Conspectus
Significant levels of the 1,N2-γ-hydroxypropano-dG adducts of the α,β-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxy-2E-nonenal (HNE) have been identified in human DNA, arising from both exogenous and endogenous exposure. They yield interstrand DNA cross-links between guanines in the neighboring C•G and G•C base pairs located in 5′-CpG-3′ sequences, as a result of opening of the 1,N2-γ-hydroxypropano-dG adducts to form reactive aldehydes that are positioned within the minor groove of duplex DNA. Using a combination of chemical, spectroscopic, and computational methods, we have elucidated the chemistry of cross-link formation in duplex DNA. NMR spectroscopy revealed that, at equilibrium, the acrolein and crotonaldehyde cross-links consist primarily of interstrand carbinolamine linkages between the exocyclic amines of the two guanines located in the neighboring C•G and G•C base pairs located in 5′-CpG-3′ sequences, that maintain the Watson–Crick hydrogen bonding of the cross-linked base pairs. The ability of crotonaldehyde and HNE to form interstrand cross-links depends upon their common relative stereochemistry at the C6 position of the 1,N2-γ-hydroxypropano-dG adduct. The stereochemistry at this center modulates the orientation of the reactive aldehyde within the minor groove of the double-stranded DNA, either facilitating or hindering the cross-linking reactions; it also affects the stabilities of the resulting diastereoisomeric cross-links. The presence of these cross-links in vivo is anticipated to interfere with DNA replication and transcription, thereby contributing to the etiology of human disease. Reduced derivatives of these cross-links are useful tools for studying their biological processing.
Introduction
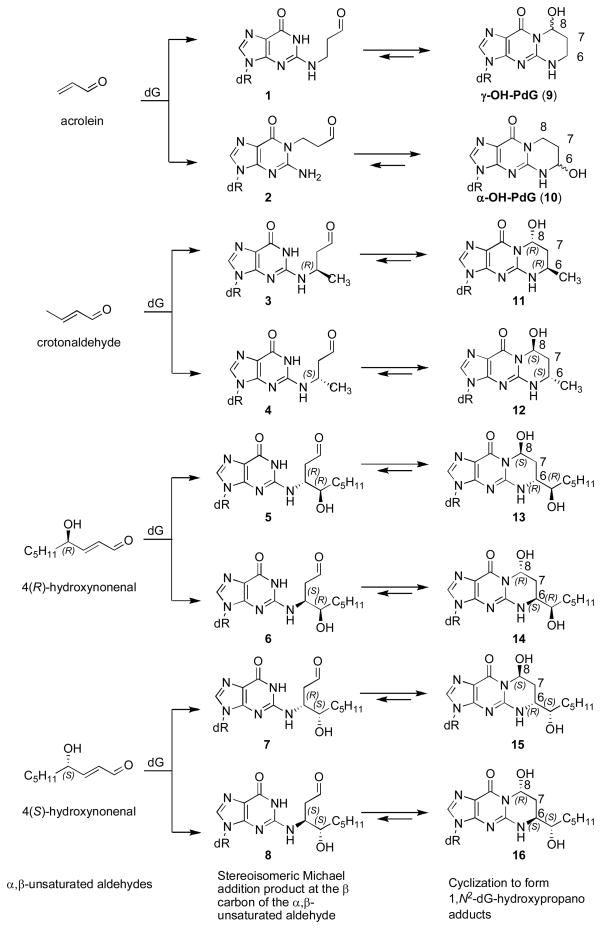
The α,β-unsaturated aldehydes (enals) acrolein, crotonaldehyde, and 4-hydroxynonenal (4-HNE) (Scheme 1) are endogenous byproducts of lipid peroxidation, arising as a consequence of oxidative stress.1–4 Acrolein and crotonaldehyde exposures also occur from exogenous sources, e.g., cigarette smoke5 and automobile exhaust.6 Enals react with DNA nucleobases to give exocyclic adducts; they also react with proteins.7 Addition of enals to dG involves Michael addition of the N2-amine to give N2-(3-oxopropyl)-dG adducts (1, 3–8), followed by cyclization of N1 with the aldehyde, yielding the corresponding 1,N2-dG products (9, 11–16). Early work is traced to Shapiro and Leonard, who independently examined the reactions of nucleosides with glyoxal, malondialdehyde, chloroacetaldehyde, and related bis-electrophiles.8,9 Galliana and Pantarotto characterized the 8-hydroxypyrimido[1,2-α]purin-10(3H)-one (γ-OH-PdG, 9) adduct from the reaction of acrolein with dG.10 Chung and Hecht concurrently reported the crotonaldehyde adduct of dG (11–12),11 and explored the reactivity of enals and enones with dG.12,13 The lipid peroxidation product 4-HNE afforded related dG-adducts (13–16).14 Identification of acrolein adducts of other nucleosides followed.15,16
Scheme 1.
1,N2-dG cyclic adducts arising from Michael addition of enals to dG.
The principal acrolein adduct is γ-OH-PdG (9),10,12 although the regioisomeric 6-hydroxypyrimido[1,2-a]purin-10(3H)-one (α-OH-PdG, 10) has also been observed.12,17 The γ-OH-PdG adduct (9) exists as a mixture of C8-OH epimers. With crotonaldehyde, addition at N2-dG creates a stereocenter at C6. Of four possible products, the two with the trans relative configurations at C6 and C8 (11,12) are observed.12,18 These are also formed through the reaction of dG with two equivalents of acetaldehyde.5,19,20 The corresponding 4-HNE-derived 1,N2-dG adducts possess an additional stereocenter on the C6-sidechain, resulting in four observable diastereomers (13–16).
The 1,N2-dG exocyclic adducts from acrolein (9,10), crotonaldehyde (11,12), and 4-HNE (13–16) exist in human and rodent DNA.2–4,17,21 The binding pattern of acrolein-DNA adducts is similar to the p53 mutational pattern in human lung cancer, implicating acrolein as a major cigarette-related lung cancer agent.22 Acrolein is mutagenic in bacterial and mammalian cells,23,24 including human,25,26 and is carcinogenic in rats.27 Crotonaldehyde is genotoxic and mutagenic in human lymphoblasts28 and induces liver tumors in rodents.29 4-HNE induces a DNA damage response in Salmonella typhimurium,30,31 but is inactive in bacterial mutagenesis assays.23 However, it causes mutations in V79 CHO cells, and DNA from liver specimens from individuals suffering from Wilson’s disease and hemochromatosis contain mutations attributed to 4-HNE-dG adducts.32 Site-specific mutagenesis reveals that these 1,N2-dG adducts induce predominantly G→T transversions in COS-7 cells.33–35
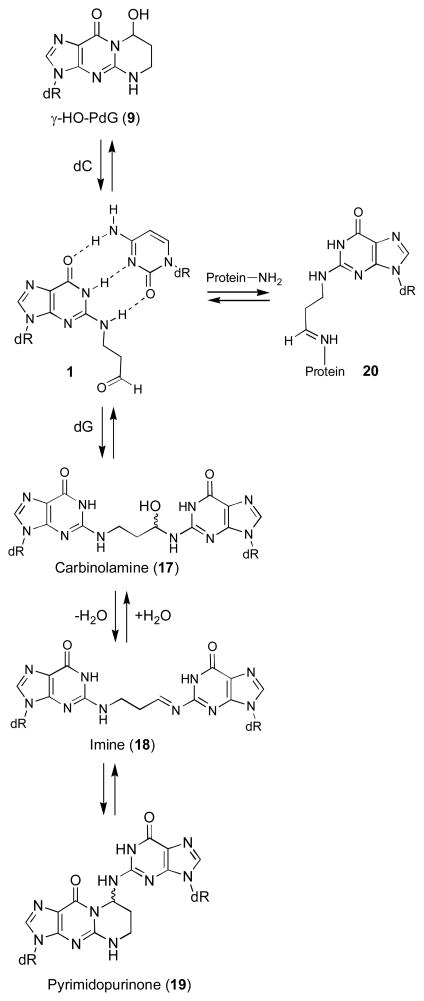
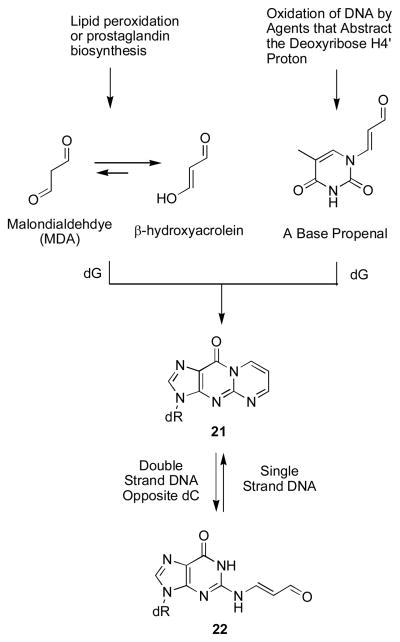
The hypothesis explored in this Account posits that in duplex DNA, 1,N2-dG enal adducts (9, 11–16) open, unmasking a reactive aldehyde (1, 3–8) in the minor groove, as shown for γ-OH-PdG (9) in Scheme 2. This hypothesis was, in part, developed from the observation that the malondialdehyde-derived adduct 21 opens to a related aldehyde 22 when placed opposite dC in DNA (Scheme 3).36–38 Enal adducts are lower oxidation state homologues of 21 and the notion that acrolein, crotonaldehyde, and 4-HNE undergo similar chemistry was confirmed by the observation that γ-OH-PdG (9) ring-opens to the N2-(3-oxopropyl)-dG aldehyde (1) when placed opposite dC.39
Scheme 2.
Enal-dG adducts mediate DNA interstrand cross-link formation.
Scheme 3.
Formation and Chemistry of the Malondialdehyde-derived M1dG Adduct.
We further hypothesized that these aldehydes react with other nucleobases in the complementary DNA strand, forming interstrand cross-links, which exist as equilibrium mixtures of carbinolamine (17), imine (18), or pyrimidopurinone (19) species. The aldehyde in structure 1 also yields peptide- and protein-DNA conjugates (20);40,41 however, analysis of this literature is beyond the scope of this Account. Interstrand DNA cross-linking was proposed based upon analysis of acrolein-treated DNA.26 Few site-specific interstrand cross-links are chemically characterized.42,43 Hecht characterized a pyrimidopurinone bis-nucleoside cross-link analogous to 19 from acetaldehyde-treated calf thymus DNA;19 the cross-link was formally derived from crotonaldehyde.
In this Account, we discuss the chemistry of interstrand cross-links that are likely to be generated in DNA as secondary dG adducts of acrolein, crotonaldehyde, and 4-HNE. These and their reduced derivatives provide tools to study the processing of interstrand cross-links and to define their roles in the etiology of human disease.
Synthesis of Oligodeoxynucleotides Containing 1,N2-dG Enal Adducts
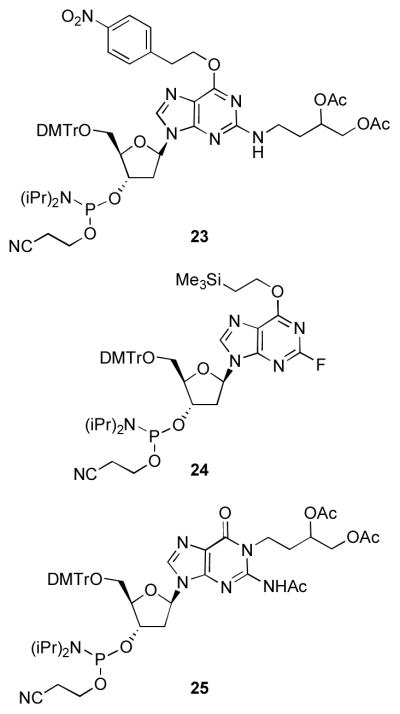
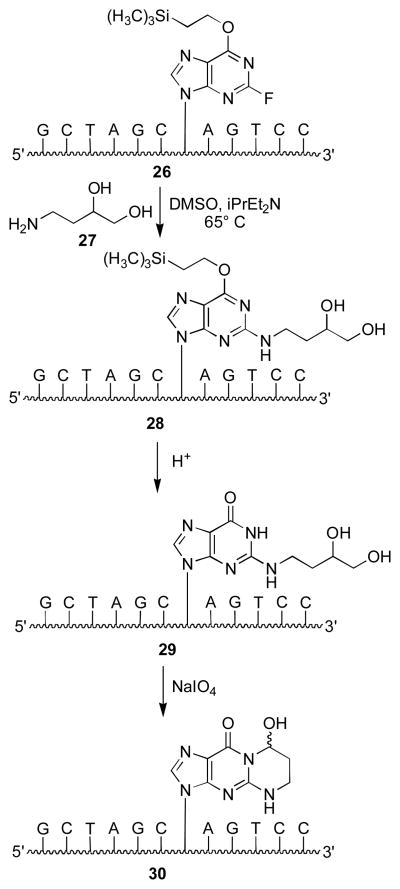
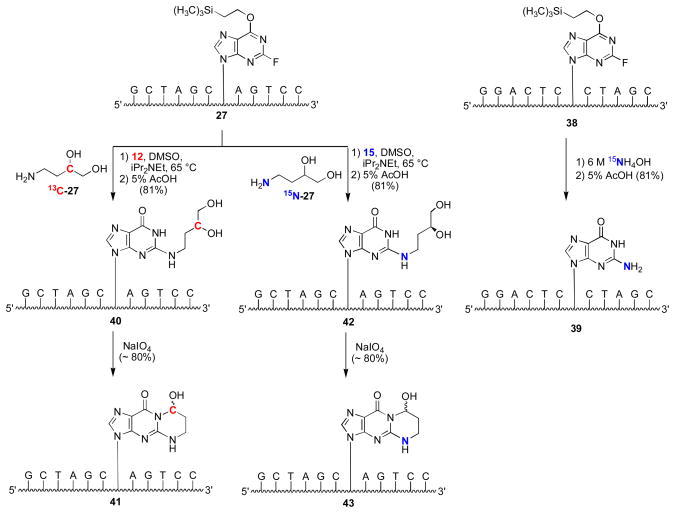
Aldehyde groups have been introduced into DNA through periodate cleavage of vicinal diols.44 Khullar et al. synthesized γ-OH-PdG (9) by condensation of 4-amino-1,2-butanediol with 3′,5′-O-bis-tert-butyldimethylsilyloxy-O6-p-nitro-phenylethyl-protected 2-fluoroinosine deoxynucleoside.45 The N2-(3,4-dihydroxy-butyl) moiety was oxidized to yield N2-(3-oxopropyl)-dG (1), which cyclized to γ-OH-PdG (9).45 Preparation of phosphoramidite 23 (Figure 1) allowed for incorporation ofγ-OH-PdG (9) into oligodeoxynucleotides; oxidative cleavage of the diol to 9 was achieved after oligodeoxynucleotide assembly and deprotection.45 Our approach introduced the N2-(3,4-dihydroxybutyl) group after oligodeoxynucleotide synthesis (Scheme 4).46 We prepared oligodeoxynucleotide 26 containing O6-[(2-trimethylsilyl)ethyl]-2-fluorohypoxanthine from phosphoramidite 24; nucleophilic aromatic substitution with amino diol 27 provided 28. Removal of the O6 protecting group under acidic conditions yielded 29, which was oxidized to oligodeoxynucleotide 30.47 Our post-synthetic modification strategy48 allowed preparation of various dG enal adducts from a single modified phosphoramidite. A challenge was the preparation of stereochemically-defined amino diols for the crotonaldehyde (31,32) and 4-HNE adducts (33–36) (Figure 2).49–52 This strategy could not be applied to oligodeoxynucleotides containing α-OH-PdG (10), which were prepared using the modified phosphoramidite 25.53,54
Figure 1.
Phosphoramidite reagents for the site-specific synthesis of oligodeoxynucleotides containing 1,N2-dG enal adducts.
Scheme 4.
Synthesis of 1,N2-γ-OH-PdG in oligodeoxynucleotides by the post-synthetic modification strategy.
Figure 2.
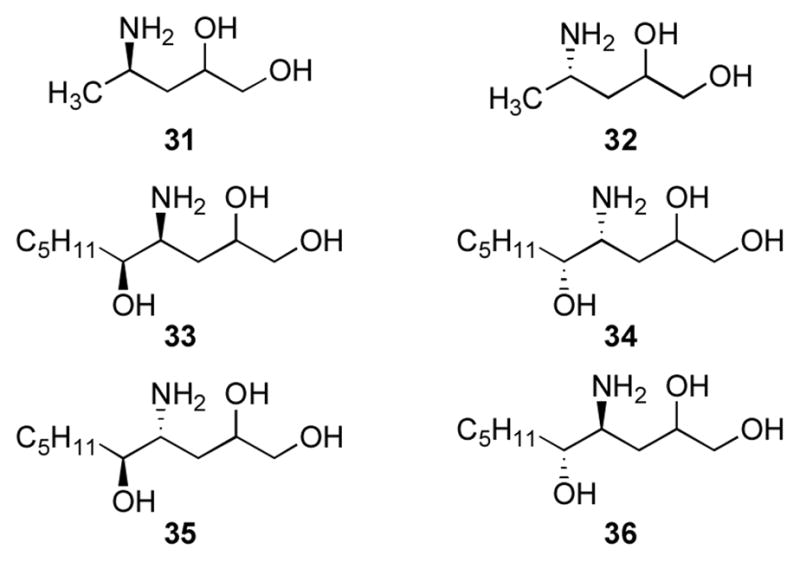
Amino alcohols for synthesis of crotonaldehyde and 4-HNE-modified oligodeoxynucleotides.
Interstrand Cross-linking by γ-OH-PdG
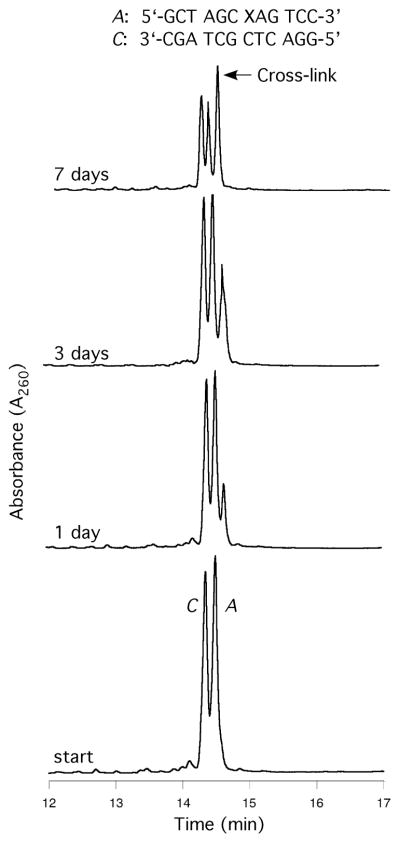
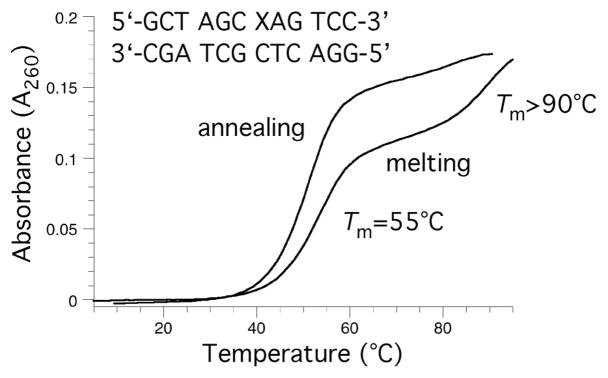
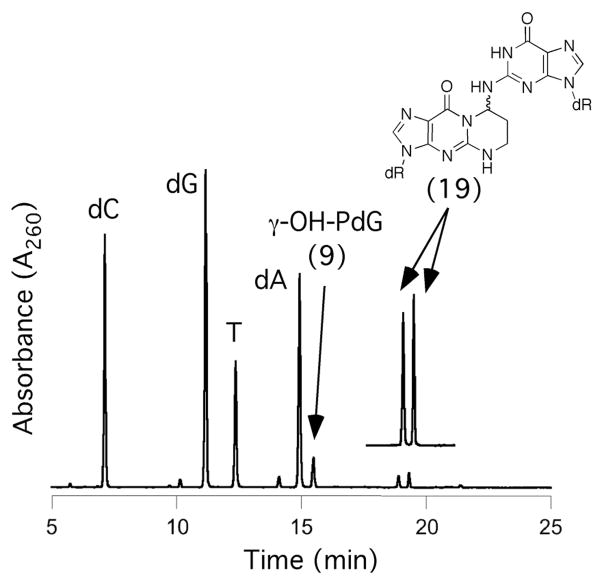
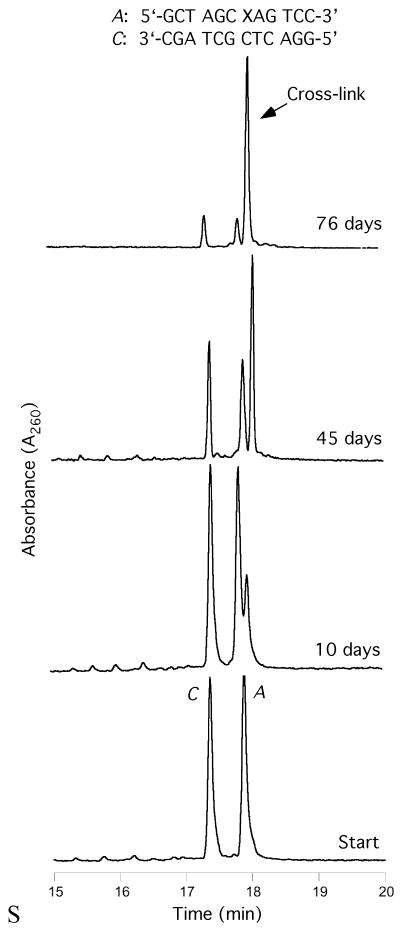
Oligodeoxynucleotide 30, containingγ-OH-PdG adduct 9, was annealed to its complement and the formation of an interstrand cross-link was monitored by capillary gel electrophoresis (CGE) (Figure 3).55,56 A new species formed and reached a level of ~ 50% yield after seven days at 25 °C. Mass spectrometric analysis suggested the chemical nature of the cross-link was a carbinolamine linkage (17), in equilibrium with either or both the imine (18) or pyrimidopurinone (19) forms.56 This cross-link exhibited a reversible melting transition (Tm) at >90° C,51,55 which was assigned as the interstrand cross-link (Figure 4); the Tm of the uncross-linked duplex containing 9 was 55° C, 10° lower than the unmodified duplex. In duplex DNA, ~20% of the cross-link reverted to uncross-linked over 16 hr at pH 7, whereas reversion occurred within 1 hr under conditions that disrupted the duplex.56 Enzymatic digestion yielded diastereomeric pyrimidopurinone bis-nucleoside cross-links 19 (Figure 5), which are structurally related to those arising from acetaldehyde-treated DNA.19 Reduction of 19 afforded N2-dG:N2-dG bis-nucleosides tethered by a trimethylene chain (37, Figure 6).56 If the cross-linked duplex was reduced with NaB(CN)H3 prior to its digestion, N2-(3- hydroxypropyl)-dG from the reduction of γ-OH-PdG (9), and crosslink 37 were observed.
Figure 3.
Cross-linking of γ-OH-PdG-adducts in the 5′-CpG-3′ sequence, monitored by CGE. The adducted and complementary strands are identified by the letters A and C, respectively; the arrows indicate interstrand cross-links.
Figure 4.
Thermal melting analysis of and acrolein modified oligonucleotide in a CpG sequence after incubation for five day. The higher melting transition is assigned to the interstrand cross-link.
Figure 5.
Digestion of the cross-linkedγ-OH-PdG-adducted duplex. The pyrimidopurinone bis-nucleosides were identified by comparison with authentic standards.
Figure 6.
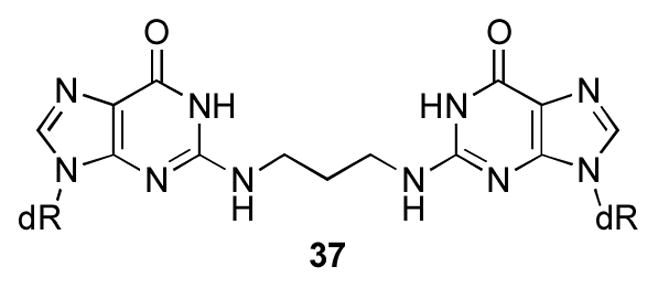
N2-dG:N2-dG trimethylene cross-link derived from the reduction of 18.
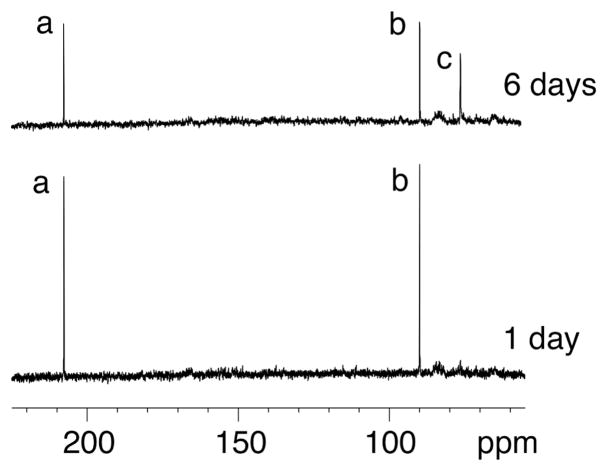
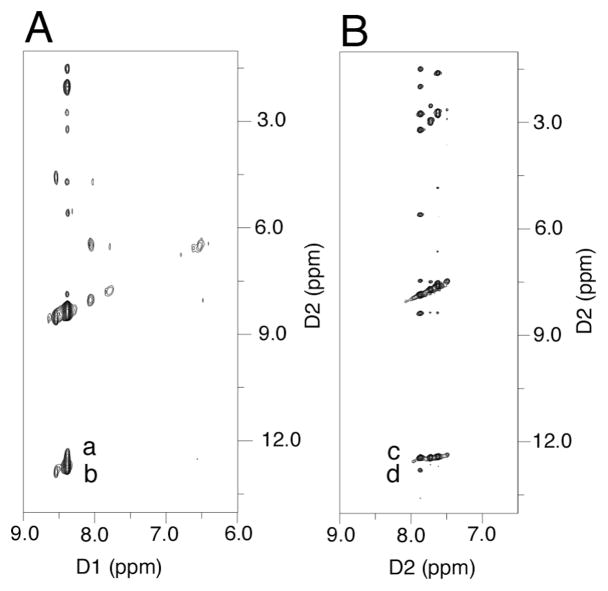
Although the cross-link could be reductively trapped, 13C NMR experiments utilizing X=γ-13C-γ-OH-PdG adducted oligodeoxynucleotide 41 (Scheme 5) failed to detect the imine linkage in duplex DNA (Figure 7).57 The identification of the cross-link in duplex DNA as the carbinolamine (17) and not the pyrimidopurinone (19)56 was accomplished by isotope-edited NMR experiments in which oligodeoxynucleotide 43 containingγ-OH-15N2-PdG was annealed with its complementary strand. A 15N-HSQC filtered spectrum revealed the NOE between X7 15N2H and the imino proton X7 N1H (Figure 8), precluding the pyrimidopurinone structure (19). A triple resonance 1H13C15N experiment conducted subsequent to annealing γ-13C-PdG-modified oligodeoxynucleotide 41 with 15N2-dG-modified oligodeoxynucleotide 39 revealed correlation between the γ-13C carbinol and the 15N amine.58
Scheme 5.
Preparation of oligodeoxynucleotides containing 13C (red) and 15N (blue) isotopes in the γ-OH-PdG adduct (41,43), and an 15N isotope (blue) in the complementary strand (39).
Figure 7.
Data from an isotopically enriched sample containing 13C-γ-OH-PdG. The imine linkage remained below the level of NMR detection. The top spectrum shows a 13C resonance assigned as the diastereomeric carbinolamine forms of the cross-link. Assignments of resonances: a, aldehyde 1; b, hydrated-aldehyde; c, diastereomeric carbinolamines 17. An imine resonance would be expected at ~130 ppm. Copyright American Chemical Society 2005.
Figure 8.
Isotope-edited NMR identified the carbinolamine linkage for the γ-OH-PdG cross-link. A. 15N- HSQC NOESY spectrum for oligodeoxynucleotide 39 annealed with oligodeoxynucleotide 31. Nucleotides are numbered 5′-d(G1C2T3A4G5C6X7A8G9T10C11C12)-3′•5′-d(G13G14A15C16T17C18Y19C20T21A22G23C24)-3′, X7=γ-OH PdG; Y19=15N2dG. Crosspeaks a, Y19 15N2H→X7 N1H (weak); b, Y19 15N2H→Y19 N1H (strong). B. 15N-HSQC NOESY spectrum for γ-OH-15N2-PdG labeled oligodeoxy-nucleotide 43 annealed with its complement. Crosspeaks c, X7 15N2H→X7 N1H (strong); d, X7 15N2H→G19 N1H (weak). Copyright American Chemical Society 2005.
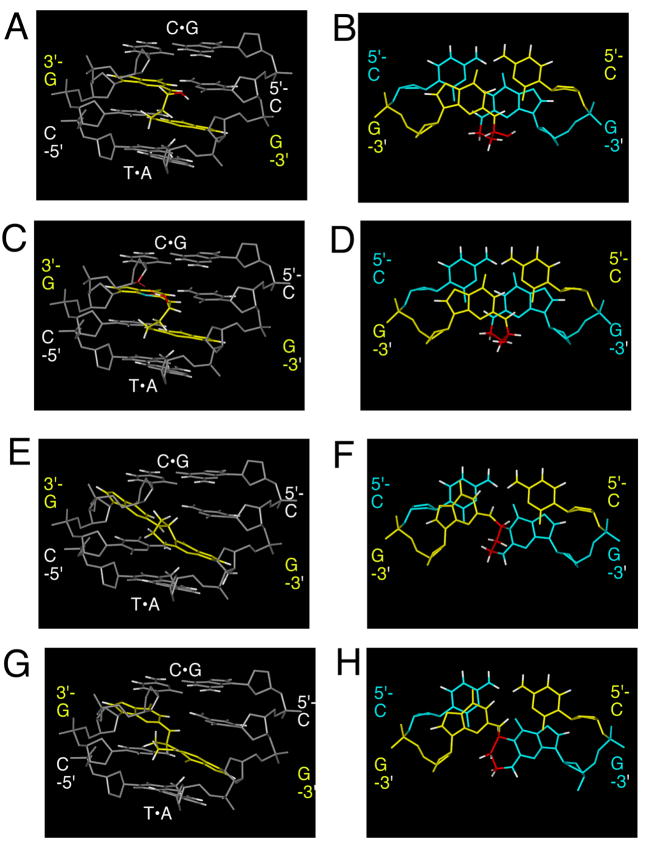
The clue as to why the cross-link preferred the carbinolamine (17),57,58 and not the pyrimidopurinone cross-link (19),56 was provided by an experiment in which a N2-dG:N2-dG trimethylene linkage 37, a surrogate for the carbinolamine cross-link (17), was constructed in 5′-d(AGGCXCCT)2; X represents the linked guanines.59 The saturated linkage caused minimal distortion.59 Additionally, modeling suggested that the carbinolamine linkage maintained Watson-Crick bonding at both of the cross-linked C•G pairs (Figure 9).58 Dehydration of carbinolamine 17 to an imine (18), or cyclization of the latter to pyrimidopurinone linkage (19), would have disrupted Watson-Crick bonding at one or both of the cross-linked base pairs.
Figure 9.
Modeling the 8R and 8S epimers of the 5′-CpG-3′ acrolein-induced cross-links. A C•G pair is 5′ and a T•A pair is 3′ to the 5′-CpG-3′ sequence. A. 8R-diastereomer of carbinolamine cross-link 17, minor groove view. B. 8R-diastereomer of cross-link 17, base-stacking. C. 8S-diastereomer of cross-link 17, minor groove view. D. 8S-diastereomer of cross-link 17, base-stacking. E. 8R-diastereomer of pyrimidopurinone cross-link 19, minor groove view. F. 8R-diastereomer of cross-link 19, base-stacking. G. 8S-diastereomer of cross-link 19, minor groove view. H. 8S-diastereomer of cross-link 19, base-stacking. Copyright American Chemical Society 2005.
Interstrand cross-linking by γ-OH-PdG was specific to the 5′-CpG-3′ sequence (Figure 9). When γ-OH-PdG (9) was engineered into 5′-d(CGTACXCATGC)-3′, containing both the 5′-CpG-3′ or 5′-GpC-3′ sequences,55 and the complement 5′-(GCATGCGTACG)-3′ was labeled with 15N2-dG (the underlined corresponds to the potential 5′-CpG-3′ cross-link), only 15N-labeled bis-nucleoside cross-link 19 was observed after enzymatic digestion and analysis by LC-ESI-MS, establishing the 5′-CpG-3′ sequence dependence for cross-linking. Other 5′-CpG-3′ interstrand cross-links are known, e.g., arising from mitomycin C,60,61 and nitrous acid.62 When the N2-dG:N2-dG trimethylene linkage (37), a surrogate for the non-observed cross-link in the 5′-GpC-3′ sequence, was constructed in d(TCCXCGGA)2, its structure was distorted, and its Tm was reduced.59,63
Interstrand Cross-linking of (6R) and (6S) Crotonaldehyde 1,N2-PdG Adducts
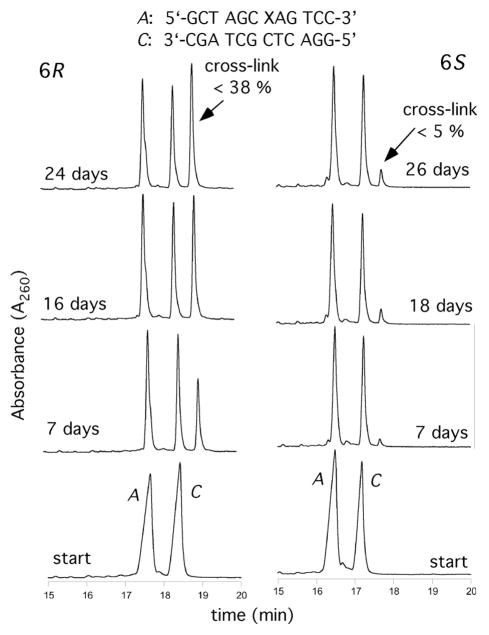
Ring-opening of the diastereomeric 1,N2-dG adducts 11 and 12 was incomplete at pH 7 when placed opposite dG in duplex DNA. The incomplete ring-opening was attributed to the positioning of the CH3 groups to avoid steric clash with N3 of guanine, which becomes significant in the N2-(1-methyl-3-oxopropyl)-dG aldehydes (3,4). The abilities of these adducts to form interstrand cross-links in the 5′-CpG-3′ sequence as oligodeoxynucleotide 30 depended upon stereochemistry at the C6 carbon. After >20 days, ~40% cross-link formation occurred for the 6R diastereomer 11 (Figure 10), whereas < 5% cross-link was observed for the 6S diasteromer 12.55 Digestion of the cross-link yielded the bis-nucleoside pyrimidopurinone,55 identical to that isolated from acetaldehyde-treated DNA.19 The presence of the imine linkage was inferred since the cross-link was reductively trapped.55 Lao and Hecht concluded the cross-link was predominetly an imine or pyrimidopurinone with some of the carbinolamine linkage present.20
Figure 10.
Cross-linking reactions of the 6R and 6S crotonaldehyde-modified duplexes in the 5′-CpG-3′ sequence monitored by CGE. The adducted and complementary strands are identified by the letters A and C, respectively; the arrows indicate the interstrand cross-links.
Although the cross-link could be reduced, NMR failed to detect the imine linkage in duplex DNA (Figure 8).57 Using isotopically labeled adducts,50 it was established that the carbinolamine form of the 6R cross-link was the only detectable cross-link species present in duplex DNA. As for the γ-OH-PdG adduct, modeling revealed that the carbinolamine linkage maintained Watson-Crick bonding at the cross-linked base pairs. Dehydration of the carbinolamine to the imine, or cyclization of the latter to form the pyrimidopurinone cross-link, would disrupt Watson-Crick bonding at one or both of the cross-linked C G base pairs, providing a rationalization for why the carbinolamine is preferred.
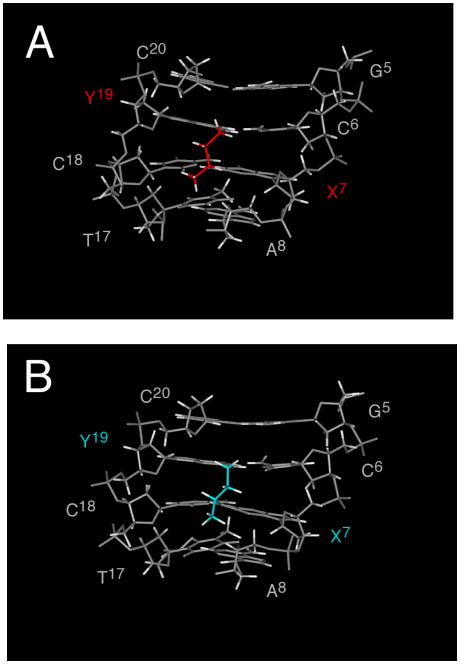
Structural studies utilizing saturated analogs of the 6R- and 6S cross-links indicated that both retained Watson-Crick hydrogen bonds at the cross-linked base pairs (Figure 12).64 However, the 6S diastereomer showed lower stability. Whereas for the 6R diastereomer, the CH3 group was positioned in the center of the minor groove, for the 6S diastereomer, it was positioned in the 3′ direction, interfering sterically with the DNA duplex structure.64 These results were consistent with modeling of the native cross-links.50 Lao and Hecht also concluded that the pyrimidopurinone cross-link arising from the 6R stereochemistry exhibited a more favorable orientation of the C6 CH3 group.20
Figure 12.
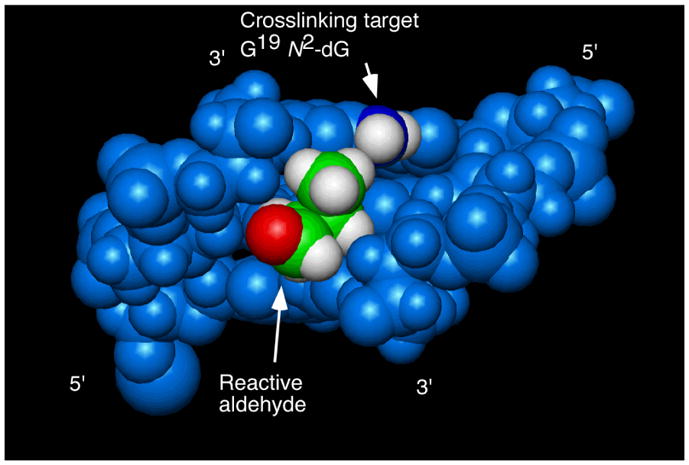
Base pairs C6•G19, X7•C18, and A8•T17 in the oligodeoxynucleotide containing the N2-(3-oxo-1S-methyl-propyl)-dG adduct 12. The orientation of the aldehyde does not favor cross-linking to the target G19 N2-dG. Nucleotides are numbered 5′-d(G1C2T3A4G5C6X7A8G9T10C11C12)-3′ 5′-d(G13G14A15C16T17C18Y19C20T21A22G23C24)-3′, X7= N2-(3-oxo-1S-methyl-propyl)-dG adduct 12. Copyright American Chemical Society 2006.
Additional studies of the 6S diastereomer 12 were performed at pH 9.3. This pH favors the ring-opened aldehyde adducts. The aldehyde group of the ring-opened 6S adduct is oriented in the 3′-direction within the minor groove (Figure 12).65 Consequently, the aldehyde was distant to the exocyclic amine of the guanine involved in cross-linking (G19), explaining why this diastereomer generated interstrand cross-links less efficiently than the 6R diastereomer.65 These observations also corroborated modeling studies.50
Interstrand Cross-linking by trans-4-HNE Adducts
Only the HNE adducts with the (6S,8R,11S) configuration (16) formed an interstrand cross-link in the 5′-CpG-3′ sequence (Figure 13). This configuration posessed the same relative stereochemistry at C6 as did the 6R configuration of the crotonaldehyde adduct (11), further supporting the role of stereochemistry at C6 in modulating interstrand cross-linking. Cross-linking proceeded slowly. However, after two months, the yield was a remarkable ~85%.51 Digestion of the DNA yielded the pyrimidopurinone bis-nucleoside cross-link.51
Figure 13.
Cross-linking of the (6S,8R,11S)-4-HNE-containing oligodeoxynucleotide.
Spectroscopic studies to delineate the cross-linking chemistry of the HNE adducts are continuing.
Potential Biological Significance
One major goal of continuing research is to demonstrate that these acrolein, crotonaldehyde, and HNE-derived interstrand cross-links are present in vivo, utilizing mass spectrometric-based analysis.17,66–68 Since they equilibrate with non-cross-linked species, and require the presence of the 5′-CpG-3′ sequence, they may be present at low levels in tissue samples. Nevertheless, it has been reported that acrolein preferentially binds at 5′-CpG-3′ sites, a consequence of cytosine methylation at these sequences.22
The potential presence of these cross-links in vivo is anticipated to interfere with DNA replication and transcription. Moreover, in humans, interstrand cross-link repair requires the cooperation of multiple proteins belonging to different biological pathways, including, but not limited to nucleotide excision repair, homologous recombination, translesion DNA synthesis, double-strand break repair, and the Fanconi anemia pathway.43,69–73 Current models suggest that interstrand cross-link repair is initiated by dual incisions around the cross-link in one of the two affected strands. This ‘unhooking’ depends on the endonucleolytic activity of the XPF/ERCC1 complex, a component of NER. The result is a gap that may be filled by pairing of the 3′ end of the pre-incised strand with the homologous sequence, followed by DNA synthesis. Alternatively, the complementary strand with the crosslink attached may be used as a template for translesion DNA synthesis. Once the integrity of one DNA strand is restored, the second strand may be repaired by conventional NER. When repair is concomitant with replication, a DNA double-strand break is formed; thus, additional biological processing would be required to tolerate interstrand cross-links.43,70
Summary
The 1,N2-dG adducts of acrolein, crotonaldehyde, and 4-HNE yield interstrand cross-links in the5′-CpG-3′ sequence. These arise via opening of the 8-hydroxypropano ring to the corresponding aldehydes, which undergo attack by the N2-amino group of the cross-strand dG in the 5′-CpG-3′ sequence. The cross-links arising from acrolein and crotonaldehyde exist in duplex DNA as carbinolamine linkages, which enable the cross-linked C•G base pairs to maintain Watson-Crick hydrogen bonding with minimal distortion of the duplex. The cross-linking chemistry depends upon the stereochemistry of the C6 carbon, which favorably orients the reactive aldehyde within the minor groove in the 5′-CpG-3′ sequence, favoring the 6R configuration for crotonaldehyde and the stereochemically equivalent 6S configuration for 4-HNE.
Figure 11.
Structures of reduced cross-links arising from crotonaldehyde. A. The 6R cross-link (red) oriented in the center of the minor groove. B. The 6S cross-link (blue) interfered sterically with the DNA and exhibited lower stability. Nucleotides are numbered 5′-d(G1C2T3A4G5C6X7A8G9T10C11C12)-3′•5′-d(G13G14A15C16T17C18Y19C20T21A22G23C24)-3′, X7= 6R or 6S-crotonaldehyde-adducted dG in the 5′-CpG-3′ sequence; Y19=cross-linked dG in the complementary strand. Copyright American Chemical Society 2007.
Acknowledgments
This work was funded by NIH program project grant PO1 ES-05355 (M.P.S., I.D.K., T.M.H., R.S.L., and C.J.R.). The Vanderbilt University Center in Molecular Toxicology is funded by NIH grant P30 ES-00267. Vanderbilt University and NIH Grant RR-05805 provided additional funding for NMR instrumentation.
Biographies
Michael P. Stone received his B.S. in Biochemistry at the University of California, Davis, and the Ph.D. in Chemistry at the University of California, Irvine, with Philip N. Borer. After postdoctoral training at the University of Rochester with Thomas R. Krugh, he joined the faculty at Vanderbilt University, where he is Professor of Chemistry and Biochemistry. His research interests include the structural consequences of DNA damage.
Carmelo J. Rizzo received his B.A in Chemistry from Temple University and the Ph. D. in Chemistry from the University of Pennsylvania under Amos B. Smith. After an NIH postdoctoral fellowship at Columbia University with Ronald Breslow, he joined the faculty at Vanderbilt University where he is currently Professor of Chemistry and Biochemistry and Co-Deputy Director of the Vanderbilt Center in Molecular Toxicology. He is interested in the chemical aspects of DNA damage.
References
- 1.Burcham PC. Genotoxic lipid peroxidation products: Their DNA damaging properties and role in formation of endogenous DNA adducts. Mutagenesis. 1998;13:287–305. doi: 10.1093/mutage/13.3.287. [DOI] [PubMed] [Google Scholar]
- 2.Chung FL, Nath RG, Nagao M, Nishikawa A, Zhou GD, Randerath K. Endogenous formation and significance of 1,N2-propanodeoxyguanosine adducts. Mutat Res. 1999;424:71–81. doi: 10.1016/s0027-5107(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 3.Chung FL, Zhang L, Ocando JE, Nath RG. Role of 1,N2-propanodeoxyguanosine adducts as endogenous DNA lesions in rodents and humans. IARC Sci Pub. 1999;150:45–54. [PubMed] [Google Scholar]
- 4.Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: A review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Hecht SS, Upadhyaya P, Wang M. Reactions of α-acetoxy-N-nitrosopyrrolidine and crotonaldehyde with DNA. IARC Sci Pub. 1999;150:147–154. [PubMed] [Google Scholar]
- 6.Nath RG, Ocando JE, Guttenplan JB, Chung FL. 1,N2-propanodeoxyguanosine adducts: Potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res. 1998;58:581–584. [PubMed] [Google Scholar]
- 7.Esterbauer H, Schaur R, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro R, Cohen B, Shiuey S, Maurer H. On the reaction of guanine with glyoxal, pyruvaldehyde, and kethoxal, and the structure of the acylguanines. A new synthesis of N2-alkylguanines. Biochemistry. 1969;8:238–245. doi: 10.1021/bi00829a034. [DOI] [PubMed] [Google Scholar]
- 9.Leonard N. Etheno-substituted nucleotides and coenzymes: Fluorescence and biological activity. CRC Crit Rev Biochem. 1984;15:125–199. doi: 10.3109/10409238409102299. [DOI] [PubMed] [Google Scholar]
- 10.Galliani G, Pantarotto C. The reaction of guanosine and 2′-deoxyguanosine with acrolein. Tetrahedron Lett. 1983;24:4491–4492. [Google Scholar]
- 11.Chung FL, Hecht SS. Formation of cyclic 1,N2-adducts by reaction of deoxyguanosine with alpha-acetoxy-N-nitrosopyrrolidine, 4-(carbethoxynitrosamino)butanal, or crotonaldehyde. Cancer Res. 1983;43:1230–1235. [PubMed] [Google Scholar]
- 12.Chung F, Young R, Hecht SS. Formation of 1,N2-propanodeoxyguanosine adducts in DNA upon reaction of acrolein or crotonaldehyde. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 13.Chung F, Roy K, Hecht S. A study of alpha, beta-unsaturated carbonyl compounds with deoxyguanosine. J Org Chem. 1988;53:14–17. [Google Scholar]
- 14.Winter C, Segall H, Haddon W. Formation of cyclic adducts of deoxyguanosine with the aldehydes trans-4-hydroxy-2-hexenal and trans-4-hydroxy-2-nonenal in vitro. Cancer Res. 1986;46:5682–5686. [PubMed] [Google Scholar]
- 15.Sodum R, Shapiro R. Reaction of acrolein with cytosine and adenosine derivatives. Bioorg Chem. 1988;16:272–282. [Google Scholar]
- 16.Chenna A, Rieger R, Iden C. Characterization of thymidine adducts formed by acrolein and 2-bromoacrolein. Carcinogenesis. 1992;13:2361–2365. doi: 10.1093/carcin/13.12.2361. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Villalta P, Wang M, Hecht S. Detection and quantitation of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol. 2007;20:565–571. doi: 10.1021/tx700023z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eder E, Hoffman C. Identification and characterization of deoxyguanosine-crotonaldehyde adducts. Formation of 7,8 cyclic adducts and 1,N2,7,8 bis-cyclic adducts. Chem Res Toxicol. 1992;5:802–808. doi: 10.1021/tx00030a012. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, McIntee EJ, Cheng G, Shi Y, Villalta PW, Hecht SS. Identification of DNA adducts of acetaldehyde. Chem Res Toxicol. 2000;13:1149–1157. doi: 10.1021/tx000118t. [DOI] [PubMed] [Google Scholar]
- 20.Lao Y, Hecht SS. Synthesis and properties of an acetaldehyde-derived oligonucleotide interstrand cross-link. Chem Res Toxicol. 2005;18:711–721. doi: 10.1021/tx0497292. [DOI] [PubMed] [Google Scholar]
- 21.Chung FL, Nath RG, Ocando J, Nishikawa A, Zhang L. Deoxyguanosine adducts of t-4-hydroxy-2-nonenal are endogenous DNA lesions in rodents and humans: Detection and potential sources. Cancer Res. 2000;60:1507–1511. [PubMed] [Google Scholar]
- 22.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, Ames BN. Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat Res. 1985;148:25–34. doi: 10.1016/0027-5107(85)90204-0. [DOI] [PubMed] [Google Scholar]
- 24.Smith RA, Cohen SM, Lawson TA. Acrolein mutagenicity in the V79 assay. Carcinogenesis. 1990;11:497–498. doi: 10.1093/carcin/11.3.497. [DOI] [PubMed] [Google Scholar]
- 25.Curren RD, Yang LL, Conklin PM, Grafstrom RC, Harris CC. Mutagenesis of xeroderma pigmentosum fibroblasts by acrolein. Mutat Res. 1988;209:17–22. doi: 10.1016/0165-7992(88)90104-2. [DOI] [PubMed] [Google Scholar]
- 26.Kawanishi M, Matsuda T, Nakayama A, Takebe H, Matsui S, Yagi T. Molecular analysis of mutations induced by acrolein in human fibroblast cells using supf shuttle vector plasmids. Mutat Res. 1998;417:65–73. doi: 10.1016/s1383-5718(98)00093-x. [DOI] [PubMed] [Google Scholar]
- 27.Cohen SM, Garland EM, St John M, Okamura T, Smith RA. Acrolein initiates rat urinary bladder carcinogenesis. Cancer Res. 1992;52:3577–3581. [PubMed] [Google Scholar]
- 28.Czerny C, Eder E, Runger TM. Genotoxicity and mutagenicity of the alpha, beta-unsaturated carbonyl compound crotonaldehyde (butenal) on a plasmid shuttle vector. Mutat Res. 1998;407:125–134. doi: 10.1016/s0921-8777(97)00069-4. [DOI] [PubMed] [Google Scholar]
- 29.Chung FL, Tanaka T, Hecht SS. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986;46:1285–1289. [PubMed] [Google Scholar]
- 30.Cajelli E, Ferraris A, Brambilla G. Mutagenicity of 4-hydroxynonenal in V79 chinese hamster cells. Mutat Res. 1987;190:169–171. doi: 10.1016/0165-7992(87)90050-9. [DOI] [PubMed] [Google Scholar]
- 31.Benamira M, Marnett LJ. The lipid peroxidation product 4-hydroxynonenal is a potent inducer of the sos response. Mutat Res. 1992;293:1–10. doi: 10.1016/0921-8777(92)90002-k. [DOI] [PubMed] [Google Scholar]
- 32.Hussain SP, Raja K, Amstad PA, Sawyer M, Trudel LJ, Wogan GN, Hofseth LJ, Shields PG, Billiar TR, Trautwein C, Hohler T, Galle PR, Phillips DH, Markin R, Marrogi AJ, Harris CC. Increased p53 mutation load in nontumorous human liver of Wilson disease and hemochromatosis: Oxyradical overload diseases. Proc Nat Acad Sci USA. 2000;97:12770–12775. doi: 10.1073/pnas.220416097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanuri M, Minko I, Nechev L, Harris T, Harris C, Lloyd R. Error prone translesion synthesis past gamma-hydroxypropano deoxyguanosine, the primary acrolein-derived adduct in mammalian cells. J Biol Chem. 2002;277:18257–18265. doi: 10.1074/jbc.M112419200. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes P, Wang H, Rizzo C, Lloyd R. Site-specific mutagenicity of stereochemically defined 1,N2-deoxyguanosine adducts of trans-4-hydroxynonenal in mammalian cells. Environ Mol Mutagen. 2003;42:68–74. doi: 10.1002/em.10174. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes P, Kanuri M, Nechev L, Harris T, Lloyd R. Mammalian cell mutagenesis of the DNA adducts of vinyl chloride and crotonaldehyde. Environ Mol Mutagen. 2005;45:455–459. doi: 10.1002/em.20117. [DOI] [PubMed] [Google Scholar]
- 36.Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 37.Mao H, Schnetz-Boutaud NC, Weisenseel JP, Marnett LJ, Stone MP. Duplex DNA catalyzes the chemical rearrangement of a malondialdehyde deoxyguanosine adduct. Proc Nat Acad Sci USA. 1999;96:6615–6620. doi: 10.1073/pnas.96.12.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riggins JN, Daniels JS, Rouzer CA, Marnett LJ. Kinetic and thermodynamic analysis of the hydrolytic ring-opening of the malondialdehyde-deoxyguanosine adduct, 3-(2′-deoxy-beta-d-erythro-pentofuranosyl)-pyrimido[1,2-alpha]purin-10(3H)-one. J Am Chem Soc. 2004;126:8237–8243. doi: 10.1021/ja040009r. [DOI] [PubMed] [Google Scholar]
- 39.de los Santos C, Zaliznyak T, Johnson F. NMR characterization of a DNA duplex containing the major acrolein-derived deoxyguanosine adduct gamma-OH-1,-N2-propano-2′-deoxyguanosine. J Biol Chem. 2001;276:9077–9082. doi: 10.1074/jbc.M009028200. [DOI] [PubMed] [Google Scholar]
- 40.Kurtz A, Lloyd R. 1,N2-deoxyguanosine adducts of acrolein, crotonaldehyde, and trans-4-hydroxynonenal cross-link to peptides via Schiff base linkage. J Biol Chem. 2003;278:5970–5976. doi: 10.1074/jbc.M212012200. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez A, Minko I, Kurtz A, Kanuri M, Moriya M, Lloyd R. Comparative evaluation of the bioreactivity and mutagenic spectra of acrolein-derived alpha-HOPdG and gamma-HOPdG regioisomeric deoxyguanosine adducts. Chem Res Toxicol. 2003;16:1019–1028. doi: 10.1021/tx034066u. [DOI] [PubMed] [Google Scholar]
- 42.Scharer OD. DNA interstrand crosslinks: Natural and drug-induced DNA adducts that induce unique cellular responses. Chembiochem. 2005;6:27–32. doi: 10.1002/cbic.200400287. [DOI] [PubMed] [Google Scholar]
- 43.Noll DM, Mason TM, Miller PS. Formation and repair of interstrand cross-links in DNA. Chem Rev. 2006;106:277–301. doi: 10.1021/cr040478b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilquin J, Dechamps M, Sonveaux E. Incorporation of an aldehyde function in oligonucleotides. Bioconjug Chem. 2001;12:451–457. doi: 10.1021/bc000060u. [DOI] [PubMed] [Google Scholar]
- 45.Khullar S, Varaprasad CV, Johnson F. Postsynthetic generation of a major acrolein adduct of 2′-deoxyguanosine in oligomeric DNA. J Med Chem. 1999;42:947–950. doi: 10.1021/jm980605u. [DOI] [PubMed] [Google Scholar]
- 46.Nechev LV, Harris CM, Harris TM. Synthesis of nucleosides and oligonucleotides containing adducts of acrolein and vinyl chloride. Chem Res Toxicol. 2000;13:421–429. doi: 10.1021/tx990167+. [DOI] [PubMed] [Google Scholar]
- 47.DeCorte BL, Tsarouhtsis D, Kuchimanchi S, Cooper MD, Horton P, Harris CM, Harris TM. Improved strategies for postoligomerization synthesis of oligodeoxynucleotides bearing structurally defined adducts at the N2 position of deoxyguanosine. Chem Res Toxicol. 1996;9:630–637. doi: 10.1021/tx9501795. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Kozekov I, Kozekova A, Tamura P, Marnett L, Harris T, Rizzo C. Site-specific synthesis of oligonucleotides containing malondialdehyde adducts of deoxyguanosine and deoxyadenosine via a postsynthetic modification strategy. Chem Res Toxicol. 2006;19:1467–1474. doi: 10.1021/tx060137o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nechev LV, Kozekov I, Harris CM, Harris TM. Stereospecific synthesis of oligonucleotides containing crotonaldehyde adducts of deoxyguanosine. Chem Res Toxicol. 2001;14:1506–1512. doi: 10.1021/tx0100690. [DOI] [PubMed] [Google Scholar]
- 50.Cho YJ, Wang H, Kozekov ID, Kurtz AJ, Jacob J, Voehler M, Smith J, Harris TM, Lloyd RS, Rizzo CJ, Stone MP. Stereospecific formation of interstrand carbinolamine DNA cross-links by crotonaldehyde- and acetaldehyde- derived alpha-CH3-gamma-OH-1,N2-propano-2′-deoxyguanosine adducts in the 5′-CpG-3′ sequence. Chem Res Toxicol. 2006;19:195–208. doi: 10.1021/tx050239z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, Kozekov ID, Harris TM, Rizzo CJ. Site-specific synthesis and reactivity of oligonucleotides containing stereochemically defined 1,N2-deoxyguanosine adducts of the lipid peroxidation product trans-4-hydroxynonenal. J Am Chem Soc. 2003;125:5687–5700. doi: 10.1021/ja0288800. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Rizzo CJ. Stereocontrolled syntheses of all four stereoisomeric 1,N2-deoxyguanosine adducts of the lipid peroxidation product trans-4-hydroxynonenal. Org Lett. 2001;3:3603–3605. doi: 10.1021/ol016810c. [DOI] [PubMed] [Google Scholar]
- 53.Nechev L, Kozekov I, Brock A, Rizzo C, Harris T. DNA adducts of acrolein: Site-specific synthesis of an oligodeoxynucleotide containing 6-hydroxy-5,6,7,8-tetrahydropyrimido[1,2-α]purin-10(3H)-one, an acrolein adduct of guanine. Chem Res Toxicol. 2002;15:607–613. doi: 10.1021/tx010181y. [DOI] [PubMed] [Google Scholar]
- 54.Huang Y, Cecilia Torres M, Iden C, Johnson F. Synthesis of the minor acrolein adducts of 2′-deoxyguanosine and their generation in oligomeric DNA. Bioorg Chem. 2003;31:136–148. doi: 10.1016/s0045-2068(02)00525-4. [DOI] [PubMed] [Google Scholar]
- 55.Kozekov ID, Nechev LV, Moseley MS, Harris CM, Rizzo CJ, Stone MP, Harris TM. DNA interchain cross-links formed by acrolein and crotonaldehyde. J Am Chem Soc. 2003;125:50–61. doi: 10.1021/ja020778f. [DOI] [PubMed] [Google Scholar]
- 56.Kozekov ID, Nechev LV, Sanchez A, Harris CM, Lloyd RS, Harris TM. Interchain cross-linking of DNA mediated by the principal adduct of acrolein. Chem Res Toxicol. 2001;14:1482–1485. doi: 10.1021/tx010127h. [DOI] [PubMed] [Google Scholar]
- 57.Kim HY, Voehler M, Harris TM, Stone MP. Detection of an interchain carbinolamine cross-link formed in a CpG sequence by the acrolein DNA adduct gamma-OH-1,N2-propano-2′-deoxyguanosine. J Am Chem Soc. 2002;124:9324–9325. doi: 10.1021/ja020333r. [DOI] [PubMed] [Google Scholar]
- 58.Cho YJ, Kim HY, Huang H, Slutsky A, Minko IG, Wang H, Nechev LV, Kozekov ID, Kozekova A, Tamura P, Jacob J, Voehler M, Harris TM, Lloyd RS, Rizzo CJ, Stone MP. Spectroscopic characterization of interstrand carbinolamine cross-links formed in the 5′-CpG-3′ sequence by the acrolein-derived gamma-OH-1,N2-propano-2′-deoxyguanosine DNA adduct. J Am Chem Soc. 2005;127:17686–17696. doi: 10.1021/ja053897e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dooley PA, Tsarouhtsis D, Korbel GA, Nechev LV, Shearer J, Zegar IS, Harris CM, Stone MP, Harris TM. Structural studies of an oligodeoxynucleotide containing a trimethylene interstrand cross-link in a 5′-(CpG) motif: Model of a malondialdehyde cross-link. J Am Chem Soc. 2001;123:1730–1739. doi: 10.1021/ja003163w. [DOI] [PubMed] [Google Scholar]
- 60.Borowy-Borowski H, Lipman R, Tomasz M. Recognition between mitomycin c and specific DNA sequences for cross-link formation. Biochemistry. 1990;29:2999–3006. doi: 10.1021/bi00464a016. [DOI] [PubMed] [Google Scholar]
- 61.Millard JT, Weidner MF, Raucher S, Hopkins PB. Determination of the DNA crosslinking sequence specificity of reductively activated mitomycin c at single-nucleotide resolution: Deoxyguanosine residues at CpG are crosslinked preferentially. J Am Chem Soc. 1990;112:3637–3641. [Google Scholar]
- 62.Kirchner J, Sigurdsson S, Hopkins P. Interstrand cross-linking of duplex DNA by nitrous acid: Covalent structure of the dG-to-dG cross-link at the sequence 5′-CG. J Am Chem Soc. 1992;114:4021–4027. [Google Scholar]
- 63.Dooley PA, Zhang M, Korbel GA, Nechev LV, Harris CM, Stone MP, Harris TM. NMR determination of the conformation of a trimethylene interstrand cross-link in an oligodeoxynucleotide duplex containing a 5′-d(GpC) motif. J Am Chem Soc. 2003;125:62–72. doi: 10.1021/ja0207798. [DOI] [PubMed] [Google Scholar]
- 64.Cho YJ, Kozekov ID, Harris TM, Rizzo CJ, Stone MP. Stereochemistry modulates the stability of reduced interstrand cross-links arising from R- and S-alpha-CH3-gamma-OH-1,N2-propano-2′-deoxyguanosine in the 5′-CpG-3′ DNA sequence. Biochemistry. 2007;46:2608–2621. doi: 10.1021/bi061381h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho YJ, Wang H, Kozekov ID, Kozekova A, Kurtz AJ, Jacob J, Voehler M, Smith J, Harris TM, Rizzo CJ, Lloyd RS, Stone MP. Orientation of the crotonaldehyde-derived N2-[3-oxo-1S-methyl-propyl]-dG DNA adduct hinders interstrand cross-link formation in the 5′-CpG-3′ sequence. Chem Res Toxicol. 2006;19:1019–1029. doi: 10.1021/tx0600604. [DOI] [PubMed] [Google Scholar]
- 66.Goodenough AK, Schut HA, Turesky RJ. Novel LC-ESI/MS/MSn method for the characterization and quantification of 2′-deoxyguanosine adducts of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-β]pyridine by 2-D linear quadrupole ion trap mass spectrometry. Chem Res Toxicol. 2007;20:263–276. doi: 10.1021/tx0601713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ricicki EM, Luo W, Fan W, Zhao LP, Zarbl H, Vouros P. Quantification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl adducts in human lymphoblastoid TK6 cells dosed with N-hydroxy-4-acetylaminobiphenyl and their relationship to mutation, toxicity, and gene expression profiling. Anal Chem. 2006;78:6422–6432. doi: 10.1021/ac0607360. [DOI] [PubMed] [Google Scholar]
- 68.Stout MD, Jeong YC, Boysen G, Li Y, Sangaiah R, Ball LM, Gold A, Swenberg JA. LC/MS/MS method for the quantitation of trans-2-hexenal-derived exocyclic 1,N2-propanodeoxyguanosine in DNA. Chem Res Toxicol. 2006;19:563–570. doi: 10.1021/tx050346t. [DOI] [PubMed] [Google Scholar]
- 69.Nojima K, Hochegger H, Saberi A, Fukushima T, Kikuchi K, Yoshimura M, Orelli BJ, Bishop DK, Hirano S, Ohzeki M, Ishiai M, Yamamoto K, Takata M, Arakawa H, Buerstedde JM, Yamazoe M, Kawamoto T, Araki K, Takahashi JA, Hashimoto N, Takeda S, Sonoda E. Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells. Cancer Res. 2005;65:11704–11711. doi: 10.1158/0008-5472.CAN-05-1214. [DOI] [PubMed] [Google Scholar]
- 70.Niedernhofer LJ, Lalai AS, Hoeijmakers JH. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Mirchandani KD, D’Andrea AD. The fanconi anemia/BRCA pathway: A coordinator of cross-link repair. Exp Cell Res. 2006;312:2647–2653. doi: 10.1016/j.yexcr.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 72.Patel KJ, Joenje H. Fanconi anemia and DNA replication repair. DNA Repair. 2007;6:885–890. doi: 10.1016/j.dnarep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Kennedy RD, D’Andrea AD. The fanconi anemia/BRCA pathway: New faces in the crowd. Genes Dev. 2005;19:2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]