Abstract
MicroRNAs are small non-coding RNAs that are directly involved in the regulation of gene expression by either translational repression or degradation of target mRNAs. Because of the high level of conservation of the target motifs, known as seed sequences, within the 3′-untranslated regions, a single microRNA can regulate numerous target genes simultaneously, making this class of RNAs a powerful regulator of gene expression. The miR200 family of microRNAs has recently been shown to regulate the process of epithelial to mesenchymal transition during tumor progression and metastasis. Here, we report that the expression of WAVE3, an actin cytoskeleton remodeling and metastasis promoter protein, is regulated by miR200 microRNAs. We show a clear inverse correlation between expression levels of WAVE3 and miR200 microRNAs in invasive versus non-invasive cancer cells. miR200 directly targets the 3′-untranslated regions of the WAVE3 mRNA and inhibits its expression. The miR200-mediated down-regulation of WAVE3 results in a significant reduction in the invasive phenotype of cancer cells, which is specific to the loss of WAVE3 expression. Re-expression of a miR200-resistant WAVE3 reverses miR200-mediated inhibition of cancer cell invasion. Loss of WAVE3 expression downstream of miR200 also results in a dramatic change in cell morphology resembling that of a mesenchymal to epithelial transition. In conclusion, a novel mechanism for the regulation of WAVE3 expression in cancer cells has been identified, which controls the invasive properties and morphology of cancer cells associated with their metastatic potential.
INTRODUCTION
MicroRNAs are small noncoding RNAs, usually 20–22 nucleotides long, which regulate gene expression, primarily at the post-transcriptional level. So far, more that 400 microRNAs have been identified in mammalian cells, with each microRNA having several target genes. The broad spectrum of genes that can be targeted by a single microRNA is attributed to the high level of conservation of the target motifs, known as seed sequences, within the 3′-untranslated region (UTR)2 of the target genes, thus making them the most powerful regulators of gene expression in complex cellular processes, including cancer cell invasion and metastasis (1–8). In fact, several microRNAs have been found to function as tumor suppressors, such as miR15a, miR16-1, and let-7 (3, 9–13), whereas others were found to possess oncogenic properties, including miR155, miR17-5p, and miR21 (3, 14, 15). Recently, the miR200 family has been found to play a central role in the regulation of the epithelial to mesenchymal transition (EMT) process during cancer progression and metastasis (2,4–8,16–20). EMT, while being a critical process during embryonic development and wound healing (21), also plays a fundamental role in cancer metastasis, where cancer cells acquire their invasive phenotype by undergoing a change from the differentiated to a more dedifferentiated state (1, 18, 21–24).
In our previously published work, we showed that WAVE3, a member of the WASP (Wiskott-Aldrich syndrome protein)/WAVE actin cytoskeleton remodeling family of proteins (25–30), is critical for the motility, migration, and invasive properties of transformed cells (31–36). We also reported a very significant correlation between the expression levels of WAVE3 and advanced stages of breast cancer (35), clearly supporting the function of WAVE3 as a metastasis promoter protein. The mechanisms of regulation of WAVE3 expression levels during tumor progression have, however, remained unsolved. In the present report, we demonstrate an inverse correlation between WAVE3 and miR200 microRNA expression levels in invasive versus non-invasive cancer cells, prompting us to hypothesize that WAVE3 expression might be regulated by miR200 microRNAs in cancer cell invasion. We show that miR200 microRNAs, the expression of which is lost during EMT and therefore cancer metastasis, directly target the 3′-UTR of the WAVE3 mRNA and inhibit its expression. We also show that the miR200-mediated inhibition of WAVE3 expression is specific for WAVE3 because it does not affect the expression levels of the other members of the WAVE family. Concordant with the phenotypic consequences of WAVE3 inhibition (33–36), we found that the miR200-mediated inhibition of WAVE3 expression results in a significant reduction in the invasive properties of cancer cells. We finally show that ZEB1/ZEB2/E-cadherin, which are critical players in the EMT process, and WAVE3 are independently regulated by miR200 microRNAs. To our knowledge, this is the first study to report the identification and characterization of a novel mechanism by which WAVE3 is regulated during cancer progression.
EXPERIMENTAL PROCEDURES
Materials
The antibodies used in this study were as follows: rabbit anti-WAVE3 from New England Peptide, Inc. (Gardner, MA); mouse anti-E-cadherin and mouse anti-vimentin from BD Biosciences; rabbit anti-green fluorescent protein from Clontech; goat horseradish peroxidase-conjugated anti-mouse IgG and goat horseradish peroxidase-conjugated anti-rabbit IgG from Calbiochem; and Alexa 488-conjugated anti-rabbit IgG and Alexa 568-conjugated anti-mouse IgG from Invitrogen. Alexa 568-conjugated phalloidin was from Invitrogen. Vecta-shield with 4′,6-diamidino-2-phenylindole was from Vector Laboratories (Burlingame, CA). Gel electrophoresis reagents were from Bio-Rad. We used the On-Targetplus SMARTpool (Thermo Scientific) siRNA L-012141-00-0010 and L-1012301-00-0010 to knock down the expression of WAVE2 and WAVE3, respectively. Pre-miRNA miR200b precursor and anti-miR200b miRNA inhibitor were from Invitrogen and were used as per the manufacturer's instructions.
Plasmid Construction, Site-directed Mutagenesis, and WAVE3 3′-UTR Reporter Analysis
The green fluorescent protein-WAVE3 fusion expression vector construction was described previously (36). The 3′-UTR of WAVE3 was PCR-amplified using WAVE3 cDNA IMAGE clone 4838122 as a template and subcloned into pmirGlo vector downstream of the firefly luciferase expression cassette (Promega, Madison, WI). The correct sequence and orientation were verified by sequencing. We used the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), following the manufacturer's instructions, to generate the WAVE3 3′-UTR variants where seed sequences that are recognized by miR200 microRNA were deleted. pmirGlo reporter plasmids (1 μg total plasmid amount) were transfected with Lipofectamine 2000 (Invitrogen) into the specified cancer cells, seeded in 12-well plates (3 × 104 cells/well). Cells were collected after 48 h for assay using the dual-luciferase reporter assay system (Promega). For co-transfection experiments, synthetic microRNA mimics or miR negative control (5 nm) or anti-miR200b microRNA inhibitor or anti-miR negative control (20 nm) were added to the transfection mix. All experiments were performed in triplicate with data averaged from at least three independent experiments.
Cell Culture and Transfections
Human breast cancer MDA-MB-231 and MCF7 cells, prostate cancer LNCaP and PC3 cells, colon cancer HT29 cells, and human embryonic kidney HEK293 cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured at 37 °C with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 4.5 g/liter glucose, 10% fetal bovine serum (Invitrogen), 2 mmol/liter glutamine, and antibiotics. Transient transfections were performed as described previously (33–35). Briefly, ∼2.5 × 105 cells were plated in either 60-mm dishes or 6-well plates in DMEM without antibiotics 24 h before transfection. Transfections were performed using Lipofectamine 2000 (Invitrogen) in OPTI-MEM (Invitrogen) medium as directed by the manufacturer. Approximately 4–5 h after transfection, medium was supplemented with DMEM containing 10% fetal bovine serum without antibiotics, and cells were incubated for an additional 16 h, after which they were harvested and used as specified.
Semiquantitative Reverse Transcription-PCR
Cells were lysed in TRIzol reagent (Invitrogen), and total RNA was extracted according to the manufacturer's instructions. RNA was quantified using a spectrophotometer (Beckman-Coulter, Fullerton, CA), and 1 μg of RNA was used to generate cDNA with the SuperScript III RT-PCR kit (Invitrogen). Reverse transcription was performed according to the manufacturer's instructions, and the cDNA generated was used as a template for semiquantitative RT-PCR as previously described (34–36) for analysis of gene expression, using a PTC-100 thermal cycler from MJ Research (Waltham, MA). Under conditions of a relatively low number of PCR cycles, 20–23 instead of the generally used 35 cycles, the amplification process is still in the exponential phase and, therefore, approximates differences in copy number between the samples analyzed (34–36).
Matrigel Invasion Assay
The invasive potential of the parental cells and the WAVE3 knockdown cells was assessed using the Matrigel invasion chambers from BD Biosciences. Cells were cultured for 48 h in complete medium, harvested by trypsinization, and then counted, and 5 × 104 cells were placed onto the top insert. One chamber consists of a cell insert and a well. The bottom of the cell insert is covered with a filter containing multiple 8-μm pores and is coated with a basement membrane matrix (Matrigel). Cells in 500 μl of serum-free DMEM were seeded in the cell insert and placed in the well, which was filled with 750 μl of DMEM supplemented with 10% fetal bovine serum. After 24 h of incubation at 37 °C and 5% CO2, the non-invasive cells present on the upper surface of the filter were removed using a sterile cotton swab. The cells that migrated through the Matrigel onto the lower surface of the filter were fixed and stained with Diff-Quick (American Scientific Products, McGraw Park, IL). The lower surface of the filter was photographed using an inverted Leica DM IRB microscope fitted with a charge-coupled device camera (Leica Microsystems, Wetzlar, Germany), and migrating cells were counted from four fields at ×200 magnification.
Immunofluorescence Microscopy
Cells were grown on glass coverslips and fixed in 4% paraformaldehyde for 20 min in phosphate-buffered saline (PBS) at room temperature and then washed with PBS. The cells were then permeabilized in 0.1% Triton X-100 in PBS for 15 min, washed again with PBS, and incubated in the blocking solution containing 4% horse serum (Invitrogen) in PBS for 2 h at room temperature. Primary as well as secondary antibodies were diluted at the recommended concentration in 4% horse serum in PBS. Coverslips were incubated with the primary antibody overnight at 4 °C. Unbound antibodies were removed by washing with PBS containing 0.1% Triton X-100, and coverslips were then incubated with an Alexa-conjugated secondary antibody (as indicated) for 1 h at room temperature. Actin filaments (F-actin) were stained with Alexa 568-conjugated phalloidin (Invitrogen) in PBS. The coverslips were mounted on object slides using Vectashield. Confocal images were collected using a Leica TCS-NT confocal laser-scanning microscope. Laser intensities were adjusted so that the excitation of the fluorochromes did not allow any cross-talk between the channels.
Immunoblotting
Cells were grown in 10-cm diameter culture dishes and washed twice with ice-cold PBS. Immediately, ice-cold lysis buffer (25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, and a mixture of protease inhibitors) was added. The cells were rapidly scraped off the plates, and the crude lysates were transferred to prechilled Eppendorf tubes and centrifuged at 15,000 × g for 20 min at 4 °C. Cleared cell lysates containing equivalent amounts of total protein (50 μg) were treated with SDS sample buffer and resolved on a 10% acrylamide gel in SDS, followed by transfer to nitrocellulose or polyvinylidene difluoride (Immobilon-P, Millipore, Billerica, MA) membranes using Bio-Rad gel and transfer apparatus. Membranes were incubated in 5% whole milk (or bovine serum albumin) for 1 h at room temperature, washed with PBS, followed by incubation with the primary antibody (as specified) overnight at 4 °C. Membranes were then washed and incubated in the appropriate secondary antibody at room temperature for 1 h, and the signals were developed using the Western Lights chemiluminescence detection kit (PerkinElmer Life Sciences).
Oligonucleotide Sequences
Sequences of the oligonucleotide primers used for genomic PCR, RT-PCR, to amplify the entire WAVE3 3′-UTR or the individual seed sequences as well as the primers used for mutagenesis were from IDT (San Diego, CA) and are listed in supplemental Table S1.
RESULTS
Inverse Correlation between Expression Levels of WAVE3 and Members of the miR200 MicroRNA Family
We used the three most commonly utilized algorithms (miRanda, TargetScan, and PITA) to predict target sites for microRNA in the 3′-UTR of the WAVE3 cDNA sequence (37–41). A total of 200 microRNAs were predicted to target WAVE3, most of which were found to have conserved seed sequences in the 3′-UTR of WAVE3. Among the 200 predicted microRNAs, only 10 were predicted by all three algorithms (supplemental Fig. S1). Interestingly, three members of the microRNA-200 family, miR200b, miR200c, and miR429, were predicted to have the highest scores (supplemental Fig. S1). The seed sequence, which is identical within these microRNAs, is perfectly conserved in three different locations in the 3′-UTR of the WAVE3 gene, the proximal, central, and distal regions (Fig. 1A and supplemental Fig. S1). Our subsequent analyses identified an additional seed sequence for miR200a and miR141 in the 3′-UTR of WAVE3 (Fig. 1A). We therefore hypothesized that WAVE3 expression may be regulated by members of the miR200 microRNA family. The five members of this family are transcribed from two separate clusters. The first cluster encodes for miR200b, -200a, and -429 on human chromosome 4 (Fig. 1B), and the second cluster, on chromosome 6, encodes for miR200c and -141. We decided to focus our study on the microRNA cluster 200a, 200b, and 429, since they have the largest number (three) of predicted seed sequences in the 3′-UTR of WAVE3.
FIGURE 1.
WAVE3 expression is elevated in cell lines with low levels of miR200 microRNAs. A, domain structure of the WAVE3 transcript showing the location of the seed sequence of the miR200 family of microRNAs within the 3′-UTR. B, schematic representation of the miR200b-200a-429 polysistronic cluster indicating the position of the putative transcription start site (TSS) and the polyadenylation signal. The locations of amplicons A–F are depicted with horizontal solid black bars. The preservation of the genomic structure and mRNA expression along the cluster was verified by genomic PCR (C) and RT-PCR (D), respectively. ORF, open reading frame; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Members of the microRNA200 family have recently been identified as powerful regulators of the epithelial-to-mesenchymal transition process (6, 20, 42). On the other hand, we have shown that WAVE3 also plays a critical role in cell motility, cell migration, and cancer cell invasion, which are all integral components of the EMT process (33–36). We therefore sought to investigate whether the activity of WAVE3 in cell invasion is regulated by members of the miR200 family. We first determined whether there is a correlation between the expression levels of WAVE3 and miR200 microRNAs. We used miR200b as a representative of the miR200 family. We used two sets of cell types based on the expression levels of miR200 microRNAs. The first set contains MDA-MB-231, LNCaP, and PC3, which are considered to be mesenchymal cell types because they do not express miR200 (6). The second set contains MCF7 and HT29, which are considered to be epithelial cell types and which express high levels of miR200 (6). We also included the HEK293 cell line, which is of embryonic origin and could be considered to be a mesenchymal cell type.
Bracken et al. (16) have recently shown that the miR200b-miR200a-miR429 cluster is transcribed from a single 7445-bp intronless transcript on human chromosome 4 (Fig. 1B). Based on those findings, we designed a set of amplicons to investigate the expression levels of these three microRNAs in the cell lines described above. Amplicons were designed from the regions upstream (A) and downstream (F) of the transcribed region, respectively, and four amplicons were designed from inside of the transcribed region (B–E) (Fig. 1B). All six amplicons were successfully amplified from the genomic DNA of all six cell lines (Fig. 1C), indicating that the genomic structure of this microRNA cluster is preserved in all six cell lines. When we used cDNAs generated from each cell line as a template, no RT-PCR product could be detected in any of the six cell lines with amplicons A and F, consistent with a complete absence of genomic DNA contamination in these cDNAs (Fig. 1D). A relatively strong RT-PCR product was generated in HT29 and MCF7 using amplicons B, C, D, and E, compared with the other four cell lines. The opposite scenario was obtained with WAVE3, where a strong WAVE3-specific RT-PCR product was generated in MDA-MB-231, LNCaP, PC3, and HEK293 cells, whereas no detectable WAVE3 transcript could be generated in HT29 or MCF7 cells. Comparable levels of WAVE2, a close relative of WAVE3, and glyceraldehyde-3-phosphate dehydrogenase were amplified from all six cell lines. These results clearly show an inverse correlation between the expression levels of miR200 microRNAs and WAVE3; the epithelial type cells, HT29 and MCF7, express more miR200 microRNAs and less WAVE3, whereas the mesenchymal type cells, MDA-MB-231, LNCaP, and PC3, show the opposite relationship. These data also suggest that the expression of WAVE3 is repressed in the cell types where high levels of miR200 microRNAs are expressed, a fact supported by the presence of multiple seed sequences of miR200 microRNAs in the 3′-UTR of WAVE3.
MicroRNA miR200 Directly Targets the 3′-UTR of WAVE3 and Represses Its Expression
To first determine whether miR200 can target endogenous WAVE3 transcript, we transiently transfected HEK293 cells with miR200b precursor and assessed WAVE3 expression levels by semiquantitative RT-PCR. Overexpression of miR200b resulted in a significant decrease in WAVE3 expression compared with the control cells, whereas no difference in expression levels of either glyceraldehyde-3-phosphate dehydrogenase or WAVE2 were detected between the control cells and miR200b-transfected cells (Fig. 2A). Hence, the decrease in WAVE3 expression levels is specific to miR200b. In animals, microRNAs regulate the expression of target genes by directly targeting and binding to specific seed sequences within the messenger RNAs, leading to either their degradation or inhibiting their translation (43, 44). To further confirm that miR200 directly binds and represses the expression of WAVE3 transcript, we used a luciferase gene reporter assay. We subcloned either the 3′-UTR of WAVE3 as a whole or DNA fragments containing each of the three seed sequences for miR200b, individually, within the 3′-UTR of the luciferase gene into the pmirGlo vector. No difference in luciferase activity was found between the cells transfected with the control vector and the cells transfected with the vector containing the 3′-UTR of WAVE3 (Fig. 2B). In contrast, overexpression of mir200b resulted in ∼50% reduction in luciferase activity in the cells transfected either with the full-length WAVE3 3′-UTR or with sequences containing each of the three individual seed sequences for miR200b (Fig. 2C). Transfection of either MDA-MB-231 or LNCaP cells, in which no miR200 microRNAs can be detected (Fig. 1D) (6), resulted in a significant decrease in WAVE3 expression to levels comparable with those obtained using WAVE3-specific siRNA (Fig. 3, A and B). MDA-MB-231 and LNCaP, which do not express miR200, did not show any change in the levels of luciferase activity when transfected with the control vector or the WAVE3 3′-UTR-containing vector (Fig. 3C). However, the miR200-expressing HT29 cells showed a significant decrease in the levels of luciferase activity when transfected with the WAVE3 3′-UTR-containing vector, owing this decrease to the presence of high endogenous levels of miR200 in the HT29 cells (Fig. 3C). Expression of exogenous miR200b in either MDA-MB-231 or LNCaP cells resulted in a 3- and 2-fold decrease in luciferase activity, respectively, compared with the control vector (Fig. 3D). These results clearly show that miR200 can specifically target and repress the expression of WAVE3. Deletion of the seed sequences of miR200 in the 3′-UTR of WAVE3, however, abrogated the effect of the exogenous miR200b in both MDA-MB-231 and LNCaP cells, as well as the endogenous miR200b in HT29 cells, further confirming that the binding of miR200b to its specific seed sequences in the 3′-UTR of WAVE3 leads to the inhibition of its expression (Fig. 3E).
FIGURE 2.
microRNA miR200b directly targets the 3′-UTR of WAVE3 and represses its expression. A, RT-PCR analysis of WAVE3 in untreated HEK293 cells or transfected with miR200b. WAVE3 expression levels were markedly reduced in the miR200b-transfected cells. WAVE2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as controls. B, firefly luciferase reporter plasmids, pmirGlo control, or pmirGlo containing the entire 3′-UTR of WAVE3 (W3–3′UTR) were transiently transfected into HEK293 cells, and luciferase activities were measured after 48 h. C, HEK293 cells were transfected with luciferase reporter plasmids or co-transfected with luciferase reporter plasmids and synthetic miR200b mimic; luciferase activities were measured after 48 h.
FIGURE 3.
Mutation of miR200 seed sequences in the WAVE3 3′-UTR abrogates miR200b effect. A and B, RT-PCR analysis of WAVE3 in MDA-MB-231 (A) and LNCaP (B) transiently transfected with either siRNA to WAVE3, synthetic miR200b, or control microRNA. C and D, MDA-MB-231, LNCaP, and HT29 cells were transfected with LR control or WAVE3–3′-UTR plasmids (C) or co-transfected with LR plasmids and synthetic miR200b precursor (D), and luciferase activities were measured after 48 h. E, MDA-MB-231, LNCaP, and HT29 cells were transfected with LR plasmids containing the WAVE3 3′-UTR with deleted miR200b seed sequences and co-transfected with or without synthetic miR200b precursor, and luciferase activities were measured after 48 h. For all luciferase activity assays, Renilla luciferase activity was used for normalization. The data are the mean ± S.D. of at least three independent transfections. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
miR200-mediated Down-regulation of WAVE3 Expression Inhibits Cancer Cell Invasion
In several of our previously published studies, we showed that WAVE3 expression is required for cell migration and cancer cell invasion during tumor progression and metastasis both in vitro and in vivo (33–35). Therefore, we evaluated the effects of miR200-mediated suppression of WAVE3 expression on invasion in vitro using the Matrigel assay. A 4-fold reduction in the number of cells invading the Matrigel-covered membrane was seen with both MDA-MB-231 and LNCaP cells treated with siRNA to WAVE3 compared with the control cells (Fig. 4, A–D). These results are consistent with our previously published studies for MDA-MB-231 cells (34, 35). Similar results were obtained with overexpression of miR200b in these two cells, clearly demonstrating that suppression of WAVE3 levels using either siRNA or miR200 effectively reduces the invasive capability of cancer cells in vitro. On the other hand, introduction of an anti-miR200b oligonucleotide in HT29 cells resulted in a small but statistically significant increase in the number of invading cells (Fig. 4, E and F).
FIGURE 4.
miR200-mediated down-regulation of WAVE3 expression inhibits cancer cell invasion. Matrigel invasion assay of MDA-MB-231 (A and B), LNCaP (C and D), and HT29 (E and F) transfected with either siRNA to WAVE3 or miR200b precursor. Shown are representative bright field microscopy images of stained cells (A, C, and E) and quantification of invasive cells (B, D, and F). At least six different fields were counted from each experiment. The data are the mean ± S.D. of at least three independent assays.
Down-regulation of WAVE3 expression by either miR200 or siRNA also resulted in a dramatic morphological change of MDA-MB-231 cells. As a result of the specific inhibition of WAVE3 expression, the number of round versus elongated cells increased from 37 to 80%. MDA-MB-231 cells switched from the fibroblastoid, elongated, and polarized shape, to a more rounded, cobblestone-like shape (supplemental Fig. S2). Although results similar to MDA-MB-231 cells were obtained in the mesenchymal-type LNCaP cells, no significant changes in the phenotype of HT29 cells was seen when these cells were transfected with the anti-miR200 oligonucleotide. This difference between the cell lines may be attributed to the high levels of endogenous miR200 in HT29 cells, where transient transfection with anti-miR200 oligonucleotide could not down-regulate miR200 to levels low enough to trigger the morphological change.
Re-expression of miR200-resistant WAVE3 Reverses miR200b-mediated Inhibition of Invasion in LNCaP Cells
Since microRNAs can regulate the expression of several genes simultaneously, we investigated whether the inhibition of cancer cell invasion is the result of the specific down-regulation of WAVE3 expression and not the broad effect of miR200b. We therefore determined whether this inhibition of cancer cell invasion could be reversed by restoring WAVE3 expression. LNCaP cells were transfected with a miR200-resistant WAVE3 construct, which does not contain the 3′-UTR of WAVE3 and therefore contains no target sequence for miR200b. In a parallel control experiment, the WAVE3 expression construct was also transfected in siWAVE3-expressing LNCaP cells. As expected, WAVE3 expression levels were significantly reduced in the siWAVE3-expressing cells, whereas no significant changes were observed in the miR200b-expressing cells or in the cells with the control transfections (Fig. 5A and supplemental Fig. S3, A and B). Also as expected, LNCaP cells expressing either endogenous or exogenous WAVE3 showed a significant reduction in their invasive activity in the presence of siRNA against WAVE3, reflective of the siWAVE3-mediated down-regulation of WAVE3 expression. Invasion of LNCaP cells was also inhibited in cells where endogenous WAVE3 was targeted by miR200b. The invasion defect associated with miR200b expression was, however, completely rescued by re-expression of WAVE3 (Fig. 5B and supplemental Fig. S3C).
FIGURE 5.
Re-expression of miR200-resistant WAVE3 reverses miR200b-mediated inhibition of LNCaP cell invasion. A, immunoblotting (IB) with the indicated antisera of LNCaP cells overexpressing green fluorescent protein or green fluorescent protein-WAVE3 fusion protein and co-transfected with either control siWAVE3 of miR200b. NS, nonspecific band. B, quantification of invasive cells for the indicated treatment. At least six different fields were counted from each experiment. The data are the mean ± S.D. of at least three independent assays.
miR200 Regulates WAVE3 and E-cadherin/ZEB1/ZEB2 through Independent Pathways
A recently published study has established a direct correlation between the expression levels of miR200 and E-cadherin in epithelial-type versus mesenchymal-type cells (6). On the other hand, several other studies have now clearly shown that the miR200 family inhibits the expression of ZEB1/ZEB2 transcription factors, which inhibit expression of E-cadherin, a key epithelial gene, thereby preventing them from triggering the EMT switch (2, 5, 6, 16, 17). Consistent with the published findings, we found that E-cadherin can barely be detected in the mesenchymal-type cells MDA-MB-231 and LNCaP (supplemental Fig. S4). In the epithelial-type HT29 cells, which grow in clusters, high levels of E-cadherin staining were detected at the cell junctions (supplemental Fig. S4). In MDA-MB-231 and LNCaP cells, which do not express high levels of E-cadherin, both the WAVE3-specific siRNA and miR200 resulted in a significant knockdown of WAVE3 expression, as expected, whereas only overexpression of miR200 and not siWAVE3 resulted in an increase in E-cadherin levels in both cell lines (Fig. 6, A–D). On the other hand, overexpression of the anti-miR200 oligonucleotide resulted in a slight decrease in E-cadherin levels in HT29, whereas WAVE3-specific siRNA did not affect the expression levels of E-cadherin (Fig. 6, E and F). These results suggest that the miR200-mediated regulation of E-cadherin is achieved through a pathway that may be independent from the pathway through which miR200 inhibits WAVE3 and that expression of E-cadherin is not regulated downstream of WAVE3. On the other hand, neither siWAVE3 nor miR200 had any effect on the expression levels of vimentin, another EMT marker (Fig. 6). No changes in the expression levels of N-cadherin were observed in any of the treatments (not shown). This difference in regulation indicates that miR200b is not the sole determinant of the changes associated with EMT.
FIGURE 6.
miR200 regulates WAVE3 and E-cadherin/ZEB1/ZEB2 through independent pathways during EMT. Western blot (A, C, and E) and RT-PCR (B, D, and F) analyses of WAVE3, E-cadherin, and vimentin in MDA-MB-231, LNCaP, and HT29, respectively, with the indicated transient treatments. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin were used as internal controls for RT-PCR and Western blot analyses, respectively.
Consistent with the effect of miR200 on E-cadherin, we found that overexpression of miR200, but not siWAVE3, resulted in down-regulation of ZEB1 and ZEB2 in both MDA-MB-231 and LNCaP cells (supplemental Fig. S4). No noticeable changes in the expression levels of either ZEB1 or ZEB2 were observed when HT29 cells were transfected with the anti-miR200 oligonucleotide (supplemental Fig. S4).
In summary, our data show that the activity of WAVE3 to control cancer cell invasion, which is an integral component of the changes associated with EMT, is regulated by the miR200 microRNAs.
DISCUSSION
WAVE3 is a member of the WASP/WAVE protein family, which regulates actin polymerization and cytoskeleton remodeling (25, 26, 29, 30, 32, 45) through the recruitment of the Arp2/3 (actin-related protein 2 and 3) protein complex, via a conserved verprolin-cofilin-acidic domain at their C terminus (29, 46). We have recently shown that WAVE3 plays a critical role in cell motility and migration of invasive cancer cells (33–36). The expression levels of WAVE3 also correlate with cancer progression and metastasis, and WAVE3 was found to be critical for the metastasis of breast cancer cells in mouse models (35, 48).
Furthermore, since WAVE3 expression levels are significantly elevated in the invasive cancer cells compared with normal tissue or low grade tumors (35), we hypothesized that the expression levels of WAVE3 during this process might be regulated at the transcriptional or post-transcriptional levels. Analysis of the genomic organization of the WAVE3 gene revealed the presence of a prominent CpG island in the promoter region, suggesting that WAVE3 expression might be regulated by means of methylation. This did not turn out to be the case, at least in the few samples that have been analyzed.3 With the exclusion of promoter methylation as a possible mechanism for the regulation of WAVE3 expression during tumor progression and with the emergence of increasing evidence confirming microRNAs as master regulators of gene expression, we sought to investigate whether microRNAs are involved in the regulation of WAVE3 expression.
We now report the identification of the miR200 family of microRNAs, which have been established as markers for epithelial cells and as powerful regulators of EMT (2, 5, 20), as being also directly involved in the regulation of WAVE3. A diverse array of signaling pathways have been shown to be activated during EMT, including those regulating the reorganization of the actin cytoskeleton, cell motility, migration and cancer cell invasion (49), processes in which WAVE3 has been implicated.
Based on these relationships, we hypothesized that WAVE3 might be regulated during EMT to promote cancer progression and metastasis. We used multiple functional assays to confirm that WAVE3 expression is regulated by members of the miR200 family. First, semiquantitative RT-PCR analysis showed a clear inverse correlation between the expression levels of WAVE3 and members of miR200 microRNA family in epithelial versus mesenchymal cells. Second, overexpression of miR200b mimics in the mesenchymal cells resulted in a significant down-regulation of WAVE3 expression levels, concomitant with a loss of the invasive potential of these cells, and a reversal to a more epithelial-like phenotype. Third, deletion of the seed sequences for miR200b in the 3′-UTR of the WAVE3 cDNA abrogated the miR200b-mediated inhibition of the reporter gene activity, clearly demonstrating that microRNA miR200 directly targets the 3′-UTR of WAVE3 and represses its expression. Finally, we showed that the loss of the invasive phenotype associated with the expression of miR200b can be completely rescued by re-expression of exogenous WAVE3, clearly demonstrating that the inhibition of cancer cell invasion is a result of the specific loss of WAVE3 and the downstream events it controls and not the effects of this microRNA on genes unrelated to WAVE3.
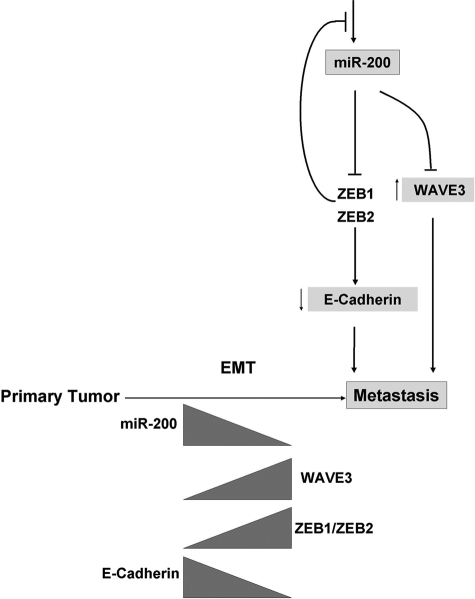
Together these studies support a model in which WAVE3 is actively involved in the invasive phenotype that is associated with the mesenchymal nature of cancer cells. Suppression of its expression by miR200 family members in cancer cells can lead to inhibition of cancer cell invasion, which is also associated with a profound change in cell morphology, consistent with a mesenchymal-to-epithelial induction-like process (Fig. 7).
FIGURE 7.
Mechanism of miR200-mediated regulation of WAVE3 during EMT. Tumor progression from the primary to the invasive/metastatic stage is associated with an increase in the expression levels of ZEB1 and ZEB2 inhibitors of transcription factors, leading to a significant decrease in the expression levels of miR200 microRNAs, which results in an increase in the expression levels of WAVE3 and a concomitant decrease in E-cadherin expression levels. Whereas down-regulation of E-cadherin leads to the loss of cell junctions, up-regulation of WAVE3 results in an increase in cell motility, both of which are required for the acquisition of the invasive phenotype. This coordinated regulation of both E-cadherin and WAVE3 by miR200 microRNAs is critical for tumor progression and metastasis.
MicroRNAs regulate gene expression by targeting specific sequences in the 3′-UTR of their cognate genes (38, 41). There are three seed sequences of miR200b in the 3′-UTR of the human WAVE3 transcript (Fig. 1). We showed that all three seed sequences are biologically functional because miR200b could invariably target each sequence and inhibit expression of the reporter gene when each seed sequence was individually subcloned within the 3′-UTR of the firefly luciferase transcription cassette (Fig. 2). It has also been reported that the level of microRNA-mediated down-regulation of its target gene correlates with the number of seed sequences present in the 3′-UTR. As a relevant example, in the case of ZEB1 and ZEB2 genes, each has five seed sequences for miR200b, and their expression is efficiently suppressed by this microRNA (6). We were not able, however, to detect any significant differences in the luciferase activity between the full-length WAVE3 3′-UTR, where all three seed sequences were present, and when only each seed sequence was tested individually (Fig. 2C), suggesting that the conservation of the seed sequences and their surrounding nucleotides might be more important in the recognition and targeting by the microRNAs than the number of seed sequences (37, 38, 40, 41). The mature sequence of miR200b and its related microRNAs are perfectly conserved among mammals, including in mice. On the other hand, the 3′-UTR of the mouse Wave3 transcript has two seed sequences of miR200b, which are identical to the seed sequences present in the 3′-UTR of the human WAVE3 transcript (not shown), suggesting that both the human and mouse Wave3 genes are regulated downstream of miR200. Therefore, when establishing a mouse model to investigate the miR200-mediated regulation of WAVE3 during the EMT process, the findings could easily be translated from mice to humans in a clinical setting.
All three members of the WAVE protein family have been shown to regulate, downstream of Rho GTPases, the remodeling of actin cytoskeleton and the formation of lamellipodia, which are crucial for cell motility, cell migration, and cancer cell invasion. The activity of the WAVE proteins has also been shown to be regulated by their inclusion in the multiprotein complex SRA1/PIR121, NAP1, ABI1/2, and HSPC300 (45). The expression profiles for the WAVE genes clearly show some overlap in embryonic and adult tissues, although WAVE3 shows a far more restricted distribution, suggesting a more tissue-specific function for this family member (28, 32, 50). However, although WAVE1 and WAVE2 are both expressed in mouse embryonic fibroblasts, they were found to have differential roles in cell migration (51). WAVE1 was found to be required for the formation of dorsal ruffles, whereas WAVE2 is required for the formation of peripheral ruffles, two membrane-based actin structures that are necessary for the initiation of cell migration (51). We have also shown that WAVE3 is involved in focal adhesions. Knockdown of WAVE3 prevents lamellipodia formation, and this phenotype cannot be compensated for by the presence of WAVE1 or WAVE2 (34). Other evidence for the non-redundant roles of the WAVE proteins in the regulation of the actin cytoskeleton comes from analysis of knock-out mice for the WAVE genes, where disruption of either WAVE1 or WAVE2 resulted in embryonic lethality associated with marked developmental delay and organ malformations (47, 52, 53). These findings, although they strongly support independent roles for the WAVE proteins, also suggest independent mechanisms for the regulation of each gene.
Our present study clearly shows that the mechanism of miR200-mediated regulation of gene expression is unique to WAVE3 in the context of the WAVE family, since the miR200-mediated knockdown to WAVE3 has no effect on the expression level of either WAVE2 (Fig. 2) or WAVE1 (not shown). The restricted specificity of miR200 to WAVE3 is consistent with the absence of miR200 seed sequences in 3′-UTRs of both WAVE1 and WAVE2.
The miR200 microRNAs have been established as master regulators of the EMT process by targeting and inhibiting the expression of ZEB1 and ZEB2 transcription factors, which in turn regulate the expression of E-cadherin (2, 5, 6, 16, 17). Inhibition of WAVE3 expression with either overexpression of miR200b or siRNA to WAVE3 both resulted in a change of cell phenotype resembling that of the mesenchymal to epithelial reversal (supplemental Fig. S2), indicating that WAVE3 might be implicated in this cellular process. On the other hand, siRNA to WAVE3 had no effect on the expression level of ZEB1, ZEB2, or E-cadherin (supplemental Fig. S4), suggesting that whereas both WAVE3 and ZEB1/ZEB2/E-cadherin are being regulated by miR200 microRNAs during EMT, WAVE3 might not be directly involved in the regulatory pathway of ZEB1/ZEB2/E-cadherin. However, it is clear that miR200 regulates both WAVE3 and ZEB1/ZEB2/E-cadherin during EMT that is associated with tumor progression and metastasis (Fig. 7). During the EMT process, down-regulation of miR200 leads to loss of E-cadherin in cell junctions, which is necessary for the dissociation of cell-cell contacts in primary tumors, allowing the release of invasive cells. Concomitantly, we have shown that increased expression of WAVE3 results in the increase of the motility, migratory, and invasiveness potentials of cancer cells, all of which are required for cancer invasion and metastasis.
In conclusion, we have identified a novel mechanism for the regulation of WAVE3 in cancer cells, which is also critical for the modulation of the EMT process during tumor progression and metastasis. Our findings also illustrate the importance of miR200 in regulating tumor cell invasion via a WAVE3-dependent mechanism and support our previously published work on the critical role played by WAVE3 in tumor metastasis. WAVE3 might, therefore, be a potential target for screening of drugs to prevent and/or inhibit cancer progression and metastasis.
Supplementary Material
Acknowledgment
We thank Tim Burke for proofreading the manuscript.
This work was supported in part by startup funds from United States Department of Defense Grant W81XWH-08-1-0236 (to K. S. A.) and by National Institutes of Health Grants P01HL073311 and P50HL077107 (to E. F. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S4.
K. Sossey-Alaoui, K. Bialkowska, and E. F. Plow, unpublished data.
- UTR
- untranslated region
- RT
- reverse transcription
- EMT
- epithelial to mesenchymal transition
- DMEM
- Dulbecco's modified Eagle's medium.
REFERENCES
- 1.Berx G., Raspé E., Christofori G., Thiery J. P., Sleeman J. P. (2007) Clin. Exp. Metastasis 24, 587–597 [DOI] [PubMed] [Google Scholar]
- 2.Burk U., Schubert J., Wellner U., Schmalhofer O., Vincan E., Spaderna S., Brabletz T. (2008) EMBO Rep. 9, 582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esquela-Kerscher A., Slack F. J. (2006) Nat. Rev. Cancer 6, 259–269 [DOI] [PubMed] [Google Scholar]
- 4.Korpal M., Kang Y. (2008) RNA Biol. 5, 115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korpal M., Lee E. S., Hu G., Kang Y. (2008) J. Biol. Chem. 283, 14910–14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S. M., Gaur A. B., Lengyel E., Peter M. E. (2008) Genes Dev. 22, 894–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peter M. E. (2009) Cell Cycle 8, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spaderna S., Brabletz T., Opitz O. G. (2009) Gastroenterology 136, 1835–1837 [DOI] [PubMed] [Google Scholar]
- 9.Akao Y., Nakagawa Y., Naoe T. (2006) Biol. Pharm. Bull. 29, 903–906 [DOI] [PubMed] [Google Scholar]
- 10.Calin G. A., Dumitru C. D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., Rassenti L., Kipps T., Negrini M., Bullrich F., Croce C. M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 15524–15529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson C. D., Esquela-Kerscher A., Stefani G., Byrom M., Kelnar K., Ovcharenko D., Wilson M., Wang X., Shelton J., Shingara J., Chin L., Brown D., Slack F. J. (2007) Cancer Res. 67, 7713–7722 [DOI] [PubMed] [Google Scholar]
- 12.Takamizawa J., Konishi H., Yanagisawa K., Tomida S., Osada H., Endoh H., Harano T., Yatabe Y., Nagino M., Nimura Y., Mitsudomi T., Takahashi T. (2004) Cancer Res. 64, 3753–3756 [DOI] [PubMed] [Google Scholar]
- 13.Yanaihara N., Caplen N., Bowman E., Seike M., Kumamoto K., Yi M., Stephens R. M., Okamoto A., Yokota J., Tanaka T., Calin G. A., Liu C. G., Croce C. M., Harris C. C. (2006) Cancer Cell 9, 189–198 [DOI] [PubMed] [Google Scholar]
- 14.He L., Thomson J. M., Hemann M. T., Hernando-Monge E., Mu D., Goodson S., Powers S., Cordon-Cardo C., Lowe S. W., Hannon G. J., Hammond S. M. (2005) Nature 435, 828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voorhoeve P. M., le Sage C., Schrier M., Gillis A. J., Stoop H., Nagel R., Liu Y. P., van Duijse J., Drost J., Griekspoor A., Zlotorynski E., Yabuta N., De Vita G., Nojima H., Looijenga L. H., Agami R. (2006) Cell 124, 1169–1181 [DOI] [PubMed] [Google Scholar]
- 16.Bracken C. P., Gregory P. A., Kolesnikoff N., Bert A. G., Wang J., Shannon M. F., Goodall G. J. (2008) Cancer Res. 68, 7846–7854 [DOI] [PubMed] [Google Scholar]
- 17.Gregory P. A., Bert A. G., Paterson E. L., Barry S. C., Tsykin A., Farshid G., Vadas M. A., Khew-Goodall Y., Goodall G. J. (2008) Nat. Cell Biol. 10, 593–601 [DOI] [PubMed] [Google Scholar]
- 18.Spaderna S., Schmalhofer O., Hlubek F., Jung A., Kirchner T., Brabletz T. (2007) Verh. Dtsch. Ges. Pathol. 91, 21–28 [PubMed] [Google Scholar]
- 19.Cano A., Nieto M. A. (2008) Trends Cell Biol. 18, 357–359 [DOI] [PubMed] [Google Scholar]
- 20.Gregory P. A., Bracken C. P., Bert A. G., Goodall G. J. (2008) Cell Cycle 7, 3112–3118 [DOI] [PubMed] [Google Scholar]
- 21.Savagner P. (2001) BioEssays 23, 912–923 [DOI] [PubMed] [Google Scholar]
- 22.Brabletz T., Jung A., Reu S., Porzner M., Hlubek F., Kunz-Schughart L. A., Knuechel R., Kirchner T. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 10356–10361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvorak H. F. (1986) N. Engl. J. Med. 315, 1650–1659 [DOI] [PubMed] [Google Scholar]
- 24.Fuchs I. B., Lichtenegger W., Buehler H., Henrich W., Stein H., Kleine-Tebbe A., Schaller G. (2002) Anticancer Res. 22, 3415–3419 [PubMed] [Google Scholar]
- 25.Cory G. O., Ridley A. J. (2002) Nature 418, 732–733 [DOI] [PubMed] [Google Scholar]
- 26.Derry J. M., Ochs H. D., Francke U. (1994) Cell 78, 635–644 [DOI] [PubMed] [Google Scholar]
- 27.Fukuoka M., Suetsugu S., Miki H., Fukami K., Endo T., Takenawa T. (2001) J. Cell Biol. 152, 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suetsugu S., Miki H., Takenawa T. (1999) Biochem. Biophys. Res. Commun. 260, 296–302 [DOI] [PubMed] [Google Scholar]
- 29.Takenawa T., Miki H. (2001) J. Cell Sci. 114, 1801–1809 [DOI] [PubMed] [Google Scholar]
- 30.Westphal R. S., Soderling S. H., Alto N. M., Langeberg L. K., Scott J. D. (2000) EMBO J. 19, 4589–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sossey-Alaoui K., Su G., Malaj E., Roe B., Cowell J. K. (2002) Oncogene 21, 5967–5974 [DOI] [PubMed] [Google Scholar]
- 32.Sossey-Alaoui K., Head K., Nowak N., Cowell J. K. (2003) Mamm. Genome 14, 314–322 [DOI] [PubMed] [Google Scholar]
- 33.Sossey-Alaoui K., Li X., Ranalli T. A., Cowell J. K. (2005) J. Biol. Chem. 280, 21748–21755 [DOI] [PubMed] [Google Scholar]
- 34.Sossey-Alaoui K., Ranalli T. A., Li X., Bakin A. V., Cowell J. K. (2005) Exp. Cell Res. 308, 135–145 [DOI] [PubMed] [Google Scholar]
- 35.Sossey-Alaoui K., Safina A., Li X., Vaughan M. M., Hicks D. G., Bakin A. V., Cowell J. K. (2007) Am. J. Pathol. 170, 2112–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sossey-Alaoui K., Li X., Cowell J. K. (2007) J. Biol. Chem. 282, 26257–26265 [DOI] [PubMed] [Google Scholar]
- 37.Farh K. K., Grimson A., Jan C., Lewis B. P., Johnston W. K., Lim L. P., Burge C. B., Bartel D. P. (2005) Science 310, 1817–1821 [DOI] [PubMed] [Google Scholar]
- 38.Friedman R. C., Farh K. K., Burge C. B., Bartel D. P. (2009) Genome Res. 19, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimson A., Farh K. K., Johnston W. K., Garrett-Engele P., Lim L. P., Bartel D. P. (2007) Mol. Cell 27, 91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis B. P., Shih I. H., Jones-Rhoades M. W., Bartel D. P., Burge C. B. (2003) Cell 115, 787–798 [DOI] [PubMed] [Google Scholar]
- 41.Lewis B. P., Burge C. B., Bartel D. P. (2005) Cell 120, 15–20 [DOI] [PubMed] [Google Scholar]
- 42.Hurteau G. J., Carlson J. A., Spivack S. D., Brock G. J. (2007) Cancer Res. 67, 7972–7976 [DOI] [PubMed] [Google Scholar]
- 43.Bagga S., Bracht J., Hunter S., Massirer K., Holtz J., Eachus R., Pasquinelli A. E. (2005) Cell 122, 553–563 [DOI] [PubMed] [Google Scholar]
- 44.Lim L. P., Lau N. C., Garrett-Engele P., Grimson A., Schelter J. M., Castle J., Bartel D. P., Linsley P. S., Johnson J. M. (2005) Nature 433, 769–773 [DOI] [PubMed] [Google Scholar]
- 45.Stovold C. F., Millard T. H., Machesky L. M. (2005) BMC Cell Biol. 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollard T. D., Borisy G. G. (2003) Cell 112, 453–465 [DOI] [PubMed] [Google Scholar]
- 47.Yan C., Martinez-Quiles N., Eden S., Shibata T., Takeshima F., Shinkura R., Fujiwara Y., Bronson R., Snapper S. B., Kirschner M. W., Geha R., Rosen F. S., Alt F. W. (2003) EMBO J. 22, 3602–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W., Goswami S., Lapidus K., Wells A. L., Wyckoff J. B., Sahai E., Singer R. H., Segall J. E., Condeelis J. S. (2004) Cancer Res. 64, 8585–8594 [DOI] [PubMed] [Google Scholar]
- 49.Thiery J. P., Sleeman J. P. (2006) Nat. Rev. Mol. Cell Biol. 7, 131–142 [DOI] [PubMed] [Google Scholar]
- 50.Oda A., Miki H., Wada I., Yamaguchi H., Yamazaki D., Suetsugu S., Nakajima M., Nakayama A., Okawa K., Miyazaki H., Matsuno K., Ochs H. D., Machesky L. M., Fujita H., Takenawa T. (2005) Blood 105, 3141–3148 [DOI] [PubMed] [Google Scholar]
- 51.Suetsugu S., Yamazaki D., Kurisu S., Takenawa T. (2003) Dev. Cell 5, 595–609 [DOI] [PubMed] [Google Scholar]
- 52.Dahl J. P., Wang-Dunlop J., Gonzales C., Goad M. E., Mark R. J., Kwak S. P. (2003) J. Neurosci. 23, 3343–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamazaki D., Suetsugu S., Miki H., Kataoka Y., Nishikawa S., Fujiwara T., Yoshida N., Takenawa T. (2003) Nature 424, 452–456 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.