Abstract
Sulfiredoxin catalyzes the ATP-dependent reduction of overoxidized eukaryotic 2-Cys peroxiredoxin PrxSO2 into sulfenic PrxSOH. Recent mechanistic studies on sulfiredoxins have validated a catalytic mechanism that includes formation of a phosphoryl intermediate on the sulfinyl moiety of PrxSO2, followed by an attack of the catalytic cysteine of sulfiredoxin on the phosphoryl intermediate that leads to formation of a thiosulfinate intermediate PrxSO-S-sulfiredoxin. Formation of this intermediate implies the recycling of sulfiredoxin into the reduced form. In this study, we have investigated how the reductase activity of the Saccharomyces cerevisiae sulfiredoxin is regenerated. The results show that an oxidized sulfiredoxin under disulfide state is formed between the catalytic Cys84 and Cys48. This oxidized sulfiredoxin species is shown to be catalytically competent along the sulfiredoxin-recycling process and is reduced selectively by thioredoxin. The lack of Cys48 in the mammalian sulfiredoxins and the low efficiency of reduction of the thiosulfinate intermediate by thioredoxin suggest a recycling mechanism in mammals different from that of sulfiredoxin from Saccharomyces cerevisiae.
INTRODUCTION
A growing number of studies have shown the importance of the versatility of Cys redox biochemistry in the regulation of various cellular processes such as catalysis, metal binding, or signal transduction (1). The typical eukaryotic 2-Cys-peroxiredoxins (Prx)3 represent a family of proteins that exemplifies these mechanisms, as these thiol peroxidases have been described under six redox states of the essential Cys residue, from −II to +IV. For example, the Cys under reduced, disulfide, and sulfenic forms (oxidation states −II, −I, and 0, respectively) is involved in the catalytic mechanism of Prx as peroxidase enzymes (2–5), the disulfide and sulfenic states in their function as redox sensor (6, 7), and the sulfinic (+II) and sulfonic (+IV) states in possible chaperon-like function (8, 9). Cysteine under the sulfinic state +II (PrxSO2) is formed by a mechanism of escape of the reactive sulfenic acid intermediate during the catalytic peroxidase cycle (5, 10). This overoxidation constitutes a post-translational modification that is thought to afford a regulation mechanism between these different functions depending on the oxidative stress conditions. In addition, Prxs are also subject to other post-translational modifications by phosphorylation and N-acetylation (11, 12).
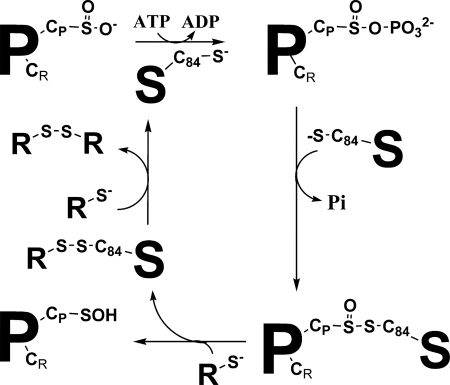
In contrast to Cys under −I and 0 oxidation states, the Cys under sulfinate state is not reducible by cellular thiols such as glutathione and thioredoxin (Trx). Therefore, regulation of Prx functions is dependent on a sulfinyl reductase referred to as sulfiredoxin (Srx), which catalyzes the ATP-dependent reduction of PrxSO2 into sulfenic Prx (PrxSOH) (13–15). Although the sestrin family of human proteins was initially proposed to possess this activity, the Srxs appear to be the only enzymes with sulfinyl reductase activity (16). Recent mechanistic studies on Srx from Saccharomyces cerevisiae and human origin support a mechanism in which the sulfinic moiety of the PrxSO2 substrate is first activated by formation of an anhydride bond with the γ-phosphate of ATP, leading to a phosphoryl sulfinic intermediate, followed by attack of Srx catalytic Cys, which results in a thiosulfinate intermediate PrxSO-SSrx (oxidation state of Prx Cys +I) (Fig. 1) (17–19). Such a mechanism implies the recycling of Srx into the reduced form.
FIGURE 1.
Srx catalytic mechanism. Srx catalyzes the reduction of the sulfinate function of PrxSO2 via a phosphotransferase and a reductase step leading to a covalent thiosulfinate intermediate with Srx. The subsequent recycling of the Srx activity involves an external reductant R. P, Prx; S, Srx; CP, peroxidatic Cys; CR, regeneration Cys; C84, Srx catalytic Cys; R, thiol reductant.
In the case of S. cerevisiae Srx, one product of the reaction with PrxSO2 and ATP in the absence of added reductant was a monomeric form of Srx oxidized under a disulfide state (17). In addition, Trx was shown to act as a reductant in the catalytic cycle at a rate that is not limiting compared with the rate of the first steps of the reaction (17). Thus, several questions have to be addressed regarding the mechanism of the recycling process of Srx. First, what is the mechanism of the recycling of Srx from S. cerevisiae? In particular, what is the intermediate species targeted by Trx? Is it the thiosulfinate species or a disulfide species? Second, if a disulfide species is involved, which of Cys48 or Cys106 forms a disulfide bond with the catalytic Cys84? Third, what is the nature of the recycling reductant for mouse and human Srxs, in which cysteine residues equivalent to Cys48 and Cys106 are not present?
To address these questions, we have done the following: 1) determined the steady-state rate of the reaction catalyzed by S. cerevisiae wild-type Srx and C48S, C106A, and C48A/C106A Srxs in the presence of dithiothreitol (DTT) and Trx; 2) shown that a disulfide bond between Cys84 and Cys48 is efficiently formed; and 3) determined the kinetic parameters of reduction of oxidized Cys48/Cys84 Srx by Trx and characterized the cysteine in oxidized Srx that is attacked by the catalytic cysteine of Trx. Together, the data, combined with previous kinetic studies, show the following: 1) oxidized Cys48/Cys84 Srx is a catalytically competent species along the Srx recycling process, and 2) Trx forms an efficient binary complex with oxidized Cys48/Cys84 Srx and reduces the oxidized Srx, likely via formation of a transient interdisulfide bond with the recycling Cys48. This cysteine is located in an extra sequence of 18 amino acids that is not present in mammalian Srxs. The data also show that Trx does not reduce efficiently oxidized Srx under the thiosulfinate state. This is consistent with the hypothesis that a reductant distinct from Trx is involved in the recycling of the mammalian Srx activity in vivo.
EXPERIMENTAL PROCEDURES
Materials
Tris was from VWR International (West Chester, PA). KCl and MgCl2 were from Merck; NADPH was obtained from Roche Applied Science; and DTT was from Euromedex (Souffelweyersheim, France). ATP, MES, iodoacetamide, 2-pyridyl disulfide (2PDS), and 5,5′-dithiobis(2 nitro) benzoate were from Sigma. Trifluoroacetic acid and methyl-7-guanosine were from Fluka.
Escherichia coli Trx1, NADPH Trx reductase (NTR), purine nucleoside phosphorylase, and S. cerevisiae Tsa1 Prx, its overoxidized form PrxSO2, S. cerevisiae wild-type, and C48A/C106A Srxs were prepared following experimental procedures described previously (17, 20, 21). Trx1 from S. cerevisiae was obtained by cloning the trx1 open reading frame amplified by PCR (with Phusion DNA polymerase, Finnzymes, Espoo, Finland) using S. cerevisiae W303 genomic DNA as template, into the pET20b(+) plasmid between NdeI and SacI sites (sequences of oligonucleotides not shown). The C33S variant of S. cerevisiae Trx1 was generated by standard PCR-mediated site-directed mutagenesis. Both wild-type and mutant S. cerevisiae Trxs were expressed and purified using the same procedure as E. coli Trx1.
The Srx variants C48S and C106A were obtained by standard PCR-mediated site-directed mutagenesis, expressed, and purified as the wild-type Srx. The C106S variant was not used because it was produced in a nonsoluble form.
Steady-state Kinetics
The reaction was followed in the steady state, as described previously, using Trx as reductant and the Trx recycling system as a coupled assay or by following the kinetics of Pi release using the purine nucleoside phosphorylase-coupled assay (17). Briefly, the decrease of the emission fluorescence intensity associated with the phosphorolysis of methyl-7-guanosine catalyzed by purine nucleoside phosphorylase was recorded at 30 °C on an SX18MV-R stopped-flow apparatus (Applied PhotoPhysics, Leatherhead, UK) fitted for fluorescence measurements, with the excitation wavelength set at 305 nm and the emitted light collected above 455 nm using a cutoff filter. The steady-state rate constant was obtained by measurement of initial rate in the presence of reductant, after calibration of the fluorescence signal against Pi concentration.
Kinetics of Formation of the Oxidized Srx Species Followed by Reversed Phase Chromatography
Reaction mixtures containing 20 μm wild-type Srx, 100 μm C171A PrxSO2, 1 mm ATP, and 1 mm MgCl2 were incubated for increasing times in 50 mm Tris, 100 mm KCl buffer, pH 7 (TK buffer), at 30 °C. Aliquots were quenched by 0.1% trifluoroacetic acid and analyzed by reverse phase liquid chromatography on an Aquapore RP-300 (C8) column, 4.6 × 100 mm, 7 μm (PerkinElmer Life Sciences), coupled to the ÄKTAexplorerTM system monitored by UV spectrophotometry (GE Healthcare), as described previously (17).
Kinetics of Reduction of the Cys48/Cys84 Srx Disulfide Bond by Trx
Oxidation of C106A Srx was carried out in TK buffer at 30 °C by incubation of 250 μm C106A Srx with 5 mm H2O2 followed by two additions of 5 mm H2O2 at 10-min intervals. The oxidized protein was then purified by gel filtration chromatography on a HiLoad 26/60 Superdex 75 prep grade (GE Healthcare) connected to a fast protein liquid chromatography system (GE Healthcare) equilibrated with buffer TK. Complete formation of the Cys48/Cys84 Srx disulfide bond was checked by titration of the protein with 5,5′-dithiobis(2-nitro)benzoate under denaturing conditions (1% SDS).
Kinetics of reduction of the Cys48/Cys84 disulfide bond by Trx were followed on a stopped-flow apparatus by monitoring the quenching of fluorescence intensity of Trx upon going from the reduced to oxidized forms (22). The excitation wavelength was set at 295 nm, and the emitted light was collected using a 320-nm cutoff filter. One syringe contained oxidized Cys48/Cys84 Srx in buffer TK (10 μm, final concentration after mixing), and the other one contained the reduced Trx at various concentrations in buffer TK (20–500 μm, final concentrations). An average of at least three runs was recorded for each concentration of Trx. Rate constants kobs were obtained by fitting fluorescence traces against Equation 1 by nonlinear regression analysis,
where c represents the end point, a represents the amplitude of the signal, and kobs represents the rate constant. Data were then fitted by nonlinear regression analysis to Equation 2,
where S represents the Trx concentration; KS the apparent affinity constant, and kobs, max the maximum rate constant at saturation.
Kinetics of Trx Reaction on Srx Activated as 2-Thiopyridine Mixed Disulfide
C48A/C106A and C84A/C106A Srxs were activated on Cys84 and Cys48, respectively, as 2-thiopyridine mixed disulfide by mixing 100 μm Srx with 500 μm 2PDS in buffer TK. Formation of the mixed disulfide on Cys48 or Cys84 was monitored by following the increase of the absorbance at 343 nm. After 5 min of incubation at room temperature, Srx-(2-thiopyridine) was purified by gel filtration chromatography on a HiLoad 26/60 Superdex 75 prep grade (GE Healthcare) connected to a fast protein liquid chromatography system (GE Healthcare), equilibrated with buffer TK, to remove 2-thiopyridine released and excess 2PDS. Fractions containing Srx activated either on Cys48 or Cys84 by a mixed disulfide with 2-thiopyridine were checked by following the amount of pyridine 2-thiolate released at 343 nm after addition of excess DTT.
The rate of formation of the intermolecular disulfide bond between Srx-(2-thiopyridine) and S. cerevisiae C33S Trx1 was measured spectrophotometrically by monitoring the release of pyridine 2-thiolate at 343 nm. Experiments were carried out at 30 °C on an SX18MV-R stopped-flow apparatus (Applied PhotoPhysics) fitted for absorbance measurements. Kinetics were measured by mixing 10 μm Srx-(2-thiopyridine) (final concentration) and variable concentration of C33S Trx (from 50 to 500 μm, final concentrations) in buffer TK. Rate constants kobs were obtained by fitting absorbance traces to Equation 1. Data were then fitted by linear regression or nonlinear regression analysis to Equation 2.
RESULTS
Steady-state Rate of the Reaction Catalyzed by Wild-type and Mutated Srxs in the Presence of Various Thiol Reductants
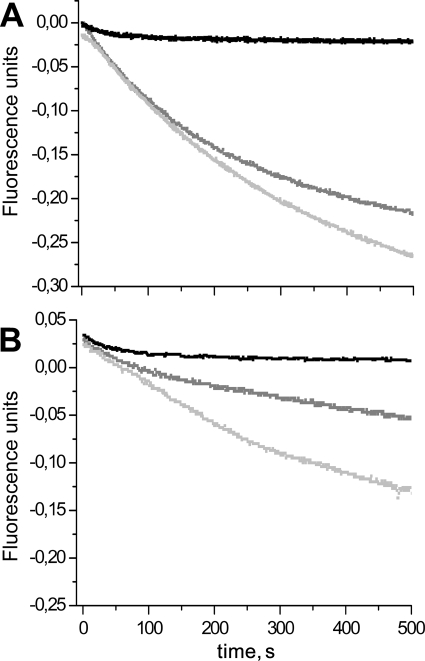
The kinetics of the reaction in the presence of various reductants were determined for the wild-type and mutated Srxs. Comparison of the traces obtained by following the release of Pi using the purine nucleoside phosphorylase-coupled assay, in the presence of Trx or DTT as reductant, is shown for wild-type and C48A/C106A Srxs in Fig. 2. The traces observed in the absence of reductant are also shown as a control. Because only the E. coli NTR was available and was not capable of recycling Trx1 efficiently from S. cerevisiae (data not shown), E. coli Trx1 was used. Therefore, unless stated otherwise, the Trx used corresponds to E. coli Trx1. As described in a previous study by our group (17), the trace is best described by a monoexponential process with a rate constant corresponding to the formation of the thiosulfinate intermediate of 2.2 ± 0.4 and 1.5 ± 0.3 min−1 for wild-type and C48A/C106A Srxs using C171A PrxSO2 as substrate, respectively. Similar results were obtained using the wild-type substrate. For wild-type Srx, in the presence of reductant, i.e. under turnover conditions, the progress curves show an initial linear phase followed by a slowing down of the process (Fig. 2A). The total amplitude of Pi production largely exceeds the Srx concentration, indicating that the process observed corresponds to several enzymatic cycles. Therefore, the steady-state rate of the reaction could be measured from the initial slope. Very similar rates were determined for wild-type Srx using Trx or DTT, i.e. 1.7 ± 0.1 and 2.2 ± 0.4 min−1, and corresponded to the rate measured using the Trx/NTR assay (1.7 ± 0.2 min−1). For the C48A/C106A Srx, a similar trace as for the wild type was observed in the presence of DTT, with an initial rate constant of 1.2 ± 0.2 min−1. In contrast, using Trx as reductant, the kinetics were best described by an exponential process with rate and amplitude similar to the control, followed by a slower steady-state linear phase with a rate constant of 0.25 ± 0.07 min−1 (Fig. 2B). The steady-state rates obtained with Trx by the Trx/NTR assay and with DTT by the purine nucleoside phosphorylase assay are summarized in Table 1 for wild-type, C48S, C106A, and C48A/C106A Srxs. In contrast to the reaction using DTT as reductant, reduction by Trx is about 10 times slower for the C48S and C48A/C106A Srxs, unlike for the wild-type and the C106A Srxs.
FIGURE 2.
Kinetics of the reaction catalyzed by Srx monitored by Pi release. A, reaction of 10 μm wild-type Srx with 100 μm C171A PrxSO2 in the presence of 1 mm ATP/MgCl2 without added reductant (black trace) or in the presence of 50 mm DTT (light gray trace) or 235 μm Trx (dark gray trace) was monitored by the purine nucleoside phosphorylase-coupled assay (see text). Reactions were carried out in buffer TK at 30 °C. The progress curves are corrected from blank traces collected in the absence of Srx. Kinetics without reductant are described by an exponential process with a rate constant of 2.2 ± 0.4 min−1 and an amplitude corresponding to 9 μm Pi released. In the presence of Trx or DTT as reductant, initial rate constants of 1.7 ± 0.1 and 2.2 ± 0.4 min−1 are measured, respectively. B, comparison of the kinetics of Pi release for the reaction of C48A/C106A Srx (10 μm) with 100 μm C171A PrxSO2 in the presence of 1 mm ATP/MgCl2 without reductant (black trace) or in the presence 50 mm DTT (light gray trace) or 235 μm Trx (dark gray trace). Kinetics without reductant are described by an exponential process with a rate constant of 1.5 ± 0.3 min−1 and an amplitude corresponding to 11 μm Pi released. The kinetics with Trx are described by an exponential process with a rate constant of 1.5 ± 0.7 min−1 and an amplitude corresponding 13 μm Pi released, followed by a slower steady-state linear phase with rate constants of 0.25 ± 0.07 min−1. In the presence of DTT as reductant, an initial rate constant of 1.2 ± 0.2 min−1 is measured.
TABLE 1.
Steady state rate of the Srx-catalyzed reaction with wild-type, C48S, C106A, and C48A/C106A Srx
Reactions were carried out in buffer TK at 30 °C with 1 mm ATP, 1 mm MgCl2, 100 μm C171A Prx, and 5–10 μm Srx. The reactions were followed with Trx (50 μm) by the Trx/NTR assay and with DTT (50 mm) by the purine nucleoside phosphorylase assay.
| Reductant | Wild type | C106A | C48S | C48A/C106A |
|---|---|---|---|---|
| Trx | 1.7 ± 0.2 | 1.9 ± 0.3 | 0.18 ± 0.2 | 0.25 ± 0.7 |
| DTT | 2.2 ± 0.4 | 2.1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 |
SDS-PAGE Analysis of the Species Formed during the Reaction of Wild-type and Mutated Srxs
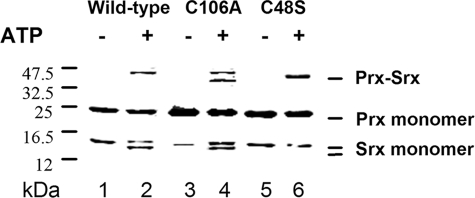
Using mass spectrometry, we have shown previously that in the absence of added thiol reductant, the Srx-catalyzed reaction released a monomeric oxidized form of Srx with an intramolecular disulfide bond between the catalytic Cys84 and another Cys. Formation of oxidized Srx is revealed by an additional band migrating slightly faster than the Srx band on SDS-polyacrylamide gels (17). To identify this residue, the products of the reaction of the single Srx variants C48S and C106A and of PrxSO2 in the presence of ATP/Mg were analyzed by SDS-PAGE in the absence of reductant (Fig. 3). In these conditions, a disulfide Prx-Srx species, which was shown to be a by-product due to the reactivity of the thiosulfinate intermediate, migrates with an apparent molecular mass of ∼45 kDa (17). The additional band corresponding to oxidized Srx is observed for the wild-type and C106A Srxs, but it is absent from the products of the reaction of C48S Srx (Fig. 3). Therefore, the monomeric oxidized form of Srx is due to a disulfide bond formed specifically between Cys84 and Cys48.
FIGURE 3.
Nonreducing SDS-PAGE analysis of the species formed during the Srx-catalyzed reaction in the absence or presence of ATP. Equimolar concentrations of C171A PrxSO2 and wild type (lanes 1 and 2), C106A (lanes 3 and 4), and C48S (lanes 5 and 6) Srxs (30 μm) were mixed in the absence (lanes 1, 3, and 5) or presence of 1 mm ATP-MgCl2 for 10 min (lanes 2, 4, and 6), immediately followed by addition of 25 mm iodoacetamide and nonreducing SDS-PAGE fractionation. Reactions were carried out in buffer TK at 30 °C. The additional band migrating between the Prx monomer and Prx·Srx complex in lane 4 corresponds to a dimer of unspecifically oxidized Srx C106A.
Kinetics of Formation of the Oxidized Cys48/Cys84 Srx Species
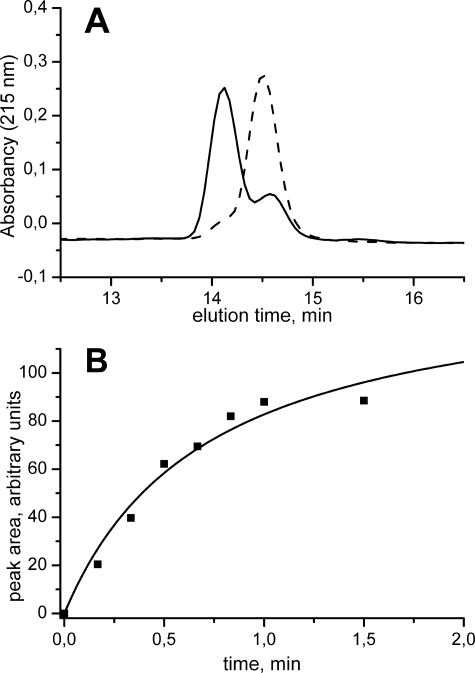
The formation of the monomeric oxidized Cys48/Cys84 Srx during the catalytic reaction suggested that the former might act as a catalytic intermediate and thus as the target of Trx. Therefore, we addressed the question of the kinetic competency of the oxidized Cys48/Cys84 Srx species, by following the kinetics of formation of this reaction product. As shown in Fig. 4A, the oxidized disulfide Srx could be resolved from the reduced form by reverse phase chromatography. Thus, the kinetics of evolution of the area of the chromatographic peak corresponding to oxidized Srx were recorded after reaction of wild-type Srx with PrxSO2 in the presence of ATP, quenched by acidification. Single turnover conditions were used, i.e. absence of reductant and excess PrxSO2 relative to Srx, to allow the determination of the rate constant of formation of the species. As shown in Fig. 4B, in these conditions, the reaction follows pseudo first-order kinetics, characterized by a rate constant of 1.8 ± 0.3 min−1.
FIGURE 4.
Kinetics of formation of Cys48/Cys84-oxidized Srx species. The reaction of 100 μm wild-type PrxSO2 with 10 μm wild-type Srx in the presence of 1 mm ATP and MgCl2 was followed at 30 °C by analysis of aliquots of the reaction mixture quenched by acidification in 0.1% trifluoroacetic acid by reverse phase chromatography and monitored by absorption spectrophotometry at 215 nm. A, chromatogram of the reaction mixture for the retention times corresponding to the Srx species after 15 s incubation (solid line) and after 1 min incubation (dashed line). B, kinetics of the evolution of the area of the peak corresponding to the Cys48/Cys84 S. cerevisiae Srx species eluted at 14.58 min (■) analyzed as a monoexponential process (solid line) with a rate constant of 1.8 ± 0.3 min−1.
Kinetics of the Reaction of Oxidized Cys48/Cys84 Srx with Trx
To study the reaction of oxidized Cys48/Cys84 Srx with Trx, preparation of oxidized Srx was set up using the C106A Srx to avoid any side reaction due to Cys106 reactivity. Oxidized Srx was prepared artificially by chemical activation of one Cys residue to promote formation of the disulfide bond. Three successive 10-min incubations of 5 mm H2O2 at 30 °C resulted in complete oxidation of Srx, mainly in a monomeric form as judged by the shift of the monomeric Srx band on SDS-PAGE, with a fraction of dimeric Srx·Srx complex. After separation by gel filtration, the monomeric fraction did not contain any free reactive thiol groups, as measured by titration using 5,5′-dithiobis(2-nitro)benzoate.
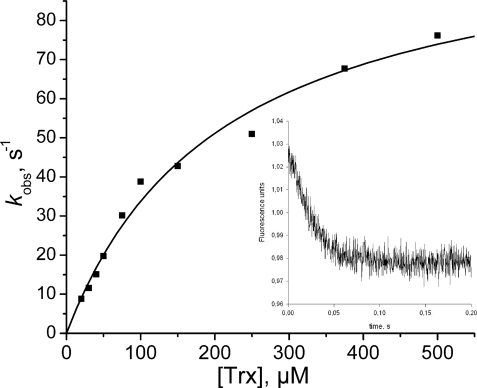
To test the potential role of Trx as reductant, the rate of reduction of oxidized Cys48/Cys84 Srx with Trx was measured by following the decrease of Trx fluorescence associated with the formation of the intramolecular disulfide bond (21). The reaction of oxidized Cys48/Cys84 Srx with an excess of Trx was followed by rapid kinetics and corresponded to a pseudo first-order process best described by a monoexponential equation with a rate constant kobs between 8 and 76 s−1 depending on Trx concentration (Fig. 5). The variation of kobs versus Trx concentration shows a hyperbolic saturation profile. Assuming binding of reduced Trx to oxidized Cys48/Cys84 is rapid equilibrium, this profile could be interpreted by nonlinear regression against Equation 2, giving a KS value of 210 ± 30 μm and a kobs, max value of 105 ± 7 s−1, corresponding to a second-order rate constant of 5.105 m−1 s−1. Similar results were obtained with the Trx1 from S. cerevisiae that gave a KS of 56 ± 15 μm and a kobs, max value of 27 ± 3 s−1. The fact that the curve passes through the origin suggested that the process up to the disulfide exchange, monitored by Trx fluorescence, can be considered as irreversible (22). This is in accord with the fact that the redox potential of oxidized/reduced Srx is −0.16 V,4 largely higher than the redox potential of Trx.
FIGURE 5.
Kinetics of reduction of the Cys48/Cys84Srx disulfide bond by E. coli Trx. The Trx fluorescence quenching associated with disulfide bond formation was recorded on a stopped-flow apparatus at 30 °C in buffer TK by mixing reduced Trx and Cys48/Cys84-oxidized C106A Srx (10 μm). Excitation wavelength was set at 295 nm, and emitted light was collected using a 320 nm cutoff filter. Data collected at various concentrations of reduced Trx were fitted to Equation 1. The deduced kobs values plotted versus Trx concentration (■) were then fitted against Equation 2, which gave kobs, max and KS values of 105 ± 7 s−1 and 210 ± 30 μm (solid line). Inset, typical time course obtained for 250 μm Trx.
The kinetics of reduction of oxidized Cys48/Cys84 Srx were also determined under steady-state conditions in the presence of NTR from E. coli. However, due to the concentration of NADPH used in the enzymatic coupled assay, which was limited by the absorbance at 340 nm, the concentrations of Trx used were not sufficiently high to be completely saturating. At the highest possible concentration of 125 μm Trx tested, the kobs value measured was of 2.0 s−1, which is 20-fold lower than the rate measured under single turnover conditions. Accordingly, the second-order rate constant k2, which is the slope of the kobs rate constant plotted against the Trx concentration, is 30-fold lower than the corresponding k2 value determined under single turnover conditions (curves not shown). Together, this supports an overall rate-limiting step of the Trx reduction occurring after the two-electron chemical process and is likely associated with the release of oxidized Trx from the binary complex with reduced Srx. Anyway, the overall rate of reduction of Cys48/Cys84 Srx by Trx under steady-state conditions remains largely higher than the following: 1) the overall rate measured for the Srx-catalyzed PrxSO2 reduction under steady-state conditions in the presence of Trx, by at least a factor of 60 (2 s−1 versus 2 min−1), and 2) the rate of reduction of the thiosulfinate intermediate by Trx, measured using C48S and C48A/C106A Srxs, by at least a factor of 600 (2 s−1 versus 0.2 min−1).
Kinetic Identification of the Cys Residue of Oxidized Srx Targeted by Trx
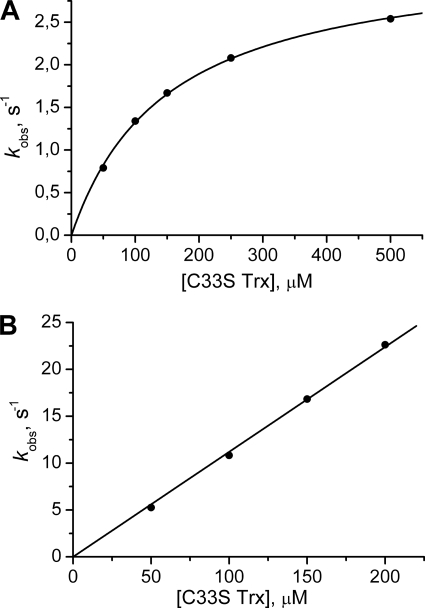
To identify which Cys in oxidized Cys48/Cys84 Srx is targeted by S. cerevisiae Trx1, we prepared two forms of Srxs activated as mixed disulfide either on Cys48 or Cys84 with 2-thiopyridine (referred to as SrxC48(2-thiopyridine) and SrxC84(2-thiopyridine)), by reacting C48A/C106A Srx or C84A/C106A Srx with excess of 2PDS. Released pyridine 2-thiolate, excess of 2PDS, and a minor fraction of Srx dimer formed were then removed by gel filtration. The C33S variant was used to avoid any nonspecific reaction of the regeneration Cys of Trx1. As shown in Fig. 6B, the rate of the reaction between the activated Srxs and C33S Trx1 depended linearly on C33S Trx concentration up to 500 μm for the SrxC48(2-thiopyridine), although a saturation effect was observed for SrxC84(2-thiopyridine). Second-order rate constants of 1.16·105 and 2.2·104 m−1·s−1 were deduced from the slope or from the kobs, max/KS ratio, respectively, for Srx activated on Cys48 and Cys84.
FIGURE 6.
Kinetics of reaction of S. cerevisiae Trx1 C33S with Srx activated by 2-thiopyridine. The reaction of 10 μm SrxC84(2-thiopyridine) (A) and SrxC48 (2-thiopyridine) (B) with variable concentrations of S. cerevisiae Trx1 C33S was monitored on a rapid kinetics spectrophotometer by the release of pyridine 2-thiolate at 343 nm. Reactions were carried out in buffer TK at 30 °C. Kinetic data (not shown) were fitted against Equation 1. The deduced kobs values were plotted versus Trx concentration (●). A, data were fitted against Equation 2, which gave kobs, max and KS constants of 3.3 s−1 and 151 μm (solid line). B, data were fitted to a linear relationship (solid line) with a slope corresponding to a k2 value of 1.16·105 m−1·s−1.
DISCUSSION
In a previous study on the Srx catalytic mechanism (17), we addressed the question of the catalysis of the first steps of the reaction and established the formation of the thiosulfinate PrxSO-SSrx species involving the catalytic Cys residues of each partner, as a catalytically competent intermediate. Because of the reactivity of this species toward thiol groups resulting in several by-products, accumulation and observation of the thiosulfinate species were possible using variants of both Prx and Srx retaining only the catalytic Cys residues and under experimental conditions devoid of any external reductant. When wild-type Srx was used, one product of the reaction with PrxSO2 and ATP was a monomeric form of Srx with an intramolecular disulfide bond, as shown by high resolution mass spectrometry analysis coupled to reverse phase liquid chromatography and by SDS-PAGE (17). Indeed, this species can be resolved by SDS-PAGE as a band migrating slightly faster than reduced Srx and by reverse phase chromatography (Fig. 3 and Fig. 4A). To address the question of the relevance of this species in catalysis, further characterization was carried out by SDS-PAGE using the mutated C48S and C106A Srxs. As shown in Fig. 3, in the absence of external reductant, a band migrating slightly faster than the reduced Srx was also observed but only with C106A Srx. This shows that a disulfide bond between Cys48 and Cys84 is specifically formed in Srx after reaction with PrxSO2 and ATP.
The question now arises regarding which of the two species, i.e. the oxidized Cys48/Cys84 Srx or the thiosulfinate species, is the catalytically competent intermediate along the Trx-recycling process. In a first approach, the catalytic competency of oxidized Cys48/Cys84 Srx was investigated by comparing the rate constant of formation of this species to the steady-state rate constant. A value of 1.8 ± 0.3 min−1 was measured for the production of this species during the reaction catalyzed by Srx in the absence of added reductant, equal to the rate of formation of the thiosulfinate intermediate. Therefore, it can be concluded that in the absence of thiol reductant Cys48 of Srx attacks Cys84 within the thiosulfinate intermediate at a rate that is limited by the rate of formation of the thiosulfinate intermediate. In other words, the thiosulfinate species does not accumulate, and as soon as it is formed, it is transformed into PrxSOH and oxidized Cys48/Cys84 Srx. The oxidized Cys48/Cys84 Srx could thus potentially behave as a catalytic intermediate in the S. cerevisiae Srx reaction.
Furthermore, comparison of the steady-state rate constants of the Srx-catalyzed reaction in the presence of DTT or Trx shows that, in contrast to the reaction using DTT as a reductant, reduction by Trx appears to be rate-limiting for the C48S and C48A/C106A Srxs, unlike for the wild-type and the C106A Srxs. Indeed, in the presence of Trx, the kcat value for the C48S and C48A/C106A Srxs is ∼0.2 min−1, which is 10-fold lower compared with the wild type. These results show that the target of Trx in the catalytic cycle of the wild-type Srx is the oxidized Cys48/Cys84 Srx and not the thiosulfinate intermediate. Indeed, assuming the latter hypothesis, the value of the overall rate for the wild-type Srx should have been 0.2 and not 2 min−1.
The fact that the rate of the recycling of the wild-type Srx activity in the presence of Trx was not limiting suggested formation of an efficient binary complex between the oxidized Cys48/Cys84 Srx and Trx. To prove that, and because the rate-limiting step in the wild type precedes the Trx-recycling process and thus prevents access to the kinetic parameters of the Trx-recycling steps, the oxidized Srx species was prepared by oxidation of the C106A Srx by H2O2. The kinetic parameters of its reduction by Trx were determined. The apparent affinity constants for S. cerevisiae and E. coli Trx1 determined under single turnover conditions are 56 and 210 μm, respectively, which is indicative of specific recognition interactions between oxidized Cys48/Cys84 Srx and Trx. Consequently, the reduction of oxidized Srx by Trx is highly efficient, with a second-order rate constant of 5·105 m−1·s−1. Moreover, the overall steady-state rate of reduction of oxidized Cys48/Cys84 Srx by E. coli Trx is largely higher than the overall rate of wild-type Srx-catalyzed reaction measured under steady-state conditions in the presence of Trx. A possible role of the glutathione/glutaredoxin1 from E. coli (GSH/Grx) was considered by comparing the rate of reduction of the oxidized Srx species by Trx and GSH/Grx measured in steady-state conditions (i.e. in the presence of excess NADPH and Trx or glutathione reductase, respectively). A 20-fold higher value was obtained for Trx (100 μm) than for GSH/Grx (5 mm/100 μm) at pH 7. This result, combined with the high second-order rate constant for the reduction of oxidized Srx by Trx (5·105 m−1·s−1), supports Trx as being the preferred cellular reductant of Srx in S. cerevisiae. This conclusion is also supported by results obtained in vivo using S. cerevisiae strains impaired in the Trx system, which show significantly modified oxidation profiles of Srx compared with the wild-type strain, although similar profiles are observed using a strain producing 100-fold less GSH than the wild type (23).
This conclusion is fully consistent with the rates of reduction of the thiosulfinate intermediate by Trx measured using C48S and C48A/C106A Srxs, which are largely lower than the corresponding value for oxidized Cys48/Cys84 Srx by a factor of 600, and definitely exclude the thiosulfinate species as a target of Trx in S. cerevisiae. In addition, the fact that Trx attacks SrxC48(2-thiopyridine) more efficiently than SrxC84(2-thiopyridine) supports an attack of Trx on Cys48 within the Srx Cys48/Cys84 disulfide bond. This is another piece of evidence, although indirect, that the thiosulfinate species, which is formed between Prx catalytic Cys and Srx Cys84, is not the specific target of Trx in the Srx mechanism.
Sequence alignments of Srx from various origins show that Cys48 is only present in some yeasts related to S. cerevisiae and is included within an extra sequence of 18 amino acids. The fact that the formation of the disulfide bond between Cys48 and Cys84 is rate-limited by the rate of formation of the thiosulfinate species supports an efficient formation of the Cys48/Cys84 disulfide bond. As the Cys84 is located at the N terminus of an α-helix, this suggests a great flexibility of the extra sequence containing Cys48. In this regard, the determination of the three-dimensional structure of Srx from S. cerevisiae, which is currently in progress, will be informative.
The lack of residue corresponding to Cys48 in the mammalian enzymes and the fact that the catalytic Cys84 is the only Cys residue in the human Srx, for example, imply that Srxs from mammalian sources operate via a catalytic recycling mechanism distinct from that of Srx from S. cerevisiae. Because an intramolecular disulfide bond cannot be formed, the recycling process of the mammalian Srxs should occur by a direct attack of the thiol reductant on the Srx Cys84 of the thiosulfinate intermediate. Consequently, this raises the question of the nature of the physiological reductant of Srx in mammalian cells. The fact that 1) the rate of reduction by Trx of the thiosulfinate species in C48S Srx from S. cerevisiae is very low, i.e. 0.2 min−1, and becomes rate-limiting compared with the wild type and 2) that the rate of reduction by Trx of the thiosulfinate species in human/mouse Srx is also very low and in the same range (24, 25)4 and is rate-limiting whereas that of formation of the thiosulfinate species is close to 2 min−1 (19)4 argues against a role of Trx in recycling the Srx activity in mammals. This is in accord with the data reported by Bondareva et al. (26) who observed that the cellular response to thioredoxin reductase gene disruption differs between yeast and mouse cells, which induce Prx versus Srx mRNA, respectively. As mentioned above, inactivation of the Trx-recycling system in yeast probably impairs Srx function, which in return would be compensated by induction of Prx expression. This is also in agreement with the fact that Trx likely recycles the activity of the Srx from S. cerevisiae via an attack on Cys48, which is not present in mammalian Srxs, and not on Cys84. Therefore, other reductants like e.g. glutathione have to be involved. With this type of reductant, the recycling of Srx activity will also occur through release of PrxOH and Srx, as a covalent SrxC84-reductant mixed disulfide species that then has to be reduced to release Srx under reduced form. Preliminary experiments using the S. cerevisiae C48S Srx as a model for mammalian Srx showed that in the presence of GSH (5 mm), Grx1 (100 μm), and its recycling system glutathione reductase/NADPH, the steady-state rate constant of the C48S Srx-catalyzed reaction was 0.5 min−1. Therefore, the GSH/Grx system might reduce the thiosulfinate intermediate more efficiently than Trx, although at a rate that would be limiting for the overall reaction. This could also explain why expression of C48S Srx in ΔSrx S. cerevisiae cells only results in a minor effect on the growth phenotype after cell treatment with H2O2 (23).
Conclusion
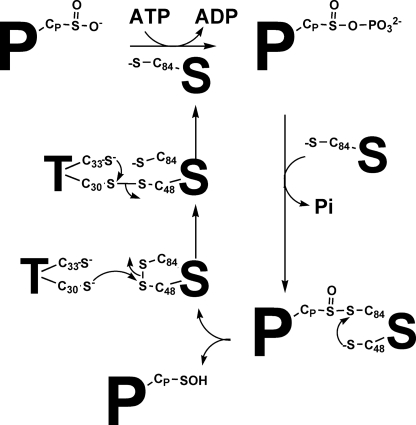
Based on the present and previous studies, we propose the following scenario for the S. cerevisiae Srx catalytic mechanism (Fig. 7): the first two steps of the reaction, which consist of phosphate transfer on PrxSO2 and of formation of the PrxSO-SSrx thiosulfinate intermediate, proceed at a limiting rate of 1.8 min−1.5 Then, an efficient recycling process, at a rate that is limited by the rate leading to formation of the thiosulfinate species, occurs as follows: 1) release of Prx under the sulfenic acid state and of oxidized Cys48/Cys84 Srx followed by 2) formation of a complex between Trx and oxidized Cys48/Cys84 Srx and reduction of Srx by Trx via an attack of the catalytic Cys30 of Trx on the Cys48 of the disulfide bond Cys48/Cys84. Such a mechanism implies the interplay of at least two protein partners with Srx in S. cerevisiae and raises the question of the dynamics that must take place within Srx upon interaction with its substrate PrxSO2 and its reductant Trx. The fact that Trx is not able to reduce efficiently the thiosulfinate intermediate from S. cerevisiae and mammalian sources argues in favor of the involvement of a distinct reductant in the recycling process of mammalian Srxs.
FIGURE 7.
Proposed catalytic mechanism of Srx from S. cerevisiae. The recycling mechanism involves the attack of the Cys48 of Srx on the sulfur atom of Cys84 of the thiosulfinate intermediate, leading to the release of the oxidized Cys48/Cys84 Srx with intramolecular disulfide bond and of the sulfenic acid PrxSOH product. The Cys48/Cys84 Srx is then reduced by attack of the Trx catalytic Cys on Cys48 to form a Trx-Srx mixed disulfide, followed by regeneration of reduced Srx by attack of the recycling Cys of Trx. P, Prx; S, Srx; CP, peroxidatic Cys; C84, Srx catalytic Cys; C48, Srx regeneration Cys; T, thioredoxin.
Acknowledgments
We gratefully thank S. Boukhenouna for excellent assistance and Professor S. Boschi-Muller, Dr. A. Gruez, and Dr. H. Mazon, for helpful discussions.
This work was supported in part by the CNRS, the University of Nancy I, the Institut Fédératif de Recherche 111 Bioingénierie, and by the French Agence Nationale de la Recherche Program ANR-06-BLAN-0369.
X. Roussel, unpublished data.
The rate-limiting step could be associated with formation of the sulfinylphosphoryl anhydride intermediate or to that of the thiosulfinate intermediate or to any conformational change of the Srx associated with these steps.
- Prx
- typical two-cysteine peroxiredoxin
- Cys48/Cys84 Srx
- oxidized Srx with Cys48/Cys84 intramolecular disulfide bond
- DTT
- 1,4-dithiothreitol
- NTR
- E. coli NADPH thioredoxin reductase
- PrxSO2
- overoxidized S. cerevisiae His-tagged peroxiredoxin Tsa1
- Srx
- sulfiredoxin
- Trx
- thioredoxin
- MES
- 4-morpholineethanesulfonic acid
- 2PDS
- 2-pyridyl disulfide.
REFERENCES
- 1.Reddie K. G., Carroll K. S. (2008) Curr. Opin. Chem. Biol. 12, 746–754 [DOI] [PubMed] [Google Scholar]
- 2.Kang S. W., Baines I. C., Rhee S. G. (1998) J. Biol. Chem. 273, 6303–6311 [DOI] [PubMed] [Google Scholar]
- 3.Kong W., Shiota S., Shi Y., Nakayama H., Nakayama K. (2000) Biochem. J. 351, 107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker L. M., Poole L. B. (2003) J. Biol. Chem. 278, 9203–9211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood Z. A., Schröder E., Robin Harris J., Poole L. B. (2003) Trends Biochem. Sci. 28, 32–40 [DOI] [PubMed] [Google Scholar]
- 6.Delaunay A., Pflieger D., Barrault M. B., Vinh J., Toledano M. B. (2002) Cell 111, 471–481 [DOI] [PubMed] [Google Scholar]
- 7.Toledano M. B., Delaunay A., Monceau L., Tacnet F. (2004) Trends Biochem. Sci. 29, 351–357 [DOI] [PubMed] [Google Scholar]
- 8.Jang H. H., Lee K. O., Chi Y. H., Jung B. G., Park S. K., Park J. H., Lee J. R., Lee S. S., Moon J. C., Yun J. W., Choi Y. O., Kim W. Y., Kang J. S., Cheong G. W., Yun D. J., Rhee S. G., Cho M. J., Lee S. Y. (2004) Cell 117, 625–635 [DOI] [PubMed] [Google Scholar]
- 9.Lim J. C., Choi H. I., Park Y. S., Nam H. W., Woo H. A., Kwon K. S., Kim Y. S., Rhee S. G., Kim K., Chae H. Z. (2008) J. Biol. Chem. 283, 28873–28880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood Z. A., Poole L. B., Karplus P. A. (2003) Science 300, 650–653 [DOI] [PubMed] [Google Scholar]
- 11.Aran M., Caporaletti D., Senn A. M., Tellez de Iñon M. T., Girotti M. R., Llera A. S., Wolosiuk R. A. (2008) FEBS J. 275, 1450–1463 [DOI] [PubMed] [Google Scholar]
- 12.Seo J. H., Lim J. C., Lee D. Y., Kim K. S., Piszczek G., Nam H. W., Kim Y. S., Ahn T., Yun C. H., Kim K., Chock P. B., Chae H. Z. (2009) J. Biol. Chem. 284, 13455–13465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biteau B., Labarre J., Toledano M. B. (2003) Nature 425, 980–984 [DOI] [PubMed] [Google Scholar]
- 14.Vivancos A. P., Castillo E. A., Biteau B., Nicot C., Ayté J., Toledano M. B., Hidalgo E. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 8875–8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozonet S. M., Findlay V. J., Day A. M., Cameron J., Veal E. A., Morgan B. A. (2005) J. Biol. Chem. 280, 23319–23327 [DOI] [PubMed] [Google Scholar]
- 16.Woo H. A., Bae S. H., Park S., Rhee S. G. (2009) Antioxid. Redox Signal. 11, 739–745 [DOI] [PubMed] [Google Scholar]
- 17.Roussel X., Béchade G., Kriznik A., Van Dorsselaer A., Sanglier-Cianferani S., Branlant G., Rahuel-Clermont S. (2008) J. Biol. Chem. 283, 22371–22382 [DOI] [PubMed] [Google Scholar]
- 18.Jönsson T. J., Murray M. S., Johnson L. C., Lowther W. T. (2008) J. Biol. Chem. 283, 23846–23851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jönsson T. J., Tsang A. W., Lowther W. T., Furdui C. M. (2008) J. Biol. Chem. 283, 22890–22894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mössner E., Huber-Wunderlich M., Glockshuber R. (1998) Protein Sci. 7, 1233–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulrooney S. B., Williams C. H., Jr. (1997) Protein Sci. 6, 2188–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olry A., Boschi-Muller S., Branlant G. (2004) Biochemistry 43, 11616–11622 [DOI] [PubMed] [Google Scholar]
- 23.Biteau B. (2005) A Sulfirédoxine, une Nouvelle Enzyme Illustrant les Deux Facettes Biologiques de l'H2O2: Toxicite et Signalisation Ph.D. thesis, University of Paris-Sud, France [Google Scholar]
- 24.Chang T. S., Jeong W., Woo H. A., Lee S. M., Park S., Rhee S. G. (2004) J. Biol. Chem. 279, 50994–51001 [DOI] [PubMed] [Google Scholar]
- 25.Jeong W., Park S. J., Chang T. S., Lee D. Y., Rhee S. G. (2006) J. Biol. Chem. 281, 14400–14407 [DOI] [PubMed] [Google Scholar]
- 26.Bondareva A. A., Capecchi M. R., Iverson S. V., Li Y., Lopez N. I., Lucas O., Merrill G. F., Prigge J. R., Siders A. M., Wakamiya M., Wallin S. L., Schmidt E. E. (2007) Free Radic. Biol. Med. 43, 911–923 [DOI] [PMC free article] [PubMed] [Google Scholar]