Abstract
Objective:
Tetrahydrobiopterin (BH4) deficiency is one of the causes of dystonia at birth. We hypothesized that BH4 is a developmental factor determining vulnerability of the immature fetal brain to hypoxic-ischemic injury and subsequent motor deficits in newborns.
Methods:
Pregnant rabbits were subjected to 40-min uterine ischemia and fetal brains were investigated for global and focal changes in BH4. Newborn kits were assessed by neurobehavioral tests following vehicle and sepiapterin (BH4-analog) treatment of dams.
Results:
Naive fetal brains at 70% gestation (E22) were severely deficient for BH4 compared to maternal and other fetal tissues. BH4 concentration rapidly increased normally in the perinatal period with the highest concentrations found in the thalamus compared to basal ganglia, frontal, occipital, hippocampus and parietal cortex. Global sustained 40-min hypoxia-ischemia depleted BH4 in E22 thalamus and to a lesser extent in basal ganglia, but not in the frontal, occipital and parietal regions. Maternal supplementation prior to hypoxia-ischemia with sepiapterin increased BH4 in all brain regions and especially in the thalamus, but did not increase the intermediary metabolite, 7,8-BH2. Sepiapterin treatment also reduced incidence of severe motor deficits and perinatal death following E22 hypoxia-ischemia.
Interpretation:
We conclude that early developmental BH4 deficiency plays a critical role in hypoxic-ischemic brain injury. Increasing brain BH4 via maternal supplementation may be an effective strategy in preventing motor deficits from antenatal hypoxia-ischemia.
Keywords: Tetrahydrobiopterin; GTP cyclohydrolase-I; dystonia; hypertonia; muscle tonus; thalamus; dopamine; hypoxia-ischemia, brain; ischemia; uterus; infant, newborn
Introduction
Hypoxia-ischemia (H-I) brain injury of the immature brain causes motor, learning and mental disabilities in surviving children1,2. Most cases of newborn H-I encephalopathy occur during the antenatal period. Currently, there is an insufficient understanding of the pathogenic H-I mechanisms in the fetal brain.
There are many similarities between dystonia and hypertonia in children resulting from H-I and those from tetrahydrobiopterin (BH4) deficiency. Defects in the BH4 biosynthetic pathway cause seizures, postural dystonia and developmental delays. Mutation of GTP-cyclohydrolase (GTPCH, Scheme 1) results in hereditary progressive dystonia3 Mutation of sepiapterin reductase (SR)4 causes motor and cognitive impairment. The most prevalent causes of BH4 deficiency are associated with mutation of 6-pyruvoyltetrahydropterin synthase (PTPS)5 and dihydropteridine reductase (DHPR)5,6. Treatment with BH4 has been found to ameliorate some cases of dystonia7.
Brain BH4 regulates tyrosine hydroxylase activity and loss of BH4 is associated with dopamine deficiency which causes early onset dystonia. It is noteworthy however that a genetic model for defective GTPCH, the hph-1 mouse8, exhibits low brain BH4, dopamine and serotonin levels, but does not have distinctive motor phenotype. The lack of behavioral models has delayed a complete understanding of the mechanisms involving BH4 deficiency and brain injury9. With the recent development of an animal model mimicking cerebral palsy10, a unique opportunity to examine the link between BH4 and motor impairments presents itself.
We hypothesized that BH4 is a developmental factor determining vulnerability of the immature fetal rabbit brain to H-I injury and the occurrence of subsequent motor disabilities. We show that normally low BH4 concentrations is a critical factor increasing the vulnerability of the immature brain to H-I injury. Maternal treatment with BH4 increased fetal levels in thalamus and basal ganglia and significantly ameliorated motor deficits and decreased stillbirths. This discovery provides fundamental information about BH4 as a target molecule in H-I fetal brain damage. Our long term goal is to establish the molecular basis of the role of BH4 in immature brain dysfunction.
Materials and Methods
Animals
This study was approved by the Animal Review Committee of the NorthShore University HealthSystems. Animals received humane care and were treated in compliance with the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals.
Surgery
The surgical procedure has been previously described10. Briefly, pregnant dams at 22 days gestation (E22) were anesthetized with intravenous fentanyl (75 μg/kg/hr) and droperidol (3.75 mg/kg/hr) and bag and mask ventilation provided to maintain normal arterial blood gases. Following spinal anesthesia with 0.75% bupivacaine, the fentanyl and droperidol was reduced to enable the dam to breathe spontaneously. The control animals were delivered by hysterotomy.
Uterine ischemia was induced by inflation of 4F Fogarty arterial embolectomy catheter introduced via the left femoral artery into the descending aorta to a level above the uterine and below the renal arteries. Balloon inflation resulting in complete ischemia to the uterus was maintained for 40 min. A subset of fetal brains was obtained after hysterotomy.
For the behavioral studies after uterine ischemia, the catheter was removed, the femoral artery reconstructed, and the dam returned to her cage and allowed to deliver spontaneously.
Neurobehavioral assessment
Newborn kits at postnatal day 1 (E32 or P1) were subjected to previously published battery of neurobehavioral tests 10. Muscle tone was assessed by active flexion and extension of the forelimbs and hindlimbs and scored (0-4)11. The surviving newborn kits were divided into severe (presence of severe hypertonia and/or postural deficits) or mild (minimal or no hypertonia, no postural deficits, but presence of other neurobehavioral deficits) or normal (no obvious deficits noted).
Biochemical Analysis
Tissue was obtained from rabbit fetuses between 22 and 29 days postconceptional age (E22-E29), corresponding to ~70 to 92% of fetal rabbit gestation respectively, and from newborn rabbit brains at P1 (E32).
Total biopterin, BH4, and BH2 analysis
Screening of all stable biopterin metabolites BH4, BH2 and biopterin (total biopterin) was quantified by HPLC with fluorescence detection as previously described12. For the quantification of BH4, 7,8-BH2 and ascorbate, a coulometric analysis using HPLC was performed13. Results were normalized to protein content.
GTP cyclohydrolase activity
Enzyme activity was measured by following the conversion of GTP to dihydroneopterin 3'-triphosphate following oxidation-dephosphorylation to neopterin as previously described13.
Dopamine, serotonin and metabolites
Analysis was performed by HPLC with a multielectrode coulometric analysis14. Concentrations were calculated using authentic standards and normalized to protein content.
Western blot analysis
Tissues were homogenized in PBS with 1% Triton X-100, 1 mM PMSF, 35 ng/mL pepstatin A, and 10 ng/mL leupeptin and protease inhibitor cocktail. Samples were resolved on 12% SDS-polyacrylamide gels and transferred to membranes. Blots were probed with a polyclonal custom GTP cyclohydrolase I antibody as described15.
Gene expression
cDNA was synthesized from 3 μg of total RNA and oligo(dT) primers using the Invitrogen Superscript First-Strand Synthesis System for RT-PCR (Invitrogen).
Statistical analysis
Results were expressed as mean±S.E.M. The analysis included ANOVA with a post hoc Student-Newman-Keuls' test for multiple group comparisons or t-test for differences between two group means. Alpha error was set at p<0.05.
Results
Developmental regulation of BH4 in late gestation and newborn period
Total biopterin concentration was quantified in E22-E32 brain homogenates, to assess the overall changes of BH4 and its metabolites during the course of biosynthesis and utilization of this cofactor in the fetal brain. There was negligible variation with gender, so the results were analyzed using age as the only dependent variable. The E22 brain had the lowest biopterin concentrations and from E22 to E32 total biopterin increased ~2.5 fold (Fig.1). The brain transition from a very low to low biopterin levels from E22 to E29 indicated that the premature brain is relatively biopterin deficient. Biopterin levels in fetal plasma also increased with age (Fig.2). In contrast, the biopterin in maternal plasma did not significantly change over the course of gestation and were higher than fetal levels at all ages. This shows a gradient in biopterin between maternal and fetal plasma. Similar differences were shown between maternal and fetal placenta (Fig.2). We then questioned whether fetal brain biopterin could be derived from maternal sources or be actively produced in the fetal brain.
Figure 1.
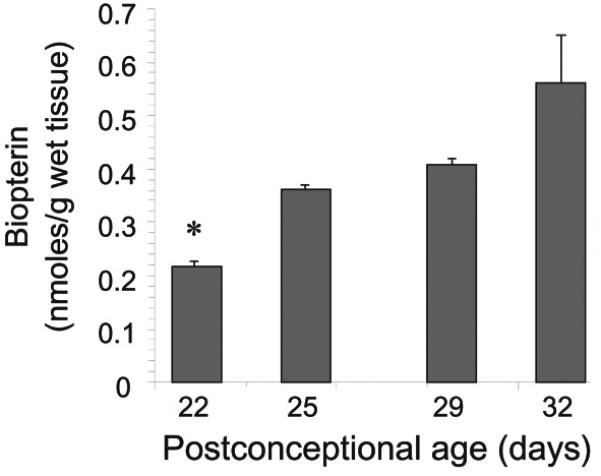
Age-dependent changes in biopterin levels in the fetal rabbit brain. Total biopterin levels were determined in whole brain homogenates and analyzed by HPLC with fluorescent detection. Values represent the mean±SE (n=4). (*) p<0.05 with respect to all other ages groups.
Figure 2.
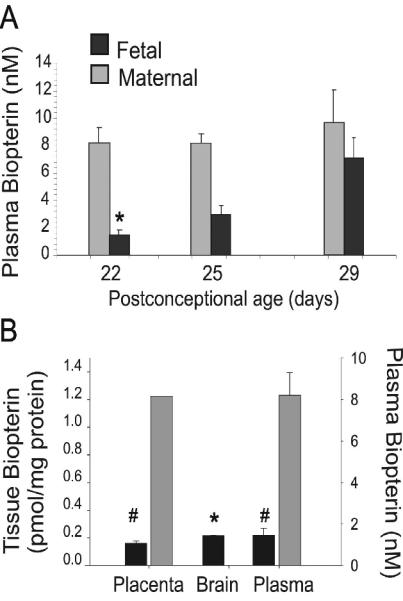
Fetal and maternal biopterin plasma and placenta gradients are maintained during perinatal period. A, Fetal and maternal biopterin levels in plasma collected at gestational 22, 25 and 29 days (E22, E25, E29). B, Placenta and brain tissue isolated from fetuses at E22. Maternal and fetal placentas were analyzed independently. Both plasma and brain E22 biopterin content are included to show the physiological gradient across maternal and fetal compartments. Values are mean±SE (n=3). Fetal plasma biopterin was significantly lower than maternal (#) p<0.01. (*) brain biopterin was significantly lower than maternal placenta and plasma values were also lower than maternal values.
BH4 biosynthesis explains fetal brain BH4 levels
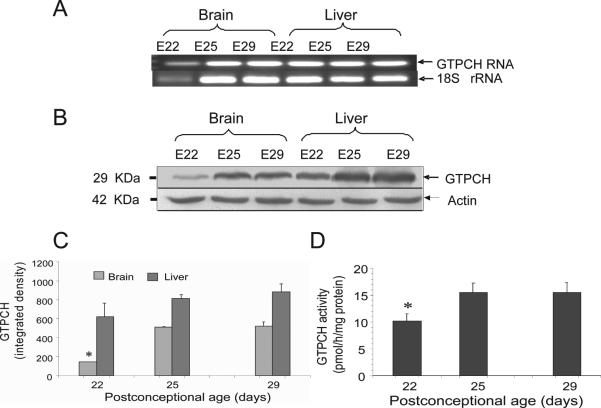
The GTPCH is the first enzyme and considered the rate limiting enzyme in the de novo biosynthesis of BH4. Fetal brain and liver expressed significant levels of both GTPCH mRNA and protein (Fig.3). Both GTPCH protein levels (Fig. 3B-3C) and enzyme activity in E22 brains, however, were significantly lower than those in E29 brains (Fig. 3D). Liver GTPCH expression was higher than the brain at all embryonic ages (Fig. 3). Thus during development, GTPCH and BH4 production in fetal brain is delayed with respect to other fetal tissues. Collectively this data shows that brain tissue is characterized by relative BH4 deficiency during early stages of development compared to newborn.
Figure 3.
GTP cyclohydrolase (GTPCH) expression and activity in brain increases during perinatal period. A, RT-PCR analysis of GTPCH in fetal brain and liver homogenates at gestational 22, 25 and 29-days (E22, E25, E29). B, Western blot analysis of GTPCH protein levels in fetal brain and liver showing increased protein expression with age; β-actin levels were used as loading control. C, GTPCH densitometry indicates that E22 brains contain the lowest level of protein. D, GTPCH enzyme activity in the fetal brain shows a significant difference between E22 and E25 with an increase of ~50% which is maintained at later times. Values represent the mean±SE (n=4). (*) p<0.05 with respect to all other ages.
BH4 is developmentally regulated in specific regions of the brain
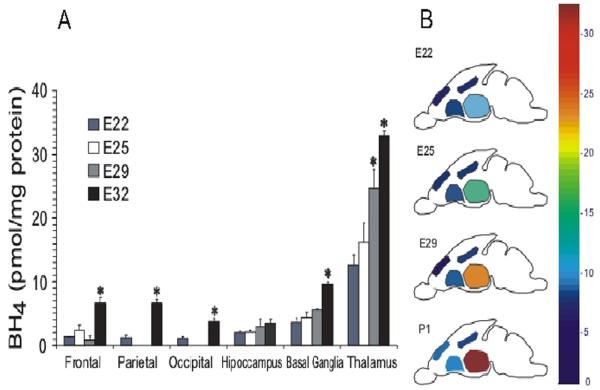
We also examined whether regional BH4 changes in normal fetal brain could indicate its role and developmental regulation. The basal ganglia and thalamus have higher BH4 concentrations than other parts of the brain such as frontal, parietal and occipital regions at all postconceptional ages (Fig.4A). The BH4 in thalamus and basal ganglia also showed a significant perinatal increase of ~54-64% between E22 and E32 (or P1). Notably, BH4 levels were highest in the thalamus at all ages (Fig.4).
Figure 4.
Thalamus is the region of the brain with highest tetrahydrobiopterin levels. Tetrahydrobiopterin (BH4) was directly measured in parts of the brain between E22 and E32 (postnatal day 1). A, Low BH4 was found in all E22 with the thalamus showing significantly higher amounts with respect to all other brain regions. Values are mean±SE (n≥5), (*) different from E22 values; p<0.05. B, Map depicting average concentration of BH4 increases with age.
BH4 as a factor in brain responses to hypoxia-ischemia
To determine whether BH4 is involved in the brain's response to one of the important etiologies of cerebral palsy, the effects of acute global H-I on BH4 in different parts of the fetal brain were examined. It was found that 40 min H-I caused a marked decrease of ~25% BH4 in the thalamus (Table 1). A less prominent yet significant decrease was found in the basal ganglia and frontal regions of the brain. Other parts of the brain showed no significant change. The uniqueness of these changes can be better appreciated when compared to the diffuse yet significant ascorbate depletion in all parts of brain (Table 1). Ascorbate is found in high concentrations in all parts of the brain and its loss in H-I signifies a global response to damage.
Table 1.
BH4 and ascorbate levels in parts of the fetal brain.
| BH4 (pmol/mg protein) |
Ascorbate (nmol/mg protein) |
|||
|---|---|---|---|---|
| Tissue | Control |
Hypoxia (E22, 40 min) |
Control |
Hypoxia (E22, 40 min) |
| Frontal | 1.28±0.07 | 0.54±0.15‡ | 136.4±7.2 | 92.2±4.5‡ |
| Parietal | 1.20±0.62 | 1.21±0.58 | 102.7±30.4 | NA |
| Thalamus | 12.62±1.50 | 9.3±1.3‡ | 131.6±8.5 | 94.4±5.85‡ |
| Basal Ganglia | 3.60±0.60 | 2.7±0.1‡ | 128.3±3.3 | 99.79±4.5‡ |
| Hippocampus | 1.96±0.34 | 1.39±0.37 | 106.3±5.4 | 81.9±3.2‡ |
Values represent mean±SE (n>4 litters).
differences were statistically significant with respect to control sham operated animals P<0.05.
NA- data not available
BH4 levels can be augmented by maternal supplementation
There is evidence that BH4 in liver, kidney and to a lesser extent brain can be increased by supplementation with exogenous BH416-18. The possibility of increasing fetal brain BH4 via maternal supplementation with BH4 was examined. Administration of BH4 (1-10mg/ml) to the pregnant dam via the distal aorta was done to maximally increase fetal levels. These doses were well tolerated and biopterin levels in maternal and fetal plasma changed with dose and time. With a freshly prepared BH4 bolus (10mg/kg) an acute increase ~130-fold (8.59±1.61 vs 1128.1±31.5nM) after 10min was found in maternal plasma, which returned to basal levels 3 hours post infusion. Fetal plasma increased ~40-fold (1.46±0.23 vs 76.56±5.24nM) but fetal brain increases were less consistent. With lower doses of BH4 changes were less pronounced and more transient.
Regional changes in BH4 levels upon maternal supplementation with sepiapterin
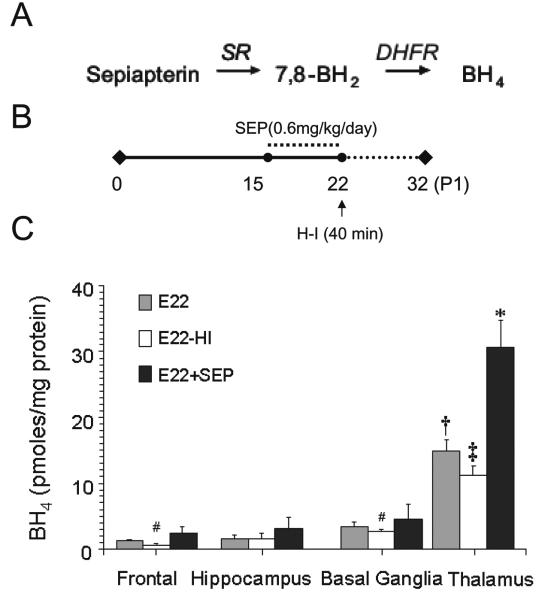
Since BH4 is not stable and stabilizing agents such as ascorbate may mask its effects, the BH4 precursor, sepiapterin, was preferred over tetrahydrobiopterin as a therapeutic agent (Scheme 1). When administered peripherally, sepiapterin is converted to BH4 by a two-step reduction catalyzed by sepiapterin reductase and dihydrofolate reductase in target tissues. Sepiapterin was delivered to E15 pregnant dams via subcutaneous osmotic mini pump that enabled a steady dosage of 0.6-mg/kg/day. After 7 days, a consistent increase of ~20-fold was observed in maternal plasma. There was also a significant increase in fetal and maternal placental BH4 (Table 2). Fetal brain levels also increased with the greatest change occurring again in the thalamus showing a >2-fold increase over basal levels (Fig.5). The sepiapterin metabolite, 7,8-BH2, was not detected in any supplemented animal indicating full conversion of sepiapterin to BH4.
Table 2. BH4 distribution in sepiapterin supplemented maternal and fetal tissues.
Sepiapterin was administered using an Alzet osmotic pump with 0.6 mg/Kg during seven days beginning at postconceptional day 17 (E17) and ending at postconceptional day 22 (E22). Tissue was harvested at the end of day E22 for BH4 analysis. Control received vehicle only for the same period of time.
| Control – Vehicle BH4 (pmoles/mg protein) |
Sepiapterin BH4 (pmoles/mg protein) |
|||
|---|---|---|---|---|
| Maternal | Fetal | Maternal | Fetal | |
| Placenta | 0.2±0.1 | 0.1±0.1 | 4.6±0.5‡ | 5.2±0.1‡ |
| Kidney | 4.1±0.8 | 1.7±0.9 | 5.6±0.4‡ | 4.6±1.4‡ |
| Liver | 8.6±1.0 | 1.9±0.3 | 10.2±1.1 | 1.2±0.3 |
Values are Mean±S.E.M (n=4).
p<0.05.
Figure 5.
Changes in BH4 concentrations in parts of the E22 fetal brain following H-I and sepiapterin supplementation. A, Stepwise conversion of sepiapterin to BH4 in reactions catalyzed by sepiapterin reductase (SR) and dihydrofolate reductase (DHFR). B, Sepiapterin treatment scheme. Treatment was initiated at E15 and finished at E22 covering from 48 to 70% term gestational period. Control dams received vehicle only. The E22 tissue from sham operated or H-I group was collected for BH4 measurements. C, Analysis of BH4 in different parts by HPLC-ECD. [E22-group]: Tissue was collected from fetuses retrieved from vehicle treated and sham operated dams at E22. [E22-H-I group]: Tissue was collected immediately after retrieving fetuses by hysterectomy from a dam that was subjected to sustained hypoxia for 40 min at E22. [E22+Sep group]: Tissue was collected from fetuses retrieved from dams receiving sepiapterin (0.6 mg/kg/day) during 5 days prior to sham-operation at E22. Values are mean±SE (n≥4). (†) p<0.05 with respect to other parts of the brain; (‡) p<0.05 with respect to E22 thalamus; (#) with respect to E22 frontal; (*) p<0.01 with respect to all experimental groups.
BH4 protects immature brain from H-I injury and decreases dystonic hypertonia
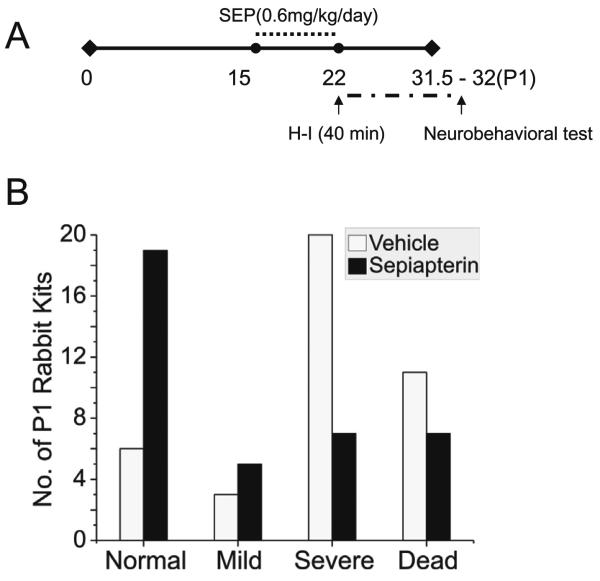
The demonstration that H-I causes a selective loss of BH4 in different regions of the fetal brain suggested that supplementation would render the fetal brain less vulnerable to H-I. Specifically, we asked whether BH4 supplementation ameliorates motor impairments caused by H-I in the fetal rabbit brain. This possibility was assessed in newborn kits supplemented with sepiapterin or vehicle before H-I at E22, when the lowest BH4 levels were observed. After H-I, the dams recovered and delivered spontaneously at term gestation. The newborn kits were examined for neurobehavioral deficits as described10. The sepiapterin treated group, showed a significant reduction in the severity of motor deficits (locomotion and righting reflex), hypertonia and dystonia score (Fig.6). Sepiapterin administration also increased the number of normal appearing kits (Fig.7 and video in supplemental material). To find out how BH4 supplementation affects motor improvement, an exploratory study was done to investigate the levels of levedopa (L-DOPA), 5-hydroxytryptamine (5-HT), homovallinic acid, and 5-hydroxyindolacetic acid in basal ganglia and thalamus.
Figure 6.
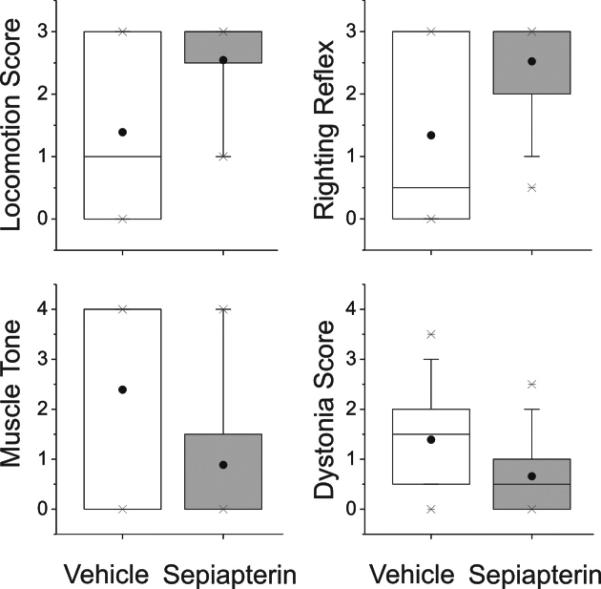
Neurobehavioral summary of scores (box-whisker plots) obtained by video analysis of vehicle and sepiapterin pre-treated newborn kits (n=37 and 44 respectively). All variables are highly significant between groups (ttest, P<0.001). Scores are quantitative estimations for all except dystonia where scoring is based on 9 point scale for %time spent on dystonic activity (0-100% of time).
Figure 7.
Hypoxia-ischemia and sepiapterin effects in neurobehavioral scoring for kits at E32 (P1). A. Dam sepiapterin treatment was initiated at E15 and finished at E22. Control dams were treated with vehicle only. At E22 dams were submitted to sustained H-I (40 min). Animals were allowed to recover as denoted by the period in broken line and to spontaneously deliver ~31.5 days term gestation. B. Number of newborn rabbit kits stillbirth and survivors having distinctive phenotypical motor performance (normal, mild, severe) after acute HI at E22. Motor function was assessed in the newborns (P1) in a blind manner and the scoring was according to Ashworth scale. Sepiapterin improved neurobehavioral outcome against any adverse outcome including death (p<0.005, Fisher exact test) and against severe motor deficits in survivors (p<0.001). Results represent analysis from 5 different litters.
Sepiapterin supplementation improves the level of biogenic amines
Dopamine changes were more marked than serotonin with H-I. Increasing BH4 with sepiapterin treatment has a beneficial effect in protecting basal glanglia and thalamus from losses in L-DOPA and 5-HT (Table 3).
Table 3.
Changes in dopamine, serotonin and metabolites in the brain after H-I injury with and without sepiapterin supplementation.
| Dopamine (ng/mg protein) | |||
| Control-Vehicle | Hypoxia | Sepiapterin | |
| Thalamus | 0.13±0.08 | 0.06±0.05# | 0.17±0.03¶ |
| Basal Ganglia | 3.18±0.95 | 0.37±0.08# | 1.19±0.42#,¶ |
| Serotonin (ng/mg protein) | |||
| Control-Vehicle | Hypoxia | Sepiapterin | |
| Thalamus | 3.20±0.90 | 2.20±0.37# | 3.10±0.26¶ |
| Basal Ganglia | 3.38±0.56 | 3.71±0.73 | 3.52±0.89 |
| HVA/5HIAAa | |||
| Control-Vehicle | Hypoxia | Sepiapterin | |
| Thalamus | 1.19±0.18 | 0.86±0.07# | 0.98±0.07¶ |
| Basal Ganglia | 1.15±0.13 | 0.52±0.14# | 0.82±0.12¶ |
Values are Mean±S.E.M. (n=3-5).
p<0.05 from controls
p<0.05 from hypoxia group.
HVA/5HIAA reference values for 0.5 years old 1.3-3.128.
Discussion
This is the first study to propose BH4 as an important pathophysiological factor in hypoxic-ischemic encephalopathy. The major findings are that typical low BH4 levels in the immature brain could determine its vulnerability and further decrease in BH4 levels with H-I could contribute to the severity of neurological outcomes from H-I.
Endogenous BH4 in the fetal brain
There is limited information about BH4 availability in the human developing brain. Early studies reported measurable changes in brain BH4 in human fetuses from 2-20 weeks of gestation with increase to ~0.16-nmol/g-wet-weight at ~20-weeks19. This amount is in the same low range observed in the E22 fetal rabbit brain (70% gestation), and lower than reported in the hph-1 mouse (0.312±0.017 nmol/g-wet-weight)20 a model for BH4 deficiency. This indicates that across different species, the brain exhibits an early developmental BH4 deficiency. The importance of maintaining this stability even at these low levels of BH4 to normal development is indicated by clinical findings in patients with different forms of BH4 disorders. Carriers of autosomal dominant form of GTPCH deficiency present with low biopterin and neopterin levels. They present with several motor signs such as poor sucking, decreased spontaneous movements, postural changes and dystonia. Those affected with sepiapterin reductase mutations also show abnormal biopterin pattern with high levels of biopterin and dihydrobiopterin, reflecting their inability to drive BH4 synthesis to term (Scheme I). These patients generally present with growth retardation, and motor and developmental delays. Additional evidence is provided by the high frequency of early (~48h) postnatal death in the homozygous mouse model for PTPS5 deficiency that is severely BH4 depleted. Premature death in the newborn period is also reported for children carrying defects in PTPS and DHPR activity21. These functional relationships support the notion that BH4 is a developmental factor involved in gain of function.
Brain BH4 and H-I injury
Maternal levels of BH4 do not appear to influence that of the fetal brain. Therefore the genetic make-up of the fetus plays a major role in determining brain tissue BH4 levels. In addition, BH4 production in the fetal brain is developmentally regulated transitioning from a BH4 deficient to a BH4 sufficient state. There are more than 90 known mutations in the GCH1 gene causing a permanent BH4 deficient state in dopa-responsive dystonia. Many other mutations are present in asymptomatic carriers, whose BH4 do not decrease to critical levels that cause alteration in brain functions. If these carriers are challenged the subclinical deficiency may be critical in determining their responses.
We showed that at BH4 deficiency such as that found in the E22 brains is critically associated with poor outcome after acute H-I injury. Since maternal levels do not influence fetal brain BH4 levels, the possibility that a population with subclinical BH4 deficiency will be more affected. This will be addressed in future studies, especially if it can explain the variability in response to a fixed H-I insult. Strategies to deplete or augment the fetal BH4 synthesis would elucidate the mechanism further but the technological challenges are daunting at present.
How BH4 deficiency alters fetal and neonatal brain development remains unclear. A disturbance in dopaminergic systems is one possible explanation. H-I further aggravates the availability of BH4 in the brain and also affects normal neurotransmitter production. Most patients with BH4 deficiency show high responsiveness to dopamine supplementation. This may be related to selective loss of TH in the brain22. Since BH4 turnover in the striatum is high even the small changes shown after H-I may be detrimental to the maintenance of normal TH activity.
We show that thalamus exhibits the most prominent changes of BH4 in normal development and H-I. It is possible that local production of neurotransmitters explain this pattern. A larger dopamine innervation in the primate thalamus than in rodents23,24 may indicate this role. Dopaminergic axons have been shown to target the human thalamus in abundance while in rodents this innervation is scanty. Thalamic dopamine has several sources suggesting a role in controlling information trafficking. Injury to more than one area of the brain may be responsible for the motor deficits observed in the rabbit complicating our understanding of the biochemical role of regional variations in BH4, TH and dopamine. We continue to investigate the responsible brain regions for motor deficits and the role of focal BH4 changes as a possible mechanism.
Brain BH4 and sepiapterin supplementation
We show that maternal-fetal BH4 supplementation by a 7 day treatment with sepiapterin resulted in a small but persistent increase in fetal brain BH4. This is a better strategy than using BH4 itself since BH4 produced small transient changes. This may be attributed to low BH4 uptake due to maternal-placental barrier effects as previously described25. Sepiapterin increased fetal and maternal placenta and kidney BH4 levels indicating that the drug is widely distributed in maternal and fetal tissues. Although we cannot establish whether sepiapterin itself reaches the brain, it is likely that sepiapterin is reduced to BH4 in peripheral tissues and BH4 then reaches the brain. Most of the enzymes involved in the salvage pathway are available in peripheral fetal tissue26. The uptake of BH4 by fetal brain has been previously shown in pregnant mice supplemented with bolus BH416. Moreover, we showed no evidence for accumulation of 7,8-BH2 further indicating the full conversion of sepiapterin to BH4 probably occurred before reaching the brain.
Neurobehavioral outcomes
Sustained H-I during perinatal period (E22) causes motor impairments in the newborn kits mostly presenting as dystonic hypertonia. While the pathogenic mechanism of impaired motor function is likely multifactorial, some of these effects are modulated by BH4. The implications of this finding may prove significant for the design of therapies. Either direct supplementation or strategies aimed at increasing endogenous BH4 production should be useful. Most importantly, the efficacy of these therapies can now be evaluated. Further studies powered enough to tease out effects of BH4 on individual behavioral tests will need to be done.
Conclusions
The hph-1 mouse has been the only animal model to study the correlation between GTPCH deficiency and neurotransmitter dysfunction. The hph-1 brain shows 90% reduction in GTPCH activity, 50% decrease in BH4, reduced dopamine and serotonin levels. However, this series of biochemical changes does not result in motor dysfunction, thus complicating the translational significance of this model and the advance of our understanding on the role of BH4 in motor disabilities. In contrast, the fetal rabbit presents a well defined and reproducible motor dysfunction that can be valuable in establishing BH4 mechanisms. Rabbit are perinatal brain developers, are as humans, whereas rodents are postnatal and other mammals including nonhuman primates are prenatal. The similarity of motor development makes the rabbit a good model for studying developmental regulators of brain functions10,27. Thus, we have defined a model that will not only allow us to more closely examine the link between BH4, and motor impairments but also explore a potential new therapy in the prevention of fetal brain injury.
Supplementary Material
Sepiapterin prevents hypertonia in hypoxia-ischemia fetal rabbit model. Neurobehavioral pattern of newborns born to dams non-supplemented and sepiapterin (0.6 mg/kg/day) supplemented for 5 days prior to hypoxia-ischemia. Sample is representative of results observed in 3 different litters.
Scheme 1.
Biochemical pathway of BH4 synthesis. GTP, guanosine triphosphate; GTPCH, GTP cyclohydrolase I; PTPS, 6-pyruvoyltetrahydropterin synthase; SR, sepiapterin reductase; CR, carbonyl reductase; AR, aldose reductase; DHFR, dihydrofolate reductase. Tetrahydrobiopterin-dependent enzymatic reactions: NOS, nitric oxide synthase, producing either nitric oxide (NO) or superoxide radical anion (O2•−) depending on BH4 levels; TH: tyrosine hydroxylase, TrH, tryptophan hydroxylase, 5-HT, 5-hydroxytryptophan.
Acknowledgements
This work was funded by the National Health Institutes, National Institutes of Neurological Disorders and Stroke award NS054017 to JVV, NS43285 and NS051402 to ST.
Footnotes
Statement of conflict of interest: None
References
- 1.Barret RD, Bennet L, Davidson J, Dean JM, et al. Destruction and reconstruction: hypoxia and the developing brain. Birth Defects (Part C) 2007;81:163–176. doi: 10.1002/bdrc.20095. [DOI] [PubMed] [Google Scholar]
- 2.Robertson C, Finner N. Term infants with hypoxic-ischemic encephalopathy: outcome at 3,5 years. Dev. Med. Child Neurol. 1985;27:473–484. doi: 10.1111/j.1469-8749.1985.tb04571.x. [DOI] [PubMed] [Google Scholar]
- 3.Ichinose H, Ohye T, Takahashi E, Seki N, et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat. Genetics. 1994;8:236–242. doi: 10.1038/ng1194-236. [DOI] [PubMed] [Google Scholar]
- 4.Blau N, Thöny B, Cotton RGH, Hyland K. Disorders of tetrahydrobiopterin and related biogenic amines. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogel-Stein B, editors. The metabolic and molecular basis of inherited diseases. 8th ed McGraw-Hill; New York: 2001. [Google Scholar]
- 5.Blau N, Dhondt JL. BIODEF international database of tetrahydrobiopterin deficiencies. doi: 10.1007/BF01799342. Available at: http://www.bh4.org/BH4DatabasesBiodef.asp. Accessed March 15, 2009. [DOI] [PubMed]
- 6.Kalkanoglu HS, Romstad A, Coşkun T, Güttler F. Evaluation of a fetus at risk for dihydropteridine reductase deficiency by direct mutation analysis using denaturing gradient gel electrophoresis. Prenat Diagn. 2001;21:868–870. doi: 10.1002/pd.161. [DOI] [PubMed] [Google Scholar]
- 7.Fink JK, Ravin P, Argoff CE, Levine RA, et al. Tetrahydrobiopterin administration in biopterin-deficient progressive dystonia with diurnal variation. Neurology. 1989;39:1393–1395. doi: 10.1212/wnl.39.10.1393. [DOI] [PubMed] [Google Scholar]
- 8.Bode VC, McDonald JD, Guenet JL, Simon D. Hph-1: a mouse mutant with hereditary hyperphenylalaninemia induced by ethylnitrosourea mutagenesis. Genetics. 1988;118:299–305. doi: 10.1093/genetics/118.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jinnah HA, Hess EJ, Ledoux MS, Sharma N. Rodent models for dystonia research: characteristics, evaluation, and utility. Movement Disorders. 2005;20:283–292. doi: 10.1002/mds.20364. [DOI] [PubMed] [Google Scholar]
- 10.Derrick M, Luo NL, Bregman JC, Jilling T, et al. Preterm fetal hypoxia-ischemia hypertonia and motor deficits in the neonatal rabbit: A model for human cerebral palsy? J. Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damiano DL, Quinlivan JM, Owen BF, Payne P, et al. What does the Ashworth scale really measure and are instrumented measures more valid and precise? Dev. Med. Child. Neurol. 2002;44:112–118. doi: 10.1017/s0012162201001761. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal. Biochem. 1980;102:176–188. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- 13.Whitsett J, Picklo MJ, Sr, Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler. Thromb. Vasc. Biol. 2007;27:2340–2347. doi: 10.1161/ATVBAHA.107.153742. [DOI] [PubMed] [Google Scholar]
- 14.Acworth IN, Cunningham ML. The measurement of monoamine neurotransmitters in microdialysis persusates using PHLC-ECD. In: Harry J, Tilton HA, editors. Methods in Molecular Medicine. Vol. 22. Humana Press; New Jersey: 1998. [DOI] [PubMed] [Google Scholar]
- 15.Pieper GM, Nilakantan V, Halligan NL, Khanna AK, et al. Nitric oxide formation in acutely rejecting cardiac allografts correlates with GTP cyclohydrolase I activity. Biochem. J. 2005;391:541–547. doi: 10.1042/BJ20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshiga M, Hatakeyama K, Watanabe M, Shimada M, Kagamiyama H. Autoradiographic distribution of [14C]tetrahydrobiopterin and its developmental change in mice. J. Pharmacol. Exp. Ther. 1993;267:971–978. [PubMed] [Google Scholar]
- 17.Canevari L, Land JM, Clarck JB, Heales SJR. Stimulation of the brain NO/cyclic GMP pathway by peripheral administration of tetrahydrobiopterin in the hph-1 mouse. J. Neurochem. 1999;73:2563–2568. doi: 10.1046/j.1471-4159.1999.0732563.x. [DOI] [PubMed] [Google Scholar]
- 18.Hasewaga H, Sawabe K, Nakanishi N, Wakasugi OK. Delivery of exogenous tetrahydrobiopterin (BH4) to cells of target organs: Role of salvage pathway and uptake of its precursor in effective elevation of tissue BH4. Mol. Gen. Metab. 2005;86:S2–S10. doi: 10.1016/j.ymgme.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Leeming RJ, Blair JA. The effects of pathological and normal physiological processes on biopterin derivative levels in man. Clin. Chim. Acta. 1980;108:103–111. doi: 10.1016/0009-8981(80)90298-3. [DOI] [PubMed] [Google Scholar]
- 20.Lam AA, Hyland K, Heales SJ. Tetrahydrobiopterin availability, nitric oxide metabolism and glutathione status in the hph-1 mouse; implications for the pathogenesis and treatment of tetrahydrobiopterin deficiency states. J Inherit Metab Dis. 2007;30:256–262. doi: 10.1007/s10545-006-0502-x. [DOI] [PubMed] [Google Scholar]
- 21.Ponzone A, Spada M, Ferreris S, et al. Dihydropteridine reductase deficiency in man: from biology to treatment. Med. Res. Rev. 2004;24:127–150. doi: 10.1002/med.10055. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Sumi-Ichinose C, Kai R, Ikemoto K, et al. Differential involvement of striosome and matrix dopamine system in a transgenic model of dopa-responsive dystonia. Proc. Natl. Acad. Sci USA. 2008;105:12551–12556. doi: 10.1073/pnas.0806065105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neurosci. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-González MA, García-Cabezas MA, Rico B, et al. The primate thalamus is a key target for brain dopamine. J. Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponzone A, Ferraris S, Biasetti S, et al. Placental barrier in mother-to-fetus transfer of tetrahydrobiopterin in humans. In: Curtius HC, Blau N, Leviev RA, editors. Unconjugated pterins and related biogenic amines. Walter de Gruyter; Berlin: 1987. [Google Scholar]
- 26.Ferrè J, Naylor EW. Sepipaterin reductase in human amniotic and skin fibroblasts, chorionic villi abd various blood fractions. Clin. Chim. Acta. 1988;174:271–282. doi: 10.1016/0009-8981(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 27.Derrick M, Drobyshevsky A, Ji X, Tan S. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007:731–735. doi: 10.1161/01.STR.0000251445.94697.64. [DOI] [PubMed] [Google Scholar]
- 28.Verbeek MM, Steenbergen-Spanjers GC, Willemsen MA, Hol FA, Smeitink J, Seeger J, Grattan-Smith P, Ryan MM, Hoffmann GF, Donati MA, Blau N, Wevers RA. Mutations in the cyclic adenosine monophosphate response element of the tyrosine hydroxylase gene. Ann Neurol. 2007;62:422–426. doi: 10.1002/ana.21199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sepiapterin prevents hypertonia in hypoxia-ischemia fetal rabbit model. Neurobehavioral pattern of newborns born to dams non-supplemented and sepiapterin (0.6 mg/kg/day) supplemented for 5 days prior to hypoxia-ischemia. Sample is representative of results observed in 3 different litters.