Abstract
MicroRNAs (miRs) participate in most cellular functions by posttranscriptional regulation of gene expression albeit with little information regarding their role in ischemic preconditioning (IP) of stem cells. We report that IP of bone marrow-derived mesenchymal stem cells (MSCs) with two cycles of 30-min ischemia/reoxygenation (I/R) supported their survival under subsequent longer exposure to anoxia and following engraftment in the infarcted heart. IP significantly reduced apoptosis in MSCs through activation of Akt (Ser473) and ERK1/2 (Thr202/Tyr204) and nuclear translocation of hypoxia-inducible factor-1α (HIF-1α). We observed concomitant induction of miR-210 in the preconditioned MSCs (PCMSCs). Inhibition of HIF-1α or of miR-210 abrogated the cytoprotective effects of preconditioning. Extrapolation of these data to in vivo studies in a rat model of acute myocardial infarction predominantly improved stem cell survival after engraftment with a role for miR-210. Notably, multiple I/R cycles more effectively regulated the miR-210 and hence promoted MSC survival compared with single-cycle hypoxia of an equal duration. Real time PCR array for rat apoptotic genes, computational target gene analyses, and luciferase reporter assay identified FLICE-associated huge protein (FLASH)/caspase-8-associated protein-2 (Casp8ap2) in PCMSCs as the target gene of miR-210. Induction of FLASH/CASP8AP2 in miR-210 knocked-down PCMSCs resulted in increased cell apoptosis. Taken together, these data demonstrated that cytoprotection afforded by IP was regulated by miR-210 induction via FLASH/Casp8ap2 suppression. These results highlighted that IP by multiple short episodes of I/R is a novel strategy to promote stem cell survival.
introduction
MicroRNAs (miRs)2 are single-stranded noncoding RNAs of 19–23 nucleotides that mechanistically participate in a variety of cellular functions (1, 2). Recent studies have successfully established a functional link between a discrete group of hypoxia-regulated miRs and cell survival signaling (i.e. miR-23, -24, -26, -27, -103, -107, -181, -210, and -213) (3–5). Hypoxia-regulated miRs are induced under low oxygen tension with a critical participation of the hypoxia-inducible factor-1α (HIF-1α) (6). Nevertheless, most studies have focused on cancer cells as a model to study the mechanistic participation of hypoxia-regulated miRs in their endeavor to escape death in the ischemic microenvironment of tumors. Fasanaro et al. (7) have shown recently that miR-210 was a crucial element of endothelial cell functioning in response to hypoxia and considerably influenced their migration, capillary network formation, and differentiation capability. Induction of miR-210 occurred as early as 4 h after exposure to hypoxia and persisted for 24 h above base-line levels. Notably, miR-210 inhibition in their experiments was associated with an increase in apoptosis of endothelial cells. Stem cells have a different biology than cancer cells and may differ in their response to hypoxia. Moreover, little is known about the role of hypoxia-regulated miRs during ischemic preconditioning (IP) of stem cells by multiple short episodes of ischemia/reoxygenation (I/R).
IP is one of the most powerful cytoprotective stimuli (8, 9). The classical methodology of IP was modified for preconditioning of stem cells and involved their single and long term exposure to hypoxia to elevate their survival (10). On the contrary, the effectiveness of intermittent hypoxia in cellular protection was shown to be a stimulus-dependent transcriptional activity of HIF-1α (11). Despite differences in the transcriptional activity of HIF-1α, these studies elucidated a pivotal role for HIF-1α in cytoprotection (12). We hypothesized that the cytoprotective effects of multiple cycles of brief I/R involved hypoxia-regulated miRs expression downstream of HIF-1α. In this study, we showed that IP-induced miR-210 plays a key role in bone marrow-derived mesenchymal stem cell (MSC) survival, and we identify caspase-8-associated protein-2 (Casp8ap2) as a functional target of miR-210. In accordance with our in vitro data, we also demonstrated that preconditioned MSCs (PCMSCs) survived better compared with nonpreconditioned MSCs (non-PCMSCs), and two cycles of I/R were more effective in cytoprotection compared with one cycle of I/R.
EXPERIMENTAL PROCEDURES
Isolation and Culture of MSCs
Bone marrow-derived MSCs were purified from young male Fischer-344 rats by flushing the cavity of femurs as described previously (13).
Preconditioning of MSCs
Native MSCs were seeded at 5 × 105 cells/60-mm cell culture dish. The cells were starved overnight for serum and glucose before preconditioning. For IP, the cells were subjected to repeated cycles of anoxia (10 or 30 min) with intermittent reoxygenation (10 min) for specific cycles (one to three cycles) in an anoxic chamber (Forma-1025 Anaerobic System). At the end of the required number of cycles, the cells were either harvested for molecular studies or were subjected to anoxia for 6 h for survival studies in vitro. Cell survival was assessed by lactate dehydrogenase (LDH) release assay using conditioned medium and transferase-mediated dUTP nick-end labeling (TUNEL) of the cells from each treatment group.
LDH Release Assay
Intracellular LDH leakage was measured using an LDH Assay Kit (Diagnostic Chemicals) according to the instructions of the manufacturer.
miR Isolation and Detection
Extraction of miRs was carried out using a mirVanaTM miR isolation kit and detected by using a mirVanaTM quantitative reverse transcription (qRT)-PCR miRNA detection kit (Ambion). Specific miRNA primers from Ambion were used during qRT-PCR according to the instructions of the manufacturer.
siRNA Transfection
Predesigned HIF-1α siRNA and scramble siRNA (Sc) were purchased from Santa Cruz Biotechnology, and Casp8ap2 siRNA was from Ambion. Transient transfection of siRNA was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocols. All transfections were carried out in triplicate.
Western Blot Analysis
MSCs were lysed in lysis buffer, pH 7.4 (50 mm HEPES, 5 mm EDTA, 50 mm NaCl), 1% Triton X-100, protease inhibitors (10 μg/ml aprotinin, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin), and phosphatase inhibitors (50 mm sodium fluoride, 1 mm sodium orthovanadate, 10 mm sodium pyrophosphate). Nuclear and cytoplasmic fractionation of MSCs was performed using an NE-PER Nuclear and Cytoplasmic Extraction Reagent kit (Pierce) according to the manufacturer's protocol. The protein samples (40 μg) were electrophoresed using SDS-polyacrylamide gel and electroimmunoblotted as described (14). The specific antibodies used for the detection of antigens of interest are listed in supplemental Table I.
TUNEL Assay
Apoptosis was detected by TUNEL according to instructions of the manufacturer (Roche Applied Science). For quantification, the numbers of TUNEL+ cells were counted in at least five randomly selected high power fields (magnification, ×200) with three independent samples.
Transfection with miR Inhibitor
To knock down miR-210, transfection was performed with anti-miR-210 (miR inhibitor; Ambion) and siPORTTM NeoFxTM transfection agent (NeoFx; Ambion) according to the instructions of the manufacturer (6). Briefly, 5 μl of NeoFx was diluted with 100 μl of Opti-MEM for each sample and incubated for 10 min at room temperature. Then, we diluted anti-miR inhibitor with 100 μl of Opti-MEM at the final concentration of 30 nm and added it to the diluted NeoFx mixture. After incubation for another 10 min, the transfection mixture was added to 2.3 ml of cell suspension (3 × 105 cells). Cell suspension containing transfection mixture was plated into a 6-well plate, and the assay was performed at 72 h after incubation.
Luciferase Reporter Assay
Precursor miR-210 expression clone was constructed in a feline immunodeficiency virus-based lentiviral vector system (pEZX-miR-210) and luciferase reporter construct containing the 3′-UTR of Casp8ap2 were designed to encompass the miR-210 binding sites (GeneCopoeia, Rockville, MD). For transfection, MSCs were plated in triplicate into 24-well plates and cotransfected with 0.8 μg of pEZX-miR-210 (or pEZX-miR-scramble) and reporter construct by using the Lipofectamine 2000TM (Invitrogen). Transfection efficiency was normalized on the basis of Renilla luciferase activity. Firefly and Renilla luciferase activities were measured by using the Dual Luciferase Reporter Assay System kit (Promega), according to the manufacturer's instructions.
Fluorescence Immunocytochemistry
Cells were fixed in 4% paraformaldehyde, permeabilized in 0.05% Triton X-100, and rinsed sequentially in PBS, 50 mm NH4Cl, and PBS. The cells were then incubated with a blocking buffer (3% bovine serum albumin in PBS) and subsequently with FLASH antibody (M-300, Santa Cruz Biotechnology), followed by a rinse in PBS and bovine serum albumin before being incubated with the appropriate species-specific secondary antibodies conjugated to Alexa Fluor 546. Nuclei were counterstained with DAPI. Cells were examined by fluorescence microscope (BX41; Olympus, Japan).
In Vivo Survival of the Transplanted PCMSCs
In vivo experiments were performed in a rat heart model of acute myocardial infarction using young female Fischer-344 rats as described earlier (14). Briefly, rats were anesthetized by intraperitoneal injection of ketamine/xylazine (24 mg/kg and 4 mg/kg body weight, respectively). After endotracheal intubation and mechanical ventilation using Harvard Apparatus Rodent Ventilator (model 683), the heart was exposed by left-sided minimal thoracotomy. The left anterior descending coronary artery was permanently ligated with Prolene 6.0 sutures. Immediately after ligation (n = 4 animals/group), 70 μl of basal Dulbecco's modified Eagle's medium without cells (group 1) or containing 1 × 106 non-PCMSCs (group 2) or PCMSCs (group 3, IP one cycle, 30 min; and group 4, IP two cycles, 30 min) were injected intramyocardially at multiple sites in and around the infarct zone. The chest was closed, and the animals were allowed to recover. During postoperative care, Buprenex (0.1 mg/kg b.i.d) was administered for 24 h to alleviate pain. For postmortem studies, the animals were killed using an overdose of sodium pentobarbitol 4 days after cell engraftment. The heart tissue was collected, washed extensively with PBS to remove blood traces, and used for molecular studies. Our study conformed to the NIH Guide for the Care and Use of Laboratory Animals and protocol approved by the Institutional Animal Care and Use Committee, University of Cincinnati.
PCR
The cell and myocardial specimens from various treatment groups of animals on day 4 after cell transplantation were used for molecular studies by PCR (15). For myocardial tissue, the specimens were snap-frozen and powdered. DNA purification was performed using Genomic DNA Isolation kit (Qiagen), and the concentration of the purified DNA was determined by spectrophotometry. The primer sequences for sry gene and actin used for PCR were: sry gene, forward 5′-GAGGCACAAGTTGGCTCAACA-3′ and reverse 5′-CTCCTGCAAAAAGGGCCTTT-3′; actin, forward 5′-AGCCATGTACGTAGCCATCC-3′ and reverse 5′- CTCTCAGCTGTGGTGGTGAA-3′.
Real-time PCR-based 84-gene array RT ProfilerTM PCR Array (SA Biosciences) for rat apoptosis genes and qPCR for Casp8ap2 using primers purchased from SA Biosciences were performed according to the instructions of the manufacturer.
All values are expressed as mean ± S.E. Comparison between two mean values was evaluated by an unpaired Student's two-tailed t test and between three or more groups was evaluated by one-way analysis of variance followed by Bonferroni's post hoc analysis. Statistical significance was accepted at p < 0.05.
RESULTS
Brief Episodes of I/R PCMSCs
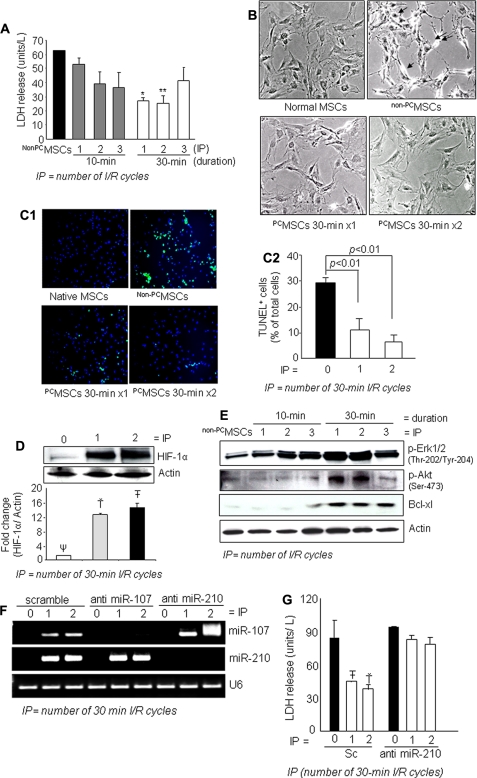
MSCs preconditioned by treatment with multiple I/R cycles of 10 or 30 min (one to three cycles) showed a significant level of protection upon subsequent exposure to 6 h of anoxia. LDH release (an indicator of cellular injury) was significantly reduced in PCMSCs both in 10-min and 30-min treatment compared with non-PCMSCs (Fig. 1A). Interestingly, two cycles of I/R were more cytoprotective compared with single hypoxic exposure. More so, two cycles of I/R were also more cytoprotective compared with three cycles of hypoxic exposure (Fig. 1A). Phase contrast microscopy revealed hypercontracted morphology of non-PCMSCs whereas IP for 30 min (one or two cycles) prevented these morphological changes (Fig. 1B). The number of TUNEL+ cells was markedly reduced in PCMSCs with two cycles of I/R under 6 h of anoxia (Fig. 1, C1 and C2), which was consistent with the LDH release data.
FIGURE 1.
A, IP attenuated cellular injury as examined by LDH release subsequent to 6 h of lethal anoxia. IP with three 30-min cycles was less effective than IP using one 30-min cycle and two 30-min cycles (* and ** versus all other groups of cells, p < 0.05). B, morphological changes of MSCs by 6 h of anoxia included rounding off and shrinkage of cells, which was prevented by IP (magnification, ×200). C1 and C2, representative fluorescence images and quantitative analysis of TUNEL positivity showing decreased number of TUNEL+ cells in PCMSCs (p < 0.01 versus non-PCMSCs) (magnification, ×200; green, TUNEL+ nuclei; blue, DAPI). D, Western blot showing higher HIF-1α in nuclear fraction (two cycles of 30-min I/R) compared with PCMSCs (one cycle of 30-min I/R) and non-PCMSCs. (Ť and T̄ vs. Ψ, p < 0.05). E, Western blot showing higher activation of ERK1/2, Akt, and Bcl.xL in PCMSCs (two cycles of 30-min I/R) compared with PCMSCs (one cycle of 30-min I/R) and non-PCMSCs. F, RT-PCR showing successful and specific knockdown of endogenous miR-210 and miR-107 by transfection with antisense molecules specific for respective miRs. Knock down of miR-107 was used as a control to show the specificity antisense interference. G, abrogation of miR-210 led to loss of cytoprotection by IP in MSCs as determined by LDH release assay. Abrogation of miR-210 completely abolished IP-induced cytoprotection (T̄ and Ť versus all other groups of cells, p < 0.05).
We also observed increased nuclear translocation of HIF-1α in PCMSC (Fig. 1D). A higher phosphorylation of Akt (Ser473) and ERK1/2 (Thr202/Tyr204) together with antiapoptotic protein Bcl.xL was observed in PCMSC (Fig. 1E and supplemental Fig. IA). Again, IP with three cycles of 30-min I/R was less effective for these molecular changes and therefore was found to be less efficient in terms of cytoprotection thereby suggesting that too many cycles of I/R were detrimental for cells (supplemental Fig. IB). Pretreatment of PCMSCs with phosphoinositide 3-kinase/Akt inhibitor (wortmannin) effectively blocked these molecular events with a concomitant reduction in cell survival upon exposure to 6 h of anoxia (supplemental Fig. I, C–E).
Induction of miR-210 during IP and Its Role in Cytoprotection
We observed marked elevation of miR-210 in PCMSCs compared with non-PCMSCs (supplemental Fig. IF). To study the mechanistic participation of miR-210 in cytoprotection, miR-210 expression was successfully abrogated in the PCMSCs by using miR-210-specific inhibitor (Fig. 1F). Using miR-107 inhibitor as a control, we observed that inhibitors of miR-107 and miR-210 specifically abrogated their respective target without showing any nonspecific interference (Fig. 1F). We observed that abrogation of miR-210 resulted in significantly higher apoptosis of these cells under 6 h of anoxia compared with controls (Fig. 1G and supplemental Fig. IIA). Pretreatment of MSCs with 50 μm wortmannin reduced miR-210 expression in PCMSCs (supplemental Fig. IIB).
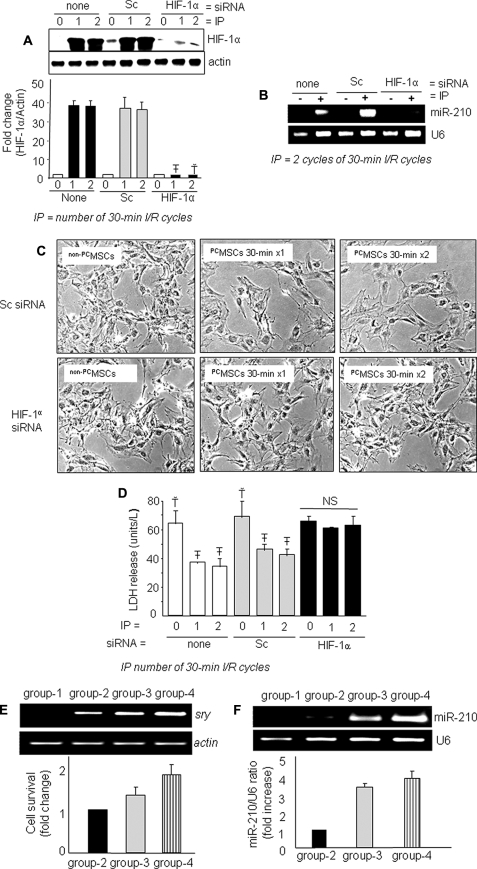
Next, we investigated a link between up-regulated expression of HIF-1α and miR-210 in PCMSCs. We successfully abrogated HIF-1α in MSCs using specific siRNA. A subsequent IP failed to induce HIF-1α in these cells with a concomitant loss of miR-210 induction (Fig. 2, A and B). Moreover, phase contrast microscopy revealed an obvious loss of morphological integrity (Fig. 2C). These molecular events and morphological changes led to an associated decreased survival of PCMSCs upon exposure to 6 h of anoxia compared with the controls (Fig. 2D and supplemental Fig. III). Native MSCs without siRNA transfection and Sc siRNA transfected MSCs were used as controls.
FIGURE 2.
A, Western blot showing successful abrogation of IP-induced HIF-1α in the nuclear fraction of PCMSCs transfected with HIF-1α-specific siRNA (T̄ & Ť versus all other groups, p < 0. 01). B, abrogation of IP-induced miR-210 was observed in PCMSCs transfected with HIF-1α-specific siRNA showing their dependence on HIF-1α. C and D, abrogation of HIF-1α and miR-210 resulted in a loss of morphologic integrity (magnification, ×200) and IP-induced cytoprotection as determined by LDH assay (Ť versus T̄, p < 0.05). NS, not significant. E, PCR for sry gene of male donor cells in the female recipient rat heart on day 4 after cell transplantation. A higher survival of transplanted cells was observed in PCMSC groups 3 and 4 (p < 0.05 versus non-PCMSCs). PCMSC survival was higher in group 4 compared with group 3 (p < 0.05). No sry gene was observed in the Dulbecco's modified Eagle's medium-injected female hearts. F, expression of miR-210 in groups 3 and 4 animal hearts was higher compared with group 2 (p < 0.05).
IP Improved MSC Survival in the Infarcted Heart
The observed in vitro relationship between preconditioning and improved survival of PCMSCs under anoxia was extrapolated in vivo using a female rat heart model of acute myocardial infarction. PCR for sry gene in the infarcted rat heart (n = 4 animals/group) on day 4 showed higher survival of the transplanted cells preconditioned by two 30-min cycles IP in group 4 (p < 0.05 with non-PCMSCs transplanted group 2 and one 30-min cycle PCMSCs in group 3) (Fig. 2E). Dulbecco's modified Eagle's medium-injected female animals hearts (group 1) were negative for sry gene expression. Improved survival of donor cells was accompanied by higher miR-210 levels in groups 3 and 4 (Fig. 2F). Of greater interest was the markedly changed expression level of miR-210 with non-PCMSC engraftment (group-2) compared with group 1, which showed that transplantation of native MSCs altered miR-210 levels in the infarcted heart. Additionally, higher survival of PCMSCs in groups and group 4 (with elevated miR-210) compared with group 2 implies a major contribution of miR-210 in cytoprotection after engraftment in accordance with our in vitro results.
IP Induced miR-210-down-regulated FLASH/Casp8ap2 in PCMSCs
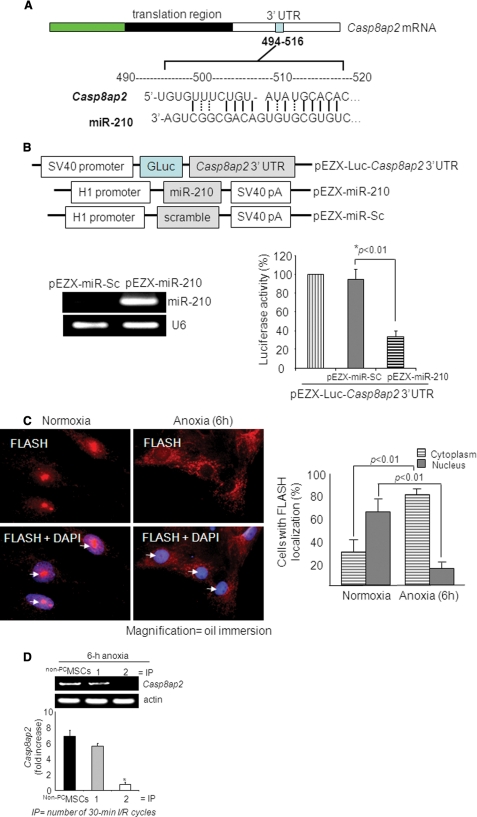
Real time PCR-based array for rat apoptotic genes analysis showed that Casp8ap2, tumor necrosis factor receptor super family-1a, DNA fragmentation factor-β, and lymphotoxin-A had more than 2-fold up-regulated expression in miR-210 knock-down MSCs compared with the control cells (supplemental Table II). To investigate further the biological relevance of miR-210 up-regulation in prosurvival signaling in PCMSCs, we examined three databases (miRANDA, Sanger MirBase, and Targetscan algorithms) to search for the potential targets of miR-210. Our in silico search resulted in a consensus putative target site of miR-210 relevant to apoptosis in the 3′-UTR of Casp8ap2 mRNAs (Fig. 3A). On the contrary, tumor necrosis factor receptor super family-1a, DNA fragmentation factor-β, and lymphotoxin-A showed poor alignment as target genes for miR-210. The role of Casp8ap2 as a target gene of miR-210 was confirmed by luciferase activity assay. Cotransfection of a precursor miR-210 expression vector (pEZX-miR-210) with the vector containing 3′-UTR of the Casp8ap2 gene led to reduced luciferase activity compared with cotransfection with the miR-Sc vector (pEZX-miR-Sc), indicating that forced expression of miR-210 can down-regulate Casp8ap2 expression via targeting the 3′-UTR of this gene (Fig. 3B).
FIGURE 3.
miR-210 targets Casp8ap2, a positive regulator of apoptosis. A, one putative target site of miR-210 highly conserved in the Casp8ap2 mRNA 3′-UTR. B, construction of pEZX-Luc-Casp8ap2 3′-UTR luciferase reporter plasmid and precursor miR-210 expression clone. qRT-PCR showed successful transfection and significantly higher expression of miR-210 in MSCs using pEZX-miR-210 plasmid compared with pEZX-miR-Sc plasmid-transfected MSCs. Cotransfection of MSCs with pEZX-Luc vector containing Casp8ap2 3′-UTR together with a plasmid encoding miR-210 showed decreased luciferase activity (p < 0.01 versus pEZX-miR-Sc-transfected cells). The ratio of luciferase activity was calculated either in the presence or absence of miR-210. C, immunofluorescence staining of MSCs stained for FLASH/Casp8ap2 (red; indicated by white arrows) and its overlay images of nuclei stained with DAPI (blue). Compared with its nuclear localization under normoxia, anoxia translocated FLASH/CASP8AP2 from the nucleus into the cytoplasm as indicated by lack of red fluorescence in the nuclei. The percentage of cells (at ×40) with FLASH/CASP8AP2-positive nuclei was significantly higher in the cells cultured under normoxia (p < 0.01 versus cells treated with anoxia for 6 h). D, RT-PCR showing the effect of preconditioning on Casp8ap2 gene expression in MSCs under 6 h of anoxia. Casp8ap2 gene expression was significantly abolished in MSCs preconditioned by two 30-min cycles of I/R compared with non-PCMSCs and MSCs preconditioned by 1 cycle of I/R (* versus other groups, p < 0.01).
CASP8AP2 in humans is FLASH homolog in mouse and has been identified for interaction with the death-effector domain of caspase-8 with a regulatory role in Fas-mediated apoptosis. Fluorescence immunocytochemistry using FLASH/ CASP8AP2-specific antibody revealed nuclear localization of FLASH/CASP8AP2 in native MSCs cultured under normoxia as was evident from colocalization of red fluorescence with blue DAPI staining (white arrows, Fig. 3C). Nevertheless, 6-h anoxic culture conditions triggered cytoplasmic translocation of FLASH/CASP8AP2 from the nucleus as indicated by the lack of red fluorescence in the nuclei. Moreover, the percentage of FLASH/CASP8AP2-positive cells with nuclear localization was higher (p < 0.01), and cytoplasmic localization was lower (p < 0.01) compared with the cells cultured under 6 h of anoxia. These results clearly indicated that FLASH/CASP8AP2 having nuclear localization in native cells was translocated into the cytoplasm under anoxia. Additionally, we also observed that FLASH/CASP8AP2 showed a concomitant up-regulated expression in non-PCMSCs exposed to anoxia that was abrogated by preconditioning of the cells (Fig. 3D).
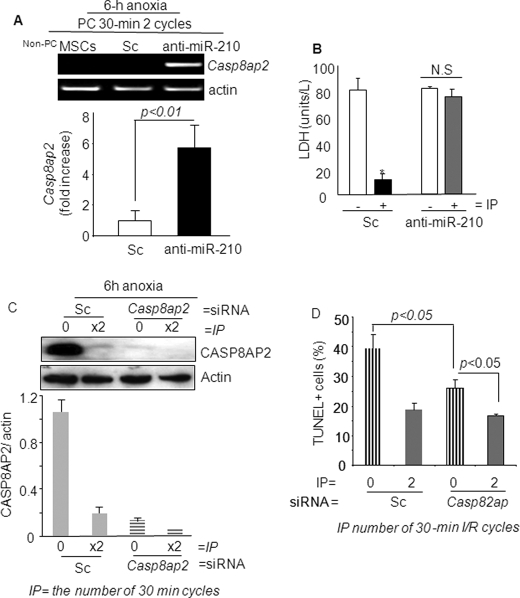
Because CASP8AP2 has a pivotal role in apoptosis, we validated this predicted gene target for its functional involvement in PCMSC survival under anoxia. IP of MSCs with two cycles of I/R was more effective in abrogation FLASH/CASP8AP2 under 6 h of anoxia (two cycles of I/R versus one cycle of I/R; p < 0.01). However, abrogation of miR-210 in PCMSCs significantly increased Casp8ap2 expression as determined by RT-PCR (Fig. 4A) and confirmed by real time PCR (supplemental Fig. IVA). LDH release assay showed that induction of Casp8ap2 was associated with increased death of PCMSCs with miR-210 knock down compared with the Sc siRNA-treated or mock-treated PCMSCs (Fig. 4B). Taken together, these data showed that Casp8ap2 gene induction was regulated downstream of miR-210 in PCMSCs.
FIGURE 4.
A, qRT-PCR for Casp8ap2 gene expression in PCMSCs exposed to 6 h of anoxia. Casp8ap2 gene expression showed a significant increase in miR-210 knock-down cells compared with native (nontransfected) and Sc siRNA-transfected MSCs. B, LDH release was significantly increased in PCMSCs with miR-210 knock-down cells compared with Sc siRNA-transfected MSCs after 6 h of anoxia (*, p < 0.01 versus non-PCMSCs). C, Western blot showing successful abrogation of FLASH/CASP8AP2 in both non-PCMSCs and PCMSCs transfected with Casp8ap2 siRNA compared with Sc siRNA-transfected non-PCMSCs. PCMSCs transfected with Sc siRNA had very low expression of CASP8AP2, thus implying that preconditioning down-regulated Casp8ap2 expression. D, TUNEL-positive MSCs were significantly reduced in PCMSCs and non-PCMSCs transfected with Casp8ap2 siRNA compared with Sc siRNA-transfected cells after 6 h of anoxia.
Casp8ap2-specific RNA Interference Improved Survival in non-PCMSCs
Finally, we investigated the role of Casp8ap2 in survival of PCMSCs and non-PCMSCs under anoxia. Casp8ap2 was successfully abrogated in MSCs by specific RNA interference using Sc siRNA transfection as a control (Fig. 4C). Abrogation of Casp8ap2 significantly improved survival of both PCMSCs and non-PCMSCs under anoxia, although the cytoprotective effects of such molecular event were more pronounced in non-PCMSCs (Fig. 4D and supplemental Fig. IVB). These results clearly showed that FLASH/Casp8ap2 plays an important role in apoptosis as a downstream target of hypoxia-induced miR-210 during IP of MSCs.
DISCUSSION
We have successfully extrapolated the classical concept of IP using multiple episodes of sublethal I/R to precondition MSCs and improve their survival upon longer exposure to anoxia. Moreover, we have also accounted for a mechanistic involvement of HIF-1α and miR-210 in IP-induced cytoprotection of MSCs. We observed that IP of MSCs with multiple cycles of brief I/R episodes was more effective in promoting stem cell survival compared with single exposure to anoxia of equivalent time duration. Moreover, we also observed that the cytoprotective effects of IP under anoxia were mediated by HIF-1α induction and its dependent expression of miR-210.
Various strategies have been adopted to prevent massive death of the donor cells in the infarcted heart after engraftment (16). We have previously reported the effectiveness of our novel strategy of preconditioning of stem cells by treatment with preconditioning mimetics or growth factors that effectively improved stem cell survival (15, 17). We report here that preconditioning of stem cells can be achieved by exposure of the cells to multiple short episodes of intermittent I/R. This involves induction of HIF-1α, which constitutes an integral part of the molecular response of a cell to hypoxia. Moreover, HIF-1α serves as a master regulator to orchestrate the expression of genes, which helps the cells to adapt to low oxygen tension (18). HIF-1α is translocated into the nucleus under hypoxia to form a functional complex (19). With better understanding of gene regulation by transcription factors with the participation of miRs (20), a novel relationship has been established between hypoxia and a select group of miRs of which miR-210 is considered a universal responder irrespective of the cell type (4, 5). Taking a cue from these studies, we elucidated miR-210 as a potent negative regulator of stem cell apoptosis during IP downstream of HIF-1α. As significantly higher levels of HIF-1α and miR-210 were observed in PCMSCs, which incidentally demonstrated better survival under lethal anoxia, abrogation of HIF-1α with specific RNAi abolished the IP-induced cytoprotection with a parallel reduction in miR-210. Likewise, transfection of MSCs with a miR-210-specific inhibitor abolished the IP-induced cytoprotection albeit without associated change in HIF-1α, thus suggesting the importance of miR-210 in antiapoptosis and HIF-1α as a regulator of miR-210 during IP. Engraftment of non-PCMSCs in group 2 insignificantly changed miR-210 compared with Dulbecco's modified Eagle's medium-injected group 1 infarcted hearts. On the contrary, PCMSC-engrafted hearts in groups 3 and 4 had significantly higher levels of miR-210 compared with other groups (highest level in group 4). Interestingly, increased donor cell survival and lower TUNEL positivity were observed in association with miR-210 levels, which clearly demonstrated its critical role in cytoprotection of the transplanted cells. Our results are consistent with the findings that HIF-1α positively regulates miR-210, inhibition of which increased endothelial cell apoptosis (5). Further studies are under way to elucidate whether the cytoprotective effects of IP can be accomplished by direct induction of miR-210. Additionally, it is highly likely the PCMSCs can serve as a source of miR-210 for delivery to the host cardiomyocytes for protection against ischemic injury. Based on our observations, we postulated that native MSCs engrafted in the infarcted heart, in response to the ischemic microenvironment, became a source of hypoxia-regulated miRs for the host myocytes. Previous studies have shown that miRs can translocate between adjacent cells (21). There is a strong possibility that hypoxia-regulated miRs from the transplanted MSCs can translocate into the myocytes to regulate cytoprotective gene expression and serve as one of the mechanisms underlying improved cardiac function after heart cell therapy.
We also observed FLASH/Casp8ap2 as the effector gene that contributed in cytoprotection downstream of miR-210 activation. Our results showed that Casp8ap2 gene expression was elevated in non-PCMSCs upon exposure to anoxia, whereas IP effectively suppressed its gene expression. On the contrary, knock down of miR-210 in PCMSCs predominantly increased Casp8ap2 expression with an associated decline in their survival, thus suggesting Casp8ap2 as one of the major targets of miR-210 during IP of MSCs. CASP8AP2 is an integral member of the apoptosis signaling complex that activates caspase-8 and facilitates Fas-induced apoptosis (22). CASP8AP2 was originally identified as a component of death-inducing signaling complex involved in Fas- and tumor necrosis factor-α-mediated apoptosis and contained a death effector domain-recruiting domain, which interacted with death effector domain of caspase-8 or Fas-associated death domain. Once activated, caspase-8 is released from the complex and participates in the activation of effector caspases including caspases-3, -4, -6, -7, -9, and -10. Recent studies indicate that FLASH, a CASP8AP2 homolog in mice and rat, is a huge multifunctional protein that is involved in S phase progression and transcriptional regulation of cells besides its critical role in apoptosis (23–24). The subcellular distribution of FLASH/CASP8AP2 may therefore be crucial for its functional participation in nonapoptosis- and apoptosis-related signaling pathways (25). In our results, nuclear-localized FLASH/CASP8AP2 was translocated into the cytoplasm subsequent to 6 h of lethal anoxia and was associated with significant loss of cell survival in non-PCMSCs. On the contrary, IP, which abolished Casp8ap2 expression in PCMSCs and also the cells with Casp8ap2 knock down, showed significantly higher survival upon exposure to lethal anoxia, thus suggesting the importance of Casp8ap2 during apoptosis.
In conclusion, because of its safety, ease, and effectiveness in reprogramming of stem cells, the IP approach is innovative to overcome stem cell attrition in the infarcted heart. Moreover, hypoxia-induced miR-210, downstream of HIF-1α is a critical regulator of stem cell survival through FLASH/Casp8ap2 suppression.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants R37-HL074272, HL-080686, and HL087246 (to M. A.) and HL087288 and HL089535 (to H. K. H).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. I–IV and Tables I and II.
- miR
- microRNA
- CASP8AP2
- caspase-8-associated protein-2
- ERK
- extracellular signal-regulated kinase
- HIF-1α
- hypoxia-inducible factor-1α
- IP
- ischemic preconditioning
- I/R
- ischemia/reoxygenation
- LDH
- lactate dehydrogenase
- MSC
- mesenchymal stem cell
- PCMSC
- preconditioned MSC
- non-PCMSC
- nonpreconditioned MSC
- PBS
- phosphate-buffered saline
- Sc
- scramble
- siRNA
- small interfering RNA
- TUNEL
- transferase-mediated dUTP nick-end labeling
- UTR
- untranslated region.
REFERENCES
- 1.Kloosterman W. P., Plasterk R. H. (2006) Dev. Cell 11, 441–450 [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. (2004) Nature 431, 350–355 [DOI] [PubMed] [Google Scholar]
- 3.Foshay K. M., Gallicano G. I. (2007) Curr. Stem Cell Res. Ther. 2, 264–271 [DOI] [PubMed] [Google Scholar]
- 4.Kulshreshtha R., Davuluri R. V., Calin G. A., Ivan M. (2008) Cell Death Differ. 15, 667–671 [DOI] [PubMed] [Google Scholar]
- 5.Kulshreshtha R., Ferracin M., Wojcik S. E., Garzon R., Alder H., Agosto-Perez F. J., Davuluri R., Liu C. G., Croce C. M., Negrini M., Calin G. A., Ivan M. (2007) Mol. Cell. Biol. 27, 1859–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng A. M., Byrom M. W., Shelton J., Ford L. P. (2005) Nucleic Acids Res. 33, 1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasanaro P., D'Alessandra Y., Di Stefano V., Melchionna R., Romani S., Pompilio G., Capogrossi M. C., Martelli F. (2008) J. Biol. Chem. 283, 15878–15883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross E. R., Gross G. J. (2007) Drug Discov. Today Dis. Mech. 4, 165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hausenloy D. J., Yellon D. M. (2006) Cardiovasc. Res. 70, 240–253 [DOI] [PubMed] [Google Scholar]
- 10.Hu X., Yu S. P., Fraser J. L., Lu Z., Ogle M. E., Wang J. A., Wei L. (2008) J. Thorac. Cardiovasc. Surg. 135, 799–808 [DOI] [PubMed] [Google Scholar]
- 11.Yuan G., Adhikary G., McCormick A. A., Holcroft J. J., Kumar G. K., Prabhakar N. R. (2004) J. Physiol. 557, 773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanduri J., Yuan G., Kumar G. K., Semenza G. L., Prabhakar N. R. (2008) Respir. Physiol. Neurobiol. 164, 277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shujia J., Haider H. K., Idris N. M., Lu G., Ashraf M. (2008) Cardiovasc, Res. 77, 525–533 [DOI] [PubMed] [Google Scholar]
- 14.Haider H. K., Jiang S., Idris N. M., Ashraf M. (2008) Circ. Res. 103, 1300–1308 [DOI] [PubMed] [Google Scholar]
- 15.Niagara M. I., Haider H. K., Jiang S., Ashraf M. (2007) Circ. Res. 100, 545–555 [DOI] [PubMed] [Google Scholar]
- 16.Haider H. K., Ashraf M. (2008) J. Mol. Cell. Cardiol. 45, 554–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu G., Haider H. K., Jiang S., Ashraf M. (2009) Circulation 119, 2587–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai Y., Xu M., Wang Y., Pasha Z., Li T., Ashraf M. (2007) J. Mol. Cell. Cardiol. 42, 1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenza G. L. (2007) Sci. STKE 2007: cm8. [DOI] [PubMed] [Google Scholar]
- 20.Hobert O. ( 2008) Science 319, 1785– 1786 [DOI] [PubMed] [Google Scholar]
- 21.Skog J., Würdinger T., van Rijn S., Meijer D. H., Gainche L., Sena-Esteves M., Curry W. T., Jr., Carter B. S., Krichevsky A. M., Breakefield X. O. (2008) Nat. Cell Biol. 10, 1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai Y., Kimura T., Murakami A., Yajima N., Sakamaki K., Yonehara S. (1999) Nature 398, 777–785 [DOI] [PubMed] [Google Scholar]
- 23.Barcaroli D., Bongiorno-Borbone L., Terrinoni A., Hofmann T. G., Rossi M., Knight R. A., Matera A. G., Melino G., De Laurenzi V. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14808–14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milovic-Holm K., Krieghoff E., Jensen K., Will H., Hofmann T. G. (2007) EMBO J. 26, 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieghoff-Henning E., Hofmann T. G. (2008) Biochim. Biophys. Acta 1783, 2185–2194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.