Abstract
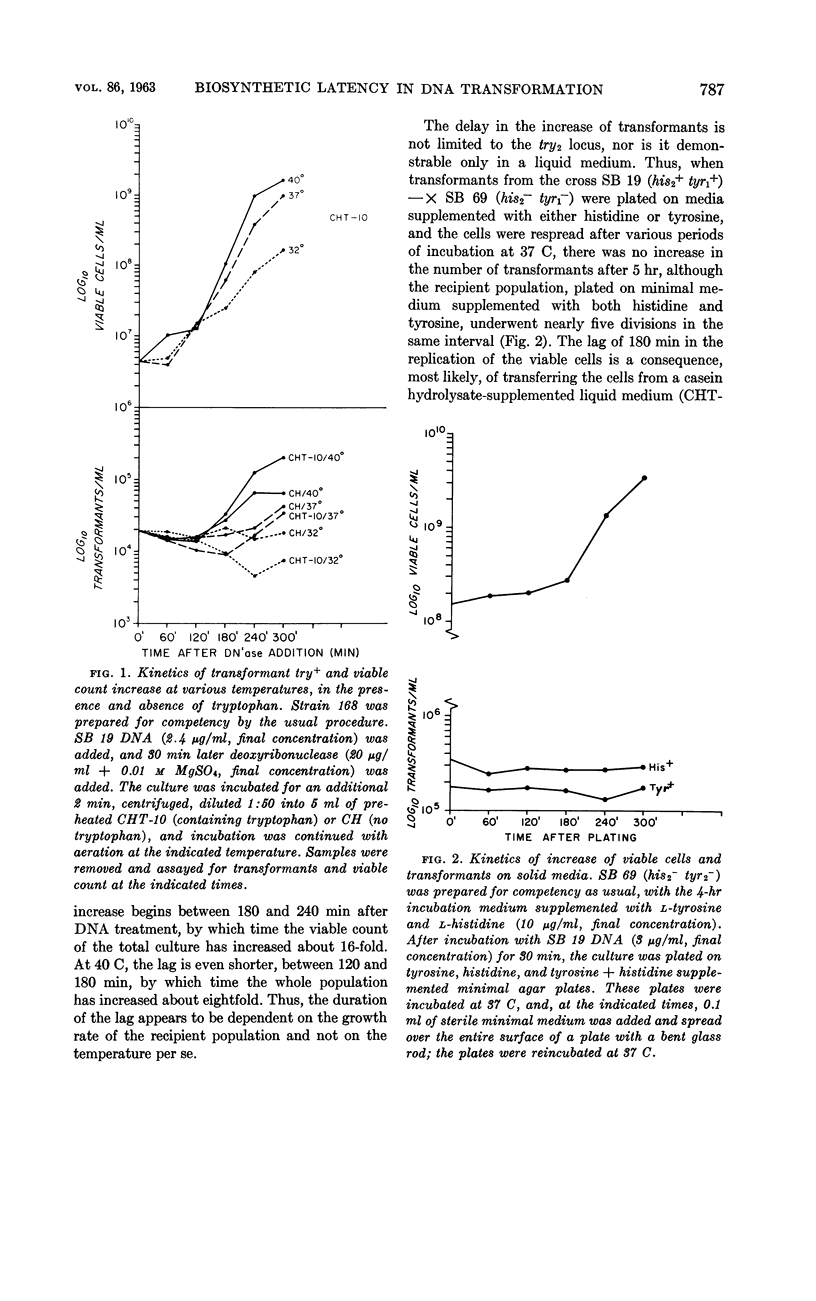
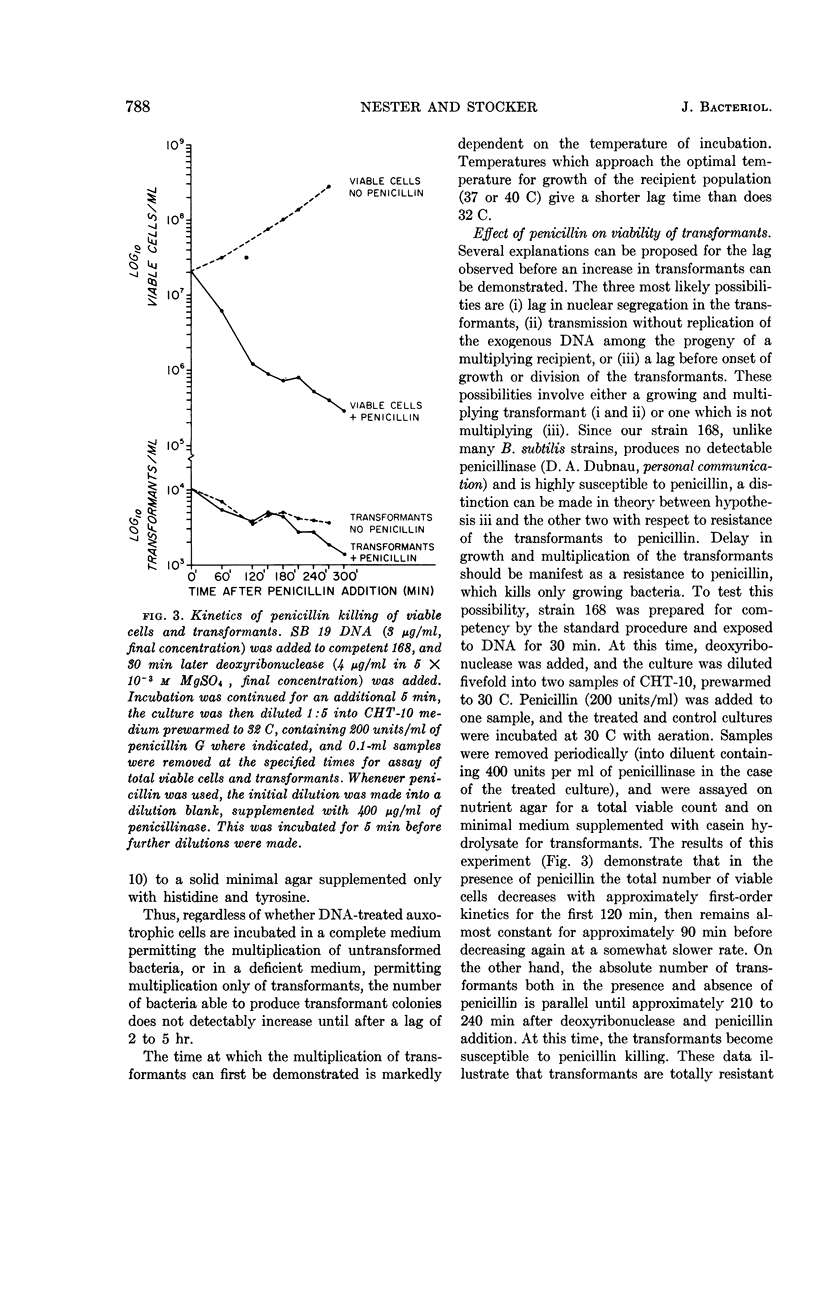
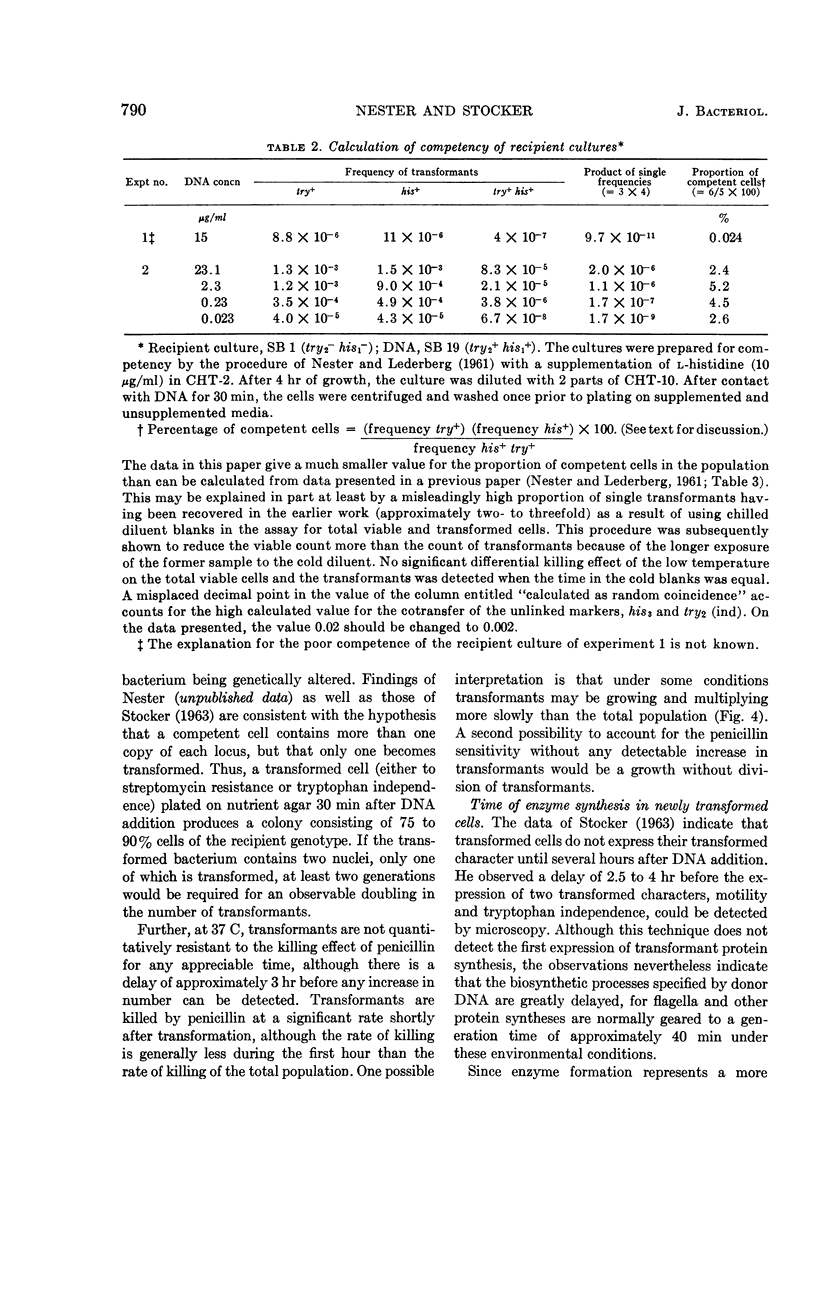
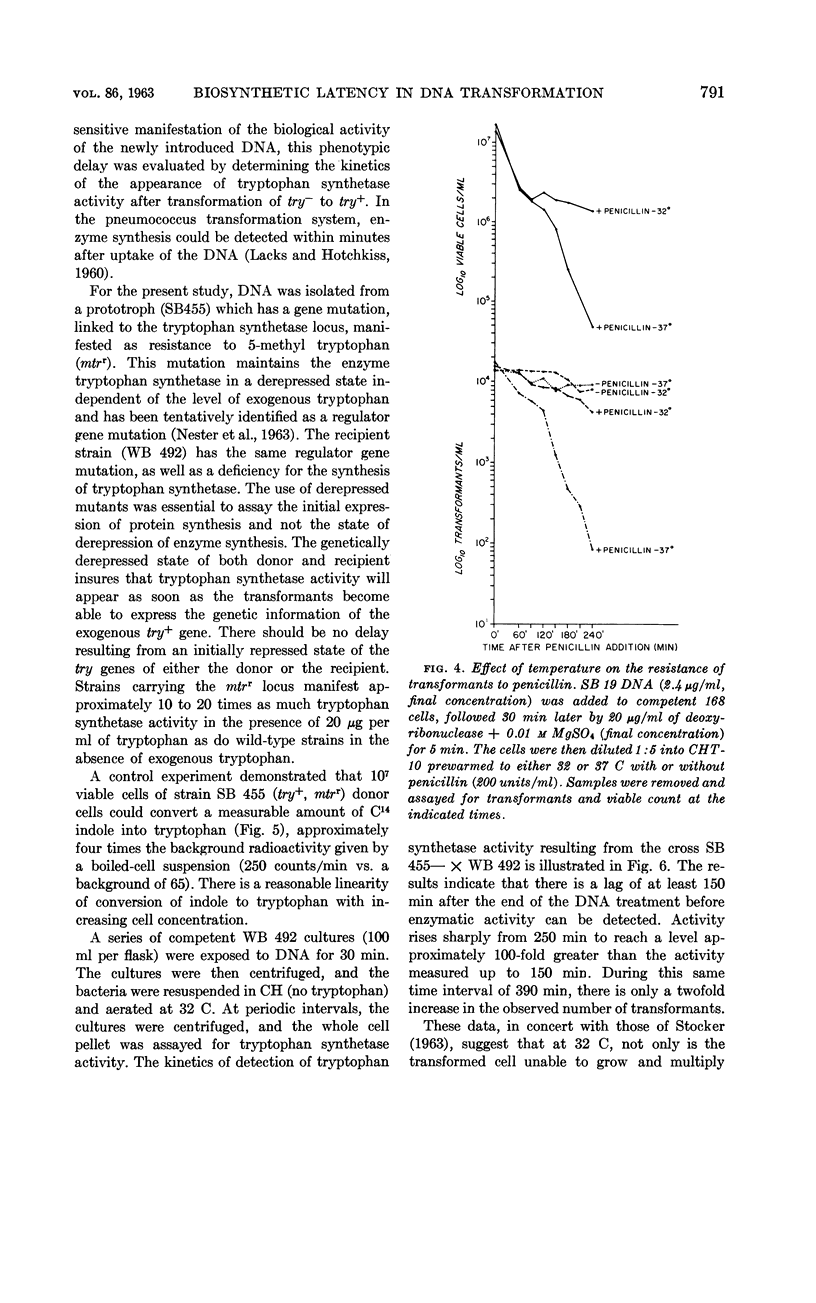
Nester, E. W. (University of Washington, Seattle) and B. A. D. Stocker. Biosynthetic latency in early stages of deoxyribonucleic acid transformation in Bacillus subtilis. J. Bacteriol. 86:785–796. 1963—In the Bacillus subtilis deoxyribonucleic acid (DNA) transformation system, transformants do not increase in number for 3 to 5 hr after the addition of DNA. During most of this period, the transformants are resistant to the bactericidal action of penicillin under conditions which result in the killing of over 90% of the recipient population. This lag in growth and nonmultiplication of the transformants (inferred from penicillin resistance) is also reflected in a lag in the synthesis of an enzyme specified by the donor DNA. Thus, when a cell population deficient in the enzyme tryptophan synthetase is transformed to tryptophan independence, activity of this enzyme cannot be detected in whole cells until 3 to 4 hr after the cells have been exposed to the DNA. Recombination between donor and recipient DNA occurs long before this. Even 30 min after exposure of a competent population of try2−his2+ cells to try2+his2− DNA, 20% of the total try2+ activity found in re-extracted DNA exists as recombinant DNA, try2+his2+. This value, the maximal linkage obtained, remains constant during incubation of the DNA-treated culture for an additional 5 hr. In addition to the heterogeneous response of a DNA-treated competent culture to penicillin killing, the recipient culture appears to be heterogeneous in ability to undergo transformation. Thus, the frequency of joint transformation of two unlinked markers is much higher than would be expected on the basis of the random coincidence of more than one DNA molecule entering the same cell in a uniformly competent recipient population. A possible relationship between these two aspects of heterogeneity of a DNA-treated recipient population is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Fate of transforming deoxyribonucleate following fixation by transformable bacteria. Nature. 1960 Sep 17;187:1002–1006. doi: 10.1038/1871002a0. [DOI] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Fate of transforming deoxyribonucleate following fixation by transformable bacteria. Nature. 1960 Sep 17;187:1002–1006. doi: 10.1038/1871002a0. [DOI] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Initiation of bacterial transformation. Nature. 1957 Jun 29;179(4574):1322–1325. doi: 10.1038/1791322a0. [DOI] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTCHKISS R. D. The genetic chemistry of the pneumococcal transformations. Harvey Lect. 1953;49:124–144. [PubMed] [Google Scholar]
- Hotchkiss R. D. CYCLICAL BEHAVIOR IN PNEUMOCOCCAL GROWTH AND TRANSFORMABILITY OCCASIONED BY ENVIRONMENTAL CHANGES. Proc Natl Acad Sci U S A. 1954 Feb;40(2):49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. Formation of amylomaltase after genetic transformation of pneumococcus. Biochim Biophys Acta. 1960 Dec 4;45:155–163. doi: 10.1016/0006-3002(60)91436-0. [DOI] [PubMed] [Google Scholar]
- NESTER E. W., LEDERBERG J. Linkage of genetic units of Bacillus subtilis in DNA transformation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:52–55. doi: 10.1073/pnas.47.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravin A W. Linked Mutations Borne by Deoxyribonucleic Acid Controlling the Synthesis of Capsular Polysaccharide in Pneumococcusx. Genetics. 1960 Oct;45(10):1387–1404. doi: 10.1093/genetics/45.10.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCKER B. A. TRANSFORMATION OF BACILLUS SUBTILIS TO MOTILITY AND PROTOTROPHY: MICROMANIPULATIVE ISOLATION OF BACTERIA OF TRANSFORMED PHENOTYPE. J Bacteriol. 1963 Oct;86:797–804. doi: 10.1128/jb.86.4.797-804.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLL M. J., GOODGAL S. H. Recombination during transformation in Hemophilus influenzae. Proc Natl Acad Sci U S A. 1961 Apr 15;47:505–512. doi: 10.1073/pnas.47.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]



