in LB and were then grown until their OD600 were 0.5–0.7 [i.e., logarithmic (Log) phase]. The cells were diluted in MgM at 1:100 and were then grown at 37 °C to stationary phase and diluted 1:100 into fresh MgM medium. They were incubated with agitation for 4 hr and harvested (i.e., MgM Dilution). The cells were harvested by centrifugation (6000 × g, 15 min) at 4 °C, washed once with ice- cold 100 mM NH4HCO3 (pH 7.8) and were used for tryptic digestion and Western blot analysis.
Lysis of S. Typhimurium Cells and Tryptic Digestion
To attain maximal proteome coverage, S. Typhimurium cells from each growth condition (i.e., LB Log, LB Stat, MgM Shock and MgM Dilution) were subjected to two different sample preparation methods applicable to peptide-level bottom-up proteomics: soluble and insoluble protein preparations (Adkins et al., 2006, Ansong et al., 2008). The combined data sets from these two sample preparation methods were then used for the proteomic analysis in this study. Briefly, S. Typhimurium cells were lysed by bead beating using 0.1-mm zirconia/silica beads in 2-mL cryovial for a total of 3 min with cooling steps. The supernatant was recovered, transferred to polycarbonate ultracentrifuge tubes and centrifuged at 356,000 × g and 4 °C for 10 min. The pellet was the insoluble fraction and the supernatant was the soluble fraction. The supernatant was transferred to a separate tube for soluble protein analysis. After protein concentrations were determined, urea, thiourea and dithiothreitol (DTT) were added to the soluble fraction at final concentrations of 7 M, 2 M and 5 mM, respectively, and incubated at 60 °C for 30 min. The samples were diluted 10-fold with 100 mM NH4HCO3 (pH 7.8) in the presence of 1 mM CaCl2 and then subjected to tryptic digestion (Promega, Madison, WI) at 1:50 (w/w) trypsin-to-protein ratio and 37 °C for 3 hr.
For insoluble protein analysis, the pellets were resuspended in 50 mM NH4HCO3 (pH 7.8) and ultracentrifuged under the same conditions as described previously. A BCA protein assay was performed with the pellets that were resuspended in water. Following the ultracentrifugation, the pellets were resuspended in ~200 μL of a solubilization buffer (7 M urea, 2 M thiourea, 9.7 mM DTT and 1% CHAPS in 50 mM NH4HCO3, pH 7.8). Samples were incubated at 60 °C for 30 min, and were then diluted and digested in the same manner as that described for the soluble protein preparation.
The resulting digested peptides from the soluble protein preparation were desalted using a C-18 solid phase extraction (SPE) column (SUPELCO, Bellefonte, PA) (Adkins et al., 2002, Ansong et al., 2008, Manes et al., 2007). Because CHAPS binds to C-18 and elutes with peptides, a strong cation-exchange SPE column was used to desalt the peptides from insoluble protein preparation (Adkins et al., 2006, Ansong et al., 2008). The resulting peptides were concentrated with a SpeedVac to a final volume of ~100 μL. A BCA protein assay was performed to determine peptide concentrations prior to capillary LC-MS/MS analysis.
Capillary LC-MS/MS Analysis
The desalted peptides were separated using an automated and reverse-phase capillary LC system designed in-house (Livesay et al., 2008). Eluate from the LC was directly electrosprayed into an LTQ-Orbitrap mass spectrometer (Thermo Fisher, San Jose, CA) using an electrospray ionization interface manufactured in-house. The heated capillary temperature and spray voltage were 200 °C and 2.2 kV, respectively. Data were acquired for 100 min, beginning 65 min after sample injection (i.e., 15 min into gradient). Orbitrap spectra (AGC 1 × 106) were collected from 400–2000 m/z at a resolution of 100 k followed by data-dependent ion trap tandem mass spectra (AGC 1 × 104) of the three most abundant ions using a collision energy of 35%. A dynamic exclusion time of 60 sec was used to discriminate against previously analyzed ions. For each culturing condition, three different samples (i.e., three biological replicates) were used for LC-MS/MS analysis, and each sample was analyzed three times (i.e., three technical replicates) with LC-MS/MS.
Data Analysis
Peptides were identified using the SEQUEST™ program (Eng et al., 1994) to search the mass spectra against the annotated S. Typhimurium strain LT2 database, which contained 4450 protein sequences (http://www.jcvi.org). The SEQUEST™ analyses included a standard parameter file with peptide_mass_tolerance=3, fragment_ion_tolerance = 0 and no amino acid modifications. These analyses also searched for all possible peptide termini (i.e., not limited to only tryptic termini). Peptides identified by SEQUEST™ were filtered using a combination of scores provided in the SEQUEST™ output files. Minimal threshold filters included those proposed by Washburn and Yates (Washburn et al., 2001). Specifically, ΔCn was = 0.1. For each parent ion charge state, the required XCorr was = 1.9 (+1), 2.2 (+2), and 3.3 (= +3), respectively. Only fully tryptic peptides were included. In addition to the above data filters, peptide identifications that corresponded to two different Salmonella proteins were discarded. The false-positive peptide identifications rate was determined using the reversed protein database approach (Qian et al., 2005a). Only proteins identified by at least two unique (i.e., chemically distinct), filter-passing peptide observations were reported. The number of peptide observations from each protein was used to estimate the relative abundance of the corresponding protein in the sample. Similar approaches have been previously described (Adkins et al., 2002; Gao et al., 2003; Ishihama et al., 2005; Jacobs et al., 2005; Liu et al., 2004; Qian et al., 2005b; VerBerkmoes et al., 2006). An arbitrary value of 1 was supplied for missing data points (i.e., no protein observed in a specific condition). Because of the assignment of 1 for missing data, those proteins with small number of observations (i.e., peptides detected) were less likely to show significant differences between conditions (Adkins et al., 2006; Ansong et al., 2008). Heat maps were generated using the software tool MeVv4.0 (JCVI, Rockville, MD) (Saeed et al., 2003). To determine the reproducibility of the MS data, a pairwise Pearson’s correlation plot was constructed to correlate protein abundance values (peptide count used as a rough measure of relative abundance) obtained for each protein in an LC-Orbitrap MS analysis to every other analysis obtained in the study.
Functional Enrichment of Proteomic Data
S. Typhimurium KEGG pathways were obtained from the KEGG website (Kanehisa and Goto, 2000), and the SPI1 (STM2854-2900) and SPI2 (STM1383-1422) protein sets were added. To identify the S. Typhimurium metabolic pathways and functional groups that were significantly affected by MgM, we assessed each pathway and functional group with more than 3 observed members for significantly higher or lower relative abundance values than background. The two-tailed Student’s t test (p = 0.05) was used to compare groups. To determine whether the expressions of the genes related to biotin biosynthesis was altered following S. Typhimurium infection of macrophages, we also reanalyzed the transcriptomic dataset of S. Typhimurium isolated from macrophages (Eriksson et al., 2003).
Genetic Manipulation of S. Typhimurium Genes
The gene tagging and deleting procedures, which were mediated by phage λ-Red recombinase, were the same as those described previously (Datsenko and Wanner, 2000; Shi et al., 2006; Uzzau et al., 2001; Shi et al., 2009). For gene tagging, the sequences encoding 3 × FLAG epitope were inserted in-frame at the 3′-end of the coding regions immediately before the stop codons of all targeted S. Typhimurium genes. Gene deletion was carried out to eliminate the entire coding region of bioB. After validation with PCR, the antibiotic resistance gene that served as a selective marker was removed to minimize polar effects. All resulting S. Typhimurium strains made in this study are listed in Table S1. The strains tagged with the 3 × FLAG epitope were used to validate the LC-MS/MS results by Western blot analysis of the expression of each tagged S. Typhimurium protein. The bioB deletion strain (ΔbioB) was tested first for biotin auxotrophy in MgM and was then used to determine whether its ability to replicate inside macrophages was affected. The primers used for tagging or deleting S. Typhimurium genes are listed in Table S2.
Cell Culture, Macrophage Infection and Isolation of S. Typhimurium Cells
The detailed procedures for maintaining a RAW 264.7 macrophage-like cell line, infecting the macrophages with S. Typhimurium and isolating S. Typhimurium cells from infected macrophages were described previously (Shi et al., 2006; Shi et al., 2009). Briefly, S. Typhimurium cultures were prepared from frozen stocks in LB and grown at 37 °C with agitation (200 rpm) for 18 hr. They were harvested, washed once with the same volume of Dulbecco’s phosphate-buffered-saline without Mg2+ or Ca2+ (DPBS), and resuspended in 1 ml of DPBS. After their concentrations were determined, S. Typhimurium cells were diluted in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum and incubated on ice for 30 min. After they were washed twice with 2 ml/well of Hanks’ buffered saline solution (HBSS), macrophage cells were infected with S. Typhimurium at multiplicity of infection of 100. To increase the uptake of S. Typhimurium, plates were centrifuged at 1000 × g for 10 min. Uptake of S. Typhimurium was allowed to occur at 37 °C in 5% CO2 for 30 min. This time point was defined as 0 hour post-infection (hpi). After washing with DMEM three times, the cells were then incubated with the DMEM medium with gentamicin to kill the S. Typhimurium cells that remained outside the macrophages. At different predetermined time points, the cells were washed twice with 2 ml/well of HBSS. The cells were lysed with 1 ml/well of cell lysis solution [0.1% (w/v) SDS, 1% (v/v) acidic phenol and 19% (v/v) ethanol in double distilled water] for 30 min. The cell lysates from one plate were pooled and centrifuged at 5,000 × g for 20 min. The pellets were washed twice with HBSS and resuspended in 100 μl of 100 mM NH4HCO3 (pH 7.8). The isolated S. Typhimurium cells were analyzed by Western blot.
Gentamicin Resistance Assay
Also as described previously (Shi et al., 2006), the RAW 264.7 macrophages, and wild-type (WT) and ΔbioB strains of S. Typhimurium were prepared 1 day before infection, with a slight modification in which RAW 264.7 macrophages were seeded at 5 × 105 cells/well in 24-well tissue culture plates. At pre-determined time points, cells were washed twice with 1 ml/well of HBSS and lysed with 0.5 ml/well of cell lysis buffer [1% (v/v) of Triton X-100, 0.1% (w/v) SDS in DPBS] at room temperature for 5 min. The lysis solution in each well was pipetted up and down 10 times and diluted by several orders of magnitude, and each dilution was plated on LB agar plates. After incubation at 37 °C for 16 hr, the numbers of colony-forming units (CFU) on each LB agar plate were counted to determine the numbers of live S. Typhimurium cells in each well, and a minimum of three wells was counted for each measurement, and three different measurements were conducted. Student’s t test was used to compare between groups.
Results
LC-MS/MS Analysis
Two different methods, which were termed MgM Shock and MgM Dilution, were used to grow S. Typhimurium cells in MgM to determine the impact of the medium on the S. Typhimurium proteome. For comparison, the S. Typhimurium cells grown under all four conditions (LB Stat, LB Log, MgM Shock and MgM Dilution) were used for LC-MS/MS analysis. Following the separation of the cell lysates into soluble and insoluble fractions, the proteins in each fraction were analyzed by LC-MS/MS independently. For each culturing condition, three different samples (i.e., three biological replicates) were used for LC-MS/MS analysis, and each sample was analyzed three times (i.e., three technical replicates) with LC-MS/MS. The identified peptides from different the fractions of the same samples were then combined to infer the proteins. A total of 1309 S. Typhimurium proteins were identified with high confidence from this study (Table S3), which corresponds to ~30% of the S. Typhimurium annotated proteome. The false-positive peptide identification rate for the entire data set used in this study was 1%. Correlation analysis showed that each LC-Orbitrap replicate analysis had a strong correlation to the other two replicate analyses for each sample, clearly demonstrating the reproducibility of our MS data. All triplicate datasets had a correlation coefficient (ρ) of 0.85 or better (Fig. 1). The identified proteins were functionally diverse with no apparent biases toward a specific functional category
Figure 1.
Reproducibility of MS data. Pearson’s pairwise correlation plot was constructed to correlate protein abundance values (peptide count used as a rough measure of relative abundance) obtained for each protein in an LC-Orbitrap MS analysis to every other analysis obtained in the study. Values on the correlation plot varied from 0.55 to 1; where 0.55 is minimal correlation (represented in black) and 1.0 was a perfect positive correlation (represented in white). All triplicate datasets had a correlation coefficient (ρ) of 0.85 or better.
Compared to LB medium, the MgM specifically induced the expression of 359 S. Typhimurium proteins (using a fivefold or greater difference between the peptide abundances detected from the bacterial cells grown in MgM and those in LB medium). Examination of the MgM-induced proteins revealed that 50 proteins were induced by both MgM Shock and MgM Dilution (Table S5 and Fig. 2A), 96 proteins were induced only by MgM Shock (Table S6 and Fig. 2A) and 213 proteins were induced only by MgM Dilution (Table S7 and Fig. 2A), demonstrating a differential induction of S. Typhimurium proteins by these two different culturing methods. Fifty-one of these MgM-induced proteins were also found in the S. Typhimurium samples isolated from RAW 264.7 macrophages (Table S5, S6 and S7) (Shi et al., 2006; Shi et al., 2009). Some of the S. Typhimurium proteins induced by both culturing methods were involved in amino acid biosynthesis (e.g., tryptophan synthase TrpA-E and histidine synthase HisB-D, G & H) and nutrient transport (Mg2+ transporter MgtB, phosphoglycerate transporter PgtE, and phosphate transporter PstB & S) (Table S5 and S8), and their inductions are most likely attributed to the limitations of these nutrients under both conditions.
Figure 2.
Heat map representations of MgM Shock- and/or MgM Dilution-induced S. Typhimurium proteins. A. Overview of the proteins induced by MgM Shock and/or MgM Dilution. A total of 359 S. Typhimurium proteins are shown, which include 50 proteins induced by both MgM Shock and MgM Dilution, 96 proteins induced only by MgM Shock, and 213 proteins induced only by MgM Dilution. B. Detailed view of differential induction of selected S. Typhimurium proteins by MgM Shock or MgM Dilution. A total of 28 S. Typhimurium proteins are shown, which include those i) involved in cobalamin (2), thiamine (7) or biotin (3) biosynthesis, ii) involved in 1,2-propanediol utilization (8), and iii) related to SPI2-T3SS (8). The numbers of identified peptides ranges from 0 (black) to =10 (red).
One of the interesting findings from the analyses of MgM Shock-induced proteins was identification of the proteins whose induction usually occurred at low O2 levels. These included anaerobic dimethyl sulfoxide reductase DmsA, cytochrome d terminal oxidase CydA-C, anaerobic sn-glycerol-3-phosphate dehydrogenase GlpA-C, cobalamin biosynthesis proteins CbiH & K and 1,2-propanediol utilization (pdu) proteins PduA, C, D-J, OP & S (Table S6, Fig. 2B) (Ailion et al., 1993; Wei and Miller, 1999; Sevcik et al., 2001). Induction of these proteins by MgM Shock but not by MgM Dilution suggests that much lower O2 level may exist under the former condition or an induction of regulatory factors that usually function under lower O2 levels. MgM shock also specifically induced flagellar-related proteins FliF & N and FljB (Table S6 and S8).
Inspections of MgM Dilution-induced proteins revealed that the abundances of a group of Salmonella proteins important for bacterial pathogenesis, including i) Salmonella plasmid virulence factors SpvBC, ii) superoxide dismutase SodA and SodCI, iii) PhoP regulated protein PagC, iv) P-type ATPase Mg2+ transporter MgtA, v) manganese transporter SitAB, and vi) SPI2-T3SS-related proteins SsaC, E, H, J & L, SscA, SseA, E and SsrA, increased under MgM Dilution conditions. Most of them are involved in S. Typhimurium intramacrophage survival (Alix et al., 2008; Zaharik et al., 2004; Chen et al., 2007; Krishnakumar et al., 2007; Uzzau et al., 2002; Browne et al., 2008; Matsui et al., 2001; Hensel, 2000; Craig and Slauch, 2009). These results indicate that, compared to MgM Shock, MgM Dilution mimics the environment within the SCV of infected macrophages better in terms of inducing these virulence factors important for S. Typhimurium survival and/or replication inside macrophages. Other MgM Dilution-induced proteins included biotin biosynthesis proteins BioAB & D, thiamine biosynthesis proteins ThiC-H & M and cysteine biosynthesis proteins CysA, CD, I-K & N (Table S7 and S8, Fig. 2B).
Metabolic Pathway and Functional Group Analysis
To determine the impacts of MgM Shock and MgM Dilution on the metabolic pathways and functional groups, we examined the enrichment of metabolic pathways and functional groups under the MgM Shock and MgM Dilution conditions. The results showed that, although they both increased the activity for histidine, phenylalanine, tyrosine and tryptophan biosynthesis and reduced the biosynthesis of the ribosomal proteins and those related to SPI1, MgM Shock and MgM Dilution affected other pathways and functional groups differently. MgM Shock positively impacted cobalamin biosynthesis and the proteins used for flagellar assembly and two-component system and had a negative impact on folate biosynthesis. In contrast, MgM Dilution decreased cobalamin biosynthesis and abundances of the proteins related flagellar assembly or two-component system and increased folate biosynthesis. MgM Shock specifically increased glycerophospholipid and glycerolipid metabolisms and decreased the abundances of the proteins regulating one carbon pool of folate. Under MgM Dilution conditions, SPI2 proteins; the metabolisms of biotin, thiamine, purine, selenoamino acid, glutamate and sulfur; the biosynthesis of siderophore and novobiocin; and ABC transporters were specifically induced. MgM Dilution also down-regulated TCA cycle, oxidative phosphorylation, benzoate degradation via CoA ligation, reductive carboxylate cycle, chemotaxis, phosphotransferase system and the metabolisms of butanoate, glyoxylate, dicarboxylate and tryptophan (Table S8). The differential impacts of MgM Shock and MgM Dilution on the S. Typhimurium metabolic pathways clearly indicate that the metabolic state of the S. Typhimurium cells grown under MgM Shock conditions is significantly different from that under MgM Dilution conditions.
Western Blot Validation
To validate the LC-MS/MS results, we directly analyzed the expression levels of a representative set of proteins by Western blot analysis. These included SsaE, SsaQ, SseE, CbiP, ThiH, BioB and BioD that were tagged with 3 × FLAG epitope via recombinant DNA techniques. Although neither of them was detected by LC-MS/MS, SsaQ and CbiP were tagged to aid investigations of the dynamic range of detection by LC-MS/MS used in this study. The presence of these recombinant proteins were measured by Western blot under three different conditions, which included MgM Shock, MgM Dilution and infection of macrophages.
As shown in Fig 3A, all three tagged SPI2-T3SS proteins were detected under MgM Dilution condition, while only SseE was detected under MgM Shock condition. Detection of SseE and SsaQ by Western blot and detection of only SseE by LC-MS/MS under MgM Dilution condition suggest that the failure to detect SsaQ by LC-MS/MS during this study is most likely attributed to the low abundance of SsaQ. Consistent with this suggestion, the relative abundance of SsaQ detected under MgM-Dilution condition was much lower than that of SseE (Fig 3B). Together with that of LC-MS/MS, our results consistently demonstrate that more SPI2-T3SS proteins are induced by MgM Dilution than by MgM Shock. The trace amount of CbiP was found under MgM Shock condition, while no CbiP was detected under MgM Dilution condition. In contrast to CbiP, more ThiH was detected under MgM Dilution condition than MgM Shock condition (Fig. 3C). While BioB could be found under both MgM Shock and MgM Dilution conditions, BioD was only detected under MgM Dilution conditions (Fig. 3D).
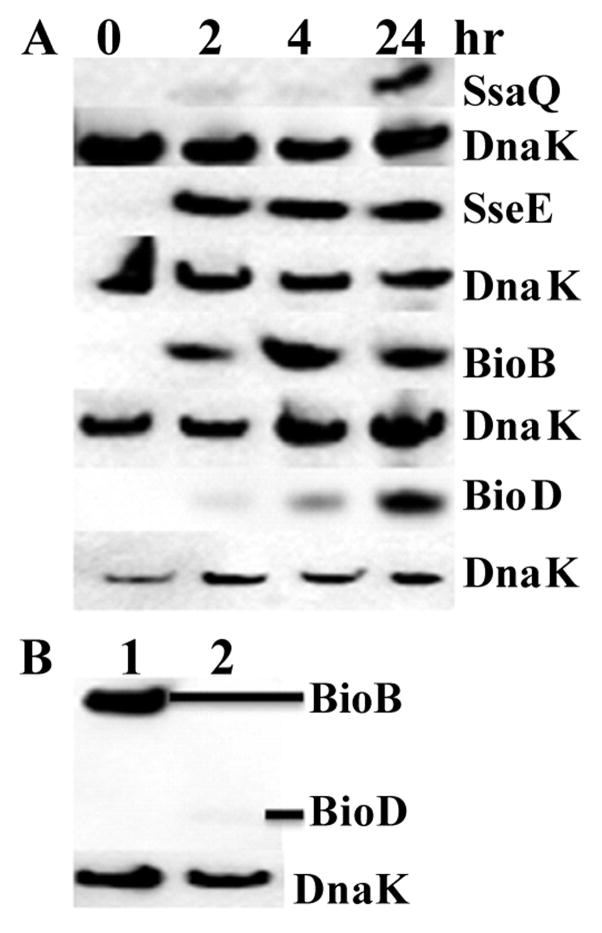
Figure 3.
Western blot confirmation of SsaE, SsaQ and SseE (panels A and B), CbiP and ThiH (panel C) and BioB and BioD (panel D). The S. Typhimurium strains with recombinant SsaE, SsaQ, SseE, CbiP, ThiH, BioB or BioD were cultured under MgM Shock (lanes S) or MgM Dilution (lanes D) conditions as described in Materials and Methods. Relative abundance between SseE (panel B, lane 1) and SsaQ (panel B, lane 2) under MgM Dilution condition was also compared. After separation by SDS-PAGE, the proteins were first probed with an anti-FLAG antibody (i.e., OctA-Probe) and then stripped and re-probed using anti-DnaK antibody. The DnaK was used as an internal control to ensure that similar amounts of proteins were loaded in each lane. Experiments were repeated three times with similar results.
As shown in Figure 4A, SsaQ, SseE, BioB and BioD were induced when the S. Typhimurium strains were used to infect RAW 264.7 macrophages. Infection of the macrophages, however, did not induce SsaE, CbiP or ThiH (data not shown). Both SsaQ and BioD were detectable at 2 hr and peaked at 24 hr following the macrophage infection. Presence of SseE and BioB could also be detected at 2, 4 and 24 hpi. The abundances of SseE at 2, 4 and 24 hpi were nearly the same, while the abundance of BioB peaked between 2 and 4 hpi. At 2 hpi, the abundance of BioB was much higher than that of BioD (Fig. 4B). Induction of BioB and BioD in infected macrophages suggests that biotin might be limited inside the SCV of RAW 264.7 macrophages under the condition tested.
Figure 4.
Western blot identification of SsaQ, SseE, BioB and BioD after S. Typhimurium cells isolated from RAW 264.7 macrophages. The S. Typhimurium strains with recombinant SsaQ, SseE, BioB or BioD were used to infect RAW 264.7 macrophages as described in Materials and Methods. Relative abundance between BioB (panel B, lane 1) and BioD (panel B, lane 2) at 2 hr after infection of RAW 264.7 macrophages was also compared. After separation by SDS-PAGE, the proteins were first probed with an anti-FLAG antibody and then stripped and re-probed using anti-DnaK antibody. Experiments were repeated three times with similar results.
Replication of ΔbioB Mutant in RAW 264.7 Macrophages
To further investigate whether biotin was limited in the SCV, the bioB gene was deleted and the resulting ΔbioB mutant was used to measure its replication rates inside RAW 264.7 macrophages (Fig. 5A). The ability of ΔbioB mutant to grow in MgM medium was impaired. Supplementing the MgM medium with 0.1 μM of biotin restored the ΔbioB mutant’s ability to grow, demonstrating the biotin auxotrophy of this mutant. Elimination of BioB had no impact on the ability of S. Typhimurium to infect macrophages, because the CFU/well were 3.4 ± 0.4 × 105 for wt and 3.9 ± 0.4 × 105 for ΔbioB mutant (n = 3) at 0 hpi. Although both WT and ΔbioB mutant began to replicate at 4 hpi, the replication rates of ΔbioB mutant were <72% of that for WT (n = 3, P< 0.01) (Fig. 5B). These findings support the notion that biotin is limited within the SCV of RAW 264.7 macrophages.
Figure 5.
Influence of deletion of bioB gene on S. Typhimurium replication in RAW 264.7 macrophages. A. Deletion of bioB gene. Agarose gel showing the PCR products of bioB amplified from WT (lane 1) and ΔbioB mutant (lane 2). The positions of DNA standards (Stds) are indicated at left. B. Relative viability of WT (■) and ΔbioB (□) cells in RAW 264.7 macrophages after compared to those at 0 hr infection, respectively (n = V3, p < 0.01). At 0 hr infection, the CFU/well values were 3.4 ± 0.4 ×105 for WT and 3.9 ± 0.4 × 105 for ΔbioB mutant (n = 3).
Discussion
Although MgM had been used to mimic certain conditions found inside the SCV, the culturing methods varied from group to group. The impact of the different culturing method on the S. Typhimurium proteome had not been fully investigated with the global proteomic methods previously. In this study, we used the medium to grow S. Typhimurium under two different culturing methods (i.e., MgM Shock & Dilution) and then used a global proteomic method to comparatively analyze their effects on the S. Typhimurium proteome. The results of proteomic as well as confirmatory Western blot analyses consistently showed that these two methods affected the S. Typhimurium proteome differently despite the same fresh medium being used for up to 4 hr. Compared to MgM dilution, MgM Shock induced a group of proteins, such as those related to cobalamin biosynthesis or pdu, whose induction is usually expected to occur only at low O2 levels where induction for fermentation may be needed. This suggests that the O2 level in solution may be lower under MgM Shock condition than MgM Dilution condition or there is the presence of regulatory factors in one of the conditions that are absent in the other. MgM Dilution, meanwhile, induced a group of Salmonella virulence factors, including those related to SPI2-T3SS that are required for intramacrophage survival of S. Typhimurium, more effectively than MgM Shock. In addition, MgM Dilution increased the abundances of the proteins related to thiamine or biotin biosynthesis. The results of metabolic pathway enrichment analysis also demonstrated that the metabolic state of the S. Typhimurium cells grown under MgM Shock condition differed significantly from that under MgM Dilution condition. Western blot analyses showed that the abundances of SPI2-T3SS proteins SsaQ and SseE and biotin biosynthesis proteins BioB and BioD increased following the infection of RAW 264.7 macrophages. Furthermore, elimination of BioB lowered the replication rates of S. Typhimurium cells within the macrophages. Induction of BioB and BioD following macrophage infection and the reduced ability of ΔbioB to replicate inside the macrophages suggest for the first time a biotin-limited environment within the SCV of RAW 264.7 macrophages and a possible mechanism that co-regulates biotin synthesis and the SPI2-T3SS secretion system.
SPI2-T3SS Proteins
MgM has been routinely used to induce SPI2-T3SS related proteins (Beuzon et al., 1999; Mazurkiewicz et al., 2008; Hansen-Wester et al., 2002). For instance, detections of SseB-D are evident when S. Typhimurium cells are grown in MgM to the Stat phase (Beuzon et al., 1999; Hansen-Wester et al., 2002). The reasons for differential induction of SPI2-T3SS proteins by the culturing methods used in this study, however, remain obscure, but might be attributed to the different metabolic states of the S. Typhimurium cells grown under these conditions. Nevertheless, to the best of our knowledge, this is the first report that change of culturing methods affects the SPI2-T3SS proteins in terms of the extent and degree of their induction by this growth medium. Increase of SsaQ and SseE abundances following infection of macrophages is consistent with the previous findings that both SsaQ and SseE help S. Typhimurium proliferate in RAW 264.7 macrophages (Hensel et al., 1998; Suvarnapunya et al., 2003). In addition, SsaQ is required for S. Typhimurium to cause systemic infection in mice (Suvarnapunya et al., 2003). Whether SsaE is involved in intramacrophage survival of S. Typhimurium is still uncertain, as it was undetected following macrophage infection.
Cobalamin and Thiamin Biosynthesis Proteins
Cobalamin is a cofactor for different types of enzymes, including isomerase, metyltransferase and dehalogenase [for review see (Brown, 2006)]. In S. Typhimurium, cobalamin serves mainly as a cofactor for B12-dependent diol dehydratase (an isomerase) that catalyzes the conversion of 1,2-propanediol to propionaldehyde, the first step of pdu pathway. For this reason, the expressions of pdu and cobalamin biosynthesis genes are usually co-regulated in S. Typhimurium (Bobik et al., 1999). Consistent with these previous observations, our results showed that the proteins used for cobalamin biosynthesis or pdu were co-induced by MgM Shock. Induction of PduB by MgM Shock was confirmed previously (Adkins et al., 2006). Also a water soluble B-type vitamin, thiamin is a cofactor for several important enzymes, such as pyruvate dehydrogenase, a-ketoglutarate dehydrogenase, transketolase and pyruvate decarboxylase, that are involved in carbohydrate metabolism (Begley et al., 1999). The reasons for failure to detect CbiP or ThiH in the S. Typhimurium cells isolated from macrophages are currently unknown. Cobalamine, thiamine and biotin are present in DMEM medium as the vitamin supplements as well as the inherent components of added fetal bovine serum and can be taken by RAW 264.7 macrophages (Baker et al., 1988). Detection of CbiP and ThiH under MgM Shock and MgM Dilution condition, respectively, but not after macrophage infection indicates that sufficient amount of cobalamine and thiamine may exist within the SCV of RAW 264.7 macrophages under the conditions used in this study, which could inhibit the induction of CbiP and ThiH.
Biotin Biosynthesis Proteins
Biotin is a covalently bound cofactor for the enzymes that catalyze carboxylation, decarboxylation and transcarboxylation reactions, such as acetyl-CoA carboxylase, pyruvate carboxylase and glutaconyl decarboxylase (Streit and Entcheva, 2003). The findings that BioB and BioD increase their abundances after S. Typhimurium infecting macrophages and that ΔbioB mutant exhibits reduced replication rates inside macrophages consistently suggest a biotin-limited condition inside the SCV of RAW 264.7 macrophages. In agreement with this suggestion, transcriptomic analysis of the S. Typhimurium strain SL1344 isolated from mouse J774-A.1 macrophage showed a moderate increase of expression of several genes involved in biotin biosynthesis at 8 hpi (Eriksson et al., 2003). Likewise, biotin biosynthesis genes were significantly up-regulated after infection of epithelial cells by the strain SL 1344. The biotin limitation inside the SCV of epithelial cells was considered as a possible cause for the up-regulation of these genes (Hautefort et al., 2008). Differences, however, exist between our results and those published previously in terms of replication of S. Typhimurium mutants deficient in biotin biosynthesis and induction of biotin biosynthesis proteins inside host cells. In previous studies, deletion of biotin biosynthesis genes did not affect the bacterial ability to replicate inside human HeLa epithelial cells (Hautefort et al., 2008). Our results showed that the replication rate of ΔbioB mutant in mouse RAW 264.7 macrophages decreased slightly. Previous studies found that neither BioB nor BioD was detected in the S. Typhi cells isolated from human THP-1 macrophages (Ansong et al., 2008). Our results clearly showed the induction of S. Typhimurium BioB and BioD following the infection of RAW 264.7 macrophages. The different host cells (human HeLa epithelial cell, mouse RAW 264.7 macrophage and human THP-1 macrophage), bacterial cells (S. Typhimurium strain 14028 and SL1344 and S. Typhi) and media (DMEM and RPMI 1640) used most likely contribute to the different results obtained from these studies. Alternatively, co-regulation of pathogenesis proteins and biotin pathways may be decoupled in S. Typhi. It should be noted that BioH of the enteric pathogen Yersinia enterocolitica was identified as a systemic infection factor (Gort and Miller, 2000). Future investigations should focus on whether biotin biosynthesis proteins are also involved in the development of S. Typhimurium-mediated systemic infection in mice.
Conclusions and Perspectives
This comparative analysis of the proteome of S. Typhimurium grown in MgM shows for the first time that MgM Shock and MgM Dilution differentially affect the S. Typhimurium proteome as well as the S. Typhimurium metabolic pathways and functional groups. Our results also show for the first time that the infection of RAW 264.7 macrophages increases BioB and BioD abundances. The general agreement between the MS and Western blot results clearly demonstrates the repeatability and reproducibility of the results as well as the robustness and accuracy of the methodology used in this study. In the future, researchers should consider the co-relationship between bacterial metabolic states and induction SPI2-T3SS proteins and the roles of biotin biosynthesis proteins in S. Typhimurium pathogenesis. This co-regulation may be more informative of the in vivo metabolic environment experienced by Salmonella. Understanding these relationships and functions should lead to improved therapeutic approaches to Salmonella infections.
Acknowledgments
This work was supported in part by the Laboratory Directed Research and Development Program of U.S. Department of Energy (DOE) to LS and by the National Institute of Allergy and Infectious Diseases, NIH/DHHS, through interagency agreements Y1-AI-4894-01 and Y1-AI-8401-01. This work used instrumentation and capabilities developed under support from the National Center for Research Resources (Grant RR 018522 to RDS) and the DOE’s Office of Biological and Environmental Research. Significant portions of this work were performed using EMSL, a national scientific user facility sponsored by the DOE’s Office of Biological and Environmental Research, located at Pacific Northwest National Laboratory. Pacific Northwest National Laboratory is operated for the DOE by Battelle Memorial Institute under Contract DE-AC05-76RLO1830.
Abbreviations
- CFU
colony-forming units
- DMEM
Dulbecco’s modified Eagle’s medium
- DPBS
Dulbecco’s phosphate-buffered-saline without Mg2+ or Ca2+
- DTT
dithiothreitol
- HBSS
Hanks’ buffered saline solution
- hpi
hour post-infection
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MgM
low Mg2+/pH defined growth medium
- pdu
1,2-propanediol utilization
- SCV
Salmonella-containing vacuole
- SPI2
Salmonella Pathogenicity Island 2
- T3SS
type III secretion system
- WT
wild-type
References
- 1.Adkins JN, Mottaz HM, Norbeck AD, Gustin JK, Rue J, et al. Analysis of the Salmonella typhimurium proteome through environmental response toward infectious conditions. Mol Cell Proteomics. 2006;5:1450–1461. doi: 10.1074/mcp.M600139-MCP200. [DOI] [PubMed] [Google Scholar]
- 2.Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, et al. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics. 2002;1:947–955. doi: 10.1074/mcp.m200066-mcp200. [DOI] [PubMed] [Google Scholar]
- 3.Ailion M, Bobik TA, Roth JR. Two global regulatory systems (Crp and Arc) control the cobalamin/propanediol regulon of Salmonella typhimurium. J Bacteriol. 1993;175:7200–7208. doi: 10.1128/jb.175.22.7200-7208.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alix E, Miki T, Felix C, Rang C, Figueroa-Bossi N, et al. Interplay between MgtC and PagC in Salmonella enterica serovar Typhimurium. Microb Pathog. 2008;45:236–240. doi: 10.1016/j.micpath.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Ansong C, Yoon H, Norbeck AD, Gustin JK, McDermott JE, et al. Proteomics snalysis of the causative agent of typhoid fever. J Proteome Res. 2008;7:546–557. doi: 10.1021/pr070434u. [DOI] [PubMed] [Google Scholar]
- 6.Baker H, DeAngelis B, Frank O. Vitamins and other metabolites in various sera commonly used for cell culturing. Experientia. 1988;44:1007–1010. doi: 10.1007/BF01939904. [DOI] [PubMed] [Google Scholar]
- 7.Begley TP, Downs DM, Ealick SE, McLafferty FW, Van Loon AP, et al. Thiamin biosynthesis in prokaryotes. Arch Microbiol. 1999;171:293–300. doi: 10.1007/s002030050713. [DOI] [PubMed] [Google Scholar]
- 8.Beuzon CR, Banks G, Deiwick J, Hensel M, Holden DW. pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol Microbiol. 1999;33:806–816. doi: 10.1046/j.1365-2958.1999.01527.x. [DOI] [PubMed] [Google Scholar]
- 9.Bobik TA, Havemann GD, Busch RJ, Williams DS, Aldrich HC. The propanediol utilization (pdu) operon of Salmonella enterica serovar Typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B(12)-dependent 1, 2-propanediol degradation. J Bacteriol. 1999;181:5967–5975. doi: 10.1128/jb.181.19.5967-5975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown KL. The enzymatic activation of coenzyme B12. Dalton Trans. 2006;7:1123–1133. doi: 10.1039/b517599m. [DOI] [PubMed] [Google Scholar]
- 11.Browne SH, Hasegawa P, Okamoto S, Fierer J, Guiney DG. Identification of Salmonella SPI-2 secretion system components required for SpvB-mediated cytotoxicity in macrophages and virulence in mice. FEMS Immunol Med Microbiol. 2008;52:194–201. doi: 10.1111/j.1574-695X.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Kodama T, Iida T, Honda T. Demonstration and characterization of manganese superoxide dismutase of Providencia alcalifaciens. Microbiol Immunol. 2007;51:951–961. doi: 10.1111/j.1348-0421.2007.tb03992.x. [DOI] [PubMed] [Google Scholar]
- 13.Craig M, Slauch JM. Phagocytic superoxide specifically damages an extracytoplasmic target to inhibit or kill Salmonella. PLoS One. 2009;4:e4975. doi: 10.1371/journal.pone.0004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass-spectral data of peptides with aminoacid-sequences in a protein database. Journal of the American Society for Mass Spectrometry. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003;47:103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- 17.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Opiteck GI, Friedrichs MS, Dongre AR, Hefta SA. Changes in the protein expression of yeast as a function of carbon source. J Proteome Res. 2003;2:643–649. doi: 10.1021/pr034038x. [DOI] [PubMed] [Google Scholar]
- 19.Gort AS, Miller VL. Identification and characterization of Yersinia enterocolitica genes induced during systemic infection. Infect Immun. 2000;68:6633–6642. doi: 10.1128/iai.68.12.6633-6642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen-Wester I, Stecher B, Hensel M. Type III secretion of Salmonella enterica serovar Typhimurium translocated effectors and SseFG. Infect Immun. 2002;70:1403–1409. doi: 10.1128/IAI.70.3.1403-1409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hautefort I, Thompson A, Eriksson-Ygberg S, Parker ML, Lucchini S, et al. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell Microbiol. 2008;10:958–984. doi: 10.1111/j.1462-5822.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hensel M. Salmonella pathogenicity island 2. Mol Microbiol. 2000;36:1015–1023. doi: 10.1046/j.1365-2958.2000.01935.x. [DOI] [PubMed] [Google Scholar]
- 23.Hensel M, Shea JE, Waterman SR, Mundy R, Nikolaus T, Banks G, et al. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 24.Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, et al. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics. 2005;4:1265–1272. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs JM, Yang X, Luft BJ, Dunn JJ, Camp DG, 2nd, et al. Proteomic analysis of Lyme disease: global protein comparison of three strains of Borrelia burgdorferi. Proteomics. 2005;5:1446–1453. doi: 10.1002/pmic.200401052. [DOI] [PubMed] [Google Scholar]
- 26.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnakumar R, Kim B, Mollo EA, Imlay JA, Slauch JM. Structural properties of periplasmic SodCI that correlate with virulence in Salmonella enterica serovar Typhimurium. J Bacteriol. 2007;189:4343–4352. doi: 10.1128/JB.00010-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 29.Livesay EA, Tang KB, Taylor K, Buschbach MA, Hopkins DF, et al. Fully automated four-column capillary LC-MS system for maximizing throughput in proteomic analyses. Anal Chem. 2008;80:294–302. doi: 10.1021/ac701727r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manes NP, Gustin JK, Rue J, Mottaz HM, Purvine SO, et al. Targeted protein degradation by Salmonella under phagosome-mimicking culture conditions investigated using comparative peptidomics. Mol Cell Proteomics. 2007;6:717–727. doi: 10.1074/mcp.M600282-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Matsui H, Bacot CM, Garlington WA, Doyle TJ, Roberts S, et al. Virulence plasmid-borne spvB and spvC genes can replace the 90-kilobase plasmid in conferring virulence to Salmonella enterica serovar Typhimurium in subcutaneously inoculated mice. J Bacteriol. 2001;183:4652–4658. doi: 10.1128/JB.183.15.4652-4658.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazurkiewicz P, Thomas J, Thompson JA, Liu M, Arbibe L, et al. SpvC is a Salmonella effector with phosphothreonine lyase activity on host mitogen-activated protein kinases. Mol Microbiol. 2008;67:1371–1383. doi: 10.1111/j.1365-2958.2008.06134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian WJ, Liu T, Monroe ME, Strittmatter EF, Jacobs JM, et al. Probability-based evaluation of peptide and protein identifications from tandem mass spectrometry and SEQUEST analysis: the human proteome. J Proteome Res. 2005a;4:53–62. doi: 10.1021/pr0498638. [DOI] [PubMed] [Google Scholar]
- 34.Qian WJ, Monroe ME, Liu T, Jacobs JM, Anderson GA, et al. Quantitative proteome analysis of human plasma following in vivo lipopolysaccharide administration using 16O/18O labeling and the accurate mass and time tag approach. Mol Cell Proteomics. 2005b;4:700–709. doi: 10.1074/mcp.M500045-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeed AI, Sharov V, White J, Li J, Liang W, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 36.Sevcik M, Sebkova A, Volf J, Rychlik I. Transcription of arcA and rpoS during growth of Salmonella typhimurium under aerobic and microaerobic conditions. Microbiology. 2001;147:701–708. doi: 10.1099/00221287-147-3-701. [DOI] [PubMed] [Google Scholar]
- 37.Shi L, Adkins JN, Coleman JR, Schepmoes AA, Dohnkova A, et al. Proteomic analysis of Salmonella enterica serovar Typhimurium isolated from RAW 264.7 macrophages: identification of a novel protein that contributes to the replication of serovar Typhimurium inside macrophages. J Biol Chem. 2006;281:29131–29140. doi: 10.1074/jbc.M604640200. [DOI] [PubMed] [Google Scholar]
- 38.Shi L, Chowdhury SM, Smallwood HS, Yoon H, Mottaz-Brewer HM, et al. Proteomic investigation of the time course responses of RAW 264.7 macrophages to infection with Salmonella enterica. Infect Immun. 2009;77:3227–3233. doi: 10.1128/IAI.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Streit WR, Entcheva P. Biotin in microbes, the genes involved in its biosynthesis, its biochemical role and perspectives for biotechnological production. Appl Microbiol Biotechnol. 2003;61:21–31. doi: 10.1007/s00253-002-1186-2. [DOI] [PubMed] [Google Scholar]
- 40.Suvarnapunya AED, Zurawski V, Guy RL, Stein MA. Molecular characterization of the prototrophic Salmonella mutants defective for intraepithelial replication. Infect Immun. 2003;71:2247–2252. doi: 10.1128/IAI.71.4.2247-2252.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uzzau S, Bossi L, Figueroa-Bossi N. Differential accumulation of Salmonella[Cu, Zn] superoxide dismutases SodCI and SodCII in intracellular bacteria: correlation with their relative contribution to pathogenicity. Mol Microbiol. 2002;46:147–156. doi: 10.1046/j.1365-2958.2002.03145.x. [DOI] [PubMed] [Google Scholar]
- 42.Uzzau S, Figueroa-Bossi N, Rubino S, Bossi L. Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci USA. 2001;98:15264–15269. doi: 10.1073/pnas.261348198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VerBerkmoes NC, Shah MB, Lankford PK, Pelletier DA, Strader MB, et al. Determination and comparison of the baseline proteomes of the versatile microbe Rhodopseudomonas palustris under its major metabolic states. J Proteome Res. 2006;5:287–298. doi: 10.1021/pr0503230. [DOI] [PubMed] [Google Scholar]
- 44.Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 45.Wei Y, Miller CG. Characterization of a group of anaerobically induced, fnr-dependent genes of Salmonella typhimurium. J Bacteriol. 1999;181:6092–6097. doi: 10.1128/jb.181.19.6092-6097.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaharik ML, Cullen VL, Fung AM, Libby SJ, Kujat Choy SL, et al. The Salmonella enterica serovar typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect Immun. 2004;72:5522–5525. doi: 10.1128/IAI.72.9.5522-5525.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]