Abstract
Stress triggers changes in gene expression mediating important adaptive and maladaptive responses. The full repertoire of genes whose expression in the adrenal medulla is altered by stress has not been previously determined. In this study, gene profiling (RAE 230 2.0 Affymetrix) was applied to elucidate global changes in gene expression in adrenal medulla of rats exposed to 2-hour immobilization stress (IMO) once or repeatedly for six consecutive days. The number of transcripts significantly (p<0.01) altered with single IMO (651 up, 487 down) was more than with repeated IMO (370 up- 195 down). The annotated transcripts were further analyzed and categorized. The largest numbers of changes were in mRNA levels in the transcription factor and cell signaling categories. Robust changes were also observed in transcripts related to growth factors, apoptosis, neurosecretion/neuropeptides, heat shock proteins, structural proteins, chemokines, cytokines, metabolism/lipid-metabolism, and proteases. Many (>80%) were uniquely induced by single IMO. About half of transcripts changed by repeated IMO were also responsive to single IMO. Pathway analysis was applied to identify direct interactions and common targets among gene products altered by single and repeated IMO. In this paper, we briefly describe the most pronounced changes observed with emphasis on those which may provide new insight into the common and distinct mechanisms whereby the adrenal medulla responses to a first encounter with stress compared to repeated exposure to the same stressor.
Keywords: stress, adrenal medulla, microarray, gene profiling
Introduction
The adrenal medulla plays a key role in the response to stress. Release of epinephrine (Epi) and norepinephrine (NE) from adrenal medulla is among the most rapid responses to stress. Epi and NE are critical in transmitting the perceived threat into action by activating the heart and muscles to prepare for the ‘fight or flight’ response1. This stimulates glycogen breakdown in muscle and liver, gluconeogenesis in the liver, and lipolysis in adipose tissue. It also inhibits insulin release while stimulating glucagons secretion, triggers vasoconstriction and enhances cardiac output [reviewed in2]. Moreover, Epi is key for memory of emotionally charged events3,4; sympathetic activation and elevated urinary NE and/or Epi concentration are consistently observed in patients with PTSD5,6.
One of the most important questions in stress research is how are the acute beneficial responses to stress converted to the prolonged detrimental effects. To this end, studies have been directed to comparing the response of the adrenal medulla to acute and chronically repeated stress. Exposure to stress, not only triggers rapid NE and Epi release, but also leads to longer lasting changes in gene expression.
The effect of various stress of different duration on gene expression of catecholamine biosynthetic enzymes in the adrenal medulla has been reviewed7–10. Even a few minutes of immobilization stress (IMO) already triggers induction and/or phosphorylation of several transcription factors and elevates transcription of catecholamine biosynthetic enzymes11–13. Moreover, exposure to single episode of immobilization stress (IMO) alters the response to subsequent exposure to the same stressor on the next day. For example, the phosphorylation of transcription factor CREB in adrenal medulla is much more pronounced and sustained on the second than on first IMO12. However, chronically repeated stress is required to manifest new steady state catecholamine levels9,14.
The full repertoire of genes whose expression in the adrenal medulla is altered under these conditions has not been previously determined. In the present study, in order to understand long-term consequences of stress, and to obtain a complete picture of changes in gene expression triggered by single and repeated stress, microarray analysis was applied. It enabled us to explore networks of connections among the genes responsive to stress. The findings revealed many previously unidentified targets and potential new biomarkers for adrenomedullary response to stress and help to delineate the mechanisms for the common and distinct responses to acute and repeated exposure to stress.
Methods
Animal procedures
All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. Male, murine pathogen-free, Sprague-Dawley rats (280–320 g) were obtained from Taconic Farms (Germantown, NY). The animals were maintained under controlled conditions of a 12-hr light-dark cycle (lights on from 06:00 AM to 18:00 PM) at 23±2 °C with food and water ad libitum. IMO was performed as previously described15–17. For repeated stress, the animals were immobilized for 2 hr daily for 6 consecutive days. Control groups were not exposed to stress. All animal manipulations were performed between 08:00 AM and 13:00 PM.
Following the last IMO, rats were euthanized by decapitation and both adrenals were dissected from the animals. To isolate the adrenal medulla, a small incision was made to the edge of the cortex and the medulla was gently squeezed out. Subsequently any cortex tissue adhering to the adrenal medulla was carefully removed. It had been estimated by immunocytochemistry that the medulla was more than 95% pure17. The right and left adrenal medullae from each individual animal was frozen separately in liquid nitrogen and kept at −80°C.
RNA Isolation
RNA was isolated from two separate immobilization experiments. To minimize sample variability caused by individual differences among animals, each sample was pooled from left adrenal medulla from 4 individual rats. There were 3-pooled samples (12 animals total) for each group. RNA was extracted using Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA). The integrity of the RNA was assessed by the A260/A280 ratio which was close to 2.0 and by electrophoresis (Agilent Bioanalyzer 2100).
Microarray
This part of experiment was performed by the NIH Neuroscience Microarray Consortium at UCLA Medical Center (Los Angeles, CA). It is described briefly as follows. Total RNA (≥ 4 μg) from each group was converted to cDNA by using superscript reverse transcriptase and the T7-Oligo (dT) promoter primer kit (Affymetrix, Inc). Following RNase H-mediated second-strand cDNA synthesis, the double-stranded cDNA were purified and served as a template in the subsequent in vitro transcription reaction (Affymetrix, Inc.). The in vitro transcription reaction was carried by T7 RNA polymerase and a biotinylated nucleotide analog/ribonucleotide mix (Affymetrix, Inc.). The biotinylated cRNA targets were cleaned up and fragmented. Each cRNA was hybridized to an individual Affymetrix GeneChip Rat Array Expression 230 2.0 (RAE 230 2.0 array) which was subsequently processed for washing and staining with the antibody stain solution with streptavidin phycoerythrin and the arrays were scanned on the GeneChip Scanner 3000. For detailed protocols see http://www.affymetrx.com.
Microarray Data Analysis
The raw pixel data were uploaded into GeneTraffic (GeneTraffic version 3.2, Iobion Informatics, La Jolla, CA), and all subsequent analyses were performed on a GeneTraffic Server at the Functional Genomics Core Facility of New York Medical College. All the microarray data were analyzed and normalized using a Robust Multi-Chip Analysis (RMA) algorithm with the control data sets as the baseline. According to Iobion Informatics, the median polishing algorithm of RMA helps minimize the effect of noise inherent in microarray data and enhances the discriminating power of the experiment. The quality and the accuracy of each hybridization were checked by Hybridization Annotation and Hybridization Statistics in GeneTraffic program. Statistical analysis was a two-class method comparing the single and repeated IMO probe sets to the probe sets for the controls. The analysis provided the significance level (p-value) for each gene. Differences with a significance of p < 0.01 and at least ± 2.0-fold change in gene expression between the respective stress group and absolute control group.
The expression data generated from GeneTraffic were imported into PathwayAssist software (version 3.0, Iobion Informatics) to provide insights into common regulatory mechanisms of the set of genes and the interactions or pathways, or for all relationships among several proteins.
Real-time Quantitative RT-PCR Assay
Changes observed for some genes in microarray analysis were individually confirmed by real-time RT-PCR. The RNA samples used for RT-PCR were the same as for microarray analysis. Mixture for reverse transcription reactions contained 1 μg total RNA, 1 μM random primer (Sigma), 1 mM dNTP mix, 2.5 units of AMV reverse transcriptase, 1 × AMV buffer, 8 units of RNase inhibitor (Roche, Indianapolis, IN) and were incubated at 42°C for 60 min. PCR reactions (25 μl) were set up with 12.5 μl RT2 Real-Time™ SYBR Green PCR Master Mix provided by SuperArray Bioscience Co. (Frecerick, MD), 10.5 μl ddH2O, 1.0 μl gene-specific 10 μM PCR primer pair stock (SuperArray Bioscience Co.), and 1.0 μl template cDNA. The real-time thermal cycler program was recommended by SuperArray manufacture protocol [95°C, 10min; 40 cycles of (95°C, 15 sec; 55°C, 35 sec; and 72°C, 30 sec)]. The specificity of the amplified target sequences was confirmed with melting curve analysis of the PCR products. For statistics, the values from triplicate pooled samples from individual animals were divided by the mean of the samples from unstressed control group to give relative values. Results were evaluated by Student’s t-test. A value of p < 0.05 was considered significant.
Results and Discussion
Microarray Profiling
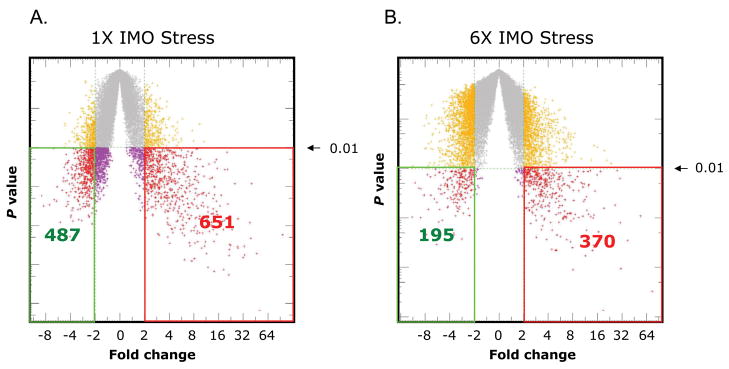
Microarray analysis was performed to obtain an unbiased characterization of changes in gene expression in adrenal medulla with single and repeated exposure to IMO stress. Analysis was performed with samples from unstressed controls and rats exposed to single (1× IMO) for 2 hrs or repeated IMO 2 hr daily for 6 consecutive days (6× IMO). The mean values of unstressed controls were taken as baseline. A scatter plot of the changes compared to control group is shown in Fig. 1 Significant changes were observed in expression of a large number of genes. Therefore, we concentrated on the changes which differed significantly (p<0.01) and were of up-or down- regulated greater than 2-fold compared to unstressed controls. Using this criteria, we identified 651 genes up-regulated, of which 160 were annotated; and 487 down regulated, of which 64 were annotated. Repeated IMO significantly induced 370 transcripts, of which 78 were annotated and 195 down regulated genes of which 22 were defined.
Figure 1.
Statistical plots of changes in gene expression with single and repeated IMO stress. Statistic plots showing the significance of changes in gene expression after single (A) and repeated (B) IMO stress. The X-axis which is the mean log2 ratio of the current chip intensity over the baseline chip intensity represents the fold change in the gene expression. The Y-axis represents the statistical significance (p value). Each spot in the plots represents one transcript. The number of transcripts that were up-regulated (right side), and down-regulated (left side) by more than 2-fold with significance of p < 0.01 are indicated.
Thus, nearly 4% of the total genome were found to be significantly (p<0.01) altered greater than 2-fold in the rat adrenal medulla by single IMO, and nearly 2% by repeated IMO stress.
Among the annotated transcripts, 35 up-regulated and 4 down-regulated were common and changed with both single and repeated IMO. Thus, most of the changes with single IMO were unique, while nearly half of the defined transcripts up-regulated by repeated IMO were also changed by single IMO.
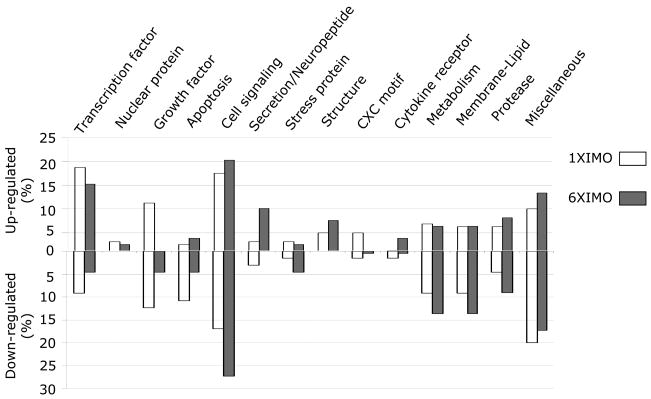
Based on Gene Ontology, and PubMed publications, combined with knowledge of the physiological function of the adrenal medulla, we organized and categorized all the defined genes changed with single and repeated IMO stress (Fig. 2). In this paper we will briefly describe the most pronounced changes observed. We particularly discuss those changes which may provide new insight into the mechanism whereby the adrenal medulla responds a new experience of stress compared to repeated exposure to the same stressor.
Figure 2.
Percent changes in gene expression with different categories with single and repeated IMO. The percent of the annotated genes up or down regulated with single and repeated IMO are indicated for each category.
Transcription Factors and Nuclear Protein Related
The greatest numbers of changes were observed with transcription factors. Table 1 shows the changes in transcription factor and nuclear protein related genes. Approximately 20% of the transcripts up-regulated by single IMO were transcription factors. Some were previously identified as transcription factors likely responsible for activation of catecholamine biosynthetic genes with IMO stress, such as Egr1 and Fra-218–20. Others were not previously recognized as IMO responsive genes in the adrenal medulla. The largest changes with single IMO were in NR4A3 (nuclear receptor subfamily 4, member 3, also know as Nor1, which was increased nearly 100-fold. The elevations of CREM (40-fold) and gonadotropin inducible ovarian transcription factor 1 (32-fold) were also very pronounced. In some cases, several members of the same transcription factor family were induced, such as: ATF3 and ATF4; Fra-1 and Fra-2; nuclear receptor subfamily 4, group A [NR4A1 (Nurr77 or NGF1-B), NR4A2 (Nurr1), and NR4A3 (Nor1)]. Furthermore, in certain transcription factor families, some members were up-regulated and others were down-regulated, such as inhibitors of DNA binding 1 and 4 which were up by nearly 4- and 3-fold respectively, while its member 2 was down by about 3-fold.
Table 1.
Transcription Factors and Nuclear Protein Related Genes
| Probe Set ID | UniGene ID | UniGene Name | 1×IMO Fold C (Up) | Probe Set ID | UniGene ID | UniGene Name | 6×IMO Fold C (Up) |
|---|---|---|---|---|---|---|---|
| 1369765_at | Rn.32936 | achaete-scute complex homolog-like 1 | 12.65 | 1369765_at | Rn.32936 | achaete-scute complex homolog-like 1 | 30.99 |
| 1369268_at | Rn.9664 | activating transcription factor 3 (ATF3) | 13.45 | 1369738_s_at | Rn.10251 | cAMP responsive element modulator | 13.07 |
| 1367624_at | Rn.2423 | activating transcription factor 4 (ATF4) | 2.29 | 1368321_at | Rn.9096 | early growth response 1 (Egr1) | 8.09 |
| 1369737_at | Rn.10251 | cAMP responsive element modulator | 39.82 | 1387306_a_at | Rn.89235 | early growth response 2 (Egr2) | 2.72 |
| 1369738_s_at | 22.5 | 1368775_at | NA | gonadotropin inducible ovarian TF 1 | 17.5 | ||
| 1368813_at | Rn.6975 | CCAAT/enhancer binding, δ | 3.57 | 1387028_a_at | Rn.2113 | inhibitor of DNA binding 1 | 3.23 |
| 1387343_at | 2.9 | 1375120_at | Rn.22987 | inhibitor of DNA binding 4 | 2.08 | ||
| 1368321_at | Rn.9096 | early growth response 1 (Egr1) | 8.23 | 1370138_at | Rn.21926 | lymphoid enhancer binding factor 1 | 6.86 |
| 1368489_at | Rn.11306 | fos-like antigen 1 (Fra-1) | 3.77 | 1368488_at | Rn.54147 | nuclear factor, interleukin 3, regulated | 10.63 |
| 1387530_a_at | Rn.10962 | fos-like antigen 2 (Fra-2) | 3.61 | 1387410_at | Rn.88129 | NR4A2 | 6.73 |
| 1368775_at | NA | gonadotropin inducible ovarian TF 1 | 32.06 | 1369067_at | Rn.62694 | NR4A3 | 54.05 |
| 1368726_a_at | NA | gonadotropin inducible ovarian TF 2 | 10.51 | 1373632_at | Rn.8139 | TAF9 RNA polymerase | 3.03 |
| 1387270_at | Rn.12188 | hematopoietically expressed homeobox | 3.58 | 1387169_at | Rn.24106 | transducin-like enhancer of split 3 | 3.76 |
| 1368546_at | Rn.9802 | HIV type I enhancer binding protein 2 | 2.64 | ||||
| 1388587_at | Rn.23638 | immediate early response 3 | 2.91 | ||||
| 1387028_a_at | Rn.2113 | inhibitor of DNA binding 1 | 3.52 | ||||
| 1394022_at | Rn.22987 | inhibitor of DNA binding 4 | 2.82 | ||||
| 1387788_at | Rn.15806 | Jun-B oncogene | 7.83 | ||||
| 1387260_at | Rn.7719 | Kruppel-like factor 4 (gut) | 10.13 | ||||
| 1370968_at | Rn.2411 | nuclear factor kappa B p105 subunit | 2.73 | ||||
| 1368488_at | Rn.54147 | nuclear factor, interleukin 3, regulated | 15.43 | ||||
| 1386935_at | Rn.10000 | NR4A1 | 8.85 | ||||
| 1369007_at | Rn.88129 | NR4A2 | 6.17 | ||||
| 1369067_at | Rn.62694 | NR4A3 | 97.62 | ||||
| 1387200_at | Rn.45339 | oligodendrocyte transcription factor 1 | 2.12 | ||||
| 1387684_at | Rn.96181 | peroxisome proliferator activated R δ | 5.15 | ||||
| 1370224_at | Rn.10247 | signal transducer and activator of T 3 | 2.38 | ||||
| 1373632_at | Rn.8139 | TAF9 RNA polymerase II | 2.05 | ||||
| 1387169_at | Rn.24106 | transducin-like enhancer of split 3 | 2.97 | ||||
| 1372211_at | Rn.3818 | v-maf | 1.97 | ||||
| 1387870_at | Rn.82737 | zinc finger protein 36 | 3.07 | ||||
| (Down) | (Down) | ||||||
| 1368870_at | Rn.3272 | inhibitor of DNA binding 2 | 2.62 | 1368376_at | Rn.10712 | NR0B2 | 5.48 |
| 1368073_at | Rn.6396 | interferon regulatory factor 1 | 2.58 | ||||
| 1373471_at | Rn.43927 | ring finger protein 166 | 2.18 | ||||
| 1387732_at | Rn.64629 | transcription termination factor | 2.13 | ||||
| 1387624_at | Rn.10845 | upstream transcription factor 1 | 3.17 | ||||
| 1399006_at | 2.58 | ||||||
| Nuclear protein related | (Up) | Nuclear protein related | (Up) | ||||
| 1382756_at | Rn.6272 | karyopherin alpha | 2.65 | 1370004_at | Rn.11098 | H2A histone family, member Y | 3.27 |
| 1368747_at | Rn.11324 | nucleoporin 98 | 3.69 | 1373032_at | Rn.9453 | musculoskeletal | 4.34 |
| 1388198_at | Rn.11099 | nucleoporin p58 | 2.55 | ||||
| 1367761_at | Rn.2947 | nudE nuclear distribution gene E | 2.19 | ||||
| 1387912_at | Rn.3436 | RNA helicase | 4.83 | ||||
Bold – Genes changed by both types of stress; Fold C – Fold change
In addition, changes were observed in some transcription factors containing basic helix-loop-helix motifs, such as achaete-scute complex homolog-like 1 (Mash1). Mash1 is a very crucial factor in the development of the sympathoadrenal lineage and noradrenergic differentiation21–23. It might be involved in stress mediated hypertrophy of the adrenal gland as described later in the growth factor section. Interestingly, transcripts for transcription termination factor and inhibitor of DNA binding 2 were down regulated, consistent with large increase in transcription with single IMO.
In the nuclear protein-related factors category, we observed induction in some genes involved in nuclear transport, such as nucleoporin subfamily member karyopherin a, as well as in RNA helicase.
Similar to single IMO, transcription factors were the main category among the changes with repeated IMO, which encompassed 16% of the up regulated gene transcripts. The largest changes were observed in NR4A3 (Nor1) (up 54 fold) an din achaete-scute complex homolog-like 1 (Mash1), (up 31 fold). Most of the transcription factors induced with repeated IMO were also elevated by 1× IMO. Only two genes (Egr2 and lymphoid enhancer binding factor I) were uniquely changed with repeated IMO (Table 1).
Cell Signaling Related
The changes in cell signaling related transcripts, including related to kinase/phosphatases, G proteins, calcium signaling, and channels are shown in Table 2. They comprised more than 16% of those up-regulated and about 14% of those down-regulated with single IMO. Among the kinases, the largest changes were observed in SNF-1 like kinase (>6-fold) and MAPK activated protein kinase 2 (4-fold). Serine/threonine kinase 10 was also elevated (3-fold), while transcript for polo-like kinase increased 3.7-fold. This kinase binds calcium and is implicated in long term synaptic plasticity24. In contrast phosphatidylinositol-3-kinase (PI-3-kinase) isoforms were down regulated. In addition to changes in kinases, we observed alterations in kinase anchor protein (PRKA) family members, with PRKA protein 12 up-regulated (4-fold) and PRKA protein 1 down-regulated (2.7-fold) with single IMO.
Table 2.
Cell Signaling Related Genes
| Probe Set ID | UniGene ID | UniGene Name | 1×IMO Fold C (Up) | Probe Set ID | UniGene ID | UniGene Name | 6×IMO Fold C (Up) |
|---|---|---|---|---|---|---|---|
| Kinase/Phosphatase related genes | Kinase/Phosphatase related genes | ||||||
| 1368869_at | Rn.122094 | A kinase (PRKA) anchor protein 12 | 4.07 | 1367942_at | Rn.3494 | acid phosphatase 5 | 2.02 |
| 1368868_at | 3.07 | 1398251_a_at | Rn.9743 | cal/cal-dependent pk II beta | 3.23 | ||
| 1388686_at | Rn.12942 | Down syndrome critical region homolog 1 | 4.44 | 1372299_at | Rn.92509 | cyclin-dependent k inhibitor 1C | 4.01 |
| 1368124_at | Rn.10877 | dual specificity phosphatase 5 | 7.01 | 1371446_at | Rn.6276 | MAPK-activated protein kinase 2 | 4.48 |
| 1371446_at | Rn.6276 | MAPK-activated protein kinase 2 | 4.28 | 1368902_at | Rn.10128 | p21-activated kinase 3 | 5.24 |
| 1368106_at | Rn.12100 | polo-like kinase 2 | 3.72 | 1382307_at | Rn.58447 | protein phosphatase 1(12A) | 2.3 |
| 1384262_at | Rn.30046 | protein phosphatase 1(3B) | 5.09 | 1384262_at | Rn.30046 | protein phosphatase 1(3B) | 5.05 |
| 1386971_at | Rn.37758 | protein phosphatase 1(10) | 3.86 | 1380045_at | Rn.30021 | PDP isoenzyme 2 | 4.19 |
| 1398807_at | Rn.4143 | protein phosphatase 1B, β | 2.73 | 1370509_at | 4.05 | ||
| 1371136_at | 2.21 | 1371943_at | Rn.4052 | Ser/Thr-like protein kinase lyk4 | 3.56 | ||
| 1380045_at | Rn.30021 | PDP isoenzyme 2 | 4.12 | ||||
| 1370509_at | 3.82 | ||||||
| 1367936_at | Rn.4190 | serine/threonine kinase 10 | 3.08 | ||||
| 1367802_at | Rn.4636 | serum/glucocorticoid regulated kinase | 2.03 | ||||
| 1368597_at | Rn.42905 | SNF1-like kinase | 8 | ||||
| 1368596_at | 6.72 | ||||||
| 1368254_a_at | Rn.18522 | sphingosine kinase 1 | 2.74 | ||||
| (Down) | (Down) | ||||||
| 1369069_at | Rn.91372 | A kinase (PRKA) anchor protein 1 | 2.66 | 1369069_at | Rn.91372 | A kinase (PRKA) anchor protein 1 | 4.24 |
| 1370923_at | Rn.36170 | expressed in non-metastatic cells 6 | 2.52 | ||||
| 1370100_at | Rn.22497 | PI-3-kinase, class 2 | 2.08 | ||||
| 1369655_at | Rn.30010 | PI-3-kinase, class 3 | 2.61 | ||||
| G-protein related genes | (Up) | G-protein related genes (6×IMO) | (Up) | ||||
| 1370649_at | Rn.9845 | bradykinin receptor b2 | 6.59 | 1370649_at | Rn.9845 | bradykinin receptor b2 | 14.24 |
| 1370650_s_at | 3.89 | 1370650_s_at | 4.97 | ||||
| 1387596_at | Rn.10543 | thrombin receptor-like 1 | 2.36 | 1369624_at | Rn.91303 | prolactin releasing hormone | 7.21 |
| 1387908_at | Rn.54720 | DEXRAS1 (Dexras1) | 4.33 | ||||
| 1384979_at | Rn.92385 | G protein-coupled receptor 50 | 2.53 | ||||
| 1368029_at | Rn.4368 | guanine nucleotide binding protein | 2.07 | ||||
| (Down) | (Down) | ||||||
| 1371635_at | Rn.3566 | transmembrane domain protein regulated | 2.27 | 1370449_at | Rn.87082G | protein-coupled receptor 105 | 2.42 |
| Calcium related genes | (Up) | Calcium related genes (6×IMO) | (Up) | ||||
| 1387276_at | Rn.80575 | ania-4 | 4.78 | 1387276_at | Rn.80575 | ania-4 | 6.08 |
| 1370050_at | Rn.7208 | ATPase | 2.56 | 1369117_at | Rn.90085 | cal/cal-related polypeptide, a | 2.87 |
| 1369116_a_at | Rn.90085 | cal/cal-related polypeptide, a | 15.17 | 1370000_at | Rn.41602 | nucleobindin | 2 |
| 1369117_at | 5.61 | ||||||
| 1370775_a_at | 3.39 | ||||||
| 1369886_a_at | Rn.23560 | calcium binding protein 1 | 2.1 | ||||
| Channel related genes (1×IMO) | (Up) | Channel related genes (6×IMO) | (Up) | ||||
| 1387477_at | Rn.64577 | potassium channel K12 | 3.01 | ||||
| 1368751_at | Rn.10878 | potassium voltage-gated S3 | 3.44 | ||||
| (Down) | (Down) | ||||||
| 1368343_at | Rn.10970 | potassium voltage-gated channel, H2 | 2.18 | 1370757_at | Rn.81221 | calcium channel, 3 | 2.3 |
| 1369674_at | Rn.10257 | purinergic receptor P2X, 5 | 3.51 | 1369059_at | Rn.86991 | ChaK | 3.29 |
| Other Signaling related genes | (Up) | Other Signaling related genes | (Up) | ||||
| 1368605_at | Rn.30041 | adaptor protein | 2.38 | ||||
| 1369468_at | Rn.48736 | frizzled homolog 4 (Drosophila) | 2.97 | ||||
| 1370454_at | Rn.37500 | homer homolog 1 (Drosophila) | 11.33 | ||||
| 1370997_at | 8.78 | ||||||
| 1370669_a_at | Rn.44869 | phosphodiesterase 10A | 10.63 | ||||
| 1368438_at | 8.96 | ||||||
| 1369044_a_at | Rn.37733 | phosphodiesterase 4B | 2 | ||||
| (Down) | (Down) | ||||||
| 1368660_at | Rn.42899 | cAMP-GEFI | 2.23 | 1398299_at | Rn.105776 | Rho GEF 11 | 2.14 |
| 1387499_a_at | Rn.51153 | phosducin-like | 2.61 | 1393955_at | Rn.79380 | WD-containing protein | 2.19 |
| 1370196_at | Rn.14548 | protein inhibitor of activated STAT 3 | 2.94 | 1371027_at | Rn.21799 | Cas-Br-M ectropic RTS b | 3.02 |
| 1387186_at | Rn.35289 | RAB9, member RAS oncogene family | 2.48 | ||||
| 1369024_at | Rn.3228 | rabaptin | 3.21 | ||||
Bold – Genes changed by both types of stress; Fold C – Fold change
The transcripts of several phosphatases were also up-regulated. The largest change was in dual specificity phosphatase 5 (up 7-fold), which can target Erk1, Erk2. Several isoforms of protein phosphatase 1, which de phosphorylate serine/threonine residues were also up-regulated (2 to 5 fold).
The largest change in G-protein related genes was in the transcript for bradykinin receptor b2 (BDKRb2), which is induced more than 6-fold. Stimulation of BDKRb2 activates PKC and triggers release of intracellular calcium25. It also is important in activation of calcium dependent NOS, formation of NO and activation of cGMP pathway26,27. Brakykinin is reported to acts as a secretagogue of medullary catecholamines28. Bradykinin and also induces NO and prostacyclin formation in adrenolmedullary endothelial cells. We have previously shown that bradykinin can elevated TH and DBH gene expression in PC12 cells29. We speculate that bradykinin, via BDKRb2, may be involved in mediating the IMO triggered elevation of TH gene expression in the adrenal medulla, which is observed even in rats which underwent hypophysectomy and splanchnic nerve section14. In regard, it is interesting to note that BRKRb2 is higher in adrenal medulla of the stress prone SHR compared to WKY rats30.
Expression of several other genes involved in G-protein coupled signaling were also altered, including: cAMP inducible exchange factor (cAMP-GEF1) (down >2-fold); Rab9, which encodes a small GTP-binding protein and rabaptin, which encodes another small protein interacting with Rab5, were reduced by 2–3-fold.
Several calcium-related genes were up-, but not down-, regulated. The greatest changes were observed with calcitonin/calcitonin-related polypeptide, α (CALCA), which codes for the peptide CGRP and neurotransmitter-induced early gene protein 4 (ania-4) which is implicated in regulation of calcium in neurons31. Two calcium channel related genes were down-regulated and two potassium channel related genes were up-regulated.
Several other signaling related genes were also changed (Table 2). The largest changes were in homer homolog 1 (up 11-fold) and in phosphodiesterase 10A (up at least 9-fold). The transcript for phosphodiesterase 14B (PDE10A, PDE4B) was also induced. These phosphodiesterases, are involved in both cAMP and cGMP turnover in many physiological and pathophysiological situations, were up-regulated more than 10- and 8-fold.
Many of the changes in signaling related transcripts with single IMO were not observed with repeated IMO. However, among the overlapping changes in response to both single and repeated IMO in kinase/phosphatase genes were MAPKAPK2 (up >4-fold) and PRKA anchor protein 1 (down > 4fold). With repeated IMO, several additional kinase- and phosphatase-related transcript were uniquely observed to be up-regulated, such as calcium/calmodulin-dependent protein kinase II beta subunit (3-fold), p21-activated kinase 3 (5-fold) and ser/thr-like protein kinase lyk 4 (3.6-fold).
Among G-protein-related only BDKRb2 was up-regulated by both types of stress. Like with single IMO, 6×IMO also induced mRNA for ania-4 (6-fold), which has similarity to calcium/calmodulin-dependent kinases and to human doublecortin and it is implicated in regulation of calcium in neurons31. With repeated IMO calcium channel, γ3 as well as ChaK, a dual function transmembrane protein which functions as both a calcium-permeant ion channel and serine/threonine protein kinase; were down regulated about 2–3 fold.
These results implicate many more signally related changes than previously studied. Marked differences in cell signaling pathways in the adrenal medulla with single and repeated exposure to IMO stress and are important to distinguish between molecular processes leading to adaptation to stress or to pathological consequences of chronically repeated stress.
Growth/Apoptosis Related
The changes in growth factor and apoptosis related genes are shown in Table 3. They comprised 11% of the up-regulated and 12% of the down regulated changes with single IMO. The largest change in this category was in brain derived neurotrophic factor (BDNF), which was elevated 18.4 fold. EGF receptor and inhibin α genes were up-regulated by about 4.5- and 4-fold. At the same time, the transcript for FGF receptor activating protein 1 and several differentiation-related genes were down-regulated by more than 2-fold. The changes in growth factor and related genes may be involved in the increased size of adrenal medulla which has been observed following various types of chronic stress32–36. However, it is perhaps surprising that even with single IMO, there were already changes in growth factor expression. Fewer growth factor-related genes are changed by the repeated compared to single IMO. However, most of the growth factor related genes changed by 6×IMO were also changed by 1×IMO. BDNF showed the largest change also with repeated IMO.
Table 3.
Other Major Changes in Gene Expression with Single and Repeated IMO Stress
| Probe Set ID | UniGene ID | UniGene Name | (1×IMO) Fold C | Probe Set ID | UniGene ID | UniGene Name | (6×IMO) Fold C |
|---|---|---|---|---|---|---|---|
| Growth factor related genes | Up | Growth factor related genes | Up | ||||
| 1386994_at | Rn.27923 | B-cell translocation gene 2 | 5.86 | 1368677_at | Rn.11266 | brain derived neurotrophic factor | 14.42 |
| 1386995_at | 4.71 | 1387244_at | Rn.87514 | cell growth regulator | 5.02 | ||
| 1370823_at | Rn.25267 | BMP and AMB inhibitor | 2.73 | 1387951_at | Rn.18841 | decay-accelarating factor | 3.1 |
| 1368677_at | Rn.11266 | brain derived neurotrophic factor | 18.36 | 1369012_at | Rn.9874 | inhibin beta-A | 3.37 |
| 1387244_at | Rn.87514 | cell growth regulator | 7.08 | ||||
| 1387951_at | Rn.18841 | decay-accelarating factor | 4.72 | ||||
| 1370830_at | Rn.37227 | epidermal growth factor receptor | 4.51 | ||||
| 1387663_at | Rn.10454 | glia maturation factor, β | 7.67 | ||||
| 1386908_at | Rn.1484 | glutaredoxin 1 (thioltransferase) | 2.72 | ||||
| 1367705_at | 2.17 | ||||||
| 1387124_at | Rn.8831 | inhibin alpha | 3.75 | ||||
| 1387922_at | Rn.4346 | late gestation lung protein 1 | 2.07 | ||||
| 1370174_at | Rn.2232 | MDP response gene 116 | 3.59 | ||||
| 1367874_at | Rn.4169 | ras homolog gene family, member Q | 4.23 | ||||
| 1386967_at | 2.76 | ||||||
| 1369867_at | Rn.96242 | sialyltransferase 8 A | 2.48 | ||||
| 1396101_at | Rn.10647 | stanniocalcin 1 | 4.49 | ||||
| 1387623_at | 2.04 | ||||||
| 1387280_a_at | Rn.32261 | tumor-associated protein 1 | 4.07 | ||||
| Down | Down | ||||||
| 1370327_at | Rn.24747 | COMM domain containing 5 | 2.55 | 1368924_at | Rn.2178 | growth hormone receptor | 5.19 |
| 1370204_at | Rn.94200 | FGF receptor activating protein 1 | 2.13 | ||||
| 1368618_at | Rn.30028 | growth factor receptor bound protein 14 | 3.03 | ||||
| 1398785_at | Rn.6775 | multiple endocrine neoplasia 1 | 2.05 | ||||
| 1392743_at | Rn.77753 | myc induced nuclear antigen | 2.64 | ||||
| 1398867_at | Rn.19573 | neuronal differentiation-related gene | 2.59 | ||||
| 1370336_at | Rn.15599 | pregnancy-induced growth inhibitor | 2.36 | ||||
| 1387427_at | Rn.51136 | RAD50 homolog (S. cerevisiae) | 2.17 | ||||
| 1387153_at | Rn.34221 | reversion induced LIM gene | 2.58 | ||||
| Apoptosis related genes | Up | Apoptosis related genes | Up | ||||
| 1367752_at | Rn.40101 | breast cancer anti-estrogen resistance 1 | 2.16 | 1395237_at | Rn.107482 | annexin V-binding protein ABP-7 | 4.09 |
| 1368860_at | Rn.40778 | pleckstrin homology-like domain, A1 | 21.8 | 1378247_at | Rn.20681 | ELL associated factor 2 | 3.68 |
| 1368025_at | Rn.9775 | DNA-damage-inducible transcript 4 | 5.41 | 1368860_at | Rn.40778 | pleckstrin homology-like domain, A1 | 5 |
| 1370319_at | Rn.2923 | peptidylprolyl isomerase F | 2.2 | ||||
| Down | Down | ||||||
| 1367842_at | Rn.19953 | amyloid beta precursor protein-binding, B1 | 2.41 | 1368652_at | Rn.32199 | caspase 9 | 2.87 |
| 1387055_at | Rn.4279 | APP-binding protein 1 | 2.55 | ||||
| 1387605_at | Rn.81078 | caspase 12 | 2.22 | ||||
| 1367890_at | Rn.1438 | caspase 2 | 2.3 | ||||
| 1370044_at | Rn.22800 | Fas apoptotic inhibitory molecule | 3.1 | ||||
| 1390434_at | Rn.18545 | TNFRSF1A-associated via death domain | 4.17 | ||||
| 1371131_a_at | Rn.2758 | upregulated by 1,25-dihydroxyvitamin D-3 | 2.34 | ||||
| Secretion/Neuropeptide related genes | Up | Secretion/Neuropeptide related genes | Up | ||||
| 1369717_at | Rn.47720 | neuromedin U | 5 | 1370028_at | Rn.10149 | angiotensin 1 converting enzyme 1 | 2.42 |
| 1370408_at | Rn.8865 | putative small membrane protein NID67 | 2.37 | 1369116_a_at | 2.59 | ||
| 1387569_at | Rn.74043 | synaptic vesicle glycoprotein 2c | 2.49 | 1370507_at | Rn.11279 | disks large-associated protein 4 | 4.87 |
| 1369619_at | Rn.153037 | urocortin 2 | 8.73 | 1369717_at | Rn.47720 | neuromedin U | 37.48 |
| 1368805_at | Rn.48886 | urotensin 2 | 3.17 | 1368369_at | Rn.87935 | prepronociceptin | 3.52 |
| 1368805_at | Rn.48886 | urotensin 2 | 3.83 | ||||
| 1390257_at | Rn.28719 | vesicle-associated membrane protein | 2.14 | ||||
| Down | Down | ||||||
| 1372950_at | Rn.90025 | blocked early in transport | 2.55 | ||||
| 1369414_at | Rn.64627 | syntaxin binding protein 3 | 2.83 | ||||
| Stress related genes | Up | Stress related genes | Up | ||||
| 1387282_at | Rn.102906 | crystallin, alpha C (Hsp22) | 6.21 | 1368247_at | NA | heat shock 70kD protein 1B | 11.87 |
| 1388721_at | 6 | 1388850_at | Rn.3277 | heat shock protein 1, alpha | 2.42 | ||
| 1368852_at | Rn.64562 | DnaJ-like protein | 1.96 | ||||
| 1370912_at | Rn.1950 | heat shock 70kD protein 1A | 7.01 | ||||
| 1368247_at | NA | heat shock 70kD protein 1B | 13.76 | ||||
| 1378002_at | Rn.58449 | osmotic stress protein 94 kDa | 3.9 | ||||
| Down | Down | ||||||
| 1367741_at | Rn.4028 | ubiquitin-like domain member 1 | 2.06 | 1382809_at | Rn.28931 | cold inducible RNA binding protein | 3.89 |
| Structural protein related genes | Up | Structural protein related genes | Up | ||||
| 1372658_at | Rn.46362 | desmuslin | 2.83 | 1370053_at | Rn.90059 | discs | 2.75 |
| 1370017_at | Rn.10968 | emerin | 4.89 | 1387031_at | Rn.32904 | endoplasmic retuclum protein 29 | 2.35 |
| 1387202_at | Rn.12 | intercellular adhesion molecule 1 | 4.11 | 1387843_at | Rn.2743 | follistatin | 7.51 |
| 1388932_at | Rn.62616 | laminin, alpha 5 | 2.55 | 1388244_s_at | Rn.999 | laminin receptor 1 | 2.47 |
| 1371682_at | Rn.3135 | microtubule-associated protein 1 LC3 α | 3.01 | 1371682_at | Rn.3135 | microtubule-associated protein 1 LC3 α | 3.68 |
| 1370478_at | Rn.48756 | myosin heavy chain Myr 8 | 5.25 | 1370478_at | Rn.48756 | myosin heavy chain Myr 8 | 12.57 |
| 1370697_a_at | Rn.107975 | nexilin | 2.19 | ||||
| 1370875_at | Rn.773 | villin 2 | 3.83 | ||||
| CXC-motif related genes | Up | CXC-motif related genes | Up | ||||
| 1367973_at | Rn.4772 | chemokine (C-C motif) ligand 2 | 7.14 | ||||
| 1367940_at | Rn.12959 | chemokine orphan receptor 1 | 33.36 | ||||
| Down | Down | ||||||
| 1370097_a_at | Rn.44431 | chemokine (C-X-C motif) receptor 4 | 8.5 | 1370097_a_at | Rn.44431 | chemokine (C-X-C motif) receptor 4 | 9.1 |
| 1373661_a_at | 6.3 | 1373661_a_at | 6.18 | ||||
| 1389244_x_at | 5.9 | 1389244_x_at | 5.13 | ||||
| Cytokine related genes | Up | Cytokine related genes | Up | ||||
| 1388233_at | Rn.14523 | cytokine inducible SH2-containing protein | 6.8 | 1387180_at | Rn.10758 | interleukin 1 receptor, type II | 3.83 |
| 1368134_a_at | Rn.10471 | interleukin 4 receptor | 5.12 | 1368134_a_at | Rn.10471 | interleukin 4 receptor | 4.51 |
| 1369584_at | Rn.81237 | suppressor of cytokine signaling 3 | 2.36 | ||||
| Down | Down | ||||||
| 1368375_a_at | Rn.2490 | interleukin 15 | 2.47 | 1368375_a_at | Rn.2490 | interleukin 15 | 4.63 |
| Metabolism related genes | Up | Metabolism related genes | Up | ||||
| 1367982_at | Rn.97126 | aminolevulinic acid synthase 1 | 7.44 | 1370375_at | Rn.10202 | glutaminase 2 (liver, mitochondrial) | 5.42 |
| 1370964_at | Rn.5078 | arginosuccinate synthetase | 6.23 | 1371350_at | Rn.41420 | methionine adenosyltransferase II, α | 2.91 |
| 1383248_at | Rn.7038 | flavin containing monooxygenase 5 | 3.13 | 1386951_at | Rn.100240 | NADH dehydrogenase 1 α 5 | 5.51 |
| 1370375_at | Rn.10202 | glutaminase 2 (liver, mitochondrial) | 5.78 | 1370354_at | Rn.13634 | poly (ADP-ribose) glycohydrolase | 4.94 |
| 1386914_at | Rn.3862 | guanosine monophosphate reductase | 7.07 | 1371762_at | Rn.108214 | retinol binding protein 4 | 4.65 |
| 1374903_at | Rn.8807 | I-branching β-1,6-ASATF | 2.32 | ||||
| 1371350_at | Rn.41420 | methionine adenosyltransferase II, α | 2.41 | ||||
| 1386951_at | Rn.100240 | NADH dehydrogenase 1 α 5 | 6.13 | ||||
| 1370191_at | Rn.6290 | ornithine decarboxylase antizyme inhibitor | 2.12 | ||||
| 1369785_at | Rn.18690 | PP amidotransferase | 2.92 | ||||
| 1370354_at | Rn.13634 | poly (ADP-ribose) glycohydrolase | 6.05 | ||||
| 1386981_at | Rn.6085 | solute carrier family member 1 | 2.07 | ||||
| Down | Down | ||||||
| 1373838_at | Rn.44467 | α 1,3-fucosyltransferase Fuc-T | 2.39 | 1388044_at | Rn.44844 | 6-PF-2-kinase/fructose-2,6-BP 2 | 2.2 |
| 1367991_at | Rn.22161 | glucosidase 1 | 2.29 | 1387973_at | Rn.10170 | cytochrome P450 4F4 | 4.31 |
| 1386983_at | Rn.11080 | hydroxymethylbilane synthase | 2.48 | 1379885_at | Rn.6404 | flavin containing monooxygenase 4 | 2.78 |
| 1387187_a_at | Rn.37420 | N-acetyltransferase 1 | 2.62 | ||||
| 1368756_at | Rn.9674 | thioesterase domain containing 1 | 3.76 | ||||
| 1389689_at | Rn.43153 | valyl-tRNA synthetase 2-like | 2.55 | ||||
| Lipid-Metabolism related genes | Up | Lipid-Metabolism related genes | Up | ||||
| 1367915_at | Rn.252 | diacylglycerol O-acyltransferase 1 | 11.93 | 1390549_at | Rn.101807 | adiponectin receptor 2 | 2.18 |
| 1387630_at | Rn.4243 | fatty acid elongase 1 | 4.83 | 1387796_at | Rn.11318 | arachidonate 12-lipoxygenase | 2.01 |
| 1394401_at | Rn.46942 | fatty acid elongase 2 | 6.32 | 1367979_s_at | Rn.107152 | cytochrome P450, subfamily 51 | 5.68 |
| 1388108_at | 4.74 | 1367915_at | Rn.252 | diacylglycerol O-acyltransferase 1 | 6.08 | ||
| 1387233_at | Rn.7040 | hydroxysteroid (17-beta) dehydrogenase 7 | 4.7 | 1368878_at | Rn.10780 | isopentenyl-diphosphate delta isomerase | 7.8 |
| 1368878_at | Rn.10780 | isopentenyl-diphosphate delta isomerase | 5.04 | 1368020_at | Rn.10288 | mevalonate (diphospho) decarboxylase | 2.89 |
| 1368570_at | Rn.54479 | lecithin-retinol acyltransferase | 17.89 | 1368015_at | Rn.7730 | prostaglandin E synthase | 10.5 |
| 1368683_at | Rn.87449 | oxidised LDL receptor 1 | 15.44 | 1368014_at | 3.05 | ||
| 1368014_at | Rn.7730 | prostaglandin E synthase | 4.97 | ||||
| 1368527_at | Rn.44369 | prostaglandin-endoperoxide synthase 2 | 12.27 | ||||
| 1367855_at | Rn.88169 | scavenger receptor class B, member 1 | 4.57 | ||||
| 1386956_at | 4.54 | ||||||
| Down | Down | ||||||
| 1367638_at | Rn.13468 | malonyl-CoA decarboxylase | 2.09 | 1388211_s_at | Rn.37524 | cytosolic acyl-CoA thioesterase 1 | 2.78 |
| 1369070_at | Rn.29982 | peroxisomal biogenesis factor 12 | 2.04 | ||||
| 1387064_at | Rn.4065 | peroxisomal membrane protein 3 | 3.23 | ||||
| 1396866_s_at | 3.00 | ||||||
| Protease related genes | Up | Protease related genes | Up | ||||
| 1368223_at | Rn.7897 | metalloprotease with thrombospondin 1,1 | 8.1 | 1387135_at | Rn.98788 | metalloproteinase domain 15 | 2.35 |
| 1392894_at | Rn.64635 | fibrinogen-like 2 | 2.45 | 1368223_at | Rn.7897 | metalloprotease with thrombospondin 1,1 | 4.77 |
| 1367800_at | Rn.107102 | plasminogen activator | 4.1 | 1368595_at | Rn.3117 | matrix metalloproteinase 24 | 2.1 |
| 1387269_s_at | Rn.82711 | plasminogen activator, urokinase receptor | 6.92 | 1368901_at | Rn.88295 | thrombomodulin | 2.41 |
| 1392264_s_at | Rn.29367 | serine/cysteine proteinase inhibitor, 1 | 10.36 | ||||
| 1368519_at | 4.37 | ||||||
| 1387812_at | Rn.950 | Subtilisin - like endoprotease | 3.3 | ||||
| 1367712_at | Rn.25754 | tissue inhibitor of metalloproteinase 1 | 2.08 | ||||
| Down | Down | ||||||
| 1368904_at | Rn.8875 | calpain 10 | 2.79 | 1368904_at | Rn.8875 | calpain 10 | 3.24 |
| 1375951_at | Rn.88295 | thrombomodulin | 7.57 | ||||
| 1368900_at | 5.95 | ||||||
| 1368901_at | 5.19 | ||||||
| 1367966_at | Rn.10902 | dipeptidylpeptidase 3 | 2.14 | 1367860_a_at | Rn.10371 | matrix metalloproteinase 14 | 4.26 |
| Miscellaneous genes | Up | Miscellaneous genes | Up | ||||
| 1368111_at | Rn.11149 | BTB domain containing 2 | 3.84 | 1368111_at | Rn.11149 | BTB domain containing 2 | 2.91 |
| 1370951_at | Rn.12038 | ER transmembrane protein Dri 42 | 2.25 | ||||
| 1370950_at | 2.42 | ||||||
| 1370177_at | Rn.10677 | poliovirus receptor | 11.92 | ||||
| Down | Down | ||||||
| 1373034_at | Rn.103351 | tryptophan rich basic protein | 2.42 | ||||
Bold – Genes changed by both types of stress; Fold C – Fold change
A substantial number of apoptosis related transcripts were also changed. In this category, the most robust changes were observed in the induction (22-fold) of pleckstrin homology-like domain family A, member 1 which can also be induced by growth factors and differentiating agents and it has been implicated in mediating programmed cell death37. Several apoptosis related transcripts were down regulated, such as caspase 2 and caspase 12. With repeated IMO there were less changes in apotosis related genes. From the changes with single IMO only pleckstrin homology-like domain family A, member 1 was also induced (up 5 fold). Caspase 9 was down-regulated by about 3 fold following repeated IMO, perhaps consistent with hypertrophy of the adrenal with repeated IMO.
Neurosecretion and Neuropeptide Related
The neurosecretion and neuropeptide related transcripts changed are also shown in Table 3. With single IMO they represent only 3% of the changes. However, several of these neuropeptide-related genes may be important in mediating the response to stress, such as urocortin 2 (Ucn2) (increased 9-fold) neuromedin (increased 5-fold), and urotensin 2 (increased 3-fold). Urocortins are reported to mediate adaptive responses of the cardiovascular system to stressful conditions [reviewed in38]. Ucn2 immunoreactivity was previously observed in TH positive cells in rat adrenal medulla and locus coeruleus39,40. Administration of Ucn2 to PC12 cells increased NE release and phosphorylation of TH41.
More of neurosecretion and neuropeptide related transcripts (8%) were changed with repeated stress than with single IMO. Neuromedin U (37 fold) and urotensin 2 (4 fold) were also increased with repeated IMO. Urotensin 2 is the most potent mammalian vasoconstrictor to date. Plasma urotensin 2 levels are elevated in congestive heart failure patients and may play a role in progression of the disease42. Central neuromedin U is implicated in the stress-induced activation of CRF-containing neurons in the paraventricular nucleus43.
In addition, transcripts of angiotensin converting enzyme (ACE) and prepronociceptin genes were induced, as well as two genes associated with synaptic vesicle function. Prepronociceptin was induced by repeated stress by more than 3-fold. Nociceptin, also known as orphanin FQ, is an endogenous ligand for the opioid receptor-like 1 (NOP) receptor and has some structural homology with the endogenous opioid peptide dynorphin A44,45. It is implicating as playing an important role in several physiological functions including pain, anxiety, locomotion, learning, and memory. It is attractive to speculate that the induction of norciceptin may participate in stress triggered nociception.
Stress Related
A number of genes related to stress were induced (Table 3) including heat shock 70kD protein 1B (up over 10-fold) with both single and repeated IMO and heat shock 70kD protein 1A (up 7 fold) with 1× IMO).
Structural
Some structural related genes (Table 3), such as desmuslin, intercellular adhesion molecule 1, myosin heavy chain Myr 8, also showed more than 2-fold change, and in some cases as much as 5-fold elevated expression. The percentage of changes in structural genes was slightly higher in response to repeated IMO than to single IMO (7.6% vs. 5%)
Immune Related
Although only a few of the changes related to immune function, these changes might be very important. As shown in Table 3, transcripts of chemokine orphan receptor 1 was induced by 33-fold and chemokine (C-C) motif ligand 2 was up-regulated by 8-fold with single IMO. One of the cytokine receptor-related genes, interleukin 4 receptor was up-regulated by 5-fold. In addition, chemokine receptor 4 (CXCR4) gene expression was more than 8-fold down-regulated. One of the ligands of cytokine receptors, interleukin 15, was regulated by more than 2-fold at the same time.
The list of immune response related genes changed by repeated IMO was similar to the genes changed by single IMO. CXCR4 was severely reduced by 9-fold and interleukin 15 by more than 4-fold.
Metabolism Related
The results also indicate that 2-hour single IMO could trigger marked changes in metabolism. We found changes of genes that related to enzymes involved in many metabolic pathways. Most of the up-regulated genes related to metabolism and also to lipid-metabolism in repeated IMO were already up-regulated with single IMO. Diacylglycerol O-acyltransferase 1, methionine adenosyltransferase II, alpha, NADH dehydrogenase 1 alpha subcomplex 5, poly (ADP-ribose) glycohydrolase (PARG) and prostaglandin E synthase (PTGES) showed large changes, as they also did with single IMO.
Protease Related
There were some changes in protease related genes, some of which were quite large, such as greater than 5-fold induction of thrombomodulin, metalloprotease with thrombospondin 1, plasminogen activator urokinase receptor, and serine/cysteine proteinase inhibitor 1.
Protease related genes, such as metalloprotease with thrombospondin 1(14.8-fold) and thrombomodulin (2.41-fold) were also increased by repeated IMO. Among the down-regulated genes that related to protease, calpain 10 was reduced by both types of stress (2.8-, 3.2-fold). Matrix metalloproteinase 14 (MMP14) was up-regulated by more than 4-fold with repeated IMO.
Others
A few of the genes were not easily categorized (Miscellaneous genes in Table 3). The undefined or genes not annotated are given in Table 4.
Validation of Changes of Selected Genes
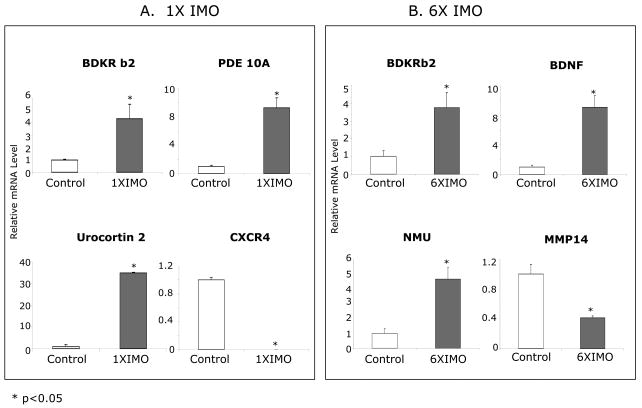
Selected genes from different categories showing robust changes by microarray analysis were chosen for validation. Real-time RT-PCR was performed to quantify the relative expression levels of mRNA for bradykinin receptor b2 (BDKRb2) in both stress groups and urocortin 2 (Ucn2), phosphodesterase (PDE) 10A, as well as chemockine receptor 4 (CXCR4) in single IMO group (Fig. 3A). To validate changes in repeated stress group, expression levels of BDKRb2, BDNF, neuromedin U, and Matrix metalloproteinase 14 (MMP14) were quantified by real-time RT-PCR as well (Fig 3B).
Figure 3.
Validation of selective microarray results from IMO stress experiment by real-time RT-PCR. Quantitative real-time RT-PCR showing the changes in mRNA levels of bradykinin receptor b2 (BDKR b2), phosphodiesterase 10A (PDE 10A), urocortin2, chemokine (C-X-C motif) receptor 4 (CXCR4), BDNF, neuromedin U (NMU) and matrix metalloproteinase 14 (MMP14) with single IMO (A) or repeated IMO (B). The mean for the control is taken as 1.0. * p < 0.05 versus unstressed controls.
There was good correspondence between the data obtained from microarray and the results from the real-time RT-PCR analysis. BDKRb2 and PDE 10A gene expression increased about 5-fold and 9-fold in the RNA from the 1× IMO group, which is consistent with the microarray results. Urocortin 2 showed a greater than 30-fold increase and CXCR4 was markedly down-regulated by single IMO, which were even greater than the extent of changes from the microarray results. With 6× IMO, BDKRb2 gene showed about 4-fold increase, neuromedin U gene expression increased 5-fold and BDNF gene expression induced 9-fold, and MMP14 about 3-fold, which are similar to the results from the microarray analysis.
Pathway Analysis
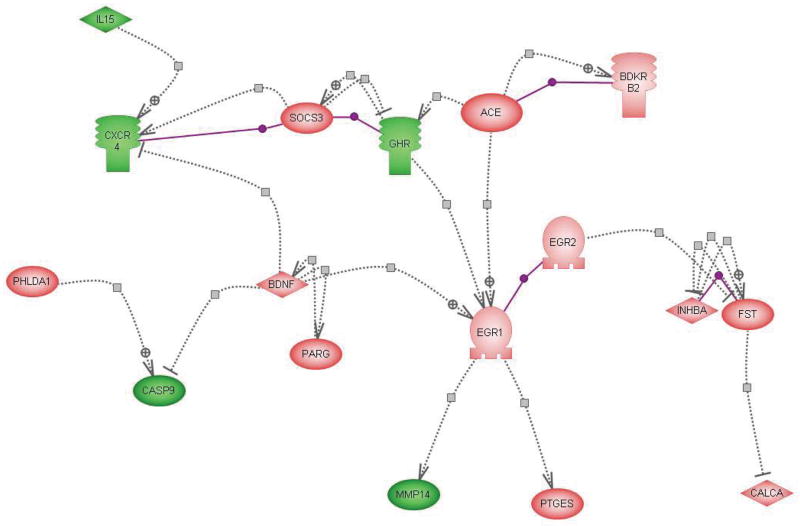
The microarray analysis, as detailed above, discovered that hundreds of genes were significantly changed in the adrenal medulla by single as well as by repeated IMO. To further understand how those genes interact with each other and if there are common factors or regulators related to those genes, PathwayAssist software was used to explore networks of connections among the genes responsive to stress. Pathway analysis of direct interactions between the genes which were up- and down-regulated by single (not shown) and repeated IMO (Fig. 4) shows that a number of genes changed by repeated IMO can regulate Egr1. Egr1 was previously shown to be induced by stress in the adrenal medulla and to be important for the regulation of TH transcription19,46,47. ACE, together with BDKRb2 and growth hormone receptor together with SOC3 (suppressor of cytokine signaling 3) and CXCR4, as well as BDNF can regulate Egr1 gene expression. BDNF can inhibit several of the down-regulated genes, such as caspase 9 and CXCR4, the later is activated by the down regulated interleukin 15 (IL15), and BDNF, which interacts with PARG. Further downstream interactions of Egr1 and Egr2 are connected to MMP14, PTGES and inhibin-beta-A (INHBA) and follistatin (FST), and subsequently to the CALCA (CGRP) gene product.
Figure 4.
Direct interaction of changes in gene expression with repeated IMO stress. Pathway analysis showing the direct interactions among genes down- regulated (green, IL15, CXCR4, GHR, CASP9, MMP14)or up-regulated (pink, all others) or by repeated IMO. The symbols are as follows: ligand:  ; transcription factor:
; transcription factor:  ; kinase:
; kinase:  ; receptor:
; receptor:  nuclear receptor:
nuclear receptor:  ;: binding::
;: binding::  positive regulations
positive regulations 
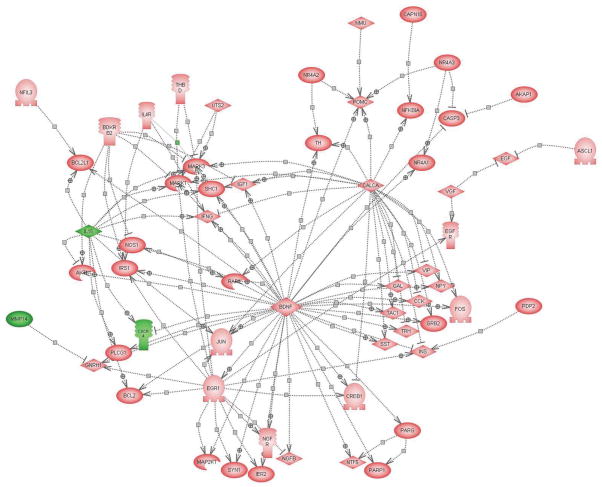
Pathway analysis also revealed common targets of the genes changed by both single and repeated IMO stress (Fig. 5). Among the changed genes, BDNF, CALCA (CGRP), and Egr1 played especially central roles for downstream activation of other target genes. As shown in Figure 5, BDNF can trigger activation of Egr1, FOS, CREB, JUN, TH, and several neuropeptides, such as vasoactive intestinal peptide (VIP) and neuropeptide Y (NPY), etc. and it inhibits CXCR4 gene expression. It is also clear that the activation of CALCA could further activate MAPK1 and MAPK3 and TH. Egr1, itself a target of BDNF, activated some growth factor and survival related genes, such as NGF receptor, insulin receptor substrate 1 (IRS1) and BCL2, several MAK kinases and MAP kinase kinases, and transcription factors such as CREB1.
Figure 5.
Putative targets of common changes in gene expression with single and repeated IMO stress. Pathway analysis showing the common targets which are activated or inhibited by the common genes which are decreased (green, IL15, MMP14, CXCR4) or elevated (pink, all others) or by both durations of IMO stress. The symbols are as described in figure above. The abbreviations are as follows: PARP1 [ADP-ribosyltransferase (NAD+; poly (ADP-ribose) polymerase)]; BCL2 (B-cell leukemia/lymphoma 2); BCL2L1 (Bcl2-like 1); BDKRB2 (bradykinin receptor B2); BDNF (brain-derived neurotrophic factor); CALCA (calcitonin/calcitonin-related polypeptide, alpha); CREB1 (cAMP responsive element binding protein 1); CASP3 (caspase 3); CD44 (CD44 antigen); CCK (cholecystokinin); EGR1 (early growth response 1); EGF (epidermal growth factor); EGFR (epidermal growth factor) FOS (FOS oncogene); GAL (galanin); GNRH1 (gonadotropin-releasing hormone 1); GRB2 (growth factor receptor bound protein 2); IER2 (immediate early response 2); INS (insulin); IRS1 (insulin receptor substrate 1); IGF1 (insulin-like growth factor 1); IFNG (interferon, gamma); IL15 (interleukin 15); IL4R (interleukin 4 receptor); JUN (Jun oncogene); MMP14 (matrix metalloproteinase 14); MMP9 (matrix metalloproteinase 9); MAPK1 (mitogen activated protein kinase 1); MAPK3 (mitogen activated protein kinase 3); MAP2K1 (mitogen activated protein kinase kinase 1); RAF1 (murine leukemia viral oncogene homolog 1); NGFR (nerve growth factor receptor); NMU (neuromedin U); NPY (neuropeptide Y); NTF5 (neurotrophin 5); NOS1 (nitric oxide synthase 1); NFIL3 (nuclear factor, interleukin 3 regulated); NR4A1 (nuclear receptor subfamily 4, group A, member 1); NR4A2 (nuclear receptor subfamily 4, group A, member 2); NR4A3 (nuclear receptor subfamily 4, group A, member 3); PARG [poly (ADP-ribose) glycohydrolase]; POMC (proopiomelanocortin); PDP2 (pyruvate dehydrogenase phosphatase isoenzyme 2); SST (somatostatin); SHC1 (src homology 2 domain-containing transforming protein C1); SYN1 (synapsin I); TAC1 (tachykinin, precursor 1); THBD (thrombomodulin); AKT1 (thymoma viral proto-oncogene 1); TRH (thyrotropin-releasing hormone); TGFB1 (transforming growth factor, beta 1); TNF (tumor necrosis factor); TH (tyrosine hydroxylase); UTS2 (urotensin 2); VEGF (vascular endothelial growth factor); VIP (vasoactive intestinal peptide); VGF (VGF nerve growth factor inducible).
Summary
The stress transcriptome of the adrenal medulla is quite extensive, especially in response to single immobilization stress. The study reveals many previously unidentified targets in the adrenal medulla. For example, extremely large changes were observed among transcription factors in nuclear receptor subfamily members (NR4A1, NR4A2, NR4A3), CREM and Mash1. Among the cell signalling related genes, changes in several kinase and protein phosphatase genes were identified, as well as down regulation of the transcript for the A kinase anchor protein. Considerable differences in cell signalling related genes was observed in response to single and repeated IMO, although bradykinin receptor b2 (BDKRb2) and transcript for CGRP (CALCA) and ania-4 were common to both single and repeated IMO. A large number of growth factor related transcripts were changed, as well as some apoptosis related genes, with several caspases down regulated. While only a few neuropeptides were identified, induction of urocortin 2 with single IMO, and urotensin 2 and neuromedin U with both durations of stress may be very meaningful. Quite a few of the changes were also observed in transcripts related to structural proteins, metabolism and especially lipid metabolism, as well as several chemokine and cytokines or their receptors. In remains to be determined if all of these changes are specifically localized to the adrenal chromaffin cell type. Pathway analyses demonstrate that Egr-1, BDNF and CALCA are likely key players which may regulate tyrosine hydroxylase transcription and thereby Epi/NE biosynthesis. Overall the findings revealed potential new biomarkers for the adrenal medullary response to stress and provide new mechanistic insights into the common and distinct responses to acute and repeated exposure to stress.
Acknowledgments
We are grateful to Dr. Caroline Ojaimi from the Department of Physiology, New York Medical College, Microarray Core Facility: Functional Genome, for guidance with microarray data analysis; and support from NIH grant NS28869, Slovak Research Agency Grant No. APVV-0148-06 and VEGA 2-0133/08; and the assistance of the NINDS/NIMH microarray consortium.
References
- 1.Cannon W. Organization of physiological homeostasis. Physiological Reviews. 1929;9:3900–431. [Google Scholar]
- 2.Goldstein DS. Stress, Catecholamines and Cardiovascular Disease. Oxford University Press; Oxford: 1995. [Google Scholar]
- 3.McCarty R, Gold PE. Plasma catecholamines: effects of footshock level and hormonal modulators of memory storage. Horm Behav. 1981;15:168–82. doi: 10.1016/0018-506x(81)90026-x. [DOI] [PubMed] [Google Scholar]
- 4.Hall JL, Gold PE. Adrenalectomy-induced memory deficits: role of plasma glucose levels. Physiol Behav. 1990;47:27–33. doi: 10.1016/0031-9384(90)90038-6. [DOI] [PubMed] [Google Scholar]
- 5.Southwick SM, et al. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 6.Yehuda R, et al. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 1998;44:56–63. doi: 10.1016/s0006-3223(98)80007-3. [DOI] [PubMed] [Google Scholar]
- 7.Kvetnansky R, Sabban EL. Stress and molecular biology of neurotransmitter-related enzymes. Ann N Y Acad Sci. 1998;851:342–56. doi: 10.1111/j.1749-6632.1998.tb09008.x. [DOI] [PubMed] [Google Scholar]
- 8.Sabban EL. Catecholamines in stress: molecular mechanisms of gene expression. Endocrine Regulations. 2007;41:61–73. [PubMed] [Google Scholar]
- 9.Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci. 2001;24:91–8. doi: 10.1016/s0166-2236(00)01687-8. [DOI] [PubMed] [Google Scholar]
- 10.Wong DL, Tank AW. Stress-induced catecholaminergic function: transcriptional and post-transcriptional control. Stress. 2007;10:121–30. doi: 10.1080/10253890701393529. [DOI] [PubMed] [Google Scholar]
- 11.Nankova BB, Tank AW, Sabban EL. Transient or sustained transcriptional activation of the genes encoding rat adrenomedullary catecholamine biosynthetic enzymes by different durations of immobilization stress. Neuroscience. 1999;94:803–8. doi: 10.1016/s0306-4522(99)00290-0. [DOI] [PubMed] [Google Scholar]
- 12.Sabban EL, et al. Stress Triggered Changes in Gene Expression in Adrenal Medulla: Transcriptional Responses to Acute and Chronic Stress. Cell Mol Neurobiol. 2006;26:845–854. doi: 10.1007/s10571-006-9069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osterhout C, Sun B, Tank AW. Regulation of Tyrosine Hydroxylase Gene Transcription Rate by Stress: Use of Transgenic Mice. In: McCarty R, et al., editors. Stress: Neural, Endocrine and Molecular Studies. Taylor & Francis; Londaon: 2002. pp. 107–113. [Google Scholar]
- 14.Nankova B, et al. Induction of tyrosine hydroxylase gene expression by a nonneuronal nonpituitary-mediated mechanism in immobilization stress. Proc Natl Acad Sci U S A. 1994;91:5937–41. doi: 10.1073/pnas.91.13.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon A, et al. Regulation of tyrosine hydroxylase and dopamine beta-hydroxylase mRNA levels in rat adrenals by a single and repeated immobilization stress. J Neurochem. 1992;58:2124–30. doi: 10.1111/j.1471-4159.1992.tb10954.x. [DOI] [PubMed] [Google Scholar]
- 16.Kvetnansky R, Mikulaj L. Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology. 1970;87:738–43. doi: 10.1210/endo-87-4-738. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, et al. Increased susceptibility to transcriptional changes with novel stressor in adrenal medulla of rats exposed to prolonged cold stress. Brain Res Mol Brain Res. 2005;141:19–29. doi: 10.1016/j.molbrainres.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Wong DL, et al. Genetic mechanisms for adrenergic control during stress. Ann N Y Acad Sci. 2004;1018:387–97. doi: 10.1196/annals.1296.048. [DOI] [PubMed] [Google Scholar]
- 19.Papanikolaou NA, Sabban EL. Sp1/Egr1 motif: a new candidate in the regulation of rat tyrosine hydroxylase gene transcription by immobilization stress. J Neurochem. 1999;73:433–6. doi: 10.1046/j.1471-4159.1999.0730433.x. [DOI] [PubMed] [Google Scholar]
- 20.Nankova BB, et al. Fos-related antigen 2: potential mediator of the transcriptional activation in rat adrenal medulla evoked by repeated immobilization stress. J Neurosci. 2000;20:5647–53. doi: 10.1523/JNEUROSCI.20-15-05647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol. 2006;298:335–43. doi: 10.1016/j.ydbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch MR, et al. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development. 1998;125:599–608. doi: 10.1242/dev.125.4.599. [DOI] [PubMed] [Google Scholar]
- 23.Guillemot F, et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–76. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- 24.Pak DT, Sheng M. Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science. 2003;302:1368–73. doi: 10.1126/science.1082475. [DOI] [PubMed] [Google Scholar]
- 25.Hecquet C, et al. Positive cooperativity between the thrombin and bradykinin B2 receptors enhances arachidonic acid release. Am J Physiol Heart Circ Physiol. 2006;290:H948–58. doi: 10.1152/ajpheart.00868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abadir PM, et al. Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension. 2006;48:316–22. doi: 10.1161/01.HYP.0000228997.88162.a8. [DOI] [PubMed] [Google Scholar]
- 27.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42:600–4. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 28.Murakumo Y, et al. Potentiation by ouabain of bradykinin-induced catecholamine secretion and calcium influx into cultured bovine adrenal chromaffin cells: evidence for involvement of Na+ influx associated with the bradykinin B2-receptor. Life Sci. 1995;57:PL259–64. doi: 10.1016/0024-3205(95)02141-5. [DOI] [PubMed] [Google Scholar]
- 29.Gebreyesus K, Kilbourne EJ, Sabban EL. Bradykinin elevates tyrosine hydroxylase and dopamine beta-hydroxylase mRNA levels in PC12 cells. Brain Res. 1993;608:345–8. doi: 10.1016/0006-8993(93)91477-a. [DOI] [PubMed] [Google Scholar]
- 30.Qadri F, et al. Kinin B2 receptor localization and expression in the hypothalamo-pituitary-adrenal axis of spontaneously hypertensive rats. Int Immunopharmacol. 2003;3:285–92. doi: 10.1016/S1567-5769(02)00269-2. [DOI] [PubMed] [Google Scholar]
- 31.Ohmae S, et al. Molecular identification and characterization of a family of kinases with homology to Ca2+/calmodulin-dependent protein kinases I/IV. J Biol Chem. 2006;281:20427–39. doi: 10.1074/jbc.M513212200. [DOI] [PubMed] [Google Scholar]
- 32.Kvetnansky R, et al. Bratislavske Lekarske Listy. 1966;46:35–41. [PubMed] [Google Scholar]
- 33.Scaria KS, Premalatha LS. Cold induced adrenal weight & volume changes in white rats. Indian J Exp Biol. 1967;5:256–7. [PubMed] [Google Scholar]
- 34.McCarty R, Horwatt K, Konarska M. Chronic stress and sympathetic-adrenal medullary responsiveness. Soc Sci Med. 1988;26:333–41. doi: 10.1016/0277-9536(88)90398-x. [DOI] [PubMed] [Google Scholar]
- 35.Wolman M, et al. Pathological changes in organs of rats chronically exposed to hypoxia. Development of pulmonary lipidosis. Histol Histopathol. 1993;8:247–55. [PubMed] [Google Scholar]
- 36.Ulrich-Lai YM, et al. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006 doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 37.Gomes I, et al. A proline- and glutamine-rich protein promotes apoptosis in neuronal cells. J Neurochem. 1999;73:612–22. doi: 10.1046/j.1471-4159.1999.0730612.x. [DOI] [PubMed] [Google Scholar]
- 38.Nazarloo HP, et al. The roles of corticotropin-releasing factor-related peptides and their receptors in the cardiovascular system. Curr Protein Pept Sci. 2006;7:229–39. doi: 10.2174/138920306777452358. [DOI] [PubMed] [Google Scholar]
- 39.Yamauchi N, et al. Distribution of urocortin 2 in various tissues of the rat. J Neuroendocrinol. 2005;17:656–63. doi: 10.1111/j.1365-2826.2005.01354.x. [DOI] [PubMed] [Google Scholar]
- 40.Reyes TM, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–8. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nemoto T, Mano-Otagiri A, Shibasaki T. Urocortin 2 induces tyrosine hydroxylase phosphorylation in PC12 cells. Biochem Biophys Res Commun. 2005;330:821–31. doi: 10.1016/j.bbrc.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 42.Gruson D, et al. Circulating urotensin II levels in moderate to severe congestive heart failure: its relations with myocardial function and well established neurohormonal markers. Peptides. 2006;27:1527–31. doi: 10.1016/j.peptides.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Yokota M, et al. Fos expression in CRF-containing neurons in the rat paraventricular nucleus after central administration of neuromedin U. Stress. 2004;7:109–12. doi: 10.1080/10253890410001727370. [DOI] [PubMed] [Google Scholar]
- 44.Meunier JC, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–5. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 45.Reinscheid RK, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–4. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 46.Papanikolaou NA, Sabban EL. Ability of Egr1 to activate tyrosine hydroxylase transcription in PC12 cells. Cross-talk with AP-1 factors. J Biol Chem. 2000;275:26683–9. doi: 10.1074/jbc.M000049200. [DOI] [PubMed] [Google Scholar]
- 47.Nakashima A, Ota A, Sabban EL. Interactions between Egr1 and AP1 factors in regulation of tyrosine hydroxylase transcription. Brain Res Mol Brain Res. 2003;112:61–9. doi: 10.1016/s0169-328x(03)00047-0. [DOI] [PubMed] [Google Scholar]