Abstract
Activation of executioner caspases during receptor-mediated apoptosis in type II cells requires the engagement of the mitochondrial apoptotic pathway. Although it is well established that recruitment of mitochondria in this context involves the cleavage of Bid to truncated Bid (tBid), the precise post-mitochondrial signaling responsible for executioner caspase activation is controversial. Here, we used distinct clones of type II Jurkat T-lymphocytes in which the mitochondrial apoptotic pathway had been inhibited to investigate the molecular requirements necessary for Fas-induced apoptosis. Cells overexpressing either Bcl-2 or Bcl-xL were protected from apoptosis induced by agonistic anti-Fas antibody. By comparison, Apaf-1-deficient Jurkat cells were sensitive to anti-Fas, exhibiting Bid cleavage, Bak activation, the release of cytochrome c and Smac, and activation of executioner caspase-3. Inhibiting downstream caspase activation with the pharmacological inhibitor Z-DEVD-fmk or by expressing the BIR1/BIR2 domains of X-linked inhibitor of apoptosis protein (XIAP) decreased all anti-Fas-induced apoptotic changes. Additionally, pretreatment of Bcl-xL-overexpressing cells with a Smac mimetic sensitized these cells to Fas-induced apoptosis. Combined, our findings strongly suggest that Fas-mediated activation of executioner caspases and induction of apoptosis do not depend on apoptosome-mediated caspase-9 activation in prototypical type II cells.
INTRODUCTION
Apoptosis is an active form of cell death that is executed by a family of cysteine proteases that cleave intracellular substrates after aspartate residues (caspases). Because apoptosis is important for cell removal during development and tissue homeostasis, disruptions in the regulation of apoptosis can play a role in the onset of numerous pathologies, including neurodegenerative disorders, autoimmunity, and cancer (1).
Apoptosis can occur through two distinct signaling pathways. One is the extrinsic (receptor-mediated) pathway in which binding of a death receptor (e.g. tumor necrosis factor (TNF)2 receptor-1, TNF-related apoptosis-inducing ligand (TRAIL) receptor-1, TRAIL-R2, and Fas) by a cognate ligand (e.g. TNFα, TRAIL, and FasL) or an agonistic antibody (e.g. CH-11) causes the recruitment of adaptor proteins (e.g. TRADD and FADD) and initiator procaspase-8 molecules to the cytosolic side of the receptor to form the death-inducing signaling complex (DISC) (2). Within the DISC, procaspase-8 molecules are activated by dimerization and subsequently undergo autoprocessing (3, 4). In most cell types (type I), active caspase-8 activates the executioner caspase-3, which, in turn, is responsible for many of the morphological and biochemical manifestations of apoptosis. In other cell types (type II), the amount of caspase-8 that is activated within the DISC is low and insufficient to directly activate downstream executioner procaspases, including caspase-3 and -7 (5). Instead, active caspase-8 in type II cells is known to cleave the cytosolic BH3-only protein Bid to truncated Bid (tBid) (6–8). In turn, tBid can activate a multidomain Bcl-2 family protein (i.e. Bax and Bak) that stimulates mitochondrial outer membrane permeabilization (MOMP) and the release of intermembrane space proteins into the cytosol (9, 10). In this regard, it is now widely accepted that the mitochondrial apoptotic pathway plays an essential role during receptor-mediated apoptosis in type II cells (5, 11–16); however, conflicting results exist regarding the precise molecular signaling requirements. In particular, some evidence obtained using prototypical type II Jurkat cells demonstrated that overexpression of XIAP could inhibit Fas-induced apoptosis in wild-type cells, and knocking down XIAP expression could sensitize Bcl-xL-transfected cells to receptor-mediated cell killing (12). A different study using a reconstituted in vitro cell-free system showed that recombinant Smac was sufficient to activate caspase-3 activity by inhibiting XIAP (13). Although these findings suggested that Smac-dependent neutralization of XIAP-mediated caspase-3 inhibition might be the most important consequence of MOMP during Fas-mediated apoptosis in type II cells, neither study examined this possibility in an experimental system in which a component of the apoptosome was absent. Intriguingly, a more recent study reported that a caspase-9-deficient Jurkat clone was highly resistant to Fas-induced caspase activation, which, in contrast to the two earlier studies, implicated an essential role for cytochrome c-dependent apoptosome formation and the activation of caspase-9 during Fas-induced apoptosis (11).
Therefore, the aim of this study was to clarify the precise signaling requirements necessary for Fas-mediated apoptosis in prototypical type II cells. Because each of the aforementioned studies was performed using Jurkat cells, we also used clones of this cell line in which the intrinsic pathway had been inhibited due to either stable overexpression of Bcl-2/Bcl-xL, the stable depletion of Apaf-1, or the stable expression of the baculoviral inhibitor of apoptosis protein repeat 1 and 2 (BIR1/BIR2) domains of XIAP (17). The data provided direct evidence that apoptosome-mediated caspase-9 activation is not required for extrinsic apoptosis induced by anti-Fas (CH-11). Instead, our findings suggested that the inhibition of XIAP was a better determinant of the susceptibility of type II cells to anti-Fas-induced apoptosis.
EXPERIMENTAL PROCEDURES
Cell Culture
Wild-type Jurkat T-lymphocytes (clone E6.1) were cultured in RPMI 1640 complete medium (Invitrogen, Carlsbad, CA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (HyClone, Logan, UT), 2% (w/v) glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified 5% CO2 incubator. For Apaf-1-deficient, Bcl-2- and Bcl-xL-overexpressing, and Myc-BIR1/BIR2-expressing Jurkat cells, 1 mg/ml Geneticin (Invitrogen) was substituted for penicillin and streptomycin. Cells were maintained in an exponential growth phase for all experiments. All cells were re-plated in fresh complete nonselective medium prior to apoptosis induction. Apoptosis was induced with anti-Fas antibody (100 ng/ml) (clone CH-11, MBL International, Woburn, MA). The caspase-3/7 inhibitor Z-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-CH2F (Z-DEVD-fmk) (Kamiya Biomedical Company, Seattle, WA) was used at a final concentration of 25 μm, and the Smac mimetic (compound 3) was used at a final concentration of 100 nm.
Western Blotting
Pelleted cells (5 × 106) were resuspended and lysed in 200 μl of ice-cold lysis buffer (10 mm Tris/HCl, pH 7.4, 10 mm NaCl, 3 mm MgCl2, 1 mm EDTA, 0.1% Nonidet P-40) supplemented with a mixture of protease inhibitors (Complete Mini EDTA-Free, Roche, Indianapolis, IN). Protein concentrations were determined using the bicinchoninic assay (Pierce) and equal amounts were mixed with Laemmli. Western blot analysis was carried out as described previously (18). The antibodies used were rabbit anti-Bak, NT (Millipore), rabbit anti-Bid (Cell Signaling), rabbit anti-caspase-3 (clone 8G10, Cell Signaling), rabbit anti-caspase-7 (Cell Signaling), mouse anti-caspase-8 (clone 1C12, Cell Signaling), rabbit anti-caspase-9 (Cell Signaling), mouse anti-cytochrome c (clone 7H8.2C12, BD Pharmingen, San Jose, CA), and mouse anti-Smac/DIABLO (Cell Signaling).
Flow Cytometry for Cell Death and Mitochondrial Membrane Potential (ΔΨ) Measurements
Phosphatidylserine exposure on the outer leaflet of the plasma membrane was detected using the annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit II (BD PharMingen) according to the manufacturer's instructions. In brief, 106 cells were pelleted following anti-Fas treatment and washed in phosphate-buffered saline (PBS). Next, the cells were resuspended in 100 μl of binding buffer containing annexin V-FITC and propidium iodide. Prior to flow cytometric analysis, 400 μl of binding buffer were added to the cells. For ΔΨ determination, the MitoProbe DiIC1(5) Kit (Invitrogen, Molecular Probes, Carlsbad, CA) was used. Briefly, cells (106) were pelleted following drug treatment, washed once in PBS, and resuspended in 1 ml of warm PBS. Next, 5 μl of 10 μm DiIC1(5) were added to the cells and incubated in a humidified 5% CO2 incubator at 37 °C for 15 min. Cells were pelleted, resuspended in 500 μl of PBS, and analyzed by flow cytometry.
In Vitro Reconstitution of Caspase-9 Activation
Cells were collected and washed twice in ice-cold PBS, resuspended in cytosolic extraction buffer (20 mm Hepes, pH 7.5, 10 mm KCl, 1.9 mm MgCl2, 1 mm EGTA, 1 mm EDTA) supplemented with a mixture of protease inhibitors (Complete Mini EDTA-Free) and allowed to swell on ice for 12 min. Plasma membrane integrity was monitored by trypan blue staining, and cell suspensions were centrifuged at 16,000 × g for 15 min at 4 °C. Resulting supernatants were collected and protein concentrations were determined using the bicinchoninic assay. Subsequently, cytosolic extract (2.5 mg of protein/ml) was incubated in the absence or presence of 50 μg/ml cytochrome c purified from horse heart (Sigma) for 1 h at 37 °C. Aliquots were collected at the indicated times and analyzed by Western blotting for the cleavage of caspase-9.
Determination of Bak Oligomerization and Activation
For detection of Bak oligomerization, cells (106) were harvested, washed, and resuspended in 80 μl of 100 mm EDTA/PBS, incubated with the cross-linking agent bismaleimidohexane (BMH) (1 mm) for 30 min at room temperature (22 °C), quenched with 100 mm dithiothreitol for 15 min, pelleted, and processed for Western blotting. For detection of activated Bak by flow cytometry, cells (106) were washed in PBS and fixed in 400 μl of 0.25% paraformaldehyde for 5 min, subsequently washed two times with 1% FBS in PBS, and incubated in 50 μl of staining buffer (1% FBS and 100 μg/ml digitonin in PBS) with a conformation-specific mouse monoclonal antibody against Bak (1:30; AM03, Calbiochem) for 30 min at room temperature (22 °C). Then, cells were washed and resuspended in 50 μl of staining buffer containing 0.25 μg Alexa Fluor 488-labeled chicken anti-mouse for 30 min in the dark. Cells were washed again and analyzed by flow cytometry. Analysis and histogram overlays were performed using FlowJo software (Tree Star, Ashland, OR).
Subcellular Fractionation
Following treatment with anti-Fas, cells (106) were washed in PBS, resuspended in 50 μl of buffer (140 mm mannitol, 46 mm sucrose, 50 mm KCl, 1 mm KH2PO4, 5 mm MgCl2, 1 mm EGTA, 5 mm Tris, pH 7.4) supplemented with a mixture of protease inhibitors (Complete Mini-EDTA Free) and permeabilized with 3 μg of digitonin (Sigma) on ice for 10 min. Plasma membrane permeabilization was monitored by trypan blue staining, and cell suspensions were centrifuged at 12,000 × g for 10 min at 4 °C. Supernatant and pellet fractions were subjected to Western blot analysis.
RESULTS AND DISCUSSION
Bcl-2 or Bcl-xL Overexpression, but Not Apaf-1 Knockdown, Confers Resistance to Fas-induced Apoptosis
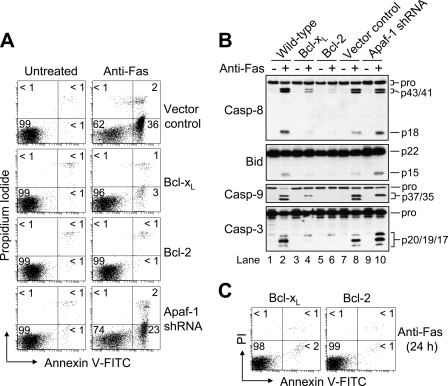
Previously, we demonstrated that Jurkat cells, in which either an anti-apoptotic Bcl-2 family protein had been overexpressed or Apaf-1 had been knocked down, were entirely resistant to mitochondria-mediated apoptosis (17). Here, we were interested in the susceptibility of the different cells to receptor-mediated killing induced by agonistic anti-Fas antibody (CH-11). As shown in Fig. 1A, only control-transfected and Apaf-1-deficient cells underwent Fas-induced apoptosis. Specifically, after treatment with a concentration of 100 ng/ml of anti-Fas, vector control and Apaf-1-silenced cells displayed ∼38 and ∼25% apoptosis, respectively, at 6 h. By comparison, when the Bcl-2- and Bcl-xL-overexpressing cells were incubated under the same conditions they exhibited ∼2 and ∼4% apoptosis, respectively. In agreement with these findings, Western blot analysis of cell lysates revealed that proteolytic processing of caspase-8 was significantly more pronounced in wild-type, vector control, and Apaf-1-deficient cells as compared with Bcl-2- and Bcl-xL-overexpressing cells (Fig. 1B). That is, more of both the cleaved intermediate (p43/p41) and active (p18) fragments of caspase-8 were evident after incubation with 100 ng/ml of anti-Fas for 6 h. Similarly, cleavage of Bid to tBid and the processing of caspase-3 to its fully active p19/p17 fragments occurred in the wild-type, vector control, and Apaf-1-deficient cells (Fig. 1B, lanes 2, 8, and 10). By comparison, only the p20 caspase-3 intermediate fragment was observed in the Bcl-xL- and Bcl-2-overexpressing cells (Fig. 1B, lanes 4 and 6) suggesting that normal caspase-3-mediated autocatalytic removal of the N-terminal prodomain had been impaired (19, 20), presumably due to inhibition by an inhibitor of apoptosis protein (IAP) (21). Cleavage of caspase-9 occurred in four out of the five cell lines, although producing two fragments (p37/p35) in wild-type and control-transfected cells, and only one fragment (p37) in Apaf-1-deficient and Bcl-xL-overexpressing cells (Fig. 1B, lanes 2 and 8 versus lanes 4 and 10). This is important because apoptosome-mediated activation and self-cleavage of caspase-9 has been reported to occur after Asp315 yielding a p35 fragment, whereas the appearance of the catalytically inactive p37 fragment (22) is most often linked to caspase-3-mediated cleavage of caspase-9 after Asp330 (23). The small amount of p37 caspase-9 that was produced in the anti-Fas-treated Bcl-xL-overexpressing cells could be due to a trace amount of autocatalytic processing of p20 caspase-3 to p19 caspase-3 that had occurred (Fig. 1B, lane 4). Nevertheless, as shown in Fig. 1C, both Bcl-2- and Bcl-xL-overexpressing cells were refractory to receptor-mediated apoptosis even after 24 h of incubation with anti-Fas. By comparison, Apaf-1-deficient cells were susceptible to apoptosis induced by anti-Fas, exhibiting ∼65% as much death as vector control cells.
FIGURE 1.
Bcl-2 or Bcl-xL overexpression, but not Apaf-1 knockdown, inhibits apoptosis in response to agonistic anti-Fas antibody (CH-11). A, control-transfected, Bcl-xL- and Bcl-2-overexpressing, and Apaf-1-deficient Jurkat clones (106/ml) were cultured in the presence or absence of 100 ng/ml anti-Fas for 6 h, harvested, and processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide (PI) staining. Quadrants are defined as live (lower left), early apoptotic (lower right), late apoptotic (upper right), and necrotic (upper left). Numbers refer to the percentage of cells in each quadrant. B, duplicate aliquots of cells were harvested and lysed for Western blotting. C, Bcl-xL- and Bcl-2-overexpressing cells were cultured in the presence of 100 ng/ml anti-Fas for 24, harvested, and processed for cell death determination as described in A. Casp, caspase.
Absence of Cytochrome c-initiated Caspase-9 Activation in Cytosolic Extracts from Apaf-1 Knockdown Cells
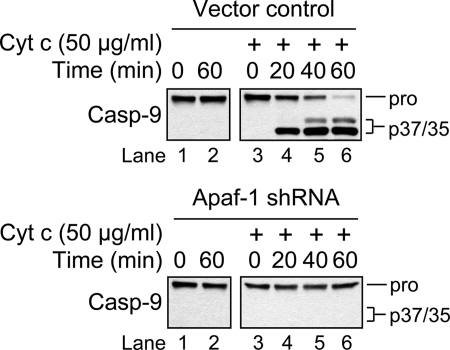
Although apoptosome-mediated activation of caspase-9 (p35) was not observed in Apaf-1-deficient cells incubated in the presence of anti-Fas (Fig. 1B, lane 10) or etoposide (17, 24), we next tried to reconstitute the caspase-9 activation pathway in vitro using cytosolic extracts from these cells. The rationale for this experiment was that, while our Apaf-1 knockdown was very efficient, it was not complete (17, 24), and we wondered whether the trace amount of Apaf-1 that remained in these cells might be sufficient to activate caspase-9 at a level that had escaped detection. However, if this were true, then we reasoned it should be possible to detect at least some activation of caspase-9 (p35) in cytosolic extracts from these cells incubated over time in the presence of a relatively high concentration of exogenous cytochrome c. Indeed, this approach has been used extensively in the literature to faithfully mimic apoptosome-mediated activation of caspase-9.
As illustrated in Fig. 2, incubation of cytosolic extract obtained from either vector control or Apaf-1-deficient cells in the absence of exogenous cytochrome c for 1 h at 37 °C resulted in no caspase-9 processing (left panels). As anticipated, the addition of cytochrome c to vector control cell cytosolic extract induced rapid, near-complete processing of procaspase-9 to its p35/p37 fragments after incubation at 37 °C for 1 h (upper right panel). By comparison, no procaspase-9 processing was observed in purified cytosolic extract from Apaf-1-deficient cells incubated under the same conditions (lower right panel). Combined, these data provide additional evidence that the residual Apaf-1 protein in the Apaf-1-deficient cells is not sufficient to promote cytochrome c-mediated activation of caspase-9.
FIGURE 2.
In vitro reconstitution of the caspase-9 activation pathway. Vector control (upper panels) or Apaf-1-deficient (lower panels) Jurkat cell cytosolic extract (2.5 mg of protein/ml) was incubated in the absence or presence of 50 μg/ml horse heart cytochrome c for 1 h at 37 °C. Aliquots were collected at the indicated times and analyzed by Western blotting for caspase-9.
Sensitivity of Apaf-1-deficient Jurkat Cells to Anti-Fas Treatment Is Associated with Extensive Mitochondrial Apoptotic Changes
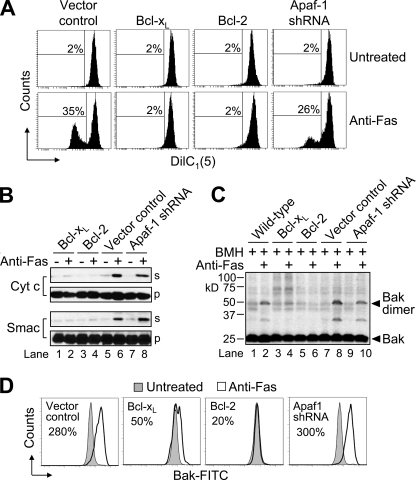
As mentioned previously, Fas-mediated apoptotic signaling in type I cells is generally thought to occur by active caspase-8 directly cleaving and activating caspase-3, whereas recruitment of the intrinsic (mitochondria-mediated) pathway is widely thought to be essential for Fas-mediated apoptosis in type II cells. Because our different gene-manipulated Jurkat cell clones with impaired intrinsic pro-apoptotic signaling were so markedly dissimilar in their sensitivity to apoptosis induced by anti-Fas, we sought to investigate the extent to which this might be due to differences in mitochondrial events. Thus, we first evaluated the different cells for changes in Δψ. Apaf-1-deficient cells experienced a drop in Δψ that was ∼74% of that exhibited by vector control cells when incubated in the presence of anti-Fas for 6 h (Fig. 3A). It is worth noting that these same Apaf-1-deficient cells were resistant to Δψ loss when incubated in the presence of etoposide (24). By comparison, overexpression of either Bcl-xL or Bcl-2 rendered cells totally refractory to any loss of Δψ induced by anti-Fas. Because a drop in Δψ is often associated with the release of mitochondrial intermembrane space proteins into the cytosol, we next investigated whether the release of cytochrome c and Smac had occurred in the different cell types. Treatment with anti-Fas led to a pronounced release of cytochrome c and Smac from vector control and Apaf-1-deficient cells (Fig. 3B) consistent with the Δψ data.
FIGURE 3.
Fas-induced apoptosis in Apaf-1-deficient cells is accompanied by extensive mitochondrial events. A, control-transfected, Bcl-xL- and Bcl-2-overexpressing, and Apaf-1-deficient Jurkat clones (106/ml) were cultured in the absence or presence of 100 ng/ml anti-Fas for 6 h and processed for mitochondrial membrane potential (ΔΨ) determination by flow cytometry. Reduced DiIC1(5) fluorescence is indicative of a loss of ΔΨ, and numbers refer to the percentage of cells that underwent a dissipation of ΔΨ. B, duplicate aliquots of cells were harvested and processed for subcellular fractionation. Supernatant (s) and pellet (p) fractions were analyzed by Western blotting. C and D, wild-type, control-transfected, Bcl-xL- and Bcl-2-overexpressing, and Apaf-1-deficient Jurkat clones (106/ml) were cultured in the absence or presence 100 ng/ml anti-Fas for 6 h and processed for determination of Bak oligomerization by Western blotting (C) or Bak activation by flow cytometric analysis (D). In D, histograms show Bak-associated fluorescence in vehicle-treated (filled histograms) and apoptotic stimulus-treated (open histograms) cells after 6 h, and numbers refer to the percentage increase in mean fluorescence intensity.
To investigate the extent to which differences in the release of cytochrome c and Smac corresponded to differences in the activation of Bak (E6.1 cells do not express Bax (7, 17)) we used two different techniques. We first investigated whether Bak underwent oligomerization using the cross-linking agent BMH. As illustrated in Fig. 3C, cross-linked Bak protein complexes were observed in wild-type and control-transfected Jurkat cells treated with 100 ng/ml of anti-Fas for 6 h. In line with the mitochondrial apoptotic changes that were observed (Fig. 3, A and B), dimerization and activation of Bak were detected in wild-type, vector control, and Apaf-1-deficient Jurkat cells, whereas cells overexpressing Bcl-2 or Bcl-xL were resistant to changes in Bak status (Fig. 3C). These findings were in agreement with results obtained using an active conformation-specific monoclonal Bak antibody and flow cytometric analysis, where Bak activation amounts to a shift to the right in the resulting histogram (Fig. 3D). Specifically, vector control and Apaf-1-deficient cells exhibited large increases in Bak-associated fluorescence of 280 and 300%, respectively, whereas Bcl-xL and Bcl-2 cells displayed more modest increases of 50 and 20%, respectively. Collectively, these findings suggest that the sensitivity of Apaf-1-deficient cells to Fas-induced apoptosis is due, at least in part, to that fact that these cells undergo MOMP.
Caspase-3/7 (DEVDase) Activity Mediates the Majority of Fas-mediated Apoptotic Events in Apaf-1-deficient Cells
As mentioned, stimulation of the Fas receptor with its corresponding ligand or an agonistic antibody leads to formation of the DISC and activation of caspase-8 (3). Because of existing evidence indicating that the amount of caspase-8 that is activated at the DISC in type II cells is too low to efficiently cleave and activate caspase-3 (16), it is widely believed that caspase-8 cleaves Bid to tBid, which, in turn, recruits the intrinsic (mitochondria-mediated) apoptotic pathway by activating Bax or Bak. In this regard, it is thought that type II cells rely heavily on the intrinsic pathway for receptor-mediated apoptosis (5, 11, 15, 16). However, such a model would not explain why the Apaf-1-deficient cells, which cannot activate caspase-9 (p35) in response to intrinsic stimuli (17, 24), extrinsic stimuli (Fig. 1B, lane 9 versus 10), or in an in vitro reconstituted cell-free system (Fig. 2), are sensitive to Fas-mediated apoptosis, exhibiting Bak activation (Fig. 3, C and D), induction of MOMP (Fig. 3B), and the robust cleavage of caspase-8 to its intermediate (p43/p41) and active (p18) fragments (Fig. 1B, lane 9 versus 10).
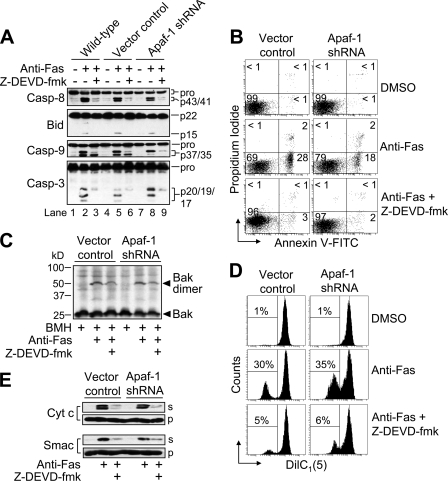
Because caspase-3 was also cleaved to its fully active p19/p17 fragments in cells lacking Apaf-1 (Fig. 1B, lane 9 versus 10), we investigated the potential importance of caspase-3/7 in producing the various apoptotic changes in anti-Fas-treated Apaf-1-deficient cells. To that end, we incubated cells with the caspase inhibitor Z-DEVD-fmk (25 μm) for 1 h prior to treatment with anti-Fas (100 ng/ml) for 6 h. As illustrated, Z-DEVD-fmk pre-treatment prevented Fas-induced autocatalytic cleavage of caspase-3 to its fully active p19/p17 fragments, whereas it was not able to eliminate the generation of the p20 caspase-3 intermediate fragment by caspase-8 (Fig. 4A, lanes 2 versus 3, 5 versus 6, and 8 versus 9). Pre-treatment of cells with Z-DEVD-fmk reduced dramatically the appearance of the p43/p41 fragments of caspase-8 suggesting that most of the Fas-mediated cleavage of procaspase-8 occurs downstream of caspase-3/7 activation (Fig. 4A). In addition, Z-DEVD-fmk markedly inhibited the appearance of tBid (Fig. 4A), arguing strongly that the majority of Fas-induced Bid cleavage occurs downstream, or independently, of the low level of caspase-8 activity that occurs initially during receptor-mediated apoptosis in type II Jurkat cells. Z-DEVD-fmk also inhibited the processing of caspase-9 (Fig. 4A). Specifically, the appearance of the p37 fragment of caspase-9 was largely prevented by pretreatment with Z-DEVD-fmk. As mentioned previously, the p37 fragment of caspase-9, which lacks proteolytic activity (22), is generated by caspase-3-mediated proteolysis, whereas the p35 fragment of caspase-9 is produced by apoptosome-mediated dimerization and autocatalysis (23). As anticipated, Z-DEVD-fmk also blocked Fas-induced apoptosis as determined by annexin V-FITC fluorescence (Fig. 4B).
FIGURE 4.
Requirement of caspase-3/7 (DEVDase) activity for Fas-induced apoptotic changes in Apaf-1-deficient cells. A, wild-type, control-transfected, and Apaf-1-deficient Jurkat cells (106/ml), preincubated with or without 25 μm Z-DEVD-fmk for 1 h, were incubated with 100 ng/ml anti-Fas for 6 h, harvested, and lysed for Western blotting. B–E, duplicate aliquots of cells were harvested and processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide staining (B), Bak oligomerization and activation (C), Δψ determination by flow cytometric analysis of DiIC1(5)-associated fluorescence (D), and subcellular fractionation followed by Western blotting of supernatant (s) and pellet (p) fractions (E). In B, quadrants are defined as live (lower left), early apoptotic (lower right), late apoptotic (upper right), and necrotic (upper left). Numbers refer to the percentage of cells in each quadrant. In D, numbers refer to the percentage of cells that underwent a dissipation of ΔΨ. Casp, caspase; Z-DEVD-fmk; Z-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-CH2F.
We next examined the effect Z-DEVD-fmk might have on Fas-induced mitochondrial apoptotic events. As shown, pretreatment of cells with Z-DEVD-fmk partially inhibited Bak oligomerization (Fig. 4C), the loss of Δψ (Fig. 4D), and the release of cytochrome c and Smac (Fig. 4E) induced by anti-Fas. Taken together, these data suggest that many of the observable apoptotic changes induced by anti-Fas treatment are mediated by caspase-3/7 (DEVDase) activity. In addition, these findings support a model of receptor-mediated apoptosis in which the intrinsic pathway per se is not required for apoptosis to occur in type II Jurkat cells.
Expression of the BIR1/BIR2 Domains of XIAP Inhibits Fas-induced Apoptosis
Because Z-DEVD-fmk pretreatment inhibited to a similar extent Fas-induced apoptotic changes in wild-type, vector control, and Apaf-1-deficient cells, and because of concerns about the specificity of so-called specific peptide-based caspase inhibitors, including Z-DEVD-fmk (25), we next took a genetic approach to inhibiting downstream caspases. Specifically, we examined the sensitivity of Jurkat cells engineered to stably express the BIR1/BIR2 domains of XIAP (17) to Fas-induced apoptosis. XIAP belongs to the inhibitor of apoptosis protein family (26) and a region of this protein that includes the BIR2 domain is known to inhibit caspase-3 and -7 (21, 27, 28).
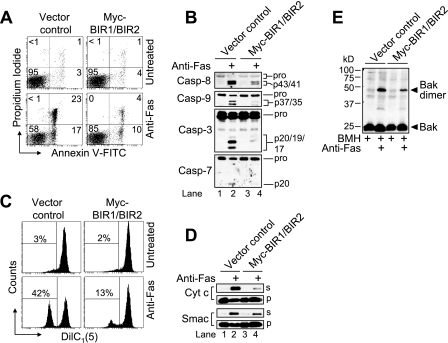
As illustrated in Fig. 5A, vector control cells underwent apoptosis (∼40%) when incubated in the presence of anti-Fas (100 ng/ml) for 6 h, whereas the BIR1/BIR2 transfectants exhibited significantly less death (∼14%). In other words, Fas-mediated apoptosis was reduced by ∼65% in BIR1/BIR2-expressing cells relative to vector control cells. In agreement with these findings, Western blot analysis of cell lysates generated at 6 h after incubation with anti-Fas showed that extensive proteolytic processing of caspase-8, -9, -3, and -7 had occurred in vector control cells, whereas caspase processing in cells expressing the BIR1/BIR2 domains of XIAP was markedly reduced (Fig. 5B).
FIGURE 5.
BIR1/BIR2-expressing Jurkat cells are resistant to early and late Fas-induced apoptotic changes. A, control-transfected and Myc-BIR1/BIR2-overexpressing Jurkat cells were incubated in the presence or absence of 100 ng of anti-Fas for 6 h and processed for cell death determination by flow cytometric analysis of annexin-V-FITC and propidium iodide staining. Quadrants are defined as live (lower left), early apoptotic (lower right), late apoptotic (upper right), and necrotic (upper left). Numbers refer to the percentage of cell in each quadrant. B–E, duplicate aliquots of cells were harvested and lysed for Western blotting (B), processed for mitochondrial membrane potential (ΔΨ) determination by flow cytometry (C), processed for subcellular fractionation followed by Western blotting of supernatant (s) and pellet (p) fractions (D), and processed for Bak oligomerization by Western blotting (E). In C, reduced DiIC1(5) fluorescence is indicative of loss of (ΔΨ), and numbers refer to the percentage of cells that underwent a dissipation of (ΔΨ). Casp, caspase.
Next, we were interested to determine whether Fas-induced mitochondrial events were altered in BIR1/BIR2-expressing cells. As shown in Fig. 5C, treatment of cells with anti-Fas for 6 h resulted in nearly half (∼42%) of the control-transfected cells displaying a loss of Δψ, while cells expressing the BIR1/BIR2 domains of XIAP underwent a more modest drop in Δψ of ∼12%. Similar findings were obtained with respect to MOMP induction as measured by Western blot analysis of cytochrome c and Smac release into the cytosol (Fig. 5D, lane 2 versus 1 and lane 4 versus 3). Finally, we were interested to investigate whether the maintenance of Δψ and reduced MOMP in the BIR1/BIR2-expressing cells corresponded with decreased Bak activation. As shown in Fig. 5E, Fas-induced Bak activation was attenuated in cells expressing the BIR1/BIR2 domains of XIAP. Overall, these findings support and extend the results presented in Fig. 4 and offer genetic evidence that executioner caspases play an important role in eliciting MOMP in response to anti-Fas in type II cells.
A Smac Mimetic Partially Sensitizes Bcl-xL-overexpressing Cells to Fas-induced Apoptosis
Because the p20 fragment of caspase-3 was detected uniformly in (i) anti-Fas-treated Apaf-1-deficient cells that had been pre-incubated with Z-DEVD-fmk (Fig. 4A), (ii) cells made to express the BIR1/BIR2 domains of XIAP (Fig. 5B), and (iii) Bcl-xL-overexpressing cells incubated with anti-Fas alone (Fig. 1B), we speculated that the reason p20 caspase-3 was not able to undergo autocatalysis to its p19/p17 fragments in cells overexpressing Bcl-xL might be due to persistent inhibition of its enzyme activity by an IAP, such as XIAP, as reported previously in vitro (13). Smac, which is a protein that is normally sequestered in the mitochondrial intermembrane space, is released together with cytochrome c in response to various apoptotic stimuli (29, 30) and inhibits XIAP function by binding XIAP second baculoviral inhibitory repeat (BIR) domain (31–33). In this respect, Smac is considered a derepressor of caspase inhibition by XIAP, and several cell-permeable Smac mimetics have been designed that can potentiate pro-apoptotic stimuli (34–38), as well as induce apoptosis independently (39).
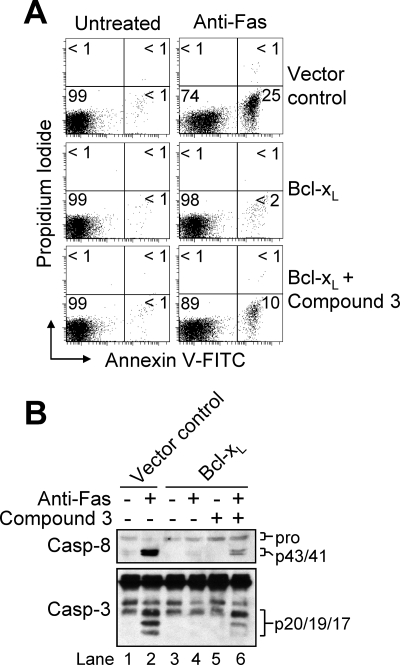
To test the extent to which Smac could sensitize Bcl-xL-overexpressing cells to anti-Fas, we obtained a Smac mimetic (compound 3) that was shown previously to bind XIAP (also cellular IAP 1(cIAP1) and cIAP2) and act synergistically with TRAIL or TNFα to induce apoptosis in cancer cell lines that are normally resistant to these two death receptor stimuli (34, 40, 41). In the current study, incubation of cells with 100 nm compound 3 for up to 16 h did not induce apoptosis (data not shown) or proteolytic processing of caspase-8 (Fig. 6B). However, when Bcl-xL-overexpressing cells were pre-treated with 100 nm compound 3 for 4 h and subsequently incubated with anti-Fas (100 ng/ml) for 12 h, ∼11% of cells underwent apoptosis compared with ∼25% of control-transfected cells incubated with anti-Fas alone (Fig. 6A). Likewise, when used in combination with anti-Fas, compound 3 caused enhanced proteolytic cleavage of caspase-8 as well as the generation of some of the p19/p17 fully active fragments of caspase-3 in Bcl-xL-overexpressing cells (Fig. 6B). Our findings are complementary to a recent report demonstrating that two distinct XIAP inhibitors (42) sensitized Bcl-2-overexpressing Jurkat cells to TRAIL-induced apoptosis (43). Significantly, accumulating lines of evidence point toward the potential use of various types of Smac mimetics as anticancer agents in both hematological and solid malignancies (35). However, the utility of Smac mimetics may ultimately be limited to cancers that display abnormally high levels of IAP, since not all malignancies studied to date were found to be sensitive to these agents. Finally, although it might seem plausible that the Smac mimetic partially sensitized Bcl-xL-overexpressing cells to anti-Fas through a caspase-8-dependent mechanism that involves TNF-α autocrine signaling (39, 44, 45), this is unlikely since incubation of cells with the Smac mimetic alone did not cause caspase proteolysis or an induction of apoptosis. Overall, our findings and other data in the literature strongly support the importance of Smac release for receptor-mediated activation of executioner caspases in type II cells, which may ultimately have clinical implications for cancers where engagement of mitochondria is needed to ensure cell death.
FIGURE 6.
A Smac mimetic (compound 3) synergizes with anti-Fas antibody to induce apoptosis in Bcl-xL-overexpressing cells. A, control-transfected and Bcl-xL-overexpressing cells (106/ml) were incubated in the absence or presence of 100 nm Smac mimetic (compound 3) for 4 h and/or anti-Fas for 12 h, harvested, and processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide staining. Quadrants are defined as live (lower left), early apoptotic (lower right), late apoptotic (upper right), and necrotic (upper left). Numbers refer to the percentage of cells in each quadrant. B, duplicate aliquots were harvested and lysed for Western blotting. Casp, caspase.
Concluding Remarks
Although the type I/II cell distinction was first described in studies involving tumor cell lines (e.g. Jurkat cells are type II, and SKW6.4 cells are type I) (5, 16), subsequent evidence has shown that the model also applies to primary cells (e.g. hepatocytes are type II, and thymocytes are type I) (46–48). As mentioned previously, type II cells are distinguished from type I cells by their reported heavy reliance on the intrinsic (mitochondria-mediated) apoptotic pathway for apoptosis induced by a death receptor ligand (e.g. TNFα, TRAIL, and FasL) or agonistic antibody. The perceived need for the intrinsic pathway in type II cells centers around the fact that formation of the DISC is impaired in these cells and that the low level of active caspase-8 that is produced within the DISC is sufficient only to cleave the BH3-only protein Bid. In turn, tBid activates a multidomain Bcl-2 protein, Bax or Bak, leading to the engagement of the mitochondrial pathway. The strongest evidence supporting the existence of a two pathway receptor-mediated model of apoptosis is the fact that overexpression of a suppressor of the intrinsic pathway, notably Bcl-2 or Bcl-xL, protects against apoptosis in some cell types (so-called type II) but not others (so-called type I) (5, 15, 16).
Recent studies examining the type I/II paradigm have produced conflicting results (11–13). The primary point of contention is the extent to which mitochondrial involvement is truly necessary for receptor-mediated executioner caspase activation and apoptosis in type II cells. Our findings, using apoptosome-deficient cells incapable of activating caspase-9, provide direct evidence that the mitochondrial apoptotic pathway per se is not essential for Fas-induced apoptosis in type II cells. In particular, we have demonstrated that Apaf-1-deficient Jurkat cells, which are equally resistant to etoposide-induced apoptosis as Jurkat cells overexpressing Bcl-2 or Bcl-xL (17), are highly susceptible to receptor-mediated apoptosis. Although the sensitivity of Apaf-1-deficient cells to anti-Fas treatment was associated with extensive cleavage of Bid and induction of MOMP, the resulting release of intermembrane space proteins failed to activate caspase-9. Additionally, our findings indicated that Fas-induced cleavage of Bid depends to a large extent on DEVDase activity and/or occurs downstream of caspase-3/7 activation. However, it is possible that the initial cleavage of Bid by caspase-8 occurs at a level that is not detectable by Western blotting but is nevertheless sufficient to cause Bak-mediated MOMP in cells other than those overexpressing Bcl-2 or Bcl-xL. Indeed, estimates have indicated that miniscule amounts of tBid are all that is needed to induce Bax/Bak-dependent cytochrome c release in some settings (49). In this way, it could be that the portion of Bid that appears to be cleaved downstream of caspase-3/7 activation functions to positively amplify MOMP in response to Fas and seal a cell's fate (7).
Because cytochrome c release following MOMP appeared to be unnecessary for Fas-induced apoptosis in Apaf-1-deficient cells since the activation of caspase-9 did not ensue, we hypothesized that Smac release might function in this context to promote executioner caspase activity by inhibiting an IAP, as reported previously for XIAP using in vitro cell-free systems (13). Indeed, we found that a cell-permeable Smac mimetic was able to partially sensitize Bcl-xL-overexpressing cells to Fas-induced apoptosis. This finding, in part, might help reconcile our results with those of Samraj et al. (11). In that study, the authors reported that a caspase-9-deficient clone of Jurkat cells they had identified was strongly resistant to Fas-mediated apoptosis, despite undergoing MOMP as indicated by cytochrome c release. However, no mention of whether Smac was released together with cytochrome c was included, and some evidence suggests the release of these proteins can be differentially regulated (50, 51). In this regard, it would be of particular interest to know whether a Smac mimetic could sensitize the authors' caspase-9-deficient cells to Fas-induced apoptosis. In our case, the fact that the Smac mimetic-induced sensitization of Bcl-xL-overexpressing cells to anti-Fas was not more pronounced suggests that the release of other mitochondrial factors, such as Omi, may contribute significantly toward alleviating the suppressive effects of IAPs. Alternatively, it is possible that Bcl-xL can exert anti-apoptotic functions in the cell that are separate from its established ability to inhibit MOMP.
Finally, while our manuscript was in initial review, another group published an article suggesting that caspase-9 may not be necessary for executioner caspase activation during Fas-mediated apoptosis in type II hepatocytes (52). In particular, the authors showed that Bid-deficient mouse hepatocytes were resistant to treatment with Fas ligand, whereas Bid/XIAP doubly-deficient hepatocytes succumbed to this form of death. Interestingly, cultured type I thymocytes obtained from Bid-deficient animals were highly sensitive to Fas-induced death, despite expressing a basal level of XIAP that was similar to that of hepatocytes. This suggests that the strong caspase-8 activation that occurs within the DISC in type I cells can, in turn, activate sufficient amounts of caspase-3 to overcome the suppressive effects of XIAP. By comparison, type II cells, such as Jurkat cells and hepatocytes, exhibit weaker initial caspase-8 activation and thus rely on mitochondrial involvement to alleviate the caspase inhibitory effects of XIAP. Overall, accumulating evidence supports the notion that inhibition of XIAP by Smac (and/or Omi) is more important than cytochrome c/Apaf-1-mediated activation of caspase-9 in determining the susceptibility of type II cells to receptor-mediated apoptosis.
Acknowledgments
We thank Dr. Joyce Slusser for expert help with flow cytometry. We thank Dr. Xiaodong Wang for the Smac mimetic, Dr. Emad Alnemri for the pcDNA3-myc-BIR1/BIR2 mammalian expression vector, and the late Dr. Stanley Korsmeyer for the pSFFV-neo, pSFFV-Bcl-2, and pSFFV-Bcl-xL mammalian expression vectors. We would also like to thank Dr. Marcus Peter for helpful discussion. The Flow Cytometry Core is supported, in part, by National Institutes of Health Grant P20 RR016443 from the NCRR.
This work was supported, in whole or in part, by National Institutes of Health Grants K22 ES011647 (to J. D. R.), P20 RR016475 from the IDeA Networks of Biomedical Research Excellence Program of the National Center for Research Resources, and T32 ES007079.
- TNF
- tumor necrosis factor
- TRAIL
- TNF-related apoptosis-inducing factor ligand
- MOMP
- mitochondrial outer membrane permeabilization
- Smac
- second mitochondria-derived activator of caspase
- Apaf-1
- apoptotic protease-activating factor-1
- tBid
- truncated Bid
- Δψ
- mitochondrial membrane potential
- XIAP
- X-linked inhibitor of apoptosis protein
- BIR
- baculoviral IAP repeat
- PBS
- phosphate-buffered saline
- PS
- phosphatidylserine
- FITC
- fluorescein isothiocyanate
- PI
- propidium iodide
- BMH
- bismaleimidohexane
- DMSO
- dimethyl sulfoxide
- Cyt c
- cytochrome c
- shRNA
- short hairpin RNA.
REFERENCES
- 1.Fadeel B., Orrenius S., Zhivotovsky B. (1999) Biochem. Biophys. Res. Commun. 266, 699–717 [DOI] [PubMed] [Google Scholar]
- 2.Kischkel F. C., Hellbardt S., Behrmann I., Germer M., Pawlita M., Krammer P. H., Peter M. E. (1995) EMBO J. 14, 5579–5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boatright K. M., Renatus M., Scott F. L., Sperandio S., Shin H., Pedersen I. M., Ricci J. E., Edris W. A., Sutherlin D. P., Green D. R., Salvesen G. S. (2003) Mol. Cell 11, 529–541 [DOI] [PubMed] [Google Scholar]
- 4.Pop C., Fitzgerald P., Green D. R., Salvesen G. S. (2007) Biochemistry 46, 4398–4407 [DOI] [PubMed] [Google Scholar]
- 5.Scaffidi C., Fulda S., Srinivasan A., Friesen C., Li F., Tomaselli K. J., Debatin K. M., Krammer P. H., Peter M. E. (1998) EMBO J. 17, 1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H., Zhu H., Xu C. J., Yuan J. (1998) Cell 94, 491–501 [DOI] [PubMed] [Google Scholar]
- 7.Shelton S. N., Shawgo M. E., Robertson J. D. (2009) J. Biol. Chem. 284, 11247–11255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. (1998) Cell 94, 481–490 [DOI] [PubMed] [Google Scholar]
- 9.Kim H., Rafiuddin-Shah M., Tu H. C., Jeffers J. R., Zambetti G. P., Hsieh J. J., Cheng E. H. (2006) Nat. Cell Biol. 8, 1348–1358 [DOI] [PubMed] [Google Scholar]
- 10.Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. (2001) Science 292, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samraj A. K., Keil E., Ueffing N., Schulze-Osthoff K., Schmitz I. (2006) J. Biol. Chem. 281, 29652–29659 [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson J. C., Cepero E., Boise L. H., Duckett C. S. (2004) Mol. Cell Biol. 24, 7003–7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X. M., Bratton S. B., Butterworth M., MacFarlane M., Cohen G. M. (2002) J. Biol. Chem. 277, 11345–11351 [DOI] [PubMed] [Google Scholar]
- 14.Li S., Zhao Y., He X., Kim T. H., Kuharsky D. K., Rabinowich H., Chen J., Du C., Yin X. M. (2002) J. Biol. Chem. 277, 26912–26920 [DOI] [PubMed] [Google Scholar]
- 15.Schmitz I., Walczak H., Krammer P. H., Peter M. E. (1999) Cell Death Differ 6, 821–822 [DOI] [PubMed] [Google Scholar]
- 16.Scaffidi C., Schmitz I., Zha J., Korsmeyer S. J., Krammer P. H., Peter M. E. (1999) J. Biol. Chem. 274, 22532–22538 [DOI] [PubMed] [Google Scholar]
- 17.Shawgo M. E., Shelton S. N., Robertson J. D. (2008) J. Biol. Chem. 283, 35532–35538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson J. D., Enoksson M., Suomela M., Zhivotovsky B., Orrenius S. (2002) J. Biol. Chem. 277, 29803–29809 [DOI] [PubMed] [Google Scholar]
- 19.Han Z., Hendrickson E. A., Bremner T. A., Wyche J. H. (1997) J. Biol. Chem. 272, 13432–13436 [DOI] [PubMed] [Google Scholar]
- 20.Liu H., Chang D. W., Yang X. (2005) J. Biol. Chem. 280, 11578–11582 [DOI] [PubMed] [Google Scholar]
- 21.Riedl S. J., Renatus M., Schwarzenbacher R., Zhou Q., Sun C., Fesik S. W., Liddington R. C., Salvesen G. S. (2001) Cell 104, 791–800 [DOI] [PubMed] [Google Scholar]
- 22.Denault J. B., Eckelman B. P., Shin H., Pop C., Salvesen G. S. (2007) Biochem. J. 405, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasula S. M., Ahmad M., Fernandes-Alnemri T., Alnemri E. S. (1998) Mol. Cell 1, 949–957 [DOI] [PubMed] [Google Scholar]
- 24.Franklin E. E., Robertson J. D. (2007) Biochem. J. 405, 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McStay G. P., Salvesen G. S., Green D. R. (2008) Cell Death Differ. 15, 322–331 [DOI] [PubMed] [Google Scholar]
- 26.Deveraux Q. L., Takahashi R., Salvesen G. S., Reed J. C. (1997) Nature 388, 300–304 [DOI] [PubMed] [Google Scholar]
- 27.Scott F. L., Denault J. B., Riedl S. J., Shin H., Renatus M., Salvesen G. S. (2005) EMBO J. 24, 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chai J., Shiozaki E., Srinivasula S. M., Wu Q., Datta P., Alnemri E. S., Shi Y. (2001) Cell 104, 769–780 [DOI] [PubMed] [Google Scholar]
- 29.Du C., Fang M., Li Y., Li L., Wang X. (2000) Cell 102, 33–42 [DOI] [PubMed] [Google Scholar]
- 30.Verhagen A. M., Ekert P. G., Pakusch M., Silke J., Connolly L. M., Reid G. E., Moritz R. L., Simpson R. J., Vaux D. L. (2000) Cell 102, 43–53 [DOI] [PubMed] [Google Scholar]
- 31.Chai J., Du C., Wu J. W., Kyin S., Wang X., Shi Y. (2000) Nature 406, 855–862 [DOI] [PubMed] [Google Scholar]
- 32.Srinivasula S. M., Hegde R., Saleh A., Datta P., Shiozaki E., Chai J., Lee R. A., Robbins P. D., Fernandes-Alnemri T., Shi Y., Alnemri E. S. (2001) Nature 410, 112–116 [DOI] [PubMed] [Google Scholar]
- 33.Wu G., Chai J., Suber T. L., Wu J. W., Du C., Wang X., Shi Y. (2000) Nature 408, 1008–1012 [DOI] [PubMed] [Google Scholar]
- 34.Li L., Thomas R. M., Suzuki H., De Brabander J. K., Wang X., Harran P. G. (2004) Science 305, 1471–1474 [DOI] [PubMed] [Google Scholar]
- 35.Chen D. J., Huerta S. (2009) Anticancer Drugs 20, 646–658 [DOI] [PubMed] [Google Scholar]
- 36.Wu T. Y., Wagner K. W., Bursulaya B., Schultz P. G., Deveraux Q. L. (2003) Chem. Biol. 10, 759–767 [DOI] [PubMed] [Google Scholar]
- 37.Park C. M., Sun C., Olejniczak E. T., Wilson A. E., Meadows R. P., Betz S. F., Elmore S. W., Fesik S. W. (2005) Bioorg. Med. Chem. Lett 15, 771–775 [DOI] [PubMed] [Google Scholar]
- 38.Sun H., Nikolovska-Coleska Z., Yang C. Y., Xu L., Tomita Y., Krajewski K., Roller P. P., Wang S. (2004) J. Med. Chem. 47, 4147–4150 [DOI] [PubMed] [Google Scholar]
- 39.Petersen S. L., Wang L., Yalcin-Chin A., Li L., Peyton M., Minna J., Harran P., Wang X. (2007) Cancer Cell 12, 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrucci E., Pasquini L., Petronelli A., Saulle E., Mariani G., Riccioni R., Biffoni M., Ferretti G., Benedetti-Panici P., Cognetti F., Scambia G., Humphreys R., Peschle C., Testa U. (2007) Gynecol. Oncol. 105, 481–492 [DOI] [PubMed] [Google Scholar]
- 41.Bockbrader K. M., Tan M., Sun Y. (2005) Oncogene 24, 7381–7388 [DOI] [PubMed] [Google Scholar]
- 42.Oost T. K., Sun C., Armstrong R. C., Al-Assaad A. S., Betz S. F., Deckwerth T. L., Ding H., Elmore S. W., Meadows R. P., Olejniczak E. T., Oleksijew A., Oltersdorf T., Rosenberg S. H., Shoemaker A. R., Tomaselli K. J., Zou H., Fesik S. W. (2004) J. Med. Chem. 47, 4417–4426 [DOI] [PubMed] [Google Scholar]
- 43.Fakler M., Loeder S., Vogler M., Schneider K., Jeremias I., Debatin K. M., Fulda S. (2009) Blood 113, 1710–1722 [DOI] [PubMed] [Google Scholar]
- 44.Vince J. E., Wong W. W., Khan N., Feltham R., Chau D., Ahmed A. U., Benetatos C. A., Chunduru S. K., Condon S. M., McKinlay M., Brink R., Leverkus M., Tergaonkar V., Schneider P., Callus B. A., Koentgen F., Vaux D. L., Silke J. (2007) Cell 131, 682–693 [DOI] [PubMed] [Google Scholar]
- 45.Varfolomeev E., Blankenship J. W., Wayson S. M., Fedorova A. V., Kayagaki N., Garg P., Zobel K., Dynek J. N., Elliott L. O., Wallweber H. J., Flygare J. A., Fairbrother W. J., Deshayes K., Dixit V. M., Vucic D. (2007) Cell 131, 669–681 [DOI] [PubMed] [Google Scholar]
- 46.Kaufmann T., Tai L., Ekert P. G., Huang D. C., Norris F., Lindemann R. K., Johnstone R. W., Dixit V. M., Strasser A. (2007) Cell 129, 423–433 [DOI] [PubMed] [Google Scholar]
- 47.McKenzie M. D., Carrington E. M., Kaufmann T., Strasser A., Huang D. C., Kay T. W., Allison J., Thomas H. E. (2008) Diabetes 57, 1284–1292 [DOI] [PubMed] [Google Scholar]
- 48.Yin X. M., Wang K., Gross A., Zhao Y., Zinkel S., Klocke B., Roth K. A., Korsmeyer S. J. (1999) Nature 400, 886–891 [DOI] [PubMed] [Google Scholar]
- 49.Zha J., Weiler S., Oh K. J., Wei M. C., Korsmeyer S. J. (2000) Science 290, 1761–1765 [DOI] [PubMed] [Google Scholar]
- 50.Adrain C., Creagh E. M., Martin S. J. (2001) EMBO J. 20, 6627–6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kandasamy K., Srinivasula S. M., Alnemri E. S., Thompson C. B., Korsmeyer S. J., Bryant J. L., Srivastava R. K. (2003) Cancer Res. 63, 1712–1721 [PubMed] [Google Scholar]
- 52.Jost P. J., Grabow S., Gray D., McKenzie M. D., Nachbur U., Huang D. C., Bouillet P., Thomas H. E., Borner C., Silke J., Strasser A., Kaufmann T. (2009) Nature 460, 1035–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]