Abstract
Angiotensin II (ANG II), acting via angiotensin type 1 receptors (AT1-R) in the brain, activates the sympathetic nervous system in heart failure (HF). We recently reported that ANG II stimulates mitogen-activated protein kinase (MAPK) to upregulate brain AT1-R in HF rats. In this study we tested the hypothesis that ANG II-activated MAPK signaling pathways contribute to sympathetic excitation in HF. Intracerebroventricular (ICV) administration of PD98059 and UO126, two selective p44/42 MAPK inhibitors, induced significant decreases in mean arterial pressure (MAP), heart rate (HR) and renal sympathetic nerve activity (RSNA) in HF rats, but had no effect on these variables in SHAM rats. Pretreatment with losartan attenuated the effects of PD98059. ICV administration of the p38 MAPK inhibitor SB203580 and the c-Jun N-terminal kinase inhibitor SP600125 had no effect on MAP, HR or RSNA in HF. The phosphatidylinositol-3 kinase inhibitor LY294002 induced a small decrease in MAP and HR, but no change in RSNA. Immunofluorescent staining demonstrated increased p44/42 MAPK activity in neurons of the paraventricular nucleus of the hypothalamus (PVN) of HF rats, co-localized with Fra-like activity (indicating chronic neuronal excitation). ICV PD98059 and UO126 reduced Fra-like activity in PVN neurons in HF rats. In confirmatory acute studies, ICV ANG II increased MAP, HR and RSNA in baroreceptor-denervated rats and Fra-LI immunoreactivity in the PVN of neurally intact rats. Central administration of PD98059 markedly reduced these responses. These data demonstrate that intracellular p44/42 MAPK activity contributes to ANG II-induced PVN neuronal excitation and augmented sympathetic nerve activity in rats with HF.
Keywords: MAPK, angiotensin II, renal sympathetic nerve activity, PI3 kinase, heart failure, paraventricular nucleus of hypothalamus
INTRODUCTION
Increased renin-angiotensin system activity and enhanced sympathetic excitation account for the predominant manifestations of chronic heart failure (HF).1, 2 These mechanisms originally serve as compensation for reduced cardiac function, but eventually contribute to left ventricular deterioration and the progression of HF.3, 4 The sustained sympathetic excitation in HF is likely multi-factorial, involving blunted inhibitory reflexes, augmented excitatory reflexes and an excess of circulating neuroactive substances (angiotensin, aldosterone and cytokines). Data from our laboratory5 and others6, 7 suggest a prominent role for angiotensin II (ANG II). Recent work in our laboratory suggests that ANG II binding to the angiotensin type 1 receptor (AT1-R) activates intracellular mitogen-activated protein kinase (MAPK) signaling pathways to upregulate AT1-R expression in cardiovascular regions of the brain,8 setting the stage for increased ANG II-mediated sympathetic drive.
Three major terminal effector kinases of the MAPK family are the p44/42 MAPK (also called extracellular signal-regulated protein kinases, Erk1/2), the stress-activated protein kinase/c-Jun NH2-terminal kinases (SAPK/JNK), and the p38 MAPK.9 ANG II-induced activation of these MAPK signaling pathways has been implicated in myocardial hypertrophy10 and inflammation11 in the periphery, and neurotransmitter catecholamine synthesis and release in the brain.12 ANG II also activates phosphatidylinositol-3 (PI3) kinase which are involved in cell growth and proliferation in vascular smooth muscle cells,13 and norepinephrine regulation in the brain of the spontaneously hypertensive rat.14 Additionally, MAPK can be activated by other factors such as pro-inflammatory cytokines15 and reactive oxygen species (ROS).16
The aim of the present study was to determine whether ANG II-triggered MAPK and PI3 kinase signaling pathways in the brain contribute to sympathetic excitation in rats with chronic heart failure. We examined the effects of centrally administered selective kinase inhibitors on renal and lumbar sympathetic nerve activity and on Fra-like (Fra-LI) activity, a marker of neuronal excitation 17 that has been used to identify chronically activated neurons in the paraventricular nucleus of the hypothalamus (PVN).18 Additional acute studies were performed to more directly assess the role of p44/42 MAPK in ANG II-driven activation of the PVN and sympathetic nerve activity. The PVN was chosen for study because it is a well known source of pre-sympathetic neurons regulating sympathetic outflow.19, 20
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats, weighing 275–325g, were obtained from Harlan Sprague Dawley. The animals were housed in temperature controlled (23 ± 2°C) rooms in University of Iowa Animal Care Facility and exposed to a normal 12-h light–dark cycle. They were provided with rat chow ad libitum. These studies were performed in accordance with the “American Physiological Society Guiding Principles for Research Involving Animals and Human Beings.” 21 The experimental procedures were approved by the University of Iowa Institutional Animal Care and Use Committee.
Experimental Protocols
Chronic Heart Failure Studies
Four weeks after coronary ligation to induce HF or a sham operation, rats were re-anesthetized and instrumented for acute electrophysiological and hemodynamic recording studies during intracerebroventricular (ICV) drug administration. At least 30 min after completion of the surgical preparations, arterial pressure (AP), heart rate (HR) and renal or lumbar sympathetic nerve activity were recorded for 15 min at baseline and then continuously during ICV drug administration (40µl/hr for 1 hour).
Renal sympathetic nerve activity (RSNA), AP and HR were recorded in sham-operated (SHAM) and HF rats treated with ICV vehicle (VEH), or the selective p44/42 MAPK inhibitors PD98059 (20 µmol/L) or UO126 (20 µmol/L). Additional HF rats were treated with the p38 MAPK inhibitor SB203580 (100 µmol/L), the JNK inhibitor SP600125 (100 µmol/L), the PI3 kinase inhibitor LY294002 (100 µmol/L), or PD98059 (20 µmol/L) administered 15 min after ICV injection of the AT1-R blocker losartan (1 mmol/L). Lumbar sympathetic nerve activity (LSNA), AP and HR were recorded only in HF rats treated with ICV PD98059 (20 µmol/L), or UO126 (20 µmol/L).
At the completion of these terminal recording sessions, some rats were transcardially perfused with 4% paraformaldehyde and the brains were harvested for immunohistochemical and immunofluorescent studies. These included VEH-treated SHAM rats, and of HF rats treated with ICV VEH, PD98059, UO126, SP600125, SB203580, and LY294002.
Acute Studies
In order to determine whether p44/42 MAPK mediates ANG II-induced activation of renal sympathetic nerve activity, animals were anesthetized to record RSNA, AP and HR during ICV injection of ANG II (100 ng/kg) before and after a one-hour ICV infusion of the p44/42 MAPK inhibitor PD98059 (20 µmol/L, 40µl/hr). These studies were conducted in baroreceptor-denervated rats to eliminate the confounding effects of baroreceptor-mediated changes on RSNA and HR.22 The recordings were carried out at least two hours after completion of the surgical preparation. Thirty minutes was allowed after the initial ICV ANG II injection for all variables to return to baseline. The second ANG II injection was made immediately after discontinuation of the ICV infusion of PD98059.
Additional studies were performed in anesthetized neurally intact rats to determine whether p44/42 MAPK mediates ANG II-induced activation of PVN neurons. These rats received a 1-hour ICV infusion of VEH, ANG II (10 ng/µL, 40 µL/h), or PD98059 (20 µmol/L, 40 µL/h) combined with ANG II (10 ng/µL, 40 µL/h). At the completion of the experiment, the brains were fixed with 4% paraformaldehyde and harvested and processed for Fra-like immunoreactivity in PVN by immunofluorescence.
Specific Methods
Please see online supplement at http://hyper.ahajournals.org.
Statistical Analysis
Electrophysiological and hemodynamic data were analyzed with Spike2 software. In studies of chronic HF and SHAM rats, responses of mean arterial pressure (MAP, mmHg), HR (bpm), RSNA and LSNA (mV) sampled over 5 min intervals during ICV infusion were compared with baseline values averaged over 5 min intervals immediately preceding each intervention. RSNA and LSNA responses are reported as percent changes in integrated voltage (mV) from baseline. In the acute studies, variables were sampled over 1-minute intervals. All values are expressed as the means ± SEM. The significance of differences among groups was analyzed by 2-way repeated-measure ANOVA followed by post hoc Fisher’s least significant difference test. Echocardiographic parameters were analyzed using 1-way ANOVA followed by Fisher’s least significant difference test. For immunohistochemical and immunofluorescent analysis, data were represented as positive cells per 104µm2 and analyzed using 1-way ANOVA followed by Fisher’s least significant difference test. The correlation coefficient of p44/42 active neurons and Fra-LI positive neurons was determined by regression analysis using SigmaPlot software. Differences were considered significant at P < 0.05.
RESULTS
Chronic Heart Failure Studies
Echocardiographic, hemodynamic and anatomical assessment of heart failure
The HF animals assigned to each group for treatment with MAPK inhibitors and PI3 kinase inhibitor versus VEH were well-matched with regard to echocardiographically defined left ventricular (LV) function (Table S1, please see http://hyper.ahajournals.org). The estimated size of the ischemic zone (%IZ) and the LV ejection fraction (LVEF) were not different in HF rats assigned to the different treatment groups. Compared with SHAM rats, LVEF was reduced and LV end-diastolic volume was increased in the rats with heart failure (Table S1, please see http://hyper.ahajournals.org).
Four weeks after myocardial infarction, LV peak systolic pressure, and maximum rate of rise of LV pressure (LV dP/dtmax) were lower, and LV end-diastolic pressure was higher, in VEH-treated HF than SHAM rats. The right ventricular weight/body weight (RV/BW) and lung weight/body weight (lung/BW) ratios were significantly higher in VEH-treated HF compared with SHAM rats. Neither the MAPK inhibitors PD98059, SP600125 and SB203580 nor the PI3 kinase inhibitor LY294002 affected these variables. (Table S1, please see http://hyper.ahajournals.org).
Effects of ICV p44/42 MAPK inhibitor PD98059 on hemodynamics and sympathetic nerve activity
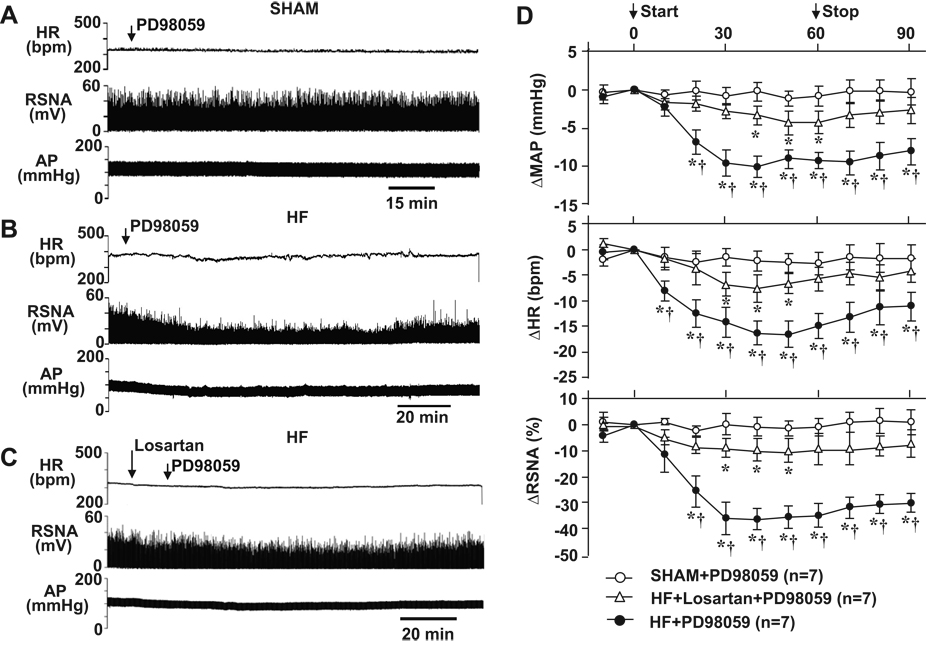
In SHAM rats (n=7), ICV infusion of PD98059, a selective p44/42 MAPK inhibitor, did not affect MAP, HR and RSNA (Figure 1A and 1D). In HF rats (n=8), however, a 1-hour ICV infusion of PD98059 substantially reduced MAP, HR and RSNA (Figure 1B and 1D). The maximum responses occurred 30–40 min after starting the ICV infusion of PD98059. MAP decreased from 99.6 ± 3.7 to 87.8 ± 3.1 mmHg, HR decreased from 367.3 ± 6.8 to 350.0 ± 5.8 bpm, and RSNA decreased by 37.2 ± 5.3 %. ICV administration of PD98059 had no effect on LSNA in the HF rats (Figure S1, please see http://hyper.ahajournals.org).
Figure 1.
Original tracings illustrating the effects of ICV p44/42 MAPK inhibitor PD98059 on AP, HR and RSNA in SHAM rats (A), HF rats (B) and HF rats pretreated with the angiotensin type-1 receptor antagonist losartan (C). (D), grouped data showing the changes from baseline in MAP, HR and RSNA elicited by ICV PD98059 in SHAM and HF rats. * P<0.05 compared with baseline; † P<0.05 compared with SHAM or losartan pretreated HF rats. AP: arterial pressure; MAP: mean arterial pressure; HR: heart rate; RSNA: renal sympathetic nerve activity (integrated voltage).
Pretreatment of HF rats with ICV AT1-R antagonist losartan significantly attenuated the inhibitory effects of PD98059. In losartan pretreated HF rats (n=7), ICV PD98059 induced a maximum responses 30–40 min after injection. MAP decreased from 94.8 ± 2.6 to 88.6 ± 2.5 mmHg, HR decreased from 343.7 ± 4.3 to 335.1 ± 4.1 bpm, and RSNA decreased by 16.8 ± 2.5 % (Figure 1C and 1D).
With both treatment protocols, MAP, HR and RSNA remained below baseline levels thirty minutes after the PD98059 infusion was stopped.
Effects of ICV p44/42 MAPK inhibitor UO126 on hemodynamics and RSNA
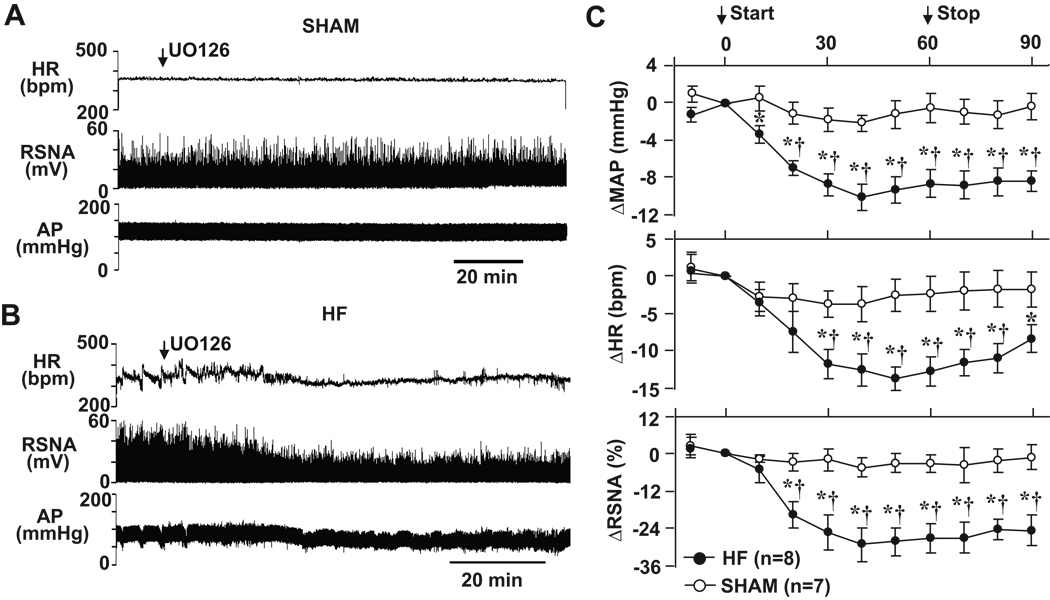
Similar to PD98059, ICV administration of UO126, another selective p44/42 MAPK inhibitor, induced a significant reduction in these variables in HF rats (n=8). The maximum changes compared to the baseline in MAP (−10.0 ± 2.3 mmHg), HR (−15.4 ± 3.2 bpm) and RSNA (−29.8 ± 6.2 %) occurred ~ 40 min after initiating the ICV infusion (Figure 2B and 2C). MAP, HR and RSNA remained below baseline levels thirty minutes after the infusion was stopped. In SHAM rats (n=7), ICV administration of UO126 had no obvious effect on MAP, HR or RSNA (Figure 2A and 2C).
Figure 2.
Original tracings illustrating the effects of ICV p44/42 MAPK inhibitor UO126 on AP, HR and RSNA in SHAM (A) and HF (B) rats. (C), grouped data showing the changes from baseline in MAP, HR and RSNA elicited by ICV UO126 in SHAM and HF rats. * P<0.05 compared with baseline; †P<0.05 compared with SHAM.
Effects of ICV p38 MAPK inhibitor, JNK and PI3 kinase inhibitors on hemodynamics and RSNA
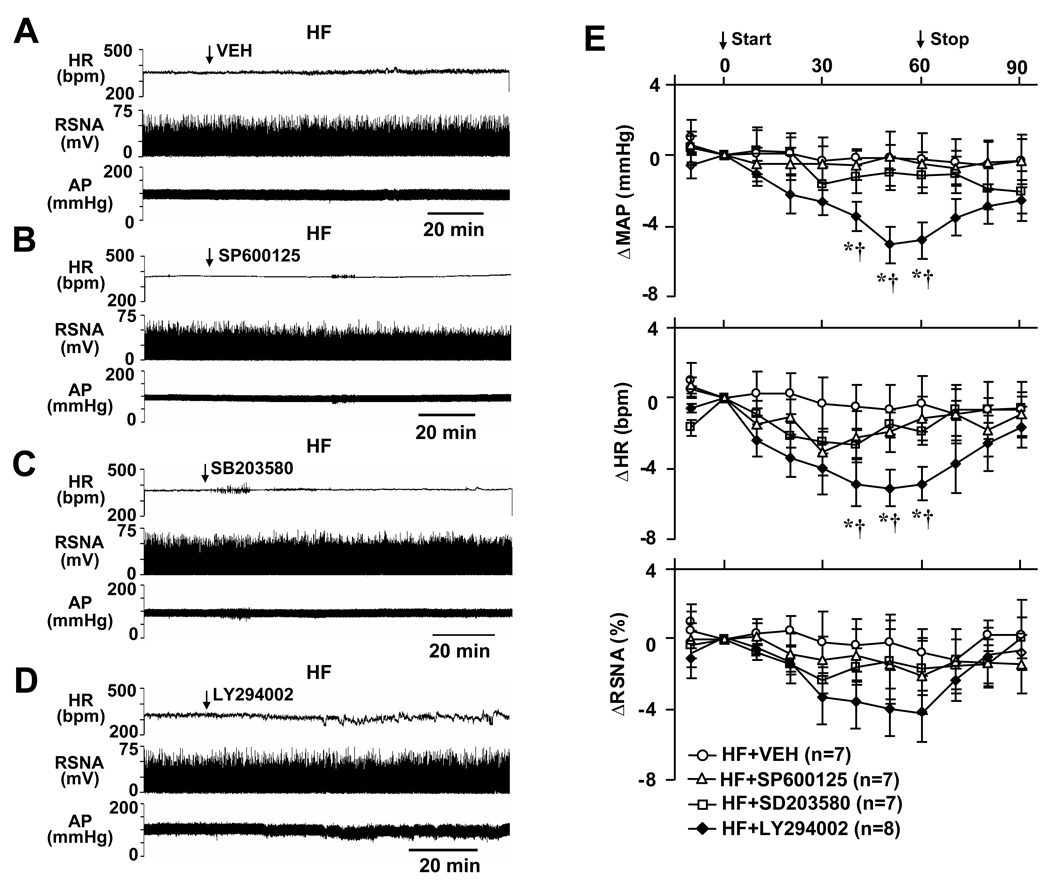
ICV administration of the VEH (n=7), the p38 MAPK inhibitor SB203580 (n=7) and c-Jun N-terminal kinase inhibitor SP600125 (n=7) had no effect on MAP, HR and RSNA in HF rats (Figure 3A, 3B, 3C and 3E). ICV injection of the PI3 kinase inhibitor LY294002 (n=8) induced a mild but significant decrease in MAP and HR 40–60 min after initiating the ICV administration, but had no effect on RSNA in HF rats (Figure 3D and 3E). Unlike the responses to PD98059 and UO126, these variables had returned to baseline level 20 min after the injection of LY294002. Since no apparent effect on RSNA was seen in HF rats, no SHAM rats were tested with these inhibitors.
Figure 3.
Original tracings showing AP, HR and RSNA in HF rats treated with ICV VEH (A), JNK inhibitor SP600125 (B), p38 MAPK inhibitor SB203580 (C) and PI3-kinase inhibitor LY294002 (D). (E), grouped data showing the changes from baseline in MAP, HR and RSNA elicited by ICV VEH, SP600125, SB203580 and LY294002 in HF rats. * P<0.05 compared with baseline; † P<0.05 compared with VEH treated HF rats.
Effects of ICV MAPK and PI3 kinase inhibitors on Fra-LI activity in PVN neurons
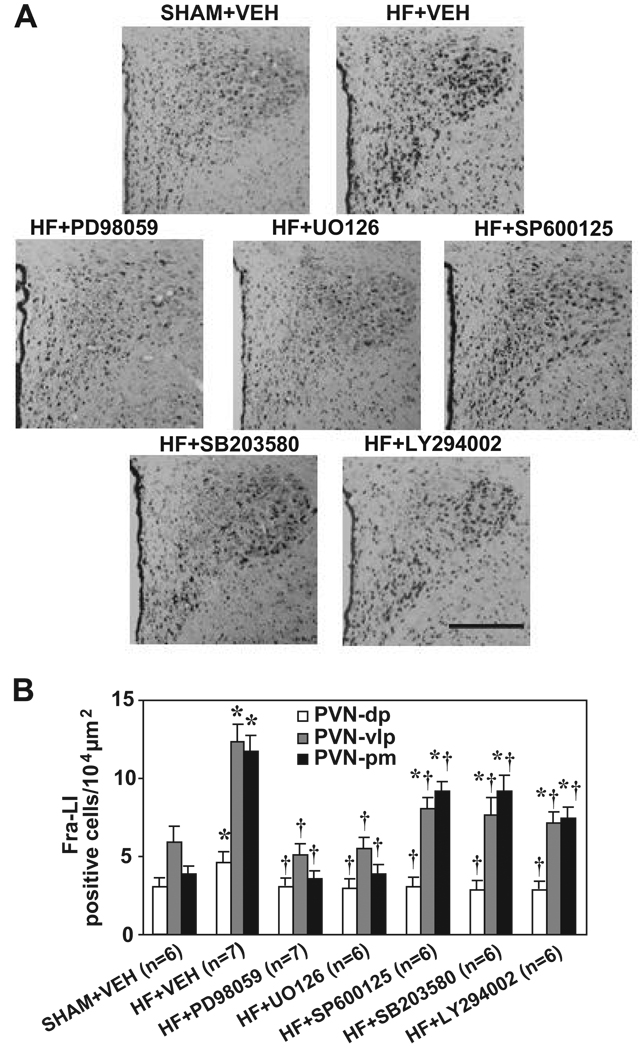
In VEH-treated HF rats (n=7), compared to SHAM rats (n=6), Fra-LI immunoreactivity was significantly increased in the dorsal parvocellular (PVN-dp), the ventrolateral parvocellular (PVN-vlp) and the posterior magnocellular (PVN-pm) subdivisions of PVN 23 (Figure 4A and 4B). The PVN-vlp and PVN-pm had a higher density of Fra-LI immunoreactivity than PVN-dp in both sham and HF rats. After treatment with p44/42 inhibitors PD98059 (n=7) or UO126 (n=6), the number of Fra-LI positive neurons in PVN-dp, PVN-vlp and PVN-pm was greatly decreased in HF rats (Figure 4A and 4B). ICV SP600125 (n=6), SB203580 (n=6) or LY294002 (n=6) also inhibited Fra-LI immunoreactivity in the PVN-dp, PVN-vlp and PVN-pm, but to a lesser degree than p44/42 MAPK inhibitors (Figure 4A and 4B).
Figure 4.
Immunohistochemical analysis of Fra-LI immunoreactivity in the PVN of VEH-treated SHAM rats, and of HF rats treated with ICV VEH, PD98059, UO126, SP600125, SB203580, and LY294002. (A) Representative sections of PVN from animals undergoing each treatment protocol (3rd ventricle to the left). Scale bar: 0.3 mm. (B) Grouped data showing numbers of Fra-LI positive neurons counted in ventrolateral parvocellular (PVN-vlp), dorsal parvocellular (PVN-dp) and posterior magnocellular (PVN-pm) subdivisions of PVN. Values are expressed as means ± SEM. * P<0.05, HF vs. SHAM; † P<0.05 Drug vs. VEH in HF rats.
Relationship between phosphorylated p44/42 MAPK and Fra-LI activity in PVN neurons
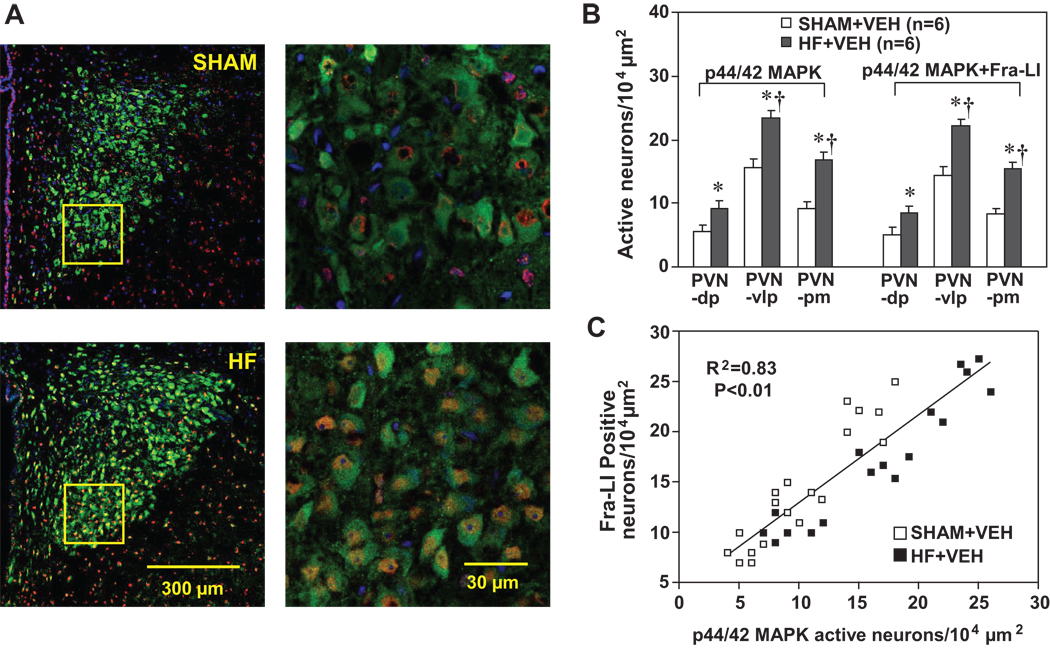
Confocal immunofluorescent images revealed an increase in p44/42 MAPK activity, indicated by the enhanced expression of phosphorylated p44/42 MAPK in the PVN (Figure 5A and 5B) in HF (n=6) compared with SHAM rats (n=6). The density of phosphorylated p44/42 MAPK-like-immunoreactivity was higher in PVN-vlp and PVN-pm than in PVN-dp in both SHAM and HF rats. The numbers of neurons in which p44/42 MAPK co-localized with Fra-LI activity were also increased in all three subdivisions of PVN in HF compared with SHAM rats (Figure 5A and 5B). Phosphorylated p44/42 MAPK-like-immunoreactivity co-localized with Fra-LI positivity in PVN neurons to a similar extent (>90%) in both SHAM and HF rats, but there were more double-labeled neurons in the PVN-vlp than PVN-pm or PVN-dp (Figure 5A and 5B). Regression analysis indicated that Fra-LI positivity correlated with p44/42 MAPK activity in PVN neurons in both SHAM and HF rats (Figure 5C).
Figure 5.
(A) Immunofluorescent images of the PVN, triple-labeled for Fra-LI (red), phosphorylated p44/42 MAPK (green) and nucleus (blue). Left panels, low power views from a SHAM (top) and a HF (bottom) rat, showing full expanse of PVN (unilateral, 3rd ventricle to the left). Right panels, high power views taken from the ventrolateral PVN of the same SHAM (top) and HF (bottom) rat, in the regions indicated by the yellow rectangles in left panels. Pink to purple appearance indicates the merge of red Fra-LI positive neurons with the blue nuclear marker.
(B) Grouped data showing numbers of PVN neurons positive for phosphorylated p44/42 MAPK (left) and for both p44/42 MAPK and Fra-LI-immunoreactivity (right) counted in ventrolateral parvocellular (PVN-vlp), dorsal parvocellular (PVN-dp) and posterior magnocellular (PVN-pm) subdivisions of PVN in SHAM and HF rats. Values are expressed as means ± SEM. * P<0.05, HF vs. SHAM; † P< 0.05, PVN-vlp, PVN-pm vs. PVN-dp in SHAM or HF.
(C) Correlation between the numbers of p44/42 MAPK active neurons and Fra-LI positive neurons in the PVN in SHAM and HF rats. The regression analysis included p44/42 MAPK active neurons and Fra-LI positive neurons in PVN-dp, PVN-vlp and PVN-pm from both SHAM and HF rats.
Acute Studies
Effects of PD98059 on ANG II-induced pressor responses in baroreceptor-denervated rats
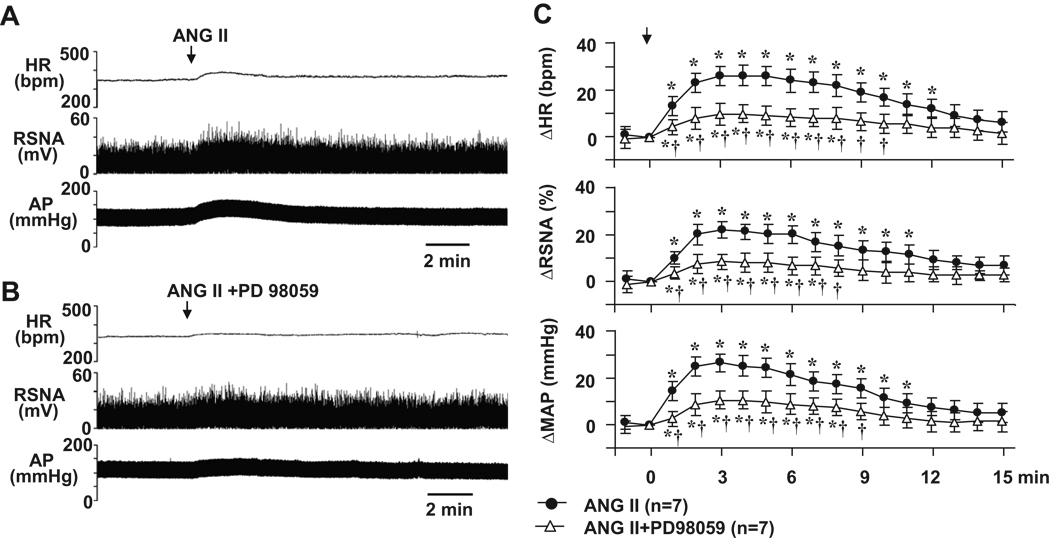
In baroreceptor-denervated rats (n=7), ICV administration of ANG II elicited significant increases in MAP, HR and RSNA (Figure 6A and 6C). The maximum increases in MAP (26.8 ± 3.7 mmHg, from baseline 101.6 ± 4.5 mmHg), HR (26.7 ± 4.2 bpm, from baseline 350.1 ± 12.8 bpm) and RSNA (22.2 ± 3.6 %) occurred 2–3 min after ICV injection of ANG II. Pre-treatment with PD98059 greatly attenuated ICV ANG II-induced pressor responses in MAP, HR and RSNA (Figure 6 B and 6C).
Figure 6.
Representative tracings showing the effects of ICV angiotensin II (ANG II) on AP, HR and RSNA in baroreceptor-denervated rats (A) and in baroreceptor-denervated rats pretreated with 1-hour ICV p44/42 MAPK inhibitor PD98059 (B). (C), grouped data demonstrating the changes from baseline in MAP, HR and RSNA elicited by ICV ANG II in baroreceptor-denervated rats before and after the ICV administration of PD98059. * P<0.05 compared with baseline; † P<0.05 compared with ICV ANG II alone in baroreceptor-denervated rats.
Effects of PD98059 on ANG II-induced Fra-LI activity in PVN in neurally intact rats
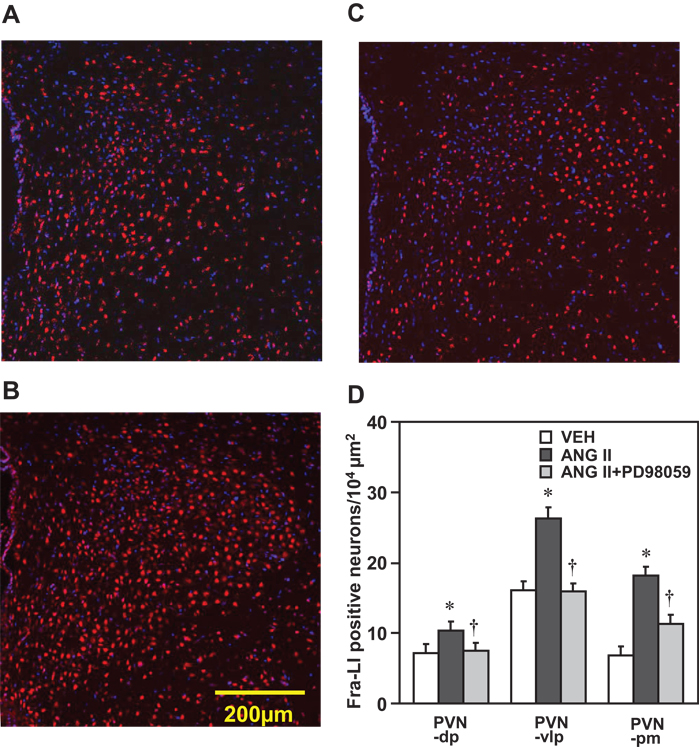
Confocal immunofluorescent images revealed that the number of Fra-LI positive neurons in the three subdivisions of PVN analyzed – PVN-dp, PVN-vlp and PVN-pm regions was significantly enhanced in the rats treated with ICV ANG II (n=6) compared with ICV VEH (n=6, Figure 7A, 7B and 7D). The increased Fra-LI activity in all three subdivisions of PVN induced by ICV administration of ANG II was attenuated by ICV p44/42 MAPK inhibitor PD98059 (n=6, Figure 7B, 7C and 7D).
Figure 7.
Confocal immunofluorescent images showing the double-labeled Fra-LI (red), nucleus (blue) in PVN (unilateral, 3rd ventricle to the left) in the rats treated with ICV administration of VEH (A), ANG II (B) and combined ANG II and PD98059 (C) for 1 hour. (D) Grouped data showing the numbers of Fra-LI positive neurons counted in ventrolateral parvocellular (PVN-vlp), dorsal parvocellular (PVN-dp) and magnocellular (PVN-pm) subdivisions of PVN in the rats treated with ICV VEH, ANG II, or combined ANG II and PD98059. Values are expressed as means ± SEM. * P<0.05, compared with VEH; † P<0.05 compared with ICV ANG II.
DISCUSSION
This study revealed an important and previously unrecognized functional role for the intracellular MAPK pathway. We found that p44/42 MAPK (ERK1/2) mediated ANG II-induced influences on sympathetic nerve activity and hemodynamics in heart failure. The data presented here are the first evidence of a critical role for the p44/42 MAPK signaling cascade in the maintenance of renal sympathetic excitation in HF rats.
In the present study, central administration of two different selective p44/42 MAPK inhibitors had the same effect in anesthetized rats with heart failure – a dramatic reduction in heart rate, arterial pressure and renal sympathetic nerve activity. In contrast, in sham animals neither of these inhibitors affected any of the measured variables. Thus, p44/42 MAPK has no apparent role in the maintenance of renal sympathetic nerve activity under basal conditions, even under the stress of anesthesia, but is a key factor mediating the augmented renal sympathetic nerve activity characteristic of heart failure. These profound differences in the responses of HF and SHAM rats are unlikely to be explained by differential effects of urethane anesthesia, but we cannot exclude that possibility.
Consistent with their effects on peripheral sympathetic nerve activity in this study, the p44/42 inhibitors also reduced neuronal excitation, as indicated by Fra-LI activity, in the PVN of HF rats. It is believed that parvocellular neurons of PVN, particularly the neurons located in the ventrolateral parvocellular subdivision that project to the spinal cord and the rostral ventrolateral medulla (RVLM), 24, 25 play an essential role in regulating sympathetic outflow. This study demonstrated co-localization of p44/42 MAPK in Fra-LI positive PVN neurons, including neurons in the ventrolateral parvocellular subdivision, and a reduction in Fra-LI positive PVN neurons in HF rats treated with the p44/42 MAPK inhibitors. These immunohistochemical data strongly support the interpretation that p44/42 MAPK signaling contributes to activation of PVN neurons and sympathetic excitation in heart failure. However, PVN is not the only nucleus in the brain containing pre-sympathetic neurons. p44/42 MAPK may also mediate the effects of ANG II on pre-sympathetic neurons in RVLM, or neurons in other cardiovascular and autonomic centers, including the subfornical organ, the median preoptic nucleus and the organum vasculosum of the lamina terminalis, 26 which impinge on pre-sympathetic neurons.
ANG II may be the primary stressor driving the p44/42 MAPK-mediated renal sympathetic excitation in heart failure. In heart failure rats, the effect of the p44/42 MAPK inhibitor is markedly diminished following pretreatment with an AT1-R antagonist. The acute studies in normal rats support this suggestion: both the pressor responses and the Fra-LI activity elicited by ICV ANG II were greatly attenuated by p44/42 MAPK inhibitor. This concept is consistent with the studies of others, demonstrating that p44/42 MAPK played an important role in maintaining high blood pressure in chronic angiotensin-infused rats,27 and that bilateral injection of PD98059 into the RVLM abolished the pressor response to exogenous ANG II in the RVLM in SHR and WKY rats.28 It is also consistent with the previous observation that AT1-R in the RVLM seem to have little impact on sympathetic drive under normal conditions but have prominent influence on sympathetic drive under conditions of stress.29 However, ANG II may not be the only signal activating MAPK in heart failure. Pretreatment with ICV losartan significantly attenuated but did not completely block the inhibitory effects of the p44/42 inhibitor PD98059. A recent study demonstrated a possible ROS-dependent intracellular MAPK pathway in RVLM neurons.30
How ANG II-driven MAPK activity elicits an increase in sympathetic drive remains a mystery. MAPK may regulate sympathetic nerve activity by modulating ion channels activity 31 in pre-sympathetic neurons. However, the time course of the change in RSNA in response to the p44/42 MAPK inhibitors suggests a longer-term modulating effect. The sustained augmentation of renal sympathetic nerve activity in heart failure 2 is more likely due to mechanisms that regulate gene expression and proteins synthesis. These might include sodium or potassium ion channel protein subunits, or angiotensin receptors. In a previous study, 8 we found that activated p44/42 MAPK upregulated AT1-R in the forebrain PVN and subfornical organ in HF. Increased ANG II binding to AT1-R might account for an increase in sympathetic excitation. The MAPK signaling cascade may also affect the synthesis and release of certain key neurotransmitters such as norepinephrine and dopamine in pre-sympathetic regions of the brain.12 Finally, p44/42 MAPK activity is linked to reactive oxygen species 32, 33 and proinflammatory cytokines,34 which are also stimulated by ANG II. A growing body of evidence indicates that ROS increases sympathetic excitation.35
Other kinases in the MAPK family, such as p38 MAPK and JNK, are activated in heart failure,8 but inhibiting these kinases did not affect renal sympathetic nerve activity even at a dose 5-fold higher than the p44/42 inhibitors. Similarly, blockade of PI3 kinase activation by its inhibitor LY294002 did not significantly change renal sympathetic nerve activity in heart failure. These findings suggest that p44/42 MAPK has an independent and distinctive influence on renal sympathetic outflow in heart failure.
The p44/42 MAPK inhibitors had no effect on lumbar sympathetic nerve activity in the heart failure rats though both heart rate and mean arterial pressure were reduced (Figure S1, please see http://hyper.ahajournals.org). This observation suggests a relative selectivity of the p44/42 MAPK activity in mediating sympathetic discharge. A preferential amplification of renal sympathetic discharge, affecting sodium and water excretion and renin release, might be expected in heart failure. A similar MAPK-dependent differential regulation is found in the regional sympathetic response to insulin:36 insulin-induced sympathetic activation to brown adipose tissue is mediated by MAPK, while the insulin-induced enhanced lumbar nervous sympathetic activity is mediated by PI3 kinase. Neither MAPK nor PI3 kinase was implicated in the insulin-mediated activation of renal or adrenal sympathetic nerve activity.36
The arterial pressure responses of the HF rats to the p44/42 MAPK inhibitors are very consistent with the findings from a previous study 28 in spontaneously hypertensive rats, showing that bilateral microinjection of PD98059 into the RVLM markedly decreased arterial pressure. However, the results in normal control rats are conflicting. Microinjection of PD98059 in RVLM lowered arterial pressure in Wistar-Kyoto rats,28 while we found no effect in SHAM Sprague-Dawley rats. It should be noted, however, that the measurements from the Wistar-Kyoto rats28 were acquired during a 14-hour infusion of PD98059. At 0.25 hour after initiating the infusion, blood pressure was not significantly decreased; the significant change in blood pressure was observed 3 hours into PD98059 administration. Our results in sham-operated Sprague-Dawley rats showed no effect of PD98059 after a 1-hour infusion, a time point not measured in that study. We observed a mild decrease in arterial pressure and heart rate in HF rats with the PI3 kinase inhibitor LY294002, less dramatic than the results reported with a different PI3 kinase inhibitor in the SHR,28 confirming some role for PI3 kinase in cardiovascular regulation. Differences in the results of these two studies may reflect differences in rat strains, experimental conditions and protocols.
Perspectives
The present study demonstrates for the first time that intracellular p44/42 MAPK signaling pathway plays a pivotal role in mediating the effects of the brain renin-angiotensin system on sympathetic nerve activity in heart failure. Since inhibition of p44/42 MAPK affects renal but not lumbar sympathetic nerve activity in heart failure rats, the central p44/42 MAPK signaling pathway may have a predominant influence on the regulation of renal function. These findings suggest that manipulating brain p44/42 MAPK signaling can ameliorate the adverse effects of the brain renin-angiotensin system on cardiovascular and renal function during the progression of heart failure.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by a Merit Review award (to RBF) from the Department of Veterans Affairs, by HL-073986 (to RBF) from the National Institutes of Health, and by institutional funds provided by the University of Iowa.
Footnotes
Disclosures
None
REFERENCES
- 1.Cohn JN. Abnormalities of peripheral sympathetic nervous system control in congestive heart failure. Circulation. 1990;82:I59–I67. [PubMed] [Google Scholar]
- 2.Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1734–R1745. doi: 10.1152/ajpregu.2001.281.5.R1734. [DOI] [PubMed] [Google Scholar]
- 3.Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248–254. doi: 10.1016/0735-1097(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 4.Pinto YM, Buikema H, van Gilst WH, Lie KI. Activated tissue renin-angiotensin systems add to the progression of heart failure. Basic Res Cardiol. 1996;91 Suppl 2:85–90. doi: 10.1007/BF00795368. [DOI] [PubMed] [Google Scholar]
- 5.Francis J, Wei SG, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Endocrinol Metab. 2004;287:H2138–H2146. doi: 10.1152/ajpheart.00112.2004. [DOI] [PubMed] [Google Scholar]
- 6.Francis GS. The relationship of the sympathetic nervous system and the renin-angiotensin system in congestive heart failure. Am Heart J. 1989;118:642–648. doi: 10.1016/0002-8703(89)90291-3. [DOI] [PubMed] [Google Scholar]
- 7.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol. 2004;84:217–232. doi: 10.1016/j.pbiomolbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Wei SG, Yu Y, Felder RB. Activated mitogen-activated protein kinase contributes to the up-regulation of angiotensin type 1 receptors in the brain of heart failure rats. Circulation. 2006;114:310. (abstract) [Google Scholar]
- 9.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 10.Pellieux C, Sauthier T, Aubert JF, Brunner HR, Pedrazzini T. Angiotensin II-induced cardiac hypertrophy is associated with different mitogen-activated protein kinase activation in normotensive and hypertensive mice. J Hypertens. 2000;18:1307–1317. doi: 10.1097/00004872-200018090-00017. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri ND, Xu ZG, Shahkarami A, Huang KT, Rodriguez-Iturbe B, Natarajan R. Role of AT-1 receptor in regulation of vascular MCP-1, IL-6, PAI-1, MAP kinase, and matrix expressions in obesity. Kidney Int. 2005;68:2787–2793. doi: 10.1111/j.1523-1755.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- 12.Lu D, Yang H, Raizada MK. Angiotensin II regulation of neuromodulation: downstream signaling mechanism from activation of mitogen-activated protein kinase. J Cell Biol. 1996;135:1609–1617. doi: 10.1083/jcb.135.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dugourd C, Gervais M, Corvol P, Monnot C. Akt is a major downstream target of PI3-kinase involved in angiotensin II-induced proliferation. Hypertension. 2003;41:882–890. doi: 10.1161/01.HYP.0000060821.62417.35. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Raizada MK. Role of phosphatidylinositol 3-kinase in angiotensin II regulation of norepinephrine neuromodulation in brain neurons of the spontaneously hypertensive rat. J Neurosci. 1999;19:2413–2423. doi: 10.1523/JNEUROSCI.19-07-02413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boone E, Vandevoorde V, De Wilde G, Haegeman G. Activation of p42/p44 mitogen-activated protein kinases (MAPK) and p38 MAPK by tumor necrosis factor (TNF) is mediated through the death domain of the 55-kDa TNF receptor. FEBS Lett. 1998;441:275–280. doi: 10.1016/s0014-5793(98)01567-1. [DOI] [PubMed] [Google Scholar]
- 16.Viedt C, Soto U, Krieger-Brauer HI, Fei J, Elsing C, Kubler W, Kreuzer J. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol. 2000;20:940–948. doi: 10.1161/01.atv.20.4.940. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- 18.Vahid-Ansari F, Leenen FH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol Heart Circ Physiol. 1998;275:H2140–H2146. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- 19.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 20.Loewy AD. Descending pathways to the sympathetic preganglionic neurons. Prog Brain Res. 1982;57:267–277. doi: 10.1016/S0079-6123(08)64133-3. [DOI] [PubMed] [Google Scholar]
- 21.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283:R281–R283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- 22.Wei SG, Felder RB. Forebrain renin-angiotensin system has a tonic excitatory influence on renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2002;282:H890–H895. doi: 10.1152/ajpheart.2002.282.3.H890. [DOI] [PubMed] [Google Scholar]
- 23.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1172–R1183. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- 24.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 25.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Dev Brain Res. 1998;801:239–243. doi: 10.1016/s0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 26.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 27.Muthalif MM, Karzoun NA, Gaber L, Khandekar Z, Benter IF, Saeed AE, Parmentier JH, Estes A, Malik KU. Angiotensin II-induced hypertension: contribution of Ras GTPase/Mitogen-activated protein kinase and cytochrome P450 metabolites. Hypertension. 2000;36:604–609. doi: 10.1161/01.hyp.36.4.604. [DOI] [PubMed] [Google Scholar]
- 28.Seyedabadi M, Goodchild AK, Pilowsky PM. Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension. 2001;38:1087–1092. doi: 10.1161/hy1101.096054. [DOI] [PubMed] [Google Scholar]
- 29.Sheriff MJ, Fontes MA, Killinger S, Horiuchi J, Dampney RA. Blockade of AT1 receptors in the rostral ventrolateral medulla increases sympathetic activity under hypoxic conditions. Am J Physiol Regul Integr Comp Physiol. 2006;290:R733–R740. doi: 10.1152/ajpregu.00410.2005. [DOI] [PubMed] [Google Scholar]
- 30.Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- 31.Schrader LA, Birnbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, Sweatt JD. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am J Physiol Cell Physiol. 2006;290:C852–C861. doi: 10.1152/ajpcell.00358.2005. [DOI] [PubMed] [Google Scholar]
- 32.Miura S, Zhang J, Matsuo Y, Saku K, Karnik SS. Activation of extracellular signal-activated kinase by angiotensin II-induced Gq-independent epidermal growth factor receptor transactivation. Hypertens Res. 2004;27:765–770. doi: 10.1291/hypres.27.765. [DOI] [PubMed] [Google Scholar]
- 33.Zucker IH, Gao L. The regulation of sympathetic nerve activity by angiotensin II involves reactive oxygen species and MAPK. Circ Res. 2005;97:737–739. doi: 10.1161/01.RES.0000188261.94569.1f. [DOI] [PubMed] [Google Scholar]
- 34.Dziennis S, Habecker BA. Cytokine suppression of dopamine-beta-hydroxylase by extracellular signal-regulated kinase-dependent and -independent pathways. J Biol Chem. 2003;278:15897–15904. doi: 10.1074/jbc.M212480200. [DOI] [PubMed] [Google Scholar]
- 35.Ye S, Zhong H, Duong VN, Campese VM. Losartan reduces central and peripheral sympathetic nerve activity in a rat model of neurogenic hypertension. Hypertension. 2002;39:1101–1106. doi: 10.1161/01.hyp.0000018590.26853.c7. [DOI] [PubMed] [Google Scholar]
- 36.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.