Abstract
Cyclin-dependent kinases (CDKs) are commonly known to regulate cell proliferation. However, previous reports suggest that in cultured postmitotic neurons, activation of CDKs is a signal for death rather than cell division. We determined whether CDK activation occurs in mature adult neurons during focal stroke in vivo and whether this signal was required for neuronal death after reperfusion injury. Cdk4/cyclin D1 levels and phosphorylation of its substrate retinoblastoma protein (pRb) increase after stroke. Deregulated levels of E2F1, a transcription factor regulated by pRb, are also observed. Administration of a CDK inhibitor blocks pRb phosphorylation and the increase in E2F1 levels and dramatically reduces neuronal death by 80%. These results indicate that CDKs are an important therapeutic target for the treatment of reperfusion injury after ischemia.
The mechanism by which stroke-induced neuronal death occurs is complex and is likely dependent upon the severity and duration of ischemic insult and an elaborate interplay between ischemic death initiators such as excitotoxicity, oxidative stress, DNA damage, and inflammatory responses (1–3). Neurons that survive the acute ischemic injury undergo a delayed cell death that exhibits some characteristics of apoptosis (1–3). This delayed cell death is dependent upon selected death-signaling elements such as caspases, poly(ADP-ribose) polymerase, and p53 (4). The identification of signaling molecules that control delayed neuronal death has led to the hope that some of these death-signaling elements may serve as useful therapeutic targets for the reduction of neuropathology and behavioral deficits associated with stroke injury. The mechanism by which stroke-evoked delayed death occurs, however, is not fully understood.
The cell cycle is a highly coordinated process regulated by the appropriate and timely activation of cyclin-dependent kinases (CDKs) (5). Regulation of CDKs is complex and includes binding to their obligate cyclin partner, activating and inhibitory phosphorylation events, and endogenous inhibitors of CDK activity. Distinct CDKs regulate progressive phases of the cell cycle. Generally, it is thought that the Cdk4/6/cyclin D1 complex regulates the G0 to G1, Cdk2/cyclin E and Cdk3 control G1 to S, and Cdk2/cyclin A and Cdk1/cyclin B control G2 and M progressions. Although the downstream targets of CDKs are not fully characterized, one important substrate is the tumor suppresser retinoblastoma protein (pRb), which is phosphorylated by activated Cdk4/6/cyclin D complex (6). Once hyperphosphorylated, pRb is released from the transcription factor complex E2F/DP, which then activates genes required for S phase transition (7–9).
Paradoxically, increasing evidence suggests that CDKs may have functions beyond that of cell cycle regulation. Numerous reports indicate the requirement of CDK signals for death of cultured postmitotic neurons exposed to select death insults. For example, inappropriate cyclin B and cyclin D1 transcripts have been observed in neuronal PC12 cells and sympathetic neurons deprived of trophic support (10, 11). In addition, cyclin D1-associated kinase activity, as well as phosphorylation of its substrate pRb, increases during the death of cultured embryonic neurons exposed to DNA damage and/or toxic forms of βamyloid (12, 13). Administration of CDK inhibitors and expression of dominant-negative forms of Cdk4/6 also block the death of neurons evoked by trophic factor deprivation (14, 15), DNA damage (16–18), extracellular K+ deprivation (19), and βamyloid (12, 20). Taken together, these observations suggest that inappropriate activation of cell cycle signals (in particular, the Cdk4/6/pRb pathway) in the environment of a postmitotic differentiated neuron results in death. However, the required role of cell cycle signaling molecules has largely been examined in cultured neuronal systems, and its involvement in the death of injured adult neurons in vivo and its potential as a therapeutic target are unknown. We show here that the Cdk4/6/pRb pathway is activated in a rat model of focal ischemia and that these signals are required for the death of neurons evoked by ischemia.
Materials and Methods
Animal Preparation.
All studies were performed in male Sprague–Dawley rats weighing 250–275 g. Rats were anesthetized with 2% halothane in a 30% oxygen/70% nitrous oxide gas mixture. Rats were allowed to breathe spontaneously during Alzet pump placement and were intubated and ventilated during focal cerebral ischemia.
Rats were fasted overnight before focal cerebral ischemia. Under anesthesia, a femoral artery catheter was placed to monitor mean arterial blood pressure and to obtain blood samples for the measurement of arterial blood gases and blood glucose. Body temperature was monitored with a rectal probe and maintained between 36 and 37°C. Brain temperature was monitored during ischemia by placement of a thermocouple probe between the temporalis and the skull. Both common carotid arteries were exposed through a midline neck incision, and the left middle cerebral artery distal to the rhinal fissure was exposed through a 2-mm-diameter craniotomy. The middle cerebral artery was occluded with a microaneurysm clip (Codman, Boston, MA), followed immediately by occlusion of both common carotid arteries with aneurysm clips. After 30 min of ischemia the clips were removed and reperfusion was visually verified in the middle cerebral artery. The incision was then infiltrated with bupivicaine gel and closed. Tylenol suppositories were given to all rats during the first 3 days of recovery. In a subset of rats receiving flavopiridol treatment, a midline sagittal incision was made in the scalp under anesthesia for placement of Alzet osmotic pumps (Alza) 24 h before focal cerebral ischemia. An Alzet cannula was stereotactically introduced into the left lateral ventricle through a 1-mm burr hole (coordinates from bregma: anterioposterior −0.4 mm, lateral 1.3 mm, depth 3.3 mm) and connected to the osmotic pump placed s.c. in the neck. Flavopiridol (a kind gift from Peter J. Worland, National Cancer Institute) at a concentration of 50 mM in DMSO was diluted to 100 or 500 μM with Krebs—Ringer's solution containing 0.01% Evans blue. DMSO alone was used with the same dilution for the vehicle-treated rats. The drug or vehicle solutions were added to the osmotic pumps, which infused at a rate of 1 μl/h, starting 24 h before ischemia. After 30 min of ischemia, rats survived for 3 h, 6 h, 24 h, or 6 days. Pump function was confirmed by weighing the pump before placement and after the experiment. Pigmentation of the cisterna magna was verified to ensure delivery of the flavopiridol or vehicle solution.
Local Cerebral Blood Flow (CBF) Measurement.
In a subset of animals treated with vehicle or flavopiridol (500 μM), CBF was measured before, during, and immediately after cerebral ischemia. CBF was measured by the H2 clearance method through a platinum wire electrode stereotactically inserted in the sensory motor cortex (coordinates from bregma: anterioposterior −1.0 mm, lateral 6.0 mm, depth 2 mm), as previously described (21).
Immunohistochemistry.
At the appropriate reperfusion intervals, rats were anesthetized and perfusion fixed with 200 ml of 0.1% heparinized saline followed by 200 ml of 4% paraformaldehyde in PBS. The brains were removed, postfixed in 10% formalin for 7 days, and then embedded in paraffin. Brain sections (7 μm) were obtained at the level of the striatum (bregma −0.2 mm) and deparaffinized. The sections were incubated with 0.3% H2O2 in PBS for 30 min to quench the endogenous peroxidase activity and washed three times in PBS. Sections were blocked in normal serum obtained from the species in which the secondary antibody was raised for 20 min and in avidin-biotin blocking reagent (Vector Laboratories) for 15 min. Sections were incubated overnight with primary antibody diluted in PBS. Primary antibodies used for immunohistochemistry were cyclin D1 [Santa Cruz Biotechnology (sc-8396); NovoCastra, Newcastle, UK (DCS-6); 1:500], Cdk4 [Transduction Laboratories, Lexington, KY (c18720), 1:500], phospho(Ser-795)Rb [New England Biolabs (9301), 1:500], E2F1 [PharMingen (KH95) 1:500], MAP2 [Upstate Biotechnology, Lake Placid, NY (05-346), 1:500], and NeuN [Chemicon (MAB377), 1:100]. Sections were then washed three times in PBS and visualized with the Vectastain elite ABC kit (Vector Laboratories). Sections were counterstained with methyl green. The number of immunopositive cells was counted in defined 650 × 500 μm2 fields at 100× magnification within specified regions of interest as follows. The region of infarcted tissue was identified, using hematoxylin/eosin (H&E) staining as described below, in vehicle-treated rats after 6 days of reperfusion after 30 min of ischemia. A 650 × 500 μm2 field was defined in the core of the ischemic area in the lateral aspect of the sensorimotor cortex. Three additional identical fields were defined superior, medial, and inferior to this first region and were separated from the first field by 200 μm. A region identical to the first field was defined in the contralateral hemisphere. This fixed geometry was then applied to all studies. The first field, or infarct region, was placed at the center of the infarcted tissue when this was evident. At short reperfusion times this field was placed at the average location of the infarct core identified after 6 days of reperfusion. The cell counts from the adjacent three regions were averaged and defined as the periinfarct region (Fig. 1B). The immunohistochemical data for the infarct, periinfarct, and contralateral regions of interest were analyzed. Double labeling with cyclin D1 or phospho-pRb and NeuN was performed as described above with the following exceptions. After incubation with anti-cyclin D1 or phospho-pRb primary antibody and biotinylated secondary antibody, sections were incubated with Alexa 594-conjugated streptavidin. The procedure was then repeated with anti-NeuN primary antibody and Alexa 488-conjugated streptavidin.
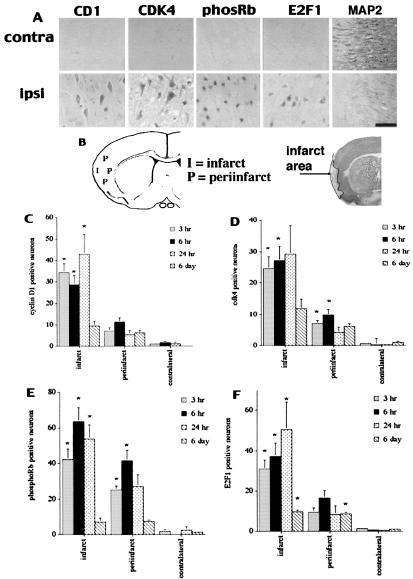
Figure 1.
(A) Cyclin D1 (CD1), Cdk4, Ser-795-phosphorylated pRb (phosRb), and E2F1 levels increase during reperfusion. Representative sections obtained from rats 24 h after reperfusion at the level of the striatum were analyzed for the indicated cell cycle markers by immunohistochemistry. The area of the ischemic core is shown. (The black bar represents 50 μm.) MAP2 staining is included as a control marker that does not increase during reperfusion. (B) Diagram indicating regions of quantitation after stroke (Left), and representative section with MAP2 staining (Right) indicating infarct. (C–F) Quantification of cyclin D1- (C), Cdk4- (D), phospho-pRb- (E), and E2F1- (F) positive cells at the indicated regions at various time points was performed as described in Materials and Methods. “Contralateral” indicates the area of uninfarcted cortex on the contralateral side. Each data point is the mean ± SEM (n = 5). * indicates significance (P < 0.05; paired t test with Bonferroni correction) as compared with contralateral control values.
Terminal Deoxynucleotidyltransferase-Mediated dUTP End Labeling (TUNEL).
TUNEL staining was performed with the In Situ Cell Death Detection kit (Roche Molecular Chemicals). After rehydration, sections were incubated with 20 μg/ml proteinase K in 10 mM Tris⋅HCl (pH 7.5) for 10 min at room temperature. Endogenous peroxidase activity was blocked by incubating with 0.3% H2O2 in methanol for 30 min. Sections were incubated in 0.1% Triton X-100 in 0.1% sodium citrate for 2 min at 4°C. After rinsing, 50 μl of TUNEL solution containing terminal deoxynucleotidyltransferase and fluorescein–dUTP mixture was applied to sections for 20 min at 37°C. After blocking with 5% serum and avidin-biotin blocking reagent (Vector Laboratories), biotin-conjugated anti-FITC antibody (1:400) in 5% normal serum/PBS was placed on sections for 30 min at room temperature. Sections were then incubated with horseradish peroxidase-conjugated avidin (Vector Laboratories) and visualized by diaminobenzidine⋅hydrochloride staining. For assessment of protection with flavopiridol, the total number of TUNEL-positive cells was determined in both the ipsilateral and contralateral cortices of the flavopiridol- or vehicle-treated rats. No TUNEL-positive cells were observed in the contralateral cortex. Double TUNEL/pRb staining was performed with the protocol described above, with the exception that only FITC-labeled dUTP was used to visualize TUNEL labeling. Immunostaining using the pRb antibody was performed as described above, with the exception that Alexa 594 dye (Molecular Probes) conjugated with streptavidin was used.
Double Immunostaining of TUNEL and pRb.
Double TUNEL/pRb staining was performed with the protocol described above, with the exception that only FITC-labeled dUTP was used to visualize TUNEL labeling. Immunostaining using the pRb antibody was performed as described above, with the exception that Alexa 594 dye (Molecular Probes) conjugated with streptavidin was used. Sections were mounted with a water-soluble 4′,6-diamidino-2-phenylindole-containing mounting medium (Vector Laboratories) and studied with a Leica confocal microscope system.
H&E Staining and Infarct Area.
Sections were stained with H&E as previously described (22). Infarcted tissue was identified by using standard criteria (23). The coronal brain sections (bregma −0.2 mm) were digitized, the area of infarct and the total area of the cortex ipsilateral to the side of occlusion were measured, and the percentage of infarct was calculated for each rat.
Statistics.
All data are presented as mean ± SEM. Individual comparisons were made by using independent t tests. Studies with data from multiple regions of interest within the brain were analyzed, using repeated-measures ANOVA with post hoc comparisons, following the method described by Howell (24). Comparisons between regions (within) used paired t tests with a Bonferroni correction, and comparisons between time points or treatments used Tukey's highly significant difference with adjusted degrees of freedom plus a Bonferroni correction for the number of regions assessed.
Results
We first attempted to determine whether CDKs and their cyclin partners are up-regulated during rat focal model of ischemia. In the distal clip model of focal injury, the cortical layers are damaged while the subcortical structures are spared (25). After distal artery occlusion for 30 min, reperfusion was allowed for 3 h, 6 h, 24 h, or 6 days. Equivalent sections at the level of the midstriatum were analyzed for cell cycle markers in the core area of damage in the cortex, areas immediately peripheral to the core region, and a cortical area equivalent to the area of core damage on the contralateral nonischemic side. As shown in Fig. 1, immunoreactivity for Cdk4 and its cyclin partner cyclin D1 increased at 3 h after reperfusion and continued to be up-regulated at 24 h. Immunoreactivity decreased at 6 days. The increase in Cdk4 protein was confirmed by Western blot analyses (data not shown). However, it is not clear whether the increase in cell cycle protein is due to increased rates of protein synthesis or decreased turnover of protein. Neuronal expression for cyclin D1 was confirmed by double labeling with the nuclear neuronal marker NeuN (Fig. 2). Neurons showed both cytoplasmic and nuclear staining for cyclin D1 and Cdk4 (Figs. 1 and 2). Interestingly, some neurons showed predominate cytoplasmic labeling for cyclin D1. Although the reason for this is unclear, cyclin D1 has been shown to reside in the cytoplasm and translocate to the nucleus with a death stimulus in neurons (19). It may be that those neurons with predominately cytoplasmic location may eventually translocate to the nucleus. The greatest number of Cdk4- and cyclin D1-positive cells was found in the ischemic core, with fewer positive cells in areas medial, superior, or inferior to this region. Cyclin E and B were also similarly up-regulated (data not shown). Few cyclin- or CDK-positive neurons were observed in the contralateral cortex at any time. In addition, no staining was observed with secondary antibody alone, and no increase was observed with MAP2 staining (Fig. 1A). In the latter case, a noticeable decrease in staining intensity was observed in the infarct core (Fig. 1B).
Figure 2.
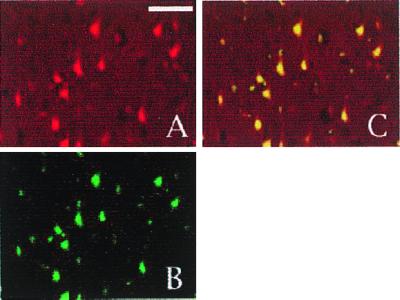
Cyclin D1 immunoreactivity is localized to neurons in a confocal image of the core infarct region 24 h after reperfusion. (A and B) Sections were double labeled with cyclin D1 (A) and the neuronal marker NeuN (B). (C) The merged image. (The white bar represents 50 μm.)
We next determined whether CDK activity was increased in neurons in the area of infarct. Activated Cdk4 as well as Cdk2 is known to phosphorylate pRb. Accordingly, we looked for an increase in pRb phosphorylation on a CDK consensus site (Ser-795), using a phospho-epitope-specific antibody. As shown in Fig. 1, the number of phospho-pRb-positive neurons also dramatically increased in the core region at 3 h after reperfusion compared with uninjured control regions. As with cyclin D1 and Cdk4 staining, fewer phospho-pRb-positive neurons were observed in areas medial, superior, and inferior to the core. Neuronal localization of phospho-pRb staining was confirmed by double labeling with NeuN (data not shown). As shown in Fig. 3, the majority of phospho-pRb-positive neurons colocalized with TUNEL-positive staining, indicating that these neurons were dying. However, there were some cells that were TUNEL positive but did not label for phospho-pRb. pRb is known to be degraded upon a death stimulus in neurons (13). Accordingly, it is possible that these neurons were in the latter stages of death. Alternatively, damage to these cells may not involve the pRb pathway. Phosphorylation of pRb is believed to be inactivating, allowing transcription mediated through E2F-containing complexes. One protein thought to be regulated by these complexes is E2F1 itself. Accordingly, we attempted to determine whether E2F1 levels increased after focal ischemia. As shown in Fig. 1, the number of E2F1-positive neurons also increased 3, 6, and 24 h after reperfusion. The distribution of E2F1-positive neurons at each time point after reperfusion was similar to the distributions observed for cyclin D1, Cdk4, and pRb staining. Taken together, these results indicate that active CDK complexes increase during reperfusion after ischemia.
Figure 3.
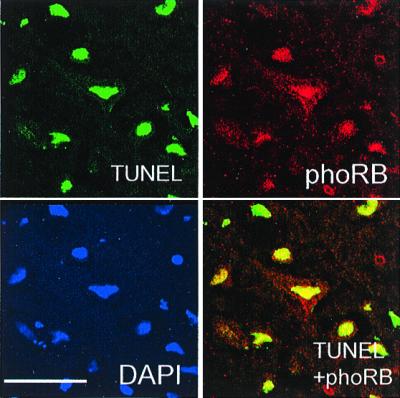
TUNEL labeling and phospho-pRb reactivity colocalize in neurons after reperfusion injury in a confocal image of the core infarct region 24 h after reperfusion. Sections were triple labeled with TUNEL, phospho-pRb (phoRb), and 4′,6-diamidino-2-phenylindole (DAPI). The merged image of TUNEL and phospho-Rb is indicated. (The white bar represents 50 μm.)
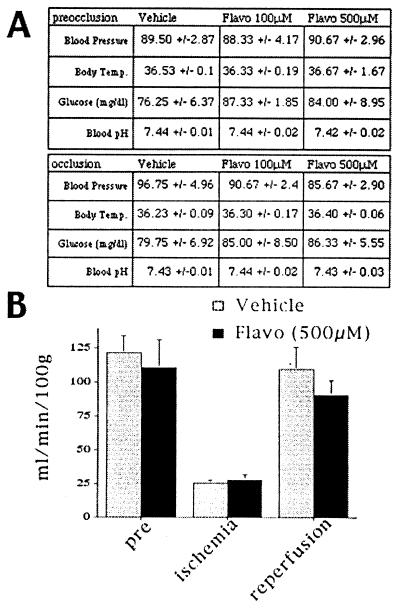
What is the function of up-regulation of CDK activity? Several possibilities exist. The up-regulation may be a protective response to reperfusion injury, a secondary stress response to damage uninvolved in death signaling, or a signaling pathway required for transduction of the ischemic death signal. To distinguish among these possibilities, we examined the neuroprotective effects of the CDK inhibitor flavopiridol. Flavopiridol, a flavone derivative, has been shown to block Cdk1, -2, and -4 with high specificity (26, 27). Flavopiridol (100 or 500 μM) was administered intracerebroventricularly to the animals 24 h before ischemic insult and throughout the duration of the reperfusion period by means of an Alzet pump. As shown in Fig. 4A, administration of the CDK inhibitor had no significant effect on physiological parameters before or during the ischemic insult. Cortical blood flow did not change detectably during the same periods, as measured by the H2 clearance method (21) (Fig. 4B).
Figure 4.
Blood flow and select physiological parameters do not change with flavopiridol treatment. (A) Table of physiological parameters of animals treated with flavopiridol or vehicle. Blood pressure is given in mmHg and body temperature in °C. (B) Flavopiridol does not affect cerebral blood flow, as measured by hydrogen clearance. Each data point represents a mean ± SEM (n = 3).
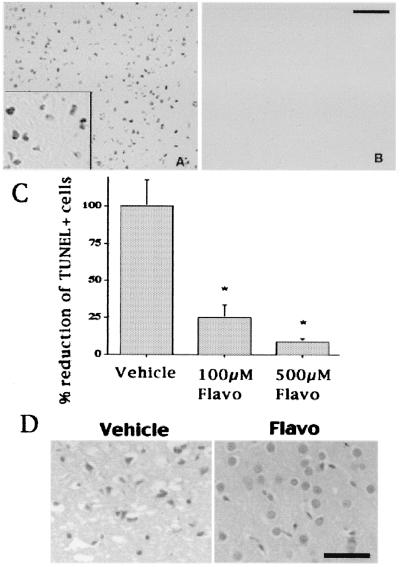
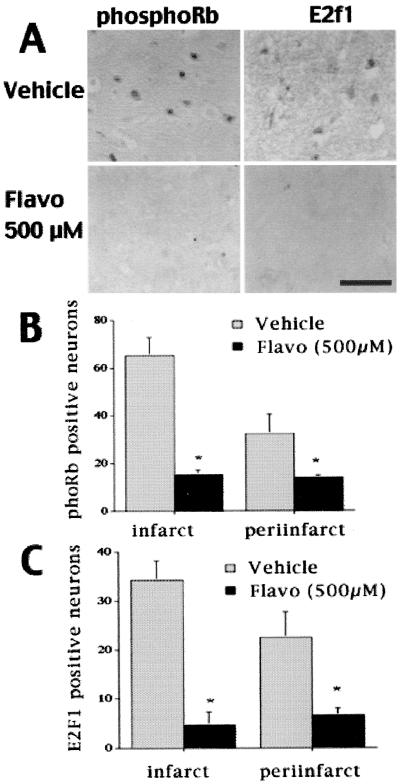
As shown in Fig. 5, TUNEL-positive neurons, which were prevalent in the core area of damage in the vehicle-treated animals at 24 h of reperfusion, were dramatically reduced (75% reduction with 100 μM or 90% with 500 μM) in flavopiridol-treated animals. By histological staining with H&E, dying neurons displayed shrunken, condensed, and crescent-shaped nuclei, whereas those protected by flavopiridol displayed the morphology of healthy nuclei. As shown in Fig. 6, treatment with flavopiridol also dramatically reduced activation of the CDK/pRb pathway as measured by pRb phosphorylation and reduced the number of E2F1-positive neurons compared with vehicle-treated controls. Finally, the analysis of the time course of TUNEL-positive cells after reperfusion indicated that TUNEL increased dramatically (more than 5.5-fold) from 3 to 24 h (Fig. 7). In contrast, the relative number of cyclin D1-positive cells changed little over that time period (Fig. 1). Taken together, these results suggest that cell cycle activation occurs before (both temporally and biochemically) neuronal damage as represented by TUNEL staining. Most importantly, the data suggest that CDK activity is required for neuronal death.
Figure 5.
Flavopiridol prevents neuronal death after 24 h of reperfusion. (A and B) Representative sections of core infarct region stained for TUNEL from vehicle-treated (A) or flavopiridol-treated (500 μM) (B) animals 24 h after reperfusion. (The black bar represents 100 μm.) (C) Quantitation of TUNEL-positive cells in the ipsilateral cortical hemisphere of animals treated with vehicle or flavopiridol. Each data point represents a mean ± SEM (n = 4) and is expressed relative to the number of TUNEL-positive cells present in vehicle-treated ischemic animals at 24 h. * indicates significance (P < 0.01 as determined by independent t test). (D) H&E analyses of sections from the core infarct area of vehicle-treated or flavopiridol-treated (500 μM) animals 24 h after reperfusion. (The black bar represents 50 μm.)
Figure 6.
Flavopiridol (500 μM) treatment inhibits pRb phosphorylation and the rise in E2F1 levels. (A) Representative sections of cortex ipsilateral to occlusion from animals treated with vehicle or flavopiridol. Sections were obtained 24 h after reperfusion. (The black bar indicates 50 μm.) (B and C) Phospho-pRb-positive (B) and E2F1-positive (C) cells were quantified as described in the legend of Fig. 1. Each point represents a mean ± SEM (n = 4). * indicates significance (P < 0.05 as determined by repeated-measures ANOVA, followed by post hoc Tukey's highly significant difference) as compared with vehicle-treated controls.
Figure 7.
Time course of TUNEL-positive neurons after reperfusion. Each data point represents a mean ± SEM (n = 5) and is expressed relative to the number of TUNEL-positive cells present in the ipsilateral hemisphere at 24 h.
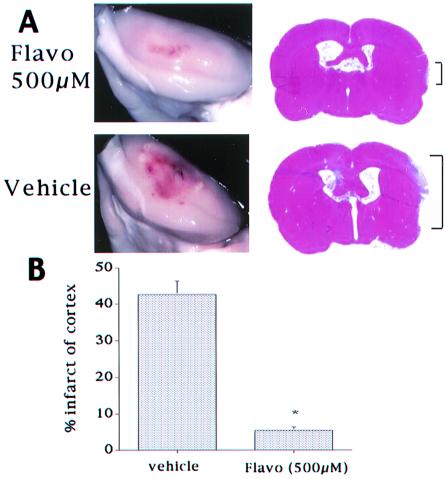
The protection observed with flavopiridol treatment at 24 h was also present 6 days after reperfusion. As shown in Fig. 8, approximately 43% of the cortex ipsilateral to the occlusion was damaged in animals treated with vehicle as measured by H&E staining. In contrast, animals treated with flavopiridol showed only a 7% area of damage. The reduction in damage was also clearly visible on the exterior of the brain, where a dramatically reduced area of damage was visible after flavopiridol treatment.
Figure 8.
Flavopiridol (500 μM) treatment reduces cortical infarct 6 days after reperfusion. (A) Photograph of exterior of the cortex and striatal sections from representative animals treated with vehicle or flavopiridol as indicated. The brackets indicate the length of infarct along the cortical surface. (B) Quantification of infarct as described in Materials and Methods. Each point represents a mean ± SEM (n = 4). * indicates significance (P < 0.01 as determined by independent t test).
Taken together, our results indicate that CDK activity is up-regulated in neurons exposed to ischemic injury and that this activity is necessary for the death of neurons after reperfusion.
Discussion
Whereas it was once thought that processes that led to neuronal death after ischemia were irreversible, the current understanding of the pathways controlling delayed neuronal death after reperfusion has provided hope for therapeutic intervention (1, 2). Although several death-signaling events have been identified, no inhibitors of these events have yet provided clear long-term therapeutic value in patients (2). The reasons for this are complex and include dosage, timing, side effects, and toxicity problems. It is anticipated, however, that improved understanding of the state of death pathways after reperfusion injury may lead to more accessible and effective therapeutic targets.
The data presented herein indicate that one such target is CDKs and suggest that the Cdk4/pRb pathway, normally associated with proliferative regulation, is required for signaling of adult neuronal death after ischemic insult. The evidence can be summarized as follows: (i) There are increased levels of cyclins and CDKs (in particular, cyclin D1 and Cdk4) in dying neurons in cortical regions exposed to ischemia. (ii) The CDKs present are most likely active, as evidenced by an increase in pRb phosphorylation on CDK consensus sites and deregulated levels of E2F1, a transcription factor regulated by pRb. (iii) Administration of the CDK inhibitor flavopiridol blocks the death of neurons as measured by TUNEL analyses and dramatically reduces neuronal death.
Our results are in accordance with numerous reports both by ourselves and by others of the involvement of CDKs in the death of in vitro cultured neurons evoked by a variety of insults, including DNA damage, trophic factor deprivation, and βamyloid toxicity. The study of CDKs in adult neurons in vivo, however, is important for several reasons. It is known that mechanisms that control neuronal death change during development and that signals mediating the death of early embryonic or postnatal neurons may differ from those for mature neurons (28). In addition, others have suggested that CDK levels also increase in some in vivo neuronal injury settings. For example, increases in Cdk4 and/or cyclin D1 have also been reported in vulnerable brain regions of Alzheimer's disease patients (29–31), the kainic acid-evoked death of hippocampal neurons (32), and in stroke (32, 33). However, it is not clear whether up-regulation of these cell cycle components results in an active complex, whether its activity is required for the death of neurons, or whether it is a protective stress response, as some have suggested (33). Our present data indicate the activation of the Cdk4/6/pRb pathway in dying neurons. Most importantly, we show that intracerebroventricular administration of the CDK inhibitor flavopiridol dramatically decreases the number of TUNEL-positive neurons at 24 h and the infarct volume at 6 days after reperfusion compared with vehicle-treated controls. Flavopiridol treatment also inhibits the increase in pRb phosphorylation and the elevation of E2F1 protein. This evidence suggests that the activation of CDKs is required for the death of adult neurons evoked by stroke in vivo. It must be noted that although the currently demonstrated effects of flavopiridol on survival are consistent with their reported ability and specificity in preventing CDK activity, the possibility exists that flavopiridol may work through alternative mechanisms. For example, flavopiridol treatment has recently been associated with a decreased vascular endothelial growth factor mRNA half-life in monocytes (34) and a reduction in metalloproteinases (35) in breast cancer cell lines. However, the observation that cultured neurons are protected both by flavopiridol and by the expression of dominant-negative Cdk4 and Cdk6 are consistent with the CDK-inhibitory and neuroprotective properties of this compound (12, 14–16, 18, 20).
The mechanism by which CDK activation controls death evoked by ischemia is unknown. Our present data suggest that one pathway may be through the phosphorylation of pRb. Consistent with this, it has been reported that pRb phosphorylation has been observed in in vitro cultured neurons exposed to apoptotic stress (12, 19, 36) and that overexpression of pRb is protective in models of embryonic cortical death evoked by DNA damage (13). In addition, there is increased developmental neuronal death in pRb-deficient mice (37, 38). The role or function of pRb itself is complex and is known to include interactions with a large number of proteins, including the E2F members. Interestingly, several lines of evidence implicate the role of E2F members in cell death. We previously showed that expression of dominant-negative DP1 protects cultured cortical embryonic neurons from death evoked by DNA damage and β-amyloid (12, 13). In addition, E2F1 expression is sufficient to promote the death of proliferating cells (39). Finally, E2F1-deficient mice are resistant to the death of cultured cortical and cerebellar granule neurons evoked by β-amyloid toxicity and low extracellular K+, respectively (12) (M. O'Hare and D.S.P., unpublished data), and to ischemic damage (40). However, in these cases, protection is transient and/or incomplete, suggesting the participation of additional, complementary death pathways. Although we currently suggest the importance of the Cdk4/pRb pathway, we cannot rule out the possibility that other CDKs may also play an important role in death. It is of particular relevance that Cdk5, a member of the CDK family important for neuronal maturation but not involved in cell cycle control, has also been implicated in neuronal death (41).
Our observations presented here suggest one signaling pathway that contributes to the death of neurons after ischemic injury. In this model, CDK-mediated signaling events, along with perhaps other stroke-relevant death signals such as p53, lead to delayed neuronal death. Accordingly, the data presented here provide compelling evidence for future examination of the CDK-mediated pathway as a potential therapeutic target for the treatment of stroke-induced neuronal injury.
Acknowledgments
This work was supported by funds from the Heart and Stroke Foundation of Canada (D.S.P. and A.M.H.) and the Medical Research Council of Canada (D.S.P.) and a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture, Japan (J.I.). D.S.P. is a recipient of a Glaxo Wellcome Fellowship.
Abbreviations
- CDK
cyclin-dependent kinase
- pRb
retinoblastoma protein
- H&E
hematoxylin/eosin
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP end labeling
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.170144197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.170144197
References
- 1.Choi D W. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 2.Dirnagl U, Iadecola C, Moskowitz M A. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 3.MacManus J P, Hill I E, Preston E, Rasquinha I, Walker T, Buchan A M. J Cereb Blood Flow Metab. 1995;15:728–737. doi: 10.1038/jcbfm.1995.93. [DOI] [PubMed] [Google Scholar]
- 4.Lipton P. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 5.Pines J. Biochem Soc Trans. 1993;21:921–925. doi: 10.1042/bst0210921. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg R A. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 7.Chellappan S P, Hiebert S, Mudryj M, Horowitz J M, Nevins J R. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 8.Bandara L R, Buck V M, Zamanian M, Johnston L H, La Thangue N B. EMBO J. 1993;12:4317–4324. doi: 10.1002/j.1460-2075.1993.tb06116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C L, Classon M, Dyson N, Harlow E. Mol Cell Biol. 1996;16:3698–3706. doi: 10.1128/mcb.16.7.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao C Y, Zelenka P S. Exp Cell Res. 1995;219:612–618. doi: 10.1006/excr.1995.1271. [DOI] [PubMed] [Google Scholar]
- 11.Freeman R S, Estus S, Johnson E M., Jr Neuron. 1994;12:343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 12.Giovanni A, Keramaris E, Morris E J, Hou S T, O'Hare M, Dyson N, Robertson G S, Slack R S, Park D S. J Biol Chem. 2000;275:11553–11560. doi: 10.1074/jbc.275.16.11553. [DOI] [PubMed] [Google Scholar]
- 13.Park D S, Morris E J, Bremner R, Keramaris E, Padmanabhan J, Rosenbaum M, Shelanski M L, Geller H M, Greene L A. J Neurosci. 2000;20:3104–3114. doi: 10.1523/JNEUROSCI.20-09-03104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park D S, Farinelli S E, Greene L A. J Biol Chem. 1996;271:8161–8169. doi: 10.1074/jbc.271.14.8161. [DOI] [PubMed] [Google Scholar]
- 15.Park D S, Levine B, Ferrari G, Greene L A. J Neurosci. 1997;17:8975–8983. doi: 10.1523/JNEUROSCI.17-23-08975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park D S, Morris E J, Greene L A, Geller H M. J Neurosci. 1997;17:1256–1270. doi: 10.1523/JNEUROSCI.17-04-01256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park D S, Morris E J, Stefanis L, Troy C M, Shelanski M L, Geller H M, Greene L A. J Neurosci. 1998;18:830–840. doi: 10.1523/JNEUROSCI.18-03-00830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park D S, Morris E J, Padmanabhan J, Shelanski M L, Geller H M, Greene L A. J Cell Biol. 1998;143:457–467. doi: 10.1083/jcb.143.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padmanabhan J, Park D S, Greene L A, Shelanski M L. J Neurosci. 1999;19:8747–8756. doi: 10.1523/JNEUROSCI.19-20-08747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Copani A, Condorelli F, Caruso A, Vancheri C, Sala A, Giuffrida Stella A M, Canonico P L, Nicoletti F, Sortino M A. FASEB J. 1999;13:2225–2234. [PubMed] [Google Scholar]
- 21.Farrar J K. In: Cerebral Blood Flow—Physiologic and Clinical Aspects. Wood J H, editor. New York: McGraw–Hill; 1987. pp. 275–287. [Google Scholar]
- 22.Stevens A. In: The Theory and Practice of Histological Techniques. Bancroft J D, editor. Edinburgh: Churchill Livingstone; 1982. pp. 109–121. [Google Scholar]
- 23.Brown A, Brierly J. Br J Exp Pathol. 1968;49:87–106. [PMC free article] [PubMed] [Google Scholar]
- 24.Howell D C. In: Statistical Methods for Psychology. 3rd Ed. Howell D C, editor. Belmont, CA: Duxbury; 1992. pp. 430–483. [Google Scholar]
- 25.Chen S T, Hsu C Y, Hogan E L, Maricq H, Balentine J D. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- 26.Losiewicz M D, Carlson B A, Kaur G, Sausville E A, Worland P J. Biochem Biophys Res Commun. 1994;201:589–595. doi: 10.1006/bbrc.1994.1742. [DOI] [PubMed] [Google Scholar]
- 27.De Azevedo W F, Jr, Mueller-Dieckmann H J, Schulze-Gahmen U, Worland P J, Sausville E, Kim S H. Proc Natl Acad Sci USA. 1996;93:2735–2740. doi: 10.1073/pnas.93.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson M D, Kinoshita Y, Xiang H, Ghatan S, Morrison R S. J Neurosci. 1999;19:2996–3006. doi: 10.1523/JNEUROSCI.19-08-02996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busser J, Geldmacher D S, Herrup K. J Neurosci. 1998;18:2801–2807. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy Z, Esiri M M, Cato A M, Smith A D. Acta Neuropathol. 1997;94:6–15. doi: 10.1007/s004010050665. [DOI] [PubMed] [Google Scholar]
- 31.McShea A, Zelasko D A, Gerst J L, Smith M A. Brain Res. 1999;815:237–242. doi: 10.1016/s0006-8993(98)01135-4. [DOI] [PubMed] [Google Scholar]
- 32.Timsit S, Rivera S, Ouaghi P, Guischard F, Tremblay E, Ben-Ari Y, Khrestchatisky M. Eur J Neurosci. 1999;11:263–278. doi: 10.1046/j.1460-9568.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Jiang N, Powers C, Chopp M. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. [DOI] [PubMed] [Google Scholar]
- 34.Melillo G, Sausville E A, Cloud K, Lahusen T, Varesio L, Senderowicz A M. Cancer Res. 1999;59:5433–5437. [PubMed] [Google Scholar]
- 35.Li Y, Bhuiyan M, Alhasan S, Senderowicz A M, Sarkar F H. Clin Cancer Res. 2000;6:223–229. [PubMed] [Google Scholar]
- 36.Gill J S, Windebank A J. J Clin Invest. 1998;101:2842–2850. doi: 10.1172/JCI1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacks T, Fazeli A, Schmitt E M, Bronson R T, Goodell M A, Weinberg R A. Nature (London) 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 38.Lee E Y, Chang C Y, Hu N, Wang Y C, Lai C C, Herrup K, Lee W H, Bradley A. Nature (London) 1992;359:288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- 39.Qin X Q, Livingston D M, Kaelin W G, Jr, Adams P D. Proc Natl Acad Sci USA. 1994;91:10918–10922. doi: 10.1073/pnas.91.23.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacManus J P, Koch C J, Jian M, Walker T, Zurakowski B. Neuroreport. 1999;10:2711–2714. doi: 10.1097/00001756-199909090-00004. [DOI] [PubMed] [Google Scholar]
- 41.Patrick G N, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai L H. Nature (London) 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]