Abstract
Background
Tobacco use is the most preventable cause of premature death and disability. Even though tobacco use is common in many Asian countries, reliable and comparable data on the burden imposed by tobacco use in this region are sparse, and surveillance systems to track trends are in their infancy.
Objective
To assess and compare the prevalence of tobacco use and its associated factors in nine selected rural sites in five Asian countries.
Methods
Tobacco use among 9,208 men and 9,221 women aged 25–64 years in nine Health and Demographic Surveillance System (HDSS) sites in five Asian countries of the INDEPTH Network were examined in 2005 as part of a broader survey of the major chronic non-communicable disease risk factors. All sites used a standardised protocol based on the WHO STEPS approach to risk factor surveillance; expanded questions of local relevance, including chewing tobacco, were also included. Multivariable logistic regression was used to assess demographic factors associated with tobacco use.
Results
Tobacco use, whether smoked or chewed, was common across all sites with some notable variations. More than 50% of men smoked daily; this applied to almost all age groups. Few women smoked daily in any of the sites. However, women were more likely to chew tobacco than men in all sites except Vadu in India. Tobacco use in men began in late adolescence in most of the sites and the number of cigarettes smoked daily ranged from three to 15. Use of both forms of tobacco, smoked and chewed, was associated with age, gender and education. Men were more likely to smoke compared to women, smoking increased with age in the four sites in Bangladesh but not in other sites and with low level of education in all the sites.
Conclusion
The prevalence of tobacco use, regardless of the type of tobacco, was high among men in all of these rural populations with tobacco use started during adolescence in all HDSS sites. Innovative communication strategies for behaviour change targeting adolescents in schools and adult men and women at work or at home, may create a mass awareness about adverse health consequences of tobacco smoking or chewing tobacco. Such efforts, to be effective, however, need to be supported by strong legislation and leadership. Only four of the five countries involved in this multi-site study have ratified the Framework Convention on Tobacco Control, and even where it has been ratified, implementation is uneven.
Keywords: tobacco smoking, tobacco chewing, cigarette, risk factor surveillance, INDEPTH Network, WHO STEPS
The World Health Organization's (WHO) report on the Global Tobacco Epidemic in 2008 highlighted that approximately 5.4 million deaths every year are related to tobacco use (1). Unless urgent attention is taken, more than 80% of tobacco-related deaths will occur in low and middle-income countries by 2030 and could kill one billion during this century (1). Global estimates of morbidity and mortality caused by smoking provide the basis for international tobacco control efforts such as the Framework Convention on Tobacco Control (FCTC) (2). Some low and middle-income countries rely on hospital-based morbidity and mortality data to estimate the health burden of tobacco, but such estimates do not reflect the level of tobacco-attributable morbidity and mortality in the population. Disaggregated estimates of tobacco use by region, age and sex can provide the evidence for choosing interventions based on their expected effectiveness in the population.
Tobacco use is the single most important preventable cause of premature death and disability from chronic non-communicable disease (NCD) conditions. Tobacco is a risk factor for six of the eight leading causes of death in the world (1). Smoking significantly contributes to chronic NCD, mainly heart disease, stroke, cancer (lung, larynx, oral cavity, pharynx and oesophagus) and chronic obstructive pulmonary diseases (COPD) (3, 4). Currently the number of premature deaths attributable to tobacco use in wealthy countries is similar to that of low and middle-income countries combined (2.41 million vs. 2.43 million). The estimate for South East Asian region for 2000 was 1.1 million deaths attributed to tobacco use, 62% in India alone (5). In this region, the production of cigarettes has increased by more than 50% in the 10 years between 1993 and 2003 (6).
Tobacco is used in many forms which vary across countries in Asia. Manufactured cigarettes and hand-rolled cigarettes are the most common forms of tobacco used in this region. Tobacco mixed with molasses in a specially devised tool, chewing betel leaf with sliced betel nut or areca nut and slaked lime, placing sun dried processed tobacco leaf with slaked lime on the gum and snuffing are also prevalent in South East Asian region (7). Use of smokeless tobacco including chewing, snuffing, sucking, rubbing on the teeth and placing on the gum have been a part of the culture in South East Asian countries. In these countries, smokeless tobacco is commonly used among illiterate people, those with low education, unemployed and unskilled workers (8). Smokeless tobacco use is associated with oral, oesophageal and respiratory cancers, adverse effects on pregnancy, cardiovascular diseases and asthma. The hazardous effects of smokeless tobacco on health in many low and middle-income countries are, however, substantially unknown.
Comparable population prevalence data on smoking and smokeless tobacco use, as well as other major risk factors for chronic diseases in Asia are sparse. In an attempt to address this gap, nine Health and Demographic Surveillance System (HDSS) of the INDEPTH Network (www.indepth-network.org) collaborated in a multi-site cross-sectional study of chronic NCD risk factors in a systematic and standardised manner to measure population levels of major risk factors, including patterns and types of tobacco use.
Study objectives
The aim of this study was to assess and compare the prevalence of cigarette smoking and smokeless tobacco use and to identify demographic factors associated with tobacco use in nine selected rural HDSS sites in Asian countries.
Methods
The multi-centre study was a cross-sectional survey conducted in nine INDEPTH HDSS in five Asian countries where subsets of the population are longitudinally followed (9). This allowed an accurate sampling frame from which an unbiased random sample of the adult population within each HDSS could be drawn for this risk factor survey (10). The sample included a minimum of 250 men and women from four age groups aged 25–64 years, to allow comparisons by age and sex. Over-sampling of individuals in each group from all sites was done to ensure that sufficient number of adults were included from each sex and age group (11).
All HDSS sites used a standardised structured instrument for data collection based on the WHO STEPwise approach to Surveillance (WHO STEPS) (11, 12). Data collection instruments were translated into local language at different sites and back-translated to ensure their validity. Information on self-reported tobacco use was gathered from all participants, including types of tobacco used (whether currently smoking manufactured or hand-rolled cigarettes, cigars, cheroots or whether tobacco is smoked, chewed, sucked or inhaled), the age at which the person started smoking daily and the frequency of cigarette smoking, snuffing or chewing in each day. The socio-demographic and economic profiles of the respondents were obtained from the HDSS database for each of the surveillance sites. The principal investigators of each HDSS met for common training. The methodology for the multi-site study together with a detailed description of the general characteristics of the sample population in each HDSS site, has been described elsewhere (13).
All questionnaires were checked immediately after each interview to ensure completeness and accuracy. Standardised data entry screens using EPIDATA were also designed to ensure uniformity of the dataset and valid values for each variable were set to ensure high quality of the data obtained. Clean datasets from all sites were merged to enable cross-site analyses. Data management and analysis were conducted in STATA Version 10. In this paper, and to ensure comparability with other surveys using the WHO STEPS approach, uses of tobacco products were measured as percentages of those who currently smoked or chewed tobacco daily; the categories were also combined to assess the full extent of tobacco use. The prevalence of tobacco use was tabulated by HDSS site, age group, gender and highest education level. The average age at taking up daily smoking and the mean number of manufactured and other forms of cigarettes smoked daily were presented. Multivariable logistic regression was undertaken to assess the association between gender, age groups, and education level and tobacco use.
Ethical approval from the Scientific Advisory Committee of INDEPTH Network and Institutional Review Board (IRB) as per norms and scientific regulations of respective HDSS site was obtained. Oral and written informed consent was obtained from every respondent before enrolment in the study. Each individual had the right to decline participation in the study at any stage.
Results
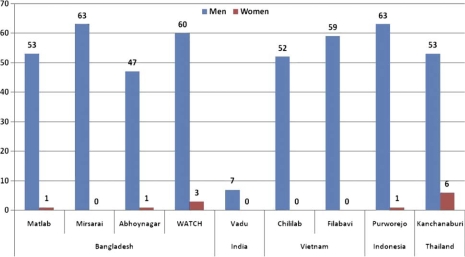
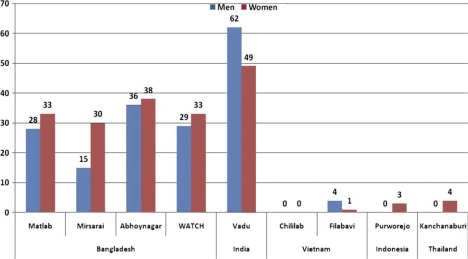
This study showed striking differences in patterns of tobacco use, both the prevalence of daily smoking (Fig. 1) and daily chewing (Fig. 2) in men and women across the nine HDSS sites. Overall, smoking was more prevalent in men than women in all HDSS sites, except in Vadu HDSS, India. The highest prevalence of daily smoking among men was observed in Mirsarai HDSS in Bangladesh and Purworejo HDSS in Indonesia where almost two-thirds of men smoked daily. The prevalence of self-reported daily smoking in women was very low. However, tobacco chewing was noted in the HDSS sites in Bangladesh and India; almost two-thirds of men and half of women in Vadu HDSS in India chewed tobacco daily. Tobacco chewing was rare in the surveillance sites in Vietnam, Indonesia or Thailand.
Fig. 1.
Proportion of daily smoking (%) in nine Asian INDEPTH HDSSs.
Fig. 2.
Proportion of daily chewing (%) in nine Asian INDEPTH HDSSs.
A more detailed analysis by age group showed highest prevalence among those between 35 and 44 years old in most of the sites except in WATCH HDSS, Vadu HDSS and Purworejo HDSS where tobacco use was more prevalent in the older age groups. In general, the prevalence of tobacco smoking among women was very low, with the exception of Kanchanaburi HDSS in Thailand where 6.3% of women reported smoking daily (Table 1).
Table 1.
Overall prevalence of current daily smoking (and its 95% CI) in nine Asian INDEPTH HDSS
| Bangladesh | India | Vietnam | Indonesia | Thailand | |||||
|---|---|---|---|---|---|---|---|---|---|
| Current daily smoker | Matlab | Mirsarai | Abhoynagar | WATCH | Vadu | Chililab | Filabavi | Purworejo | Kanchanaburi |
| Men | 1,039 | 1,019 | 991 | 997 | 1,037 | 1,090 | 989 | 990 | 1,056 |
| 25–34 | 46.6 | 54.1 | 38.2 | 53.6 | 3.1 | 55.1 | 57.7 | 58.8 | 46.1 |
| (40.3–52.8) | (47.8–60.3) | (32–44.4) | (47.4–59.8) | (1–5.1) | (49.1–61) | (51.4–63.9) | (52.7–65) | (40–52.2) | |
| 35–44 | 59 | 66.8 | 55 | 57 | 5.9 | 57.5 | 66.9 | 63.6 | 57.9 |
| (52.9–65.1) | (61–72.6) | (48.9–61.1) | (50.9–63.2) | (3.1–8.6) | (51.6–63.4) | (61.2–72.6) | (57.6–69.6) | (51.9–63.8) | |
| 45–54 | 56.3 | 67.8 | 50.6 | 73.6 | 10.9 | 47.7 | 55.2 | 61 | 54 |
| (50.4–62.3) | (62.1–73.6) | (44.3–56.9) | (68.1–79.1) | (7.1–14.7) | (42.1–53.3) | (48.9–61.5) | (55–67.1) | (48–60) | |
| 55–64 | 45.4 | 66.4 | 43.5 | 60 | 13.5 | 35.4 | 52.4 | 68.5 | 57.4 |
| (39.5–51.3) | (60.7–72.1) | (37.4–49.6) | (53.9–66.1) | (9.2–17.7) | (29.4–41.3) | (46.2–58.7) | (62.8–74.3) | (51.5–63.2) | |
| Total | 52.5 | 62.6 | 46.6 | 59.7 | 7.1 | 51.5 | 59.5 | 62.7 | 53.4 |
| (49.3–55.7) | (59.5–65.7) | (43.4–49.8) | (56.4–63) | (5.5–8.6) | (48.4–54.6) | (56.3–62.7) | (59.7–65.8) | (50.3–56.5) | |
| Women | 1,022 | 1,009 | 992 | 1,000 | 1,037 | 1,104 | 1,011 | 956 | 1,090 |
| 25–34 | 0 | 0 | 0.8 | 0.4 | 0 | 0.4 | 0.8 | 0.4 | 2.3 |
| (−0.3 to 2) | (−0.4 to 1.2) | (−0.4 to 1.1) | (−0.3 to 1.8) | (−0.4 to 1.2) | (0.5–4) | ||||
| 35–44 | 0.8 | 0 | 2 | 3.2 | 0 | 0.4 | 0 | 0.8 | 7.7 |
| (−0.3 to 1.8) | (0.3–3.6) | (1–5.4) | (−0.4 to 1.1) | (−0.3 to 2) | (4.5–10.8) | ||||
| 45–54 | 1.6 | 0.8 | 1.2 | 4.8 | 0.4 | 0.3 | 0.8 | 0.4 | 7.2 |
| (0–3.1) | (−0.3 to 1.9) | (−0.2 to 2.6) | (2.1–7.5) | (−0.4 to 1.1) | (−0.3 to 1) | (−0.3 to 1.8) | (−0.4 to 1.2) | (4.2–10.2) | |
| 55–64 | 2 | 0.8 | 1.6 | 7.6 | 0.4 | 0.4 | 0.4 | 4.3 | 9.9 |
| (0.3–3.7) | (−0.3 to 1.9) | (0–3.2) | (4.3–10.9) | (−0.4 to 1.2) | (−0.4 to 1.2) | (−0.4 to 1.2) | (1.7–7) | (6.4–13.5) | |
| Total | 0.8 | 0.3 | 1.4 | 2.7 | 0.1 | 0.4 | 0.5 | 1.4 | 6.3 |
| (0.3–1.3) | (0–0.6) | (0.6–2.1) | (1.8–3.6) | (−0.1 to 0.3) | (0–0.7) | (0–0.9) | (0.7–2.1) | (4.9–7.8) | |
Women in the four HDSS in Bangladesh and the HDSS in India reported a high prevalence of tobacco chewing especially in the older age groups (Table 2). Except for men in Vadu HDSS where tobacco chewing was high in all age groups, the prevalence of tobacco chewing in the oldest age group were 2–6 times higher than the prevalence in the youngest age group in other HDSSs in Bangladesh.
Table 2.
Overall prevalence of current tobacco chewing (and its 95% CI) in nine Asian INDEPTH HDSS
| Bangladesh | India | Vietnam | Indonesia | Thailand | |||||
|---|---|---|---|---|---|---|---|---|---|
| Current tobacco chewer | Matlab | Mirsarai | Abhoynagar | WATCH | Vadu | Chililab | Filabavi | Purworejo | Kanchanaburi |
| Men | 1,039 | 1,019 | 991 | 997 | 1,037 | 1,090 | 989 | 990 | 1,056 |
| 25–34 | 8.9 | 5.7 | 25.3 | 16.9 | 52.7 | 0 | 3.3 | 0 | 0 |
| (5.4–12.5) | (2.8–8.6) | (19.7–30.9) | (12.3–21.6) | (46.6–58.7) | (1.1–5.6) | ||||
| 35–44 | 28.3 | 16.8 | 33.5 | 29.7 | 61.5 | 0 | 4.2 | 0 | 0 |
| (22.7–33.9) | (12.2–21.4) | (27.7–39.2) | (24–35.4) | (55.8–67.3) | (1.8–6.6) | ||||
| 45–54 | 41.8 | 21.6 | 46.1 | 37.6 | 72.4 | 0 | 5.0 | 0 | 0.4 |
| (35.9–47.7) | (16.5–26.6) | (39.9–52.4) | (31.6–43.6) | (66.9–77.8) | (2.2–7.8) | (–0.4 to 1.1) | |||
| 55–64 | 51.3 | 23.3 | 50.6 | 45.6 | 69.0 | 0.4 | 3.7 | 0.4 | 1.5 |
| (45.3–57.2) | (18.2–28.4) | (44.4–56.8) | (39.4–51.8) | (63.2–74.8) | (−0.4 to 1.2) | (1.3–6) | (−0.4 to 1.2) | (0–2.9) | |
| Total | 28.0 | 15.1 | 36.3 | 28.6 | 62.0 | 0 | 4.1 | 0.1 | 0.3 |
| (25.2–30.8) | (12.9–17.3) | (33.2–39.4) | (25.6–31.5) | (58.9–65.1) | (0–0.1) | (2.8–5.3) | (−0.1 to 0.2) | (0–0.6) | |
| Women | 1,022 | 1,009 | 992 | 1,000 | 1,037 | 1,104 | 1,011 | 956 | 1,090 |
| 25–34 | 12.3 | 10.7 | 11.8 | 21.2 | 28.8 | 0 | 0.4 | 0 | 0.8 |
| (8.3–16.3) | (6.8–14.6) | (7.7–15.9) | (16.1–26.3) | (23.2–34.4) | (−0.4 to 1.1) | (−0.3 to 1.8) | |||
| 35–44 | 30.6 | 30.9 | 38.3 | 32.8 | 49.4 | 0 | 0 | 0 | 1.5 |
| (25–36.2) | (25.3–36.5) | (32.3–44.2) | (27–38.6) | (43.3–55.5) | (0–2.9) | ||||
| 45–54 | 52.6 | 45.7 | 58.2 | 50.4 | 64.8 | 0 | 0 | 4.0 | 5.8 |
| (46.4–58.7) | (39.5–51.8) | (52.1–64.4) | (44.2–56.6) | (59.2–70.5) | (1.6–6.5) | (3–8.5) | |||
| 55–64 | 67.3 | 49.4 | 69.5 | 57.2 | 66.7 | 0.8 | 4.6 | 10.0 | 14.7 |
| (61.5–73.1) | (43.2–55.6) | (63.8–75.2) | (51.1–63.3) | (60.9–72.5) | (−0.3 to 1.9) | (1.9–7.2) | (6.1–13.9) | (10.5–18.9) | |
| Total | 32.5 | 29.6 | 37.7 | 33.5 | 48.8 | 0.1 | 0.7 | 3.2 | 4.3 |
| (29.6–35.5) | (26.8–32.5) | (34.6–40.8) | (30.3–36.6) | (45.6–52) | (0–0.2) | (0.3–1.1) | (2.1–4.2) | (3.2–5.4) | |
Men started daily smoking before they turned 20, except in Vadu HDSS in India where daily smoking started on average at 28 years of age (Table 3). Among daily smokers, the proportion who smoked manufactured cigarettes varied widely across sites from 33% in Kanchanaburi HDSS in Thailand to 78% in Matlab HDSS in Bangladesh. A large proportion of men who smoked daily used both manufactured and other forms of cigarettes. With the exception of the low average number of cigarettes smoked (2.7 cigarettes) by men in Vadu HDSS, the number of manufactured cigarettes smoked daily ranged from 5.4 cigarettes in Abhoynagar HDSS in Bangladesh to 13.6 cigarettes in another HDSS in Bangladesh (Mirsarai).
Table 3.
Characteristics of men who currently smoked (and its 95% CI) in nine Asian INDEPTH HDSS
| Bangladesh | India | Vietnam | Indonesia | Thailand | |||||
|---|---|---|---|---|---|---|---|---|---|
| Current men smoker | Matlab | Mirsarai | Abhoynagar | WATCH | Vadu | Chililab | Filabavi | Purworejo | Kanchanaburi |
| Age when started smoking daily | 19.8 | 18.6 | 19.4 | 20.2 | 27.9 | 20.7 | 19.8 | 18 | 19.4 |
| (19.4–20.3) | (18.1–19) | (18.8–20.1) | (19.7–20.7) | (25–30.8) | (20.3–21.2) | (19.3–20.2) | (17.6–18.4) | (18.9–19.8) | |
| Proportion who smoked manufactured cigarettes | 78.4 | 73.7 | 36.2 | 40 | 40.2 | 70.1 | 41.7 | 57 | 33.2 |
| (74.9–82) | (70.3–77.1) | (31.6–40.8) | (35.8–44.2) | (28.9–51.5) | (66.1–74.1) | (37.5–46) | (53.1–61) | (29.1–37.2) | |
| Number of manufactured cigarettes smoked daily | 9.6 | 13.6 | 5.4 | 9.2 | 2.7 | 11.4 | 8.8 | 8 | 11.6 |
| (9–10.2) | (12.7–14.4) | (4.8–5.9) | (8.3–10) | (1.6–3.8) | (10.7–12.2) | (7.9–9.6) | (7.5–8.5) | (10.6–12.6) | |
| Proportion who smoked other forms of cigarettes | 24.9 | 26.8 | 78.3 | 66 | 62.7 | 36.8 | 70.6 | 55.8 | 80.1 |
| (21.1–28.6) | (23.4–30.3) | (74.3–82.2) | (61.9–70.1) | (51.5–73.9) | (32.6–41) | (66.7–74.6) | (51.9–59.8) | (76.6–83.5) | |
| Number of other forms of cigarettes smoked daily | 15.4 | 18.9 | 10.1 | 14.5 | 3.2 | 13.8 | 14.4 | 4.2 | 13.2 |
| (13.9–16.9) | (17.2–20.7) | (9.3–10.8) | (13.7–15.3) | (1.7–4.7) | (12.5–15.1) | (13.5–15.3) | (3.8–4.6) | (12.5–14) | |
Table 4 shows the association between age, sex and education. Tobacco use was significantly associated with being male, being older and having a low level of education. The odds ratio of tobacco use (smoking and/or chewing) increased steadily with age in the HDSSs in Bangladesh and India. The odds of tobacco use was, however, not significantly different across age-groups in many of South East Asian HDSS. People with no formal education and those who did not graduate from primary school consistently showed significantly higher odds of tobacco use compared to those who graduated from high school or university in all HDSSs.
Table 4.
Association (95% CI) between demographic variables and tobacco use (smoking and/or chewing) in nine Asian INDEPTH HDSS
| Bangladesh | India | Vietnam | Indonesia | Thailand | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tobacco Use | Matlab | Mirsarai | Abhoynagar | WATCH | Vadu | Chililab | Filabavi | Purworejo | Kanchanaburi |
| Sex | |||||||||
| Men | 5.63 | 6.93 | 4.98 | 6.13 | 2.93 | 211.38 | 101.73 | 39.39 | 10.98 |
| (4.51–7.02) | (5.55–8.64) | (3.97–6.26) | (4.89–7.7) | (2.37–3.64) | (93.41–478.32) | (60.11–172.15) | (28.18–55.05) | (8.68–13.9) | |
| Women | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Age groups (years) | |||||||||
| 25–34 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 35–44 | 2.02 | 2.12 | 2.36 | 1.45 | 1.45 | 1.18 | 1.5 | 1.01 | 1.25 |
| (1.52–2.68) | (1.59–2.81) | (1.78–3.14) | (1.1–1.91) | (1.12–1.89) | (0.84–1.67) | (1.05–2.14) | (0.71–1.42) | (0.91–1.71) | |
| 45–54 | 4.48 | 3.26 | 3.91 | 3.42 | 2.49 | 0.8 | 1.01 | 0.96 | 1.1 |
| (3.36–5.98) | (2.43–4.36) | (2.91–5.24) | (2.56–4.56) | (1.88–3.3) | (0.57–1.13) | (0.71–1.45) | (0.67–1.37) | (0.8–1.51) | |
| 55–64 | 5.93 | 3.29 | 5.64 | 3.76 | 1.99 | 0.49 | 0.97 | 1.74 | 1.98 |
| (4.4–7.99) | (2.46–4.41) | (4.16–7.64) | (2.8–5.03) | (1.47–2.68) | (0.34–0.7) | (0.68–1.39) | (1.21–2.51) | (1.46–2.71) | |
| Highest education levels | |||||||||
| No schooling or not graduated | 4.86 | 4.56 | 6.73 | 4.54 | 3.38 | 2.48 | 2.38 | 2.38 | 4.3 |
| from primary school | (3.42–6.92) | (3.07–6.78) | (4.2–10.79) | (3.08–6.69) | (2.54–4.51) | (1.43–4.33) | (1.36–4.18) | (1.61–3.52) | (2.89–6.41) |
| Graduated from primary school | 2.6 | 2.73 | 3.57 | 2.23 | 2.4 | 2.04 | 1.01 | 1.76 | 2.2 |
| (1.8–3.76) | (1.69–4.39) | (2.06–6.16) | (1.41–3.52) | (1.7–3.41) | (1.38–3) | (0.65–1.55) | (1.25–2.47) | (1.52–3.18) | |
| Graduated from secondary school | 1.32 | 2.01 | 2.56 | 1.43 | 1.66 | 1.4 | 0.83 | 1.26 | 1.67 |
| (0.77–2.27) | (1.34–3.02) | (1.58–4.14) | (0.88–2.34) | (1.24–2.23) | (1.08–1.84) | (0.59–1.16) | (0.86–1.85) | (1.02–2.73) | |
| Graduated from high school or university | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Discussions
This multi-site study conducted in nine rural surveillance sites in five countries used the same methodology and definitions. Overall, men and women in these rural Asian INDEPTH HDSS reported a high prevalence of tobacco use, cigarette smoking and/or use of smokeless tobacco. Reported prevalence of smoking was high for men in most HDSS sites, and the majority of smokers started smoking in adolescence. In addition, the prevalence of tobacco chewing was uniformly high among men and women, particularly among older people, in all four sites in Bangladesh, as well as the surveillance site in India. The pattern of tobacco use varied by HDSS, sex, age and education levels. Vadu HDSS in India had the lowest prevalence of smoking and the highest prevalence of tobacco chewing, and the majority of smokers in Vadu started smoking in their late 20s.
As with all INDEPTH HDSS sites, the sample in this study is not necessarily representative of the population in each country. The high response rate, as expected in these HDSS settings, however, provided an unbiased sample to report valid estimates in these rural settings (13). The limitation of self-reported data on tobacco use is acknowledged; self-reported smoking rates are usually underreported, particularly for women where a social taboo on smoking is present (14–17). No objective measure of tobacco use (cotonin, or carbon monoxide measurements) was used to confirm self-reporting.
The widespread cultural and socio-economic associations, including gender, occupation and education, with tobacco use (14–16) have been confirmed in our study. Smoking by men in front of the elders is socially unacceptable as is smoking by women in Bangladesh and other South East Asian countries (17). This cultural constraint may be weakening and population health is increasingly threatened by the aggressive expansion of multinational tobacco industries in the region (18). Tobacco chewing is gradually being replaced by tobacco smoking. Taking these cultural differences into account, recommendations for tobacco control strategies may need to be developed differently for men and women. For men, tobacco control should focus on reducing tobacco use and promoting smoking cessation, for women, it should focus on preventive measures to forestall smoking initiation.
Availability of locally reliable population-based data on tobacco use burden and tobacco-attributable morbidity and mortality, as well as their predictors, are helpful in advocating for national tobacco control strategies. Comparable data on tobacco use are often hampered by different study designs, samples and definitions of tobacco use (19) although the increasing use of the STEPS methodology, now carried out in over 100 low and middle-income countries, will go some way to address this problem (20). In addition, the Global Tobacco Surveillance System (GTSS) collects data through five surveys and was developed to enhance the capacity of countries to design, implement and evaluate their national comprehensive tobacco action plan and to monitor the key articles of the WHO FCTC (21). Understanding the population burden of tobacco use contributes to the development of effective interventions and policies to reduce tobacco use; measuring changes in population levels through systematic cross-sectional surveys, can help measure the effective of such policies (22).
Use of smokeless tobacco is common in South Asian countries, particularly among women where tobacco smoking is seen as socially unacceptable. Another cultural belief concerns the remedial effects of smokeless tobacco for common illnesses; promotion of its use in children has been reported (7). The different patterns of tobacco use in Asian countries, as shown in this study, combined with the influence of the tobacco industry in these countries, calls for comprehensive approaches to tobacco control which take into account country-specific tobacco consumption patterns. Asian countries are also at different stages of tobacco control efforts. Over the past decade, tobacco consumption has increased in Vietnam and Bangladesh, remained unchanged in India and declined in Thailand (1, 6). Even though most of the countries represented in this study have ratified the FCTC, there is a clear gap in enforcement and implementation (23). At present, Indonesia is not a signatory to the FCTC and little improvement in tobacco control has been observed in Indonesia during the last few years, in contrast to Thailand where tobacco control efforts have shown some positive achievements (1). In our study, the highest prevalence of tobacco smoking was in Indonesian men.
Implementation of elements of the FCTC such as increasing tobacco taxes, banning smoking in public places, including restaurants and other indoor areas in some countries, have succeeded in decreasing the overall prevalence of smoking (1, 2, 24). Restrictions on tobacco products advertisements, sales and access, and prohibition of smoking in public have resulted in declining tobacco use in Singapore (25). Progressive increases in excise taxes in tobacco production have contributed to reduction in tobacco consumption (26). Comprehensive and legible health warnings on packages of manufactured cigarettes are associated with higher quitting attempts in smokers (27). However, text warning on cigarette packages may go unnoticed by smokers with low literacy, and the possibility of buying cigarettes in single sticks in India, Bangladesh and Indonesia reduces the effectiveness of health warnings in reaching smokers. Educational interventions on tobacco use have increased the quitting rate in some parts of India (7). Each of these measures can yield different results and effectiveness in different settings, therefore, all these measures are required to achieve optimal tobacco control effects.
Countries which have ratified the FCTC are obliged to embark on comprehensive tobacco control efforts involving, besides the Ministry of Health, support of other key ministries and sectors such as education, trade, labour and finance; all are essential for a successful tobacco control programme. Print and electronic media can help raise the population's awareness on the hazardous effects of tobacco consumption by disseminating educational materials. NGOs and civil society can put pressure on government to enforce tobacco legislation and regulation at national and local levels. Broader engagement of the community will eventually strengthen law enforcing tobacco regulations. All key players need to be mobilised and efforts intensified to expedite the achievement of the FCTC. The INDEPTH Network has an important role to play in leading tobacco control interventions in the populations they serve, and in undertaking surveillance to measure the impact of these efforts in rural populations in Asia.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgements
The authors would like to acknowledge the INDEPTH Network for financing this work, Dr. Anand Krishnan and Dr. S.K. Kapoor from Ballabgarh HDSS for organising training workshop for this project, the Umeå Centre for Global Health Research, Umeå University, Sweden for supporting the coordination of this supplement, and Dr. Ruth Bonita, who as guest editor for this series of papers, provided substantial and critical scientific input into earlier drafts of this paper.
References
- 1.World Health Organization. Geneva: World Health Organization; 2008. WHO report on the global tobacco epidemic. [Google Scholar]
- 2.World Health Organization. Geneva: World Health Organization; 2003. WHO Framework Convention on Tobacco Control. [Google Scholar]
- 3.Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116:963–71. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
- 4.Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation. 2005;112:489–97. doi: 10.1161/CIRCULATIONAHA.104.521708. [DOI] [PubMed] [Google Scholar]
- 5.Ezzati M, Lopez AD. Regional, disease specific patterns of smoking-attributable mortality in 2000. Tob Control. 2004;13:388–95. doi: 10.1136/tc.2003.005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafey O, Dolwick S, Guindon GE. The tobacco control country profiles. 2nd ed. Atlanta: American Cancer Society, World Health Organization, International Union Against Cancer; 2003. [Google Scholar]
- 7.Gupta PC, Ray CS. Smokeless tobacco and health in India and South Asia. Respirology. 2003;8:419–31. doi: 10.1046/j.1440-1843.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen G, Gupta PC, Pednekar MS. Social disparities in tobacco use in Mumbai, India: the roles of occupation, education, and gender. Am J Public Health. 2005;95:1003–8. doi: 10.2105/AJPH.2004.045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.INDEPTH Network. Ottawa: INDEPTH Network, IDRC; 2002. Population and health in developing countries. [Google Scholar]
- 10.Ng N, Minh HV, Tesfaye F, Bonita R, Byass P, Stenlund H, et al. Combining risk factors and demographic surveillance: potentials of WHO STEPS and INDEPTH methodologies for assessing epidemiological transition. Scand J Public Health. 2006;34:199–208. doi: 10.1080/14034940500204506. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Geneva: World Health Organization; 2005. WHO STEPS surveillance manual: the WHO STEPwise approach to chronic disease risk factor surveillance. [Google Scholar]
- 12.Armstrong T, Bonita R. Capacity building for an integrated noncommunicable disease risk factor surveillance system in developing countries. Ethn Dis. 2003;13:S13–8. [PubMed] [Google Scholar]
- 13.Ng N, Minh HV, Juvekar S, Razzaque A, Bich TH, Kanungsukkasem U, et al. Using the INDEPTH HDSS to build capacity for chronic non-communicable disease risk factor surveillance in low and middle-income countries. Global Health Action. 2009;(Supplement 1) doi: 10.3402/gha.v2i0.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichter M. Smoking: what does culture have to do with it? Addiction. 2003;98:139–45. doi: 10.1046/j.1360-0443.98.s1.9.x. [DOI] [PubMed] [Google Scholar]
- 15.Gupta R. Smoking, educational status and health inequity in India. Indian J Med Res. 2006;124:15–22. [PubMed] [Google Scholar]
- 16.Ng N, Weinehall L, Ohman A. ‘If I don't smoke, I'm not a real man’ – Indonesian teenage boys’ views about smoking. Health Educ Res. 2007;22:794–804. doi: 10.1093/her/cyl104. [DOI] [PubMed] [Google Scholar]
- 17.Mackay J, Amos A. Women and tobacco. Respirology. 2003;8:123–30. doi: 10.1046/j.1440-1843.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 18.Stillman F, Hoang M, Linton R, Ritthiphakdee B, Trochim W. Mapping tobacco industry strategies in South East Asia for action planning and surveillance. Tob Control. 2008;17:e1. doi: 10.1136/tc.2006.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO Global NCD Infobase. 2009. Available from: http://www.who.int/ncd_surveillance/infobase/en/ [cited 30 May 2009]
- 20.World Health Organization. STEPwise approach to surveillance (STEPS) Available from: http://who.int/chp/steps [cited 18 August 2009]
- 21.World Health Organization. Global Tobacco Surveillance System. Available from: http://who.int/tobacco/surveillance [cited 18 August 2009]
- 22.World Health Organization Regional Office for the South-East Asia. Scaling up prevention and control of chronic non communicable diseases in South East Asian Region. 2007. Available from: http://www.searo.who.int/LinkFiles/Provisional_Agenda_SEA-RC60-7_Agenda_Item_9.pdf [cited 10 May 2009]
- 23.Leowski J, Krishnan A. Capacity to control noncommunicable diseases in the countries of South-East Asia. Health Policy. 2009;92:43–8. doi: 10.1016/j.healthpol.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Pierce JP. Tobacco industry marketing, population-based tobacco control, and smoking behavior. Am J Prev Med. 2007;33:S327–34. doi: 10.1016/j.amepre.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Morrow M, Barraclough S. Tobacco control and gender in south-east Asia. Part II: Singapore and Vietnam. Health Promot Int. 2003;18:373–80. doi: 10.1093/heapro/dag403. [DOI] [PubMed] [Google Scholar]
- 26.van Walbeek C. Tobacco excise taxation in South Africa: tools for advancing tobacco control in the XXIst century: success stories and lessons learned. Geneva: World Health Organization; 2003. [Google Scholar]
- 27.Borland R, Yong HH, Wilson N, Fong GT, Hammond D, Cummings KM, et al. How reactions to cigarette packet health warnings influence quitting: findings from the ITC Four-Country survey. Addiction. 2009;104:669–75. doi: 10.1111/j.1360-0443.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]