Abstract
The purpose of this study was to test the hypothesis that, in idiopathic minimal lesion nephrotic syndrome (IMLNS), the T regulatory (T reg) cell suppressor mechanism is deficient, thereby enhancing cytokine release by T effector cells. Twenty-one patients with IMLNS, eight healthy controls and two patients with nephrotic syndrome and membranoproliferative glomerulonephritis were studied. The percentage of T reg cells was similar in the healthy controls and in patients with IMLNS in relapse or in remission. Thymidine incorporation in autologous T effector cells, as well as expression of the regulatory cytokine interleukin (IL)-10, was significantly reduced in patients in relapse when compared with patients in remission and healthy subjects. IL-2 expression was also reduced in patients in relapse but did not achieve statistical significance. In a different set of experiments, T cells, from subjects with IMLNS in remission, when stimulated with antiCD3-antiCD28 antibodies, secreted increased levels of cytokines. No such increase in cytokines was observed when cells from healthy controls were stimulated with same mitogen. The impaired T reg cell function observed in these patients may have pathogenic and therapeutic implications, because it could explain the persistence of the proposed pathogenic cytokines observed in the patients with IMLNS.
Keywords: Minimal lesion nephrotic syndrome, T regulatory cell, Cytokines
Introduction
Idiopathic minimal lesion nephrotic syndrome (IMLNS), the most common type of nephrotic syndrome in children and adolescents, is currently considered an immune mediated disease [1]. In 1974 Shalhoub proposed the hypothesis that IMLNS was a T cell disorder [2]. Circulating T cells were postulated to release cytokine(s) that reached the glomerulus and induced an increase in permeability to plasma proteins. Indirect evidence for this hypothesis was supported by the absence of humoral (immunoglobulins and complement) components in glomeruli, the often prompt response to treatment with agents known to inhibit T cell function (corticosteroids, cyclosporine, cyclophosphamide, mycophenolate), the association of remission following measles infection (which is known to depress T cell immunity), and the association with T cell disorders, such as Hodgkin’s lymphoma [2].
A specific pathogenic cytokine has not yet been identified, but several cytokines known to be elevated in the serum of patients with IMLNS during relapse have been shown to increase glomerular permeability to plasma proteins, among them interleukin (IL)-8 [3], 100 kDa glycoprotein [4], IL-13 [5], and a cytokine described by Koyama et al. [6]. These latter authors were able to immortalize T cells from patients with IMLNS and show that the T cell culture supernatants could induce massive proteinuria in rats.
Normally, the expression and release of cytokines by T cells is transient, due to the activation of T regulatory (T reg) cells that act on the T effector (T eff) cell to suppress their production of cytokines [7–9]. The purpose of this study was to test the hypothesis that, in IMLNS, the T reg cells suppressor mechanism is deficient, thereby allowing the T eff cells, after stimulation, to secrete excessive amounts of cytokines. The impaired T reg cell function in these patients may have pathogenic and therapeutic implications, because it could explain the persistence of the proposed pathogenic cytokines observed in patients with IMLNS.
Subjects and methods
Subjects
The study included two different sets of tests involving two different groups of patients. A total of 31 individuals participated in the study. Twenty-two patients participated in T cell suppression studies, and nine individuals were included in the cytokine production analyses.
-
Suppression studies (Table 1). Sixteen patients with biopsy proven IMLNS (eight in relapse and eight in remission), four healthy controls and two patients with nephrotic syndrome and membranoproliferative glomerulonephritis were included in this phase of the study.
Immunosuppressive therapy at the time of the study is shown for each patient in Table 1. Relapse was defined as proteinuria [> 3.0 urinary protein (in milligrams)/creatinine (in milligrams) ratio or 3 + or greater by colorimetric dipstick test with tetrabromophenol–citrate buffer] and a concomitant serum albumin level < 3.0 g/l. Remission was defined as a urinary protein/creatinine ratio <0.2 and a serum albumin level> 3.5 g/l. Definitions of glomerular diseases were based on established criteria according to the International Study for Kidney Diseases in Children [10].
-
Cytokine production by mononuclear cells in peripheral blood of patients with IMLNS and in that of controls (Table 2). Five patients (aged 38–57 years) with IMLNS in remission and off immunosuppressive therapy and four healthy controls (aged 23–48 years) were studied.
The study was approved by the Institutional Review Board of the University of Florida, USA, and informed consent was obtained from each patient.
Table 1.
Clinical data of patients undergoing suppression studies (Up/Uc urinary protein/creatinine ratio, M male, F female, MPGN membranoproliferative glomerulonephritis, Pred prednisone, TAC tacrolimus, MMF mycophenolate mofetil, CsA cyclosporine A, N/A not applicable)
| Patients | Age (years) | Gender | Diagnosis | Up/Uc | Albumin (g/dl) | Therapy |
|---|---|---|---|---|---|---|
| 1 | 7 | M | Control | Negative | None | |
| 2 | 5 | M | Control | 0.06 | 5 | None |
| 3 | 11 | M | Control | Negative | None | |
| 4 | 6 | F | Control | Negative | 4.1 | None |
| 5 | 3 | F | IMLNS remission | 0.2 | 4.1 | None |
| 6 | 50 | F | IMLNS remission | 0.03 | Pred 1 mg every other day | |
| 7 | 20 | F | IMLNS remission | 0.2 | 4.4 | Pred 10 mg every day, TAC 2 mg twice a day |
| 8 | 6 | M | IMLNS remission | 0.4 | 4.4 | None |
| 9 | 3 | M | IMLNS remission | 0.15 | 3.1 | Pred 30 mg every day |
| 10 | 3 | F | IMLNS remission | 0.26 | 4.3 | Pred 3 mg every other day, CsA 40 mg twice a day |
| 11 | 21 | F | IMLNS remission | 0.05 | 4.8 | Pred 10 mg every other day, MMF 500 mg twice a day, TAC 2 mg twice a day |
| 12 | 9 | M | IMLNS remission | 0.08 | 4.8 | Pred 10 mg every other day |
| 13 | 12 | M | IMLNS relapse | 14.9 | 1.9 | None |
| 14 | 5 | M | IMLNS relapse | 16.7 | 2.7 | None |
| 15 | 5 | F | IMLNS relapse | 3+ | N/A | Pred 5 mg every other day |
| 16 | 3 | M | IMLNS relapse | 41 | 1 | None |
| 17 | 7 | F | IMLNS relapse | 6.57 | 1.9 | None |
| 18 | 9 | F | IMLNS relapse | 4.4 | 2.9 | Pred 45 mg every day |
| 19 | 2 | F | IMLNS relapse | 17 | 2.6 | None |
| 20 | 14 | M | IMLNS relapse | 2.25 | 3.3 | None |
| 21 | 7 | F | MPGN | 1.5 | 2.8 | Methylprednisolone 10 mg/kg |
| 22 | 7 | F | MPGN | 2.1 | 3.8 | None |
Table 2.
Clinical data of patients undergoing cytokine production studies (Up/Uc urinary protein/creatinine ratio, F female, M male, Pred prednisone, ND none detected)
| Patients | Age (years) | Gender | Diagnosis | Up/Uc | Albumin (g/dl) | Therapy |
|---|---|---|---|---|---|---|
| 1 | 24 | F | Control | Negative | ND | None |
| 2 | 38 | M | Control | Negative | ND | None |
| 3 | 35 | F | Control | Negative | ND | None |
| 4 | 33 | M | Control | Negative | ND | None |
| 5 | 44 | F | IMLNS remission | 0.12 | 4.7 | Pred 50 mg every other day |
| 6 | 48 | F | IMLNS remission | 6.35 | 1.8 | None |
| 7 | 57 | F | IMLNS remission | 4.01 | 3.1 | None |
| 8 | 46 | F | IMLNS remission | Negative | 4.1 | None |
Methods
Flow cytometric analysis was undertaken and forkhead box p3 (Foxp3) expression was investigated (Fig. 1) [11]. For flow cytometry, whole blood was collected in K-EDTA S-Monovette tubes (Sarstedt, Newton, NC, USA) and immediately subjected to cellular staining. Whole blood (100 μl) was measured (per tube), together with 20 μl each of appropriate test antibody, fluorescein isothiocyanate anti-CD3 (clone HIT3a), allophycocyanin (APC) anti-CD4 (SK3), phycoerythrin (PE) anti-CD25 (M-A251), and allophycocyanin (APC)-FOXP3 (clone PCH101). The following isotype control antibodies were used: fluorescein isothiocyanate mouse IgG1 (MOPC-21), PerCP mouse IgG1 (MOPC-21), PE mouse IgG1 (MOPC-21), APC-labeled mouse immunoglobulin (Ig)G1 (MOPC-31C), mouse IgG2A (G155-78), and mouse IgG2B (clone 27–35). All antibodies for cytometric analyses were purchased from BD Biosciences (San Jose, CA, USA), with the exception of FOXP3 (eBioscience, San Diego, CA, USA). After surface staining for 30 min (4°C), erythrocytes were lysed and cells were fixed for 10 min at room temperature (BD FACS lysing solution) followed by two washes with stain buffer containing 0.2% bovine serum albumin (BSA). Surface-stained cells then underwent intracellular FOXP3 staining with the anti-human FOXP3 staining kit, according to the manufacturer’s recommendations. Stained cells were then subjected to flow cytometric analysis with a BD FACSCalibur flow cytometer with 1.5 × 105 cells acquired per test. FCS Express (version 2.200.0023; De Novo Software, Thornhill, ON, Canada) was used for analysis of cytometric data.
Fig. 1.
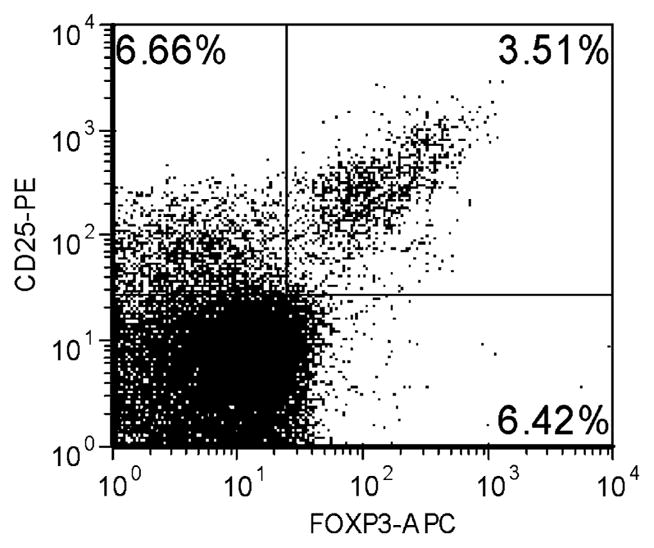
Representative flow cytometric plot of a healthy control subject showing expression of FOXP3
Cell purification
Peripheral blood was collected in Vacutainer tubes containing sodium heparin. An accessory cell population (>98% T cell depleted) was produced by incubation of an aliquot of blood with a T cell depletion antibody cocktail (StemCell, Vancouver, BC, Canada) followed by density gradient centrifugation and subsequent irradiation (3,300 rad). The CD4+ T cell population was purified by negative selection using a CD4+ T enrichment cocktail (StemCell). After purification and washing in phosphate-buffered saline (PBS) solution containing 2% AB serum, the ‘untouched’ CD4+ population underwent a positive selection for CD4+CD25+ T reg cells (90% pure) using CD25 microbeads (Mitenyi Biotech, Bergisch, Germany) with separation on the AutoMACS sorter (Mitenyi). The unlabeled CD4+CD25− population (>98% pure) provided the CD4+CD25− T eff cell population for use in suppression assays.
Suppression assay
A suppression assay was developed to test the capacity of CD4+CD25+ T reg cells to suppress the proliferation of co-cultured T eff cells [11]. Regulatory T cells were added in decreasing ratios (1:0, 1:1, and 0:1) to a constant number of T eff cells (5×103 cells per well). A combination of 5 μg/ml soluble anti-CD3 and 2.5 μg/ml soluble anti-CD28 (eBioscience) antibodies provided the polyclonal stimulus for proliferation over a 5-day culture period. The 5×104 irradiated T cell-depleted accessory cells were also added to each well in a total volume of 200 μl. One γCi 3H-thymidine (Amersham Biosciences, Piscataway, NJ, USA) was added at the end of the 5-day period for the final 16 h culture to assess proliferation. Supernatants from three to six replicate wells were collected for each condition at day 5, just before the addition of 3H-thymidine to assess cytokine production. Suppression was determined by the reduction of 3H-thymidine incorporation in the combination of cells and was calculated by the following equation: percent suppression = [1− (mean counts per minute T reg + T eff)/(mean counts per minute T eff × 100%)].
Cytokine profile
The cytokine profile from the suppression assay was assessed on supernatants collected at the end of the 5-day incubation period with a commercially available multiplex kit (Beadlyte human multi-cytokine detection system 3, Upstate Biotechnology, Waltham, VA, USA) and the Luminex (100) LabMAP system (Austin, TX, USA). Levels of IL-2 and IL-10 were simultaneously determined. Transforming growth factor-beta 1 (TGF-β1) levels were determined by standard enzyme-linked immunoassay (Bioscience, Camarillo, CA, USA).
Cytokine production by mononuclear cells in peripheral blood of patients with IMLNS and in that of controls
Mononuclear cells in peripheral blood were separated by Ficoll-Hypaque gradient and cultured in triplicate in Roswell Park Memorial Institute (RPMI) culture medium with 5% AB serum at the concentration of 1×105 cells/ml per 200 μl per well. Cells were stimulated with antiCD3-antiCD28 antibodies (5 μg/ml and 2.5 μg/ml, respectively) (eBioscience). Twenty-four hours later, culture was terminated and the supernatant was recovered. The following cytokines were measured with the Luminex (100) system (LabMAP): IL-2, IL-8, IL-13, interferon-gamma (IFN)-γ, and tumor necrosis factor-alpha (TNF-α).
Statistical analysis
Statistical analyses were performed by nonparametric tests, including the Kruskal–Wallis one-way analysis of variance and the Mann–Whitney U test. For paired data, the Wilcoxon test was used. Receiver operator characteristic curve analysis was used to determine possible cut off values to differentiate patients with IMLNS from healthy subjects. All data are presented as means ± standard errors of the means (SEMs).
Results
-
Suppression studies
T reg cells frequency. Four healthy controls and six patients with IMLNS demonstrated similar percentages of T reg cells (4.0±0.5 and 4.3±0.6, respectively).
Thymidine incorporation. In contrast, the ability of T reg cells to suppress thymidine incorporation in autologous T eff cells was significantly reduced in patients with IMLNS in relapse when compared with that in control subjects (P<0.01). The suppressive capacity of T reg cells obtained from patients with IMLNS in remission was not significantly different from that observed in control subjects (P=0.23). The two patients with membranoproliferative glomerulonephritis (MPGN) and nephrotic syndrome had a suppressive capacity of T reg cells similar to that observed in healthy subjects (Fig. 2).
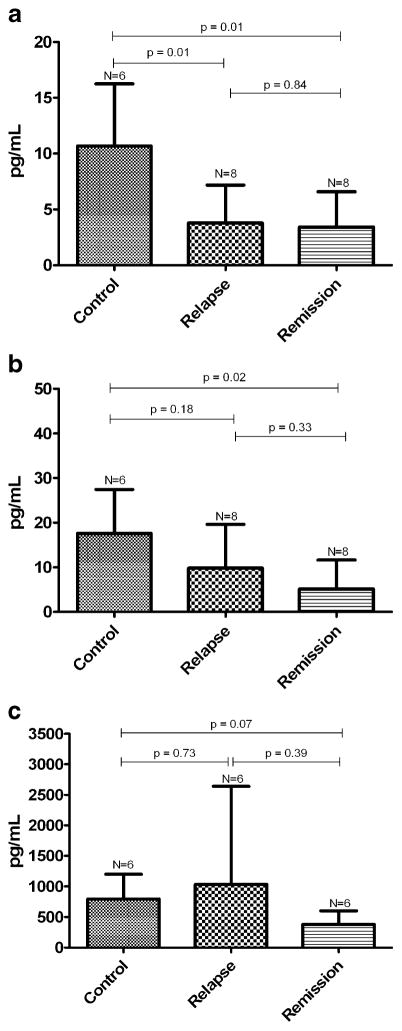
Release of regulatory cytokines by T cell co-cultures. After 5 days of incubation, the supernatant concentration of IL-10, IL-2, and TGF-β by T cells from patients with IMLNS in relapse was compared with that observed in healthy controls and patients with IMLNS in remission. Figure 3 shows supernatant concentrations of the tested cytokines (in picograms per milliliter) after the co-culturing of T reg cells and autologous T eff cells (1:1) for 5 days. A significant reduction in IL-10 (P<0.0191) was observed in supernatants from patients with IMLNS in relapse and in remission when compared with those from healthy subjects (Fig. 3a). No differences were found between patients with IMLNS in relapse and in remission. Receiver operator curve (ROC) analyses were used to establish a cut-off value for IL-10 in the differentiation between patients with IMLNS and control subjects. A value less than 7.6 pg/ml, which represented the upper limit found in patients with IMLNS, had 84% sensitivity and 83% specificity for differentiating individuals with IMLNS from healthy individuals (likelihood ratio 5.08, P=0.001).
When IL-2 response was studied, IL-2 was not significantly reduced in patients with IMLNS in relapse when compared with that in healthy controls (P=0.18) or when compared with that in patients with IMLNS in remission (P=0.33). However, IL-2 concentration was significantly reduced in patients in remission (P=0.02) when compared with normal controls (Fig. 3b).
When TGF-β supernatant concentrations were compared between patients in relapse and normal subjects or patients in remission, no significant differences were found among the groups (Fig. 3c).
Cytokine production by mononuclear cells in peripheral blood of patients with IMLNS and of controls (Table 2). The reduction in regulatory cytokines observed in the supernatant of co-cultured T reg and T eff cells in previous experiments contrasted with the cytokine pattern observed in supernatants from peripheral blood mononuclear cell (PBMC) cultures from patients with IMLNS and normal controls stimulated with antiCD3- antiCD28 antibodies. In these studies, stimulated PBMC supernatants from IMLNS patients in remission displayed significantly higher levels of IL-8, TNF-α and IL-2 after stimulation. No such statistically significant increase in these cytokine levels was observed when PBMCs from healthy control subjects were stimulated with antiCD3- antiCD28 antibodies (Table 3). No statistically significant differences were seen before and after stimulation in both nephrotic patients and controls with respect to IFN-γ and IL-13.
Fig. 2.
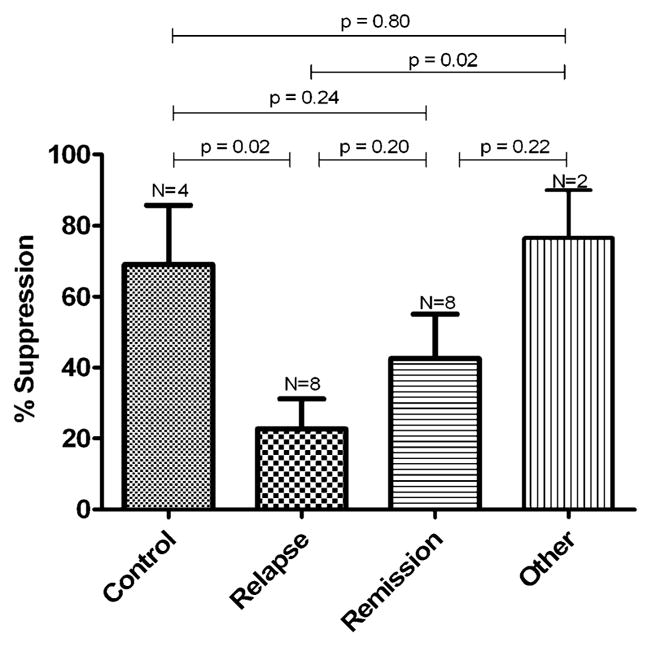
Percentage of suppression of T reg 3H-thymidine incorporation in healthy subjects, patients with IMLNS in relapse and remission, and nephrotic syndrome from other glomerulopathies
Fig 3.
a IL-10 in supernatants of T reg and T eff cell co-cultures from patients with IMLNS (relapse and remission) and healthy controls. b IL-2 in supernatants of T reg and T eff cell co-cultures from IMLNS patients (relapse and remission) and healthy controls. c TGF-β in supernatants of T reg and T eff cell co-cultures from IMLNS patients (relapse and remission) and healthy controls
Table 3.
IFN-γ, IL-13, IL-2, IL-8 and TNF-α levels in PBMC cultures after stimulation with anti-CD3/CD28 antibody, from patients with IMLNS in relapse and in healthy controls. The study included five patients with IMLNS in remission and four healthy subjects
| Cytokine | Patients with IMLNS |
Healthy subjects |
||||
|---|---|---|---|---|---|---|
| Cells only | AntiCD3/CD28 | P | Cells only | AntiCD3/CD28 | P | |
| INF-γ | 14.2±1.9 | 2982±2474 | 0.09 | 38±20 | 482±729 | 0.30 |
| IL-13 | 12.7±0.3 | 165±184 | 0.19 | 12.62±0.06 | 50.50±38.18 | 0.39 |
| IL-2 | 10.8±0.7 | 1275±411 | 0.01 | 3.65±5.45 | 558±991 | 0.20 |
| IL-8 | 3952±508 | 5160±525 | 0.01 | 3025±2409 | 2977±2411 | 0.60 |
| TNF-α | 15.1±3.5 | 681±250 | 0.01 | 37.2±37.9 | 245±353 | 0.22 |
Discussion
Regulatory T cells are a specialized subpopulation of T cells that actively suppress activation of the immune system [12]. Natural T reg cells are phenotypically recognized by their constitutive expression of CD4 and CD25 (alpha chain of the receptor for interleukin-2) and the forkhead family transcription factor FoxP3 (forkhead box p3) [13, 14]. They are cells of the adaptive immune system and represent 2–3% of CD4+ cells in humans [15]. We have previously demonstrated that the frequency of CD4+CD25+FOXP3+ T-cells is age independent [11].
This is the first report on T reg cell function in patients with IMLNS. Previous studies on T lymphocytes in IMLNS have only demonstrated that the distribution of T and B cells in these patients is not different from that in healthy controls [16–19]. Only two reports present data on CD4+ CD25+ T cells, but neither distinguished between activated T eff cells (which express both receptors) and natural T reg cells (which express both receptors and Foxp3) [20–22].
Our study indicated that T reg cells from patients with IMLNS in relapse have an impaired ability to suppress T eff cell proliferation. This defect was corrected when the patients were in remission, as T eff cell proliferation was significantly greater than that observed in patients with IMLNS in relapse and was not significantly different from that seen in healthy controls. Moreover, it was not secondary to the nephrotic milieu, because patients with membranoproliferative glomerulonephritis and nephrotic syndrome demonstrated a normal capacity to suppress T eff cell proliferation.
This impaired ability to suppress T eff cell proliferation did not seem to be due to an overall reduction in the number of T reg cells, since the frequency of these cells did not differ among the groups. However, as long as there is no precise identification of the clone of T reg cells involved in IMLNS, the effect could still have been due to a decrease in the number of specific T reg cells.
The mechanism of the impaired regulatory function could be related to the fact that IL-10 release [23] was reduced in the co-culture of T eff and T reg cells. The molecular mechanism by which T reg cells exert their suppressor/regulatory activity has not been definitively defined. There are divergent opinions regarding the importance of cell–cell contact versus secreted cytokines in their suppressive functions [24]. In vitro experiments have suggested the need for cell-to-cell contact rather than soluble mediators [25]. In vivo studies have suggested a pivotal role for interleukin 10 in T reg cell function [24]. Activated T reg cells secrete large amounts of IL-10 and often TGF-β [26]. Most models implicating IL-10 also associate TGF-β [24]. IL-10 may act locally at the site of inflammation, while TGF-β seems to have a more systemic effect in the immune response [24].
Associated with this impaired suppressor effect, we observed that PBMCs from patients with nephrotic syndrome had the capability to release more cytokine than those from healthy controls when stimulated. We used antiCD3-antiCD28 as stimuli because these were the same stimuli as in our suppression assay. A non-specific increase in cytokine secretion by PBMCs from patients with IMLNS has been previously reported in several studies using different mitogens [27–32]. IL-2, IL8, and TNF-α were found to be increased or unchanged in these studies [27, 33]. However, no reports on the use of antiCD3-antiCD28 as mitogens to evaluate cytokine production in nephrotic patients have been published. It is difficult, therefore, to compare our results with those previously reported.
The subjects in this phase of the study were all young or middle-aged adults, and, therefore, the results may not be applicable to children. There are no reports that specifically studied the age-related changes after antiCD3-antiCD28 stimulation. However, comparisons between elderly and young adults (aged 18–22 years) using antiCD3 as a mitogen gives inconclusive results for the cytokines measured in this study. Some of the reports observed no changes, while others showed decreased or increased cytokine production in the two groups studied.
In patients with IMLNS the impaired T reg cell function may elucidate the mechanism for the elevated serum levels of the pathogenic cytokine. We have previously shown that chronic infusion of IL-8 induced proteinuria in rats. In addition, in comparison with that in healthy subjects, IL-8 mRNA half life [t(1/2)] is prolonged in these patients, due to altered post-transcriptional regulation [34], leading to persistent secretion of cytokine. Owing to the impaired negative feedback mechanism because of deficient IL-10 release by the T reg cell, the T eff cell may continue secreting IL-8, among other cytokines, including the elusive pathogenic cytokine postulated in patients with IMLNS.
In this study all patients with IMLNS in relapse were studied while they were off immunosuppressive therapy, except for two patients, one of whom was receiving 5 mg prednisone every other day. Therefore, the observed T reg cell dysfunction cannot be attributed to the effect of steroids on T cell function. Moreover, immunosuppressive therapy given to IMLNS patients may induce remission by inducing recovery of the T reg cell suppressor function, as seen in our patients. Corticosteroids have been shown to stimulate the generation of IL-10-producing suppressive T cells [35]. In our patients, both IL-2 and IL-10 concentrations were not increased in supernatants of T reg and T eff cell co-cultures of the patients with IMLNS in remission when compared with those seen in patients in relapse, and they were reduced when compared with those observed in healthy subjects.
In summary, we present the first evidence of impaired T reg cell function in patients with IMLNS. This finding was associated with increased production of some cytokines by IMLNS PBMCs in response to stimuli. The deficient suppressor T reg cell activity could have pathogenic and therapeutic implications, because it could explain the increased levels and persistent activity of the postulated pathogenic cytokine in patients with IMLNS.
References
- 1.International Study of Kidney Disease in Children. Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at the time of diagnosis. A report rom the International Study of Kidney Disease in Children. Kidney Int. 1978;13:159–165. doi: 10.1038/ki.1978.23. [DOI] [PubMed] [Google Scholar]
- 2.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–603. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 3.Garin EH, Blanchard DK, Matsushima K, Djeu JY. IL-8 production by peripheral blood mononuclear cells in nephrotic patients. Kidney Int. 1994;45:1311–1317. doi: 10.1038/ki.1994.171. [DOI] [PubMed] [Google Scholar]
- 4.Garin EH, Laflam PF, Muffly K. Proteinuria and fusion of foot processes in rats after infusion of cytokine from patients with idiopathic minimal lesion nephrotic syndrome. Nephron Exp Nephrol. 2006;102:105–112. doi: 10.1159/000089689. [DOI] [PubMed] [Google Scholar]
- 5.Lai KW, Wei CL, Tan LK, Tan PH, Chiang GSC, Lee CGL, Jordan SC, Yap HK. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol. 2007;18:1476–1485. doi: 10.1681/ASN.2006070710. [DOI] [PubMed] [Google Scholar]
- 6.Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40:453–460. doi: 10.1038/ki.1991.232. [DOI] [PubMed] [Google Scholar]
- 7.Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions. J Immunol. 2004;172:34–39. doi: 10.4049/jimmunol.172.1.34. [DOI] [PubMed] [Google Scholar]
- 8.Finger EB, Bluestone JA. When ligand becomes receptor—tolerance via B7 signaling on DCs. Nat Immunol. 2002;3:1056–1057. doi: 10.1038/ni1102-1056. [DOI] [PubMed] [Google Scholar]
- 9.Oaks MK, Hallet KM. Cutting edge: a soluble form of CTLA-4 in patients with autoimmune thyroid disease. J Immunol. 2000;164:5015–5018. doi: 10.4049/jimmunol.164.10.5015. [DOI] [PubMed] [Google Scholar]
- 10.Churg J, Habib R, White HRH. Pathology of the nephrotic syndrome in children: a report for the International Study of Kidney Disease in Children. Lancet. 1970;i:1299–1302. doi: 10.1016/s0140-6736(70)91905-7. [DOI] [PubMed] [Google Scholar]
- 11.Brusko TN, Wasserfall CH, McGrail K, Schatz R, Viener HL, Schatz DA, Haller M, Rockell J, Gotlieb P, Clare-Salzler M, Atkinson MA. No alterations in the frequency of FOXP3 + regulatory T-cells in type 1 diabetes. Diabetes. 2007;56:604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- 12.Shimon S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune response. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 14.Fontenot JD, Gavin MA, Rudensky AY. FoxP3 programs the development and function of CD4 + CD25 + regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 15.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4 + CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama H, Kida H, Tani Y, Abe T, Tomosugi N, Koshino Y, Hattori N. Immunodynamics of minimal change nephrotic syndrome in adults T and B lymphocytes subsets and serum immunoglobulin levels. Clin Exp Immunol. 1985;61:601–617. [PMC free article] [PubMed] [Google Scholar]
- 17.Lama G, Luongo I, Tirino G, Borriello A, Carangio C, Salsano ME. T-lymphocyte populations and cytokines in childhood nephrotic syndrome. Am J Kidney Dis. 2002;39:958–965. doi: 10.1053/ajkd.2002.32769. [DOI] [PubMed] [Google Scholar]
- 18.Daniel V, Trautmann Y, Konrad M, Nayir A, Scharer K. T-lymphocyte populations, cytokines and other growth factors in serum and urine of children with idiopathic nephrotic syndrome. Clin Nephrol. 1997;47:289–297. [PubMed] [Google Scholar]
- 19.Kemper MJ, Meyer-Jark T, Lilova M, Muller-Wiefel DE. Combined T- and B-cell activation in childhood steroid-sensitive nephrotic syndrome. Clin Nephrol. 2003;60:242–247. doi: 10.5414/cnp60242. [DOI] [PubMed] [Google Scholar]
- 20.Topaloglu R, Saatci U, Arikan M, Canpinar H, Bakkaloglu A, Kansu E. T-cell subsets, interleukin-2 receptor expression and production of interleukin-2 in minimal change nephrotic syndrome. Pediatr Nephrol. 1994;8:649–652. doi: 10.1007/BF00869075. [DOI] [PubMed] [Google Scholar]
- 21.Hulton SA, Shah V, Byrne MR, Morgan G, Barratt TM, Dillon MJ. Lymphocyte subpopulations, interleukin-2, and interleukin-2 receptor expression in childhood nephrotic syndrome. Pediatr Nephrol. 1994;8:135–139. doi: 10.1007/BF00865458. [DOI] [PubMed] [Google Scholar]
- 22.Lan RY, Selmi C, Gershwin ME. The regulatory, inflammatory, and T cell programming roles of interleukin-2(IL-2) J Autoimmun. 2008;31:7–12. doi: 10.1016/j.jaut.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Wakkach A, Augier S, Breittmayer JP, Blin-Wakkach C, Carle GF. Characterization of IL-10 secreting T cells derived from regulatory CD4+CD25+ cells by the TIRC7 surface marker. J Immunol. 2008;180:6054–6063. doi: 10.4049/jimmunol.180.9.6054. [DOI] [PubMed] [Google Scholar]
- 24.Mottet C, Golshayan D. CD4+CD25+Foxp3+ regulatory T cells: from basic research to potential therapeutic use. Swiss Med Wkly. 2007;137:625–634. doi: 10.4414/smw.2007.11916. [DOI] [PubMed] [Google Scholar]
- 25.Thornton AM, Shevach EM. CD4 + CD25 + immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4 + CD25 + T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho BS, Yoon SR, Jang JY, Pyun KH, Lee CE. Up-regulation of interleukin-4 and CD23/FcepsilonRII in minimal change nephrotic syndrome. Pediatr Nephrol. 1999;13:199–204. doi: 10.1007/s004670050592. [DOI] [PubMed] [Google Scholar]
- 28.Bustos C, Gonzalez E, Muley R, Alonso JL, Egido J. Increase of tumor necrosis factor alpha synthesis and gene expression in peripheral blood mononuclear cells of children with idiopathic nephrotic syndrome. Eur J Clin Invest. 1994;24:799–805. doi: 10.1111/j.1365-2362.1994.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 29.Suranyi MG, Guasch A, Hall BM, Myers BD. Elevated levels of tumor necrosis factor alpha in the nephrotic syndrome in humans. Am J Kidney Dis. 1993;21:251–259. doi: 10.1016/s0272-6386(12)80742-6. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto K, Kanmatsuse K. Increased IL-2 release by monocytes in nephrotic syndrome. Clin Exp Immunol. 1999;117:361–367. doi: 10.1046/j.1365-2249.1999.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxena S, Mittal A, Andal A. Pattern of interleukins in minimal change nephrotic syndrome of childhood. Nephron. 1993;65:56–61. doi: 10.1159/000187441. [DOI] [PubMed] [Google Scholar]
- 32.Neuhaus TJ, Wadhwa M, Callard R, Barrat TM. Increased IL-2, IL-4 and interferon-gamma (IFN-gamma) in steroid-sensitive nephrotic syndrome. Clin Exp Immunol. 1995;100:475–479. doi: 10.1111/j.1365-2249.1995.tb03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yap HK, Cheung W, Murugasu B, Sim SK, Seah CC, Jordan SC. Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: evidence for increased IL-13 mRNA expression in relapse. J Am Soc Nephrol. 1999;10:529–537. doi: 10.1681/ASN.V103529. [DOI] [PubMed] [Google Scholar]
- 34.Laflam PF, Haragushi S, Garin EH. Cytokine mRNA profile in lipoid nephrosis: evidence for increased IL-8 increased IL-8 mRNA stability. Nephron. 2002;91:620–626. doi: 10.1159/000065022. [DOI] [PubMed] [Google Scholar]
- 35.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefvt R, Coffman RL, Hawrylowicz CM, O’Garra A. In Vitro generation of interleukin 10- producing regulatory CD4(+) Tcells is induced by immunosuppressive drugs and inhibited by T- helper type-1 (Th1) and Th2-inducing cytokines. J Exp Med. 2002;195:603–606. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]