Abstract
Chronic hepatitis C virus (HCV) infection is associated with altered lipid metabolism and hepatocellular steatosis. Virus-induced steatosis is a cytopathic effect of HCV replication. The goal of this study was to examine the mechanisms underlying HCV-induced lipid metabolic defects in a transgenic mouse model expressing the full HCV protein repertoire at levels corresponding to natural human infection. In this model, expression of the HCV full-length open reading frame was associated with hepatocellular steatosis and reduced plasma triglyceride levels. Triglyceride secretion was impaired, whereas lipogenesis was activated. Increased lipogenic enzyme transcription was observed, resulting from maturational activation and nuclear translocation of sterol regulatory element-binding protein 1c (SREBP1c). However, endoplasmic reticulum (ER) stress markers were expressed at similar levels in both HCV transgenic mice and their wild type counterparts, suggesting that SREBP1c proteolytic cleavage in the presence of HCV proteins was independent of ER stress. In conclusion, transgenic mice expressing the HCV full-length polyprotein at low levels have decreased plasma triglyceride levels and develop hepatocellular steatosis in the same way as HCV-infected patients. In these mice, SREBP1c activation by one or several HCV proteins induces de novo triglyceride synthesis via the lipogenic pathway, in a manner independent of ER stress, whereas triglyceride secretion is simultaneously reduced.
INTRODUCTION
Chronic hepatitis C virus (HCV)5 infection affects ∼170 million individuals worldwide and is associated with liver inflammation and fibrosis. Approximately 20% of patients develop cirrhosis, with a subsequent risk of lethal complications such as end stage liver disease and hepatocellular carcinoma (1). Chronic HCV infection is now the leading indication for liver transplantation in industrialized countries. Chronic hepatitis C is curable in about 50% of cases with a combination of pegylated interferon-α and ribavirin (1). New drugs, and particularly specific inhibitors of HCV functions, are in preclinical and clinical development (2).
Chronic HCV infection is also associated with altered lipid metabolism, resulting in low serum cholesterol, triglyceride, and betalipoprotein levels (3, 4). These abnormalities generally resolve after viral eradication (3, 5). Hepatocellular steatosis, i.e. excessive triglyceride accumulation within lipid droplets in the hepatocyte cytoplasm, is frequent in chronic hepatitis C (6). Steatosis may be due to metabolic disorders in overweight patients or those with diabetes, dyslipidemia, and/or chronic alcohol consumption (metabolic steatosis). It may also be directly related to virus replication (virus-induced steatosis), and both forms may be present together. Metabolic steatosis behaves in a similar way as in non-HCV-infected patients with nonalcoholic fatty liver disease or alcoholic liver disease and appears to influence the outcome of chronic liver disease and undermine the effectiveness of antiviral therapy (7, 8). Virus-induced steatosis is a cytopathic effect of HCV replication, but the precise underlying mechanisms are controversial. The influence of virus-induced steatosis on the outcome of liver disease and the response to therapy is unclear. Although HCV-induced steatosis is mostly seen in patients with HCV genotype 3a infection, in whom it correlates directly with the level of viral replication, cases have been reported in patients infected with other genotypes (9, 10). The reasons for these genotype differences are unclear. The level of virus expression required for excessive triglyceride accumulation in hepatocytes may be lower in genotype 3 HCV infection.
Viral interaction with host lipid metabolism appears to be essential for the HCV cell cycle. Indeed, HCV replication occurs in association with endoplasmic reticulum (ER)-derived membranes and lipid droplets, and localization of the HCV core and nonstructural 5A (NS5A) proteins to lipid storage organelles plays an important role (11–14). We recently showed that HCV core protein alters lipid droplet morphology (15). Whether or not these effects play a role in hepatocellular steatosis is unclear. However, HCV protein expression has been shown to induce steatosis in transgenic mice. In particular, we have previously shown that expression of the full HCV open reading frame at physiological levels triggers hepatocellular steatosis in C57BL6 mice (16). HCV protein expression thus appears sufficient to induce hepatocellular steatosis in the absence of infectious virus production. HCV protein expression might: (i) induce de novo triglyceride synthesis; (ii) reduce cellular triglyceride excretion; (iii) alter triglyceride oxidation; and/or (iv) increase triglyceride uptake from serum.
Sterol regulatory element-binding proteins (SREBP) belong to the basic helix-loop-helix-leucine zipper family of transcription factors. They are synthesized as precursor proteins bound to the ER. The SREBP1c isoform is predominant in the liver and enhances the transcription of genes involved in fatty acid synthesis. Foreign protein synthesis inside host cells creates nonspecific stress that triggers the unfolded protein response (UPR). During the UPR, SREBP1c is matured by two successive cleavage steps, and its transcription factor domain relocates to the nucleus, where it up-regulates numerous genes involved in lipid synthesis and maturation. However, the effect of HCV proteins on these processes is largely unknown. The goal of this study was to examine the mechanisms underlying HCV-induced lipid metabolic defects in a transgenic mouse model expressing the full HCV protein repertoire at levels corresponding to chronic human infection.
EXPERIMENTAL PROCEDURES
Animals
Animal housing was conducted in accordance with the Direction des Services Vétérinaires, Ministère de l'Agriculture of France, with European Communities Council Directive 86/609/EEC and with the Federation of European Laboratory Animal Science Associations for the health monitoring recommendations. Eight- to nine-month-old C57BL6 male mice transgenic for the HCV full-length open reading frame (FL-N/35 lineage) (16) were used in this study. Age-matched wild type male littermates were used as controls. The animals were housed in a temperature-controlled environment with a 12-h light/dark cycle and had free access to water and a regular diet (D04 from SAFE, Augy, France: 6.1% carbohydrate, 3.1% fat, and 15.8% protein). All of the procedures conformed to official French guidelines for the care and use of experimental animals. After sacrifice by CO2 intoxication, the liver tissue fragments were either immediately snap-frozen in liquid nitrogen and stored at −80 °C for analysis or fixed overnight (∼16 h) in neutral buffered formalin before transfer to 70% ethanol and embedding in paraffin. Sections 4–5 μm thick were deparaffinized and stained with hematoxylin and eosin for histological analysis.
Assessment of Hepatic Triglyceride and Apolipoprotein Content
Frozen tissue sections were stained with Oil-Red-O dye to identify neutral lipids. As previously described (17), serum triglycerides and apolipoprotein B (apoB) were quantified immediately before and 4 h after intraperitoneal injection of 30 mg of Triton WR 1339 (Tyloxapol; Sigma) in 24 h fasted mice by using the Trinder Enzymatic method for triglycerides (Biotrol, Earth City, MO) and kit 357 for apoB (Sigma).
Assessment of Microsomal Triglyceride Transfer Protein Activity
Microsomal triglyceride transfer (MTP) protein activity was measured with a fluorometric assay (MTP kit; Roar Biomedical, New York, NY) that detects MTP-mediated transfer of neutral lipids in cell lysates or tissue homogenates, following the manufacturer's instructions. The liver samples were homogenized with a Dounce homogenizer in 15 mm Tris, pH 7.4, 40 mm NaCl, 1 mm EDTA, plus protease inhibitors (phenylmethylsulfonyl fluoride (1 mm), benzamidine (1 mm), dithiothreitol (1 mm), leupeptin (2 μg/ml), and aprotinin (2 μg/ml). MTP assay was performed by incubating 100 μg of liver protein homogenate (MTP source) with donor and acceptor solutions for 4 h at 37 °C. MTP activity was determined by measuring fluorescence at 465 nm (excitation) and 538 nm (emission).
Total RNA Isolation and RT-qPCR
Total RNA was isolated from mouse livers as previously described (18). RNA quality and quantity were determined using a 2100 Bioanalyser and RNA Nano Chips (Agilent Technologies, Santa Clara, CA). RNA integrity number was calculated using the Agilent software, and samples displaying a RNA integrity number below 6 were discarded (see supplemental Table S1). One μg of RNA was reverse-transcribed with the Superscript IITM enzyme (Invitrogen). Real time quantitative PCR was performed with 25 ng of cDNA and 250 nm sense and antisense primers (Eurogentec, Seraing, Belgium) in a final reaction volume of 25 μl, using the qPCR Core Kit (Eurogentec) and the MyiQ real time PCR detection system (Bio-Rad). Specific primers were designed with Primer Express software (Table 1). The expression level of each studied gene was normalized to that of 18 S ribosomal RNA by using the comparative CT method.
TABLE 1.
Sequences of the primers used for quantitative real time PCR
| SREBP-1c | 5′-ggagccatggattgcacatt-3′ |
| 5′-ggcccgggaagtcactgt-3′ | |
| ChREBP | 5′-gtccgatatctccgacacactctt-3′ |
| 5′-cattgccaacataagcgtcttctg-3′ | |
| FAS | 5′-tgctcccagctgcaggc-3′ |
| 5′-gcccggtagctctgggtgta-3′ | |
| Stearoyl-CoA desaturase 1 | 5′-acctgcctcttcgggatttt-3′ |
| 5′-gtcggcgtgtgtttctgaga-3′ | |
| Malic enzyme | 5′-gggcatccctgtgggtaaa-3′ |
| 5′-gaaggcgtcatactcagggc-3′ | |
| ATP citrate-lyase | 5′-ctccaaactgtaccgccca-3′ |
| 5′-ggagtgtcctggtagcgcag-3′ | |
| Acetyl-CoA carboxylase | 5′-tgggcacagaccgtggtag-3′ |
| 5′-gtcttaaatgcagagtctgggaa-3′ | |
| GRP78 | 5′-gaaaggatggttaatgatgctgag-3′ |
| 5′-gtcttcaatgtccgcatcctg-3′ | |
| TRB3 | 5′-ctctgaggctccaggacaag-3′ |
| 5′-ggctcaggctcatctctcac-3′ | |
| EDEM | 5′-ggatcccctatccctcgggt-3′ |
| 5′-gttgctccgcaagttccag-3′ | |
| CHOP | 5′-catacaccaccacacctgaaag-3′ |
| 5′-ccgtttcctagttcttccttgc-3′ | |
| Protein-disulfide isomerase | 5′-aacgggagaagccattgta-3′ |
| 5′-aggtgtcatccgtcagctct-3′ |
XBP1 RT-PCR Splicing Assay
To analyze XBP1 mRNA splicing, 200 ng of cDNA was amplified with a pair of primers specific for the mouse XBP1 gene (sense, 5′-GGCCTTGTGGTTGAGAACCAGGAG-3′; antisense, 5′-GAATGCCCAAAAGGATATCAGACTC-3′). The PCR conditions are described in the Ron lab website. PCR products were separated by electrophoresis on 2.5% agarose gels and visualized by ethidium bromide staining.
Preparation of Cytoplasmic, Microsomal, and Nuclear Extracts and Immunoblot Analysis
Nuclear and cytoplasmic extracts were prepared from livers of wild type and transgenic mice by using the NE-PER nuclear and cytoplasmic extraction reagent kit (Pierce), following the manufacturer's instructions. For microsomal extracts, mouse liver was homogenized in sucrose buffer (0.25 m sucrose, 10 mm HEPES, 3 mm MgCl2 protease inhibitors). The homogenate was centrifuged (500 × g for 5 min at 4 °C), and the resulting supernatant was centrifuged again at 100,000 × g for 45 min at 4 °C. Isolated microsomes were resuspended in microsomal extraction buffer (20 mm HEPES, 100 mm NaCl2,1 mm EDTA, 1 mm EGTA, protease inhibitors).
The protein concentration was measured in the extracts by means of the Bradford method (Bio-Rad) with bovine serum albumin as standard. Proteins (20–70 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes (GE Healthcare). The following primary antibodies were used: mouse monoclonal antibodies against SREBP1 clone 2A4 (Thermo Fisher Scientific, Waltham, MA), activating transcription factor 6 (ATF6) (Imgenex, San Diego, CA), and X-box-binding protein 1 (XBP1) (Prosci Inc., Poway, CA); rat polyclonal antibodies against immunoglobulin heavy chain-binding protein glucose-regulated protein 78 (GRP78); and rabbit polyclonal antibodies against phosphoprotein kinase-like endoplasmic reticulum kinase (PERK) (Santa Cruz Biotechnology, Santa Cruz, CA), and phospho-(Ser51)-eukaryotic initiation factor 2α (eIF2α), total Akt/protein kinase B, and eIF2α (Cell Signaling Technology, Beverly, MA). The membranes were incubated with the corresponding secondary antibodies coupled to horseradish peroxidase. Labeled antibodies were detected with the ECL Plus or Advance detection kits (GE Healthcare). A polyclonal mouse lamin A/C antibody (BD Biosciences, Franklin Lakes, NJ), calnexin, and β-actin were used as loading controls for nuclear extracts, microsomal fractions, and total lysates, respectively.
Statistical Analysis
The results are expressed as the means ± S.E. The Fisher-Yates Terry or Mann-Whitney nonparametric rank tests were used, as appropriate. Quantitative results are presented as box plots (median and 95th percentiles).
RESULTS
Expression of the HCV Full-length Open Reading Frame Is Associated with Hepatocellular Steatosis and Reduced Plasma Triglyceride Concentrations in Transgenic Mice
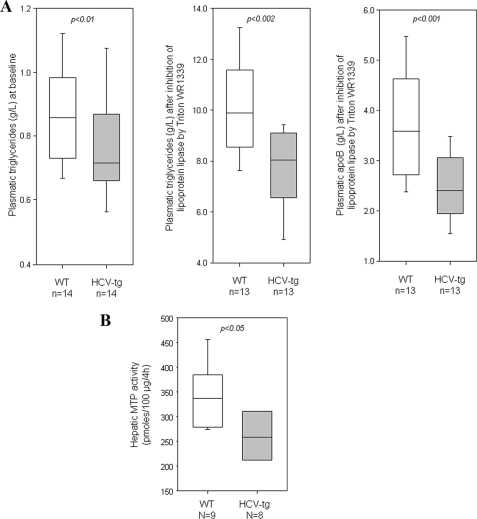
Steatosis was scored after hematoxylin-eosin and Oil-Red-O staining of liver tissues. Steatosis was considered absent, mild, moderate, and severe when 0, <30, 30–60, and >60%, respectively, of hepatocytes were positively stained. Although C57Bl6 mice have a natural propensity to develop liver steatosis (19), moderate to severe hepatocellular steatosis was more frequent in FL-N/35 transgenic mice than in controls (78% versus 29%, p < 0.01). This finding confirmed that the colony housed and fed as described above developed the same phenotype as initially reported (16). In addition, plasma triglyceride levels were moderately but significantly lower in transgenic mice than in controls (0.77 ± 0.04 versus 0.86 ± 0.04 g/liter, p < 0.01; Fig. 1A, left panel).
FIGURE 1.
A, hepatic secretion of VLDL, based on plasma triglyceride levels in fasted wild type (WT) males and HCV transgenic age-matched male littermates (HCV-tg) at base line (left panel) and after lipoprotein lipase inhibition by Triton WR 1339 (middle panel) and plasma levels of apoB after Triton WR 1339 treatment (right panel). B, MTP activity in crude liver extracts from WT and HCV-tg mice. Box-and-whisker graphs are used; the line in the middle is the median, the box extends from the 25th to the 75th percentile, and the whiskers extend to the lowest and highest values. The values in WT and HCV-tg mice were compared with the Mann and Whitney test.
Triglyceride Secretion Is Impaired in Mice Transgenic for the HCV Full-length Open Reading Frame
Triglycerides are secreted from liver cells as very low density lipoprotein (VLDL) particles. Hepatic VLDL secretion was assessed in fasted mice by measuring the increase in plasma triglyceride and apoB levels after lipoprotein lipase inhibition by Triton WR 1339. As shown in Fig. 1A, Triton treatment substantially increased the plasma triglyceride level. Plasma triglyceride levels were ∼30% lower in FL-N/35 transgenic mice than in controls (7.7 ± 0.4 versus 10.0 ± 0.5 g/liter, p < 0.002), pointing to defective VLDL secretion (Fig. 1A). This was confirmed by the significantly lower plasma level of apoB (a protein mainly present in VLDL and LDL) in transgenic mice than in controls after Triton treatment (2.5 ± 0.2 versus 3.7 ± 0.3 g/L, p < 0.001; Fig. 1A).
MTP plays a key role in cotranslocational lipidation of apoB as it enters the ER lumen (thus preventing apoB degradation) and participates in the conversion of a precursor particle to VLDL by the addition of bulk triglycerides. As shown in Fig. 1B, MTP activity was significantly lower in crude liver extracts from FL-N/35 transgenic mice than from controls (p < 0.05), confirming the impaired triglyceride excretion.
Lipogenesis Is Activated in Mice Transgenic for the HCV Full-length Open Reading Frame
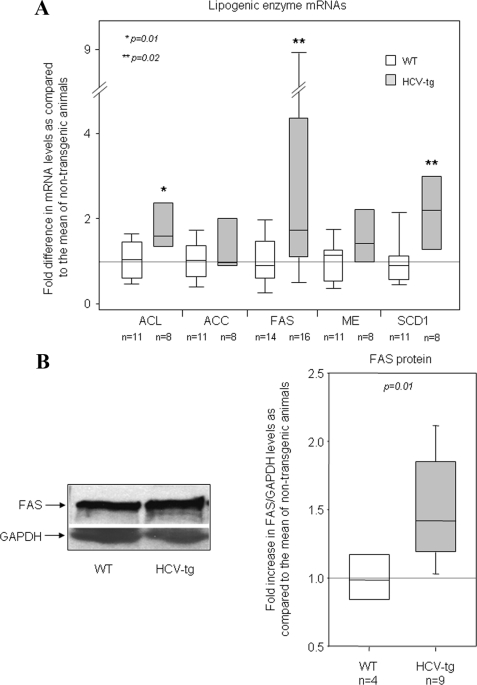
To determine whether the lipogenic pathway is activated in HCV transgenic mice, hepatic transcriptional levels of genes coding for various enzymes involved in lipogenesis were compared with values in nontransgenic controls. These genes included ATP citrate-lyase, fatty acid synthase (FAS), acetyl-CoA carboxylase, malic enzyme, and hepatic stearoyl-CoA desaturase 1. Transcript levels of all these enzymes were higher in transgenic mice than in controls, as measured by RT-qPCR (Fig. 2A). The difference was statistically significant for ATP citrate-lyase, FAS, and stearoyl-CoA desaturase 1 (fold increase compared with the mean values in nontransgenic animals: 2.2 ± 0.6, p = 0.01; 2.9 ± 0.7, p = 0.02; and 2.0 ± 0.4, p = 0.02 respectively), whereas a consistent but nonsignificant trend was observed for acetyl-CoA carboxylase and malic enzyme. FAS protein expression, quantified by Western blotting of mouse liver extracts, was significantly higher in the liver of FL-N/35 transgenic mice than in controls (Fig. 2B). Together, these results suggest that the lipogenic pathway is activated in the liver of transgenic mice expressing the HCV full-length open reading frame.
FIGURE 2.
A, gene expression of ATP citrate-lyase (ACL), acetyl-CoA carboxylase (ACC), FAS, malic enzyme (ME), and hepatic stearoyl-CoA desaturase 1 (SCD1), assessed by RT-qPCR in the livers of WT mice and HCV transgenic (HCV-tg) littermates (all males, 8–9 months old). The results were normalized to the mean expression level in WT liver. B, FAS protein expression, assessed by Western blotting. The left-hand panel shows a representative blot. Variations between samples were normalized on the basis of GAPDH expression. The results were normalized to the mean expression level in WT liver. Box-and-whisker graphs are used. The values in WT and HCV-tg mice were compared with the Mann and Whitney test.
SREBP1c Is Activated in Mice Transgenic for the HCV Full-length Open Reading Frame
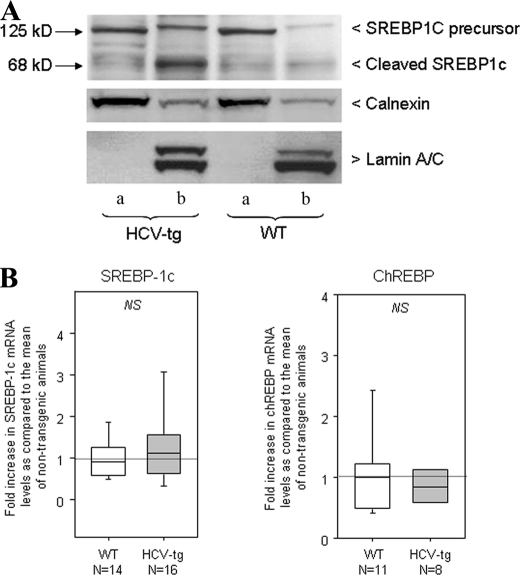
In the liver, lipogenic enzymes are transcriptionally regulated by insulin and glucose via the actions of two transcription factors: SREBP1c and carbohydrate response element-binding protein (ChREBP). To assess SREBP1c expression, nuclear and microsomal protein fractions were purified from liver extracts of transgenic and nontransgenic animals and were analyzed by Western blotting. Lamin A/C and calnexin served as loading controls for the nuclei and microsomes, respectively. As shown in Fig. 3A, microsomal fractions from transgenic and nontransgenic animals contained similar amounts of SREBP1c precursor. In contrast, mature cleaved SREBP1c was substantially more abundant in nuclear extracts from transgenic animals than from controls (Fig. 3A), suggesting that increased lipogenic enzyme transcription in HCV transgenic mice resulted from activation and nuclear translocation of SREBP1c. The expression of ChREBP, a transcription factor involved in the activation of glycolytic and lipogenic genes, was not modulated by the HCV transgene (data not shown).
FIGURE 3.
A, Western blot analysis showing precursor and cleaved SREBP1c expression in WT mice and HCV transgenic littermates (HCV-tg) in liver cell microsome extracts (lanes a) and liver cell nuclear extracts (lanes b). Lamin A/C and calnexin were used as loading and fraction purity controls. B, SREBP1c and ChREBP mRNA levels assessed by RT-qPCR in the liver of WT and HCV-tg mice. The results were normalized to the mean expression level in WT liver. Box-and-whisker graphs are used. The values in WT and HCV-tg mice were compared with the Mann and Whitney test.
As shown in Fig. 3B, the level of SREBP1c mRNA was slightly but not significantly higher in the liver of transgenic mice than in controls (fold increase compared with the mean control value: 1.3 ± 0.2 versus 1.0 ± 0.1, p = 0.39), suggesting that SREBP1c activation by HCV proteins occurs during protein maturation rather than through increased transcription. Similarly, the expression of ChREBP transcripts was not modulated by the HCV transgene (Fig. 3B).
ER Stress Is Not Induced in Mice Transgenic for the HCV Full-length Open Reading Frame
Eukaryotic cells respond to the accumulation of misfolded, unfolded or foreign proteins (including viral proteins) in the ER by activating a signaling pathway known as the UPR. Because HCV proteins are synthesized on ER membranes and remain closely associated with ER-derived membranous webs, we examined whether HCV protein expression could trigger an unfolded protein response in HCV transgenic mice.
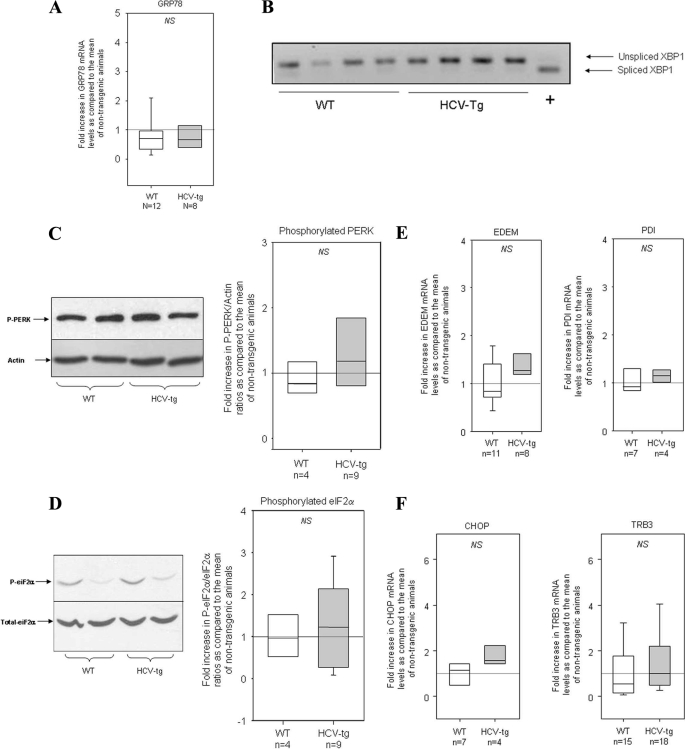
We first measured GRP78 mRNA levels. As shown in Fig. 4A, no significant difference was found between transgenic and nontransgenic mice. IRE1 release from GRP78 activates IRE1 RNase activity and initiates mRNA splicing of XBP1, thus generating a spliced mRNA encoding a transcription factor that activates UPR target genes. We screened total RNA extracts from transgenic and nontransgenic livers for spliced XBP1 mRNA by means of RT-PCR and observed no modulation of XBP1 splicing by the HCV transgene (Fig. 4B).
FIGURE 4.
A, GRP78 mRNA levels assessed by RT-qPCR in the liver of WT mice and HCV transgenic littermates (HCV-tg). The results were normalized to the mean expression level in WT liver. Box-and-whisker graphs are used. Values in WT and HCV-tg mice were compared with the Mann and Whitney test. B, unspliced and spliced XBP1 mRNA transcripts in liver RNA extracts from WT and HCV-tg mice, as assessed by RNA size discrimination on agarose gel. A positive control (+) showing spliced XBP1 was migrated in parallel. C, respective amounts of the phosphorylated form of PERK, as assessed by Western blot, in WT and HCV-tg mice. Left panel, representative Western blot; right panel, box-and-whisker graphs. The values in WT and HCV-tg mice were compared with the Mann and Whitney test. D, respective amounts of the phosphorylated form of eIF2α, as assessed by Western blot, in WT and HCV-tg mice. Left panel, representative Western blot; right panel, box-and-whisker graphs. The values in WT and HCV-tg mice were compared with the Mann and Whitney test. E, EDEM and protein-disulfide isomerase mRNA levels assessed by RT-qPCR in the liver of WT and HCV-tg mice. The results were normalized to the mean expression level in WT liver. Box-and-whisker graphs are used. The values in WT and HCV-tg mice were compared with the Mann and Whitney test. F, CHOP and TRB3 mRNA levels assessed by RT-qPCR in the liver of WT and HCV-tg mice. The results were normalized to the mean expression level in WT liver. Box-and-whisker graphs are used. The values in WT and HCV-tg mice were compared with the Mann and Whitney test.
PERK release from GRP78 induces PERK autophosphorylation and activation. Activated PERK in turn phosphorylates eIF2α, thereby reducing mRNA translation initiation. We measured phosphorylated PERK and eIF2α by Western blotting with specific antibodies, normalizing the results to the respective total amounts of the two proteins. As shown in Fig. 4 (C and D), respectively, we found no difference in the levels of phosphorylated PERK and eIF2α between transgenic and nontransgenic animals.
Protein-disulfide isomerase is another chaperone involved in the ER-associated degradation pathway. ER degradation-enhancing mannosidase-like (EDEM) proteins target UPR-inducing proteins and trigger their degradation. As shown in Fig. 4E, quantitative RT-PCR revealed no significant differences in EDEM or protein-disulfide isomerase mRNA levels between HCV transgenic and nontransgenic mice. C/EBP (CCAAT- enhancer-binding protein) homologous protein (CHOP), also known as GADD153 (growth arrest- and DNA damage-inducible gene 153), is a component of the ER stress-mediated apoptosis pathway. TRB3 is induced by ER stress downstream of CHOP and was recently identified as a potential pro-apoptotic protein that can modulate the Akt/protein kinase B-dependent signaling pathway. As shown in Fig. 4F, CHOP and TRB3 expression levels did not differ between HCV transgenic and nontransgenic mice, although there was a trend toward higher expression of CHOP transcripts in transgenic mice (p < 0.06).
Thus, none of the ER stress markers tested here was expressed at significantly higher levels in HCV transgenic mice than in their wild type counterparts. This suggests that SREBP1c proteolytic cleavage in transgenic mice expressing the HCV full-length open reading frame is independent of ER stress.
DISCUSSION
The mechanisms by which chronic HCV infection perturbs lipid metabolism are poorly understood. Intracellular virion production appears to use metabolic pathways involved in lipid metabolism (11–15). Chronically HCV-infected patients often have low plasma cholesterol, triglyceride, and betalipoprotein levels (3, 20). In addition, virus-induced steatosis appears to be a direct cytopathic lesion induced preferentially but not exclusively by genotype 3a HCV (21, 22). A direct role of HCV is supported by the disappearance of these abnormalities when infection is cured by antiviral therapy and by their reappearance after HCV infection of the donor liver in HCV-infected transplant recipients (23–25). The precise role of HCV protein expression in these phenomena is unclear.
The transgenic mice used here, which expresses the HCV full-length open reading frame, are a particularly relevant model for studying HCV protein-induced abnormalities, because all of the HCV proteins are produced at the same time at physiologically relevant levels, without infectious virus production (16, 26–28). We have previously reported that two lineages of transgenic mice expressing the HCV full-length open reading frame develop more frequent and more severe hepatocellular steatosis than their nontransgenic littermates (16). In the present study, chronic expression of the entire panel of HCV proteins in mice receiving a normal diet was associated with lower plasma triglyceride levels and with microvesicular steatosis preferentially located in the central vein area, as previously demonstrated in human HCV infection (29, 30).
Several mutually nonexclusive mechanisms could explain these observations. HCV protein expression could (i) induce de novo triglyceride synthesis; (ii) reduce cellular triglyceride secretion; (iii) alter triglyceride degradation; and/or (iv) increase triglyceride uptake from serum. Here we tested the first two hypotheses in transgenic mice expressing the HCV full-length open reading frame. Taken together, our results suggest that HCV protein expression induces de novo triglyceride secretion independently of ER stress, while concurrently reducing triglyceride secretion.
Triglyceride synthesis from acetyl-CoA is a complex multistep process that includes lipogenesis, desaturation, elongation, and esterification. These steps are catalyzed by different enzymes, expression of which was higher in the transgenic mice expressing the HCV full-length open reading frame than in nontransgenic controls (Fig. 3A). These findings suggest that de novo triglyceride synthesis induced by lipogenic pathway activation could at least partly explain the intracytoplasmic triglyceride accumulation observed in hepatocytes expressing HCV proteins. FAS also plays a central role in triglyceride synthesis. The increased FAS expression observed here in vivo, at both the mRNA and protein levels, is in keeping with recent results obtained with cellular models. Indeed, FAS has been shown to be up-regulated in Huh-7 cells after transient expression of HCV core protein, after transfection with a full-length genome genotype 1b HCV replicon, and during infection by genotype 2a JFH1 virus (31, 32). In addition, a Tet-regulated HeLa cell model stably expressing the HCV core protein has been developed.6 Core expression in this model was associated with microvesicular steatosis. Importantly, core protein expression did not increase lipid uptake in HeLa cells, which do not express MTP.
FAS expression is mainly regulated at the transcriptional level by SREBP1. FAS-SREBP1 pathway alteration has been shown to be associated with steatosis in vivo (33). We observed increased levels of mature SREBP1c in the nucleus of HCV transgenic mice, compared with nontransgenic controls, possibly explaining the enhanced FAS mRNA expression in the transgenic mice. This is in keeping with the recent report that SREBP1c proteolytic cleavage is induced by JFH1 infection in Huh-7 cells (34). Likewise, a recent study comparing liver biopsy specimens from HCV-infected and uninfected patients showed no difference in hepatic SREBP1c mRNA levels, but SREBP1c protein levels were not studied (35). A role of HCV core, NS2 and NS4B proteins in SREBP1c proteolytic cleavage has been suggested based on transient overexpression of sequences derived from different HCV genotypes (34, 36, 37). However, the viral protein(s) responsible for SREBP1c activation remain to be identified. Finally, contrary to SREBP1c, ChREBP, another potent regulator of FAS expression at the transcriptional level (38), was not involved in lipogenic pathway activation in our HCV transgenic mice.
SREBP-1c is a potent negative regulator of MTP expression (39). HCV protein activation of SREBP1c could thus down-regulate MTP activity, thereby explaining the decreased MTP activity that we observed in transgenic mice expressing the HCV full-length open reading frame. Reduced MTP activity could in turn be responsible for the defective VLDL secretion which, in combination with increased triglyceride synthesis, participates in hepatocyte triglyceride accumulation. It is noteworthy in this respect that reduced plasma MTP activity has also been reported in HCV-infected patients (40). Transgenic mice overexpressing HCV core protein also show reduced MTP activity (17), but MTP mRNA levels and activity are also reduced in the HCV subgenomic replicon model, which lacks HCV core protein (41).
Before concluding that de novo triglyceride synthesis mediated by enhanced FAS expression results from direct SREBP1c activation by HCV protein(s), it was important to eliminate a potential role of viral protein-induced ER stress. The unfolded protein response is triggered by the accumulation of unfolded, misfolded, or foreign proteins within the ER. Thus, HCV protein expression and accumulation in transgenic hepatocytes could trigger ER stress, triggering nonspecific SREBP1c cleavage and activation. We therefore studied the three principal ER stress pathways, driven by PERK, ATF6, and IRE1, under the control of GRP78. None was found to be activated in transgenic hepatocytes: GRP78 expression, PERK and eIF2α phosphorylation, and XBP-1 mRNA splicing were similar in HCV transgenic and nontransgenic livers. These findings appear to conflict with previous reports. Indeed, ectopic expression of HCV envelope glycoproteins E1 and E2 has been reported to increase GRP78 expression (42). However, in these experiments, a large proportion of E1 and E2 proteins were trapped in the ER as aggregates, a phenomenon that does not occur during natural infection. Likewise, GRP78 expression has been reported to be induced in the replicon system, a replicative model based on Huh-7 cells. However, GRP78 induction was three times higher than in the same cells expressing HCV nonstructural proteins in the absence of replication, and HCV replication contributed to stimulating ER chaperone expression (43). Finally, the E2 envelope glycoprotein has been reported to bind PERK as a pseudosubstrate, to inhibit its autophosphorylation, and to sequester it from its substrate eIF2α, potentially masking downstream ER stress events (44). However, high E2 expression was required for this inhibition to occur. In addition, The FL-N/35 lineage is characterized by its very low level of HCV protein expression. Indeed, we were only able to demonstrate transgene expression within the mouse liver by using very sensitive immunochemical methods (26). Therefore, our findings conclusively show that HCV protein expression stimulates triglyceride synthesis independently of nonspecific ER stress activation.
In conclusion, transgenic mice expressing the HCV full-length polyprotein at low, physiological levels have decreased plasma triglyceride levels and develop hepatocellular steatosis in the same way as HCV-infected patients (summarized in Fig. 5). In these transgenic mice, de novo triglyceride synthesis is induced by direct SREBP1c activation by one or several HCV proteins through induction of the lipogenic pathway, independently of ER stress, whereas triglyceride secretion is simultaneously reduced. The HCV protein(s) responsible for these perturbations of lipid metabolism remain to be identified, along with the underlying molecular mechanisms.
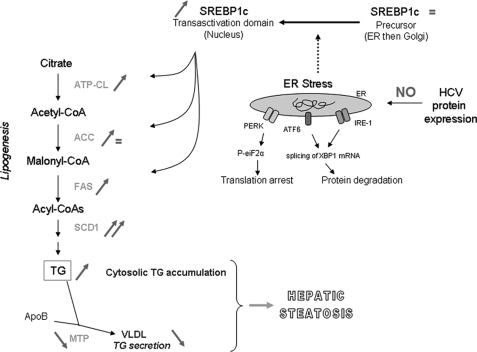
FIGURE 5.
Schematic representation of lipogenesis induction and VLDL secretion default in HCV transgenic mice. Red signs show HCV protein expression effects. We demonstrated for the first time, in a single expression model, that increased MTP activity, VLDL secretion default, lipogenesis stimulation, and SREBP1c cleavage and relocation into the nucleus occur specifically and concurrently in livers expressing HCV proteins. ER stress has been demonstrated to induce the cleavage of SREBP1c (dotted line), although we found no evidence of such stress in our studies. The basis of SREPB1c cleavage in the context of HCV protein expression remains unclear.
Supplementary Material
Acknowledgment
We thank the Center for Exploration and Experimental Functional Research (Genopole, Evry, France) for animal housing and care.
This work was supported, in whole or in part, by National Institutes of Health Grant U19-AI40035.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
K. Li and S. M. Lemon, unpublished data.
- HCV
- hepatitis C virus
- SREBP
- sterol regulatory element-binding protein(s)
- ER
- endoplasmic reticulum
- UPR
- unfolded protein response
- apoB
- apolipoprotein B
- MTP
- microsomal triglyceride transfer protein
- RT-qPCR
- reverse transcription-quantitative PCR
- XBP
- X-box-binding protein
- PERK
- phosphoprotein kinase-like endoplasmic reticulum kinase
- eIF
- eukaryotic initiation factor
- VLDL
- very low density lipoprotein
- FAS
- fatty acid synthase
- ChREBP
- carbohydrate response element-binding protein
- EDEM
- ER degradation-enhancing mannosidase-like
- CHOP
- CCAAT-enhancer-binding protein (C/EBP) homologous protein
- WT
- wild type.
REFERENCES
- 1.The NIH Consensus Development Panel (2002) Hepatology 36, (Suppl. 1) S3–S2012407572 [Google Scholar]
- 2.Pawlotsky J. M., Chevaliez S., McHutchison J. G. (2007) Gastroenterology 132, 1979–1998 [DOI] [PubMed] [Google Scholar]
- 3.Serfaty L., Andreani T., Giral P., Carbonell N., Chazouillères O., Poupon R. (2001) J. Hepatol. 34, 428–434 [DOI] [PubMed] [Google Scholar]
- 4.Siagris D., Christofidou M., Theocharis G. J., Pagoni N., Papadimitriou C., Lekkou A., Thomopoulos K., Starakis I., Tsamandas A. C., Labropoulou-Karatza C. (2006) J. Viral Hepatitis 13, 56–61 [DOI] [PubMed] [Google Scholar]
- 5.Tada S., Saito H., Ebinuma H., Ojiro K., Yamagishi Y., Kumagai N., Inagaki Y., Masuda T., Nishida J., Takahashi M., Nagata H., Hibi T. (2009) Hepatol. Res. 39, 195–199 [DOI] [PubMed] [Google Scholar]
- 6.Bach N., Thung S. N., Schaffner F. (1992) Hepatology 15, 572–577 [DOI] [PubMed] [Google Scholar]
- 7.Castéra L., Hézode C., Roudot-Thoraval F., Bastie A., Zafrani E. S., Pawlotsky J. M., Dhumeaux D. (2003) Gut 52, 288–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serfaty L., Mathurin P., Cadranel J. F., Tran A. (2007) Gastroenterol. Clin. Biol. 31, 4S40–43 [PubMed] [Google Scholar]
- 9.Castera L., Chouteau P., Hezode C., Zafrani E. S., Dhumeaux D., Pawlotsky J. M. (2005) Am. J. Gastroenterol. 100, 711–715 [DOI] [PubMed] [Google Scholar]
- 10.Hezode C., Roudot-Thoraval F., Zafrani E. S., Dhumeaux D., Pawlotsky J. M. (2004) J. Viral Hepatitis 11, 455–458 [DOI] [PubMed] [Google Scholar]
- 11.Boulant S., Targett-Adams P., McLauchlan J. (2007) J. Gen. Virol. 88, 2204–2213 [DOI] [PubMed] [Google Scholar]
- 12.Miyanari Y., Atsuzawa K., Usuda N., Watashi K., Hishiki T., Zayas M., Bartenschlager R., Wakita T., Hijikata M., Shimotohno K. (2007) Nat. Cell Biol. 9, 1089–1097 [DOI] [PubMed] [Google Scholar]
- 13.Shavinskaya A., Boulant S., Penin F., McLauchlan J., Bartenschlager R. (2007) J. Biol. Chem. 282, 37158–37169 [DOI] [PubMed] [Google Scholar]
- 14.Appel N., Zayas M., Miller S., Krijnse-Locker J., Schaller T., Friebe P., Kallis S., Engel U., Bartenschlager R. (2008) PLoS Pathogens 4, e1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piodi A., Chouteau P., Lerat H., Hézode C., Pawlotsky J. M. (2008) Hepatology 48, 16–27 [DOI] [PubMed] [Google Scholar]
- 16.Lerat H., Honda M., Beard M. R., Loesch K., Sun J., Yang Y., Okuda M., Gosert R., Xiao S. Y., Weinman S. A., Lemon S. M. (2002) Gastroenterology 122, 352–365 [DOI] [PubMed] [Google Scholar]
- 17.Perlemuter G., Sabile A., Letteron P., Vona G., Topilco A., Chrétien Y., Koike K., Pessayre D., Chapman J., Barba G., Bréchot C. (2002) FASEB J. 16, 185–194 [DOI] [PubMed] [Google Scholar]
- 18.Hegarty B. D., Bobard A., Hainault I., Ferré P., Bossard P., Foufelle F. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blake W. L., Ulrich R. G., Marotti K. R., Melchior G. W. (1994) Biochem. Biophys. Res. Commun. 205, 1257–1263 [DOI] [PubMed] [Google Scholar]
- 20.Petit J. M., Benichou M., Duvillard L., Jooste V., Bour J. B., Minello A., Verges B., Brun J. M., Gambert P., Hillon P. (2003) Am. J. Gastroenterol. 98, 1150–1154 [DOI] [PubMed] [Google Scholar]
- 21.Kumar D., Farrell G. C., Fung C., George J. (2002) Hepatology 36, 1266–1272 [DOI] [PubMed] [Google Scholar]
- 22.Negro F. (2002) Hepatology 36, 1050–1052 [DOI] [PubMed] [Google Scholar]
- 23.Poynard T., Ratziu V., McHutchison J., Manns M., Goodman Z., Zeuzem S., Younossi Z., Albrecht J. (2003) Hepatology 38, 75–85 [DOI] [PubMed] [Google Scholar]
- 24.Rubbia-Brandt L., Giostra E., Mentha G., Quadri R., Negro F. (2001) J. Hepatol. 35, 307. [DOI] [PubMed] [Google Scholar]
- 25.Baiocchi L., Tisone G., Palmieri G., Rapicetta M., Pisani F., Orlando G., Casciani C. U., Angelico M. (1998) Liver Transpl. Surg. 4, 441–447 [DOI] [PubMed] [Google Scholar]
- 26.Keasler V. V., Lerat H., Madden C. R., Finegold M. J., McGarvey M. J., Mohammed E. M., Forbes S. J., Lemon S. M., Hadsell D. L., Grona S. J., Hollinger F. B., Slagle B. L. (2006) Virology 347, 466–475 [DOI] [PubMed] [Google Scholar]
- 27.Disson O., Haouzi D., Desagher S., Loesch K., Hahne M., Kremer E. J., Jacquet C., Lemon S. M., Hibner U., Lerat H. (2004) Gastroenterology 126, 859–872 [DOI] [PubMed] [Google Scholar]
- 28.Erdtmann L., Franck N., Lerat H., Le Seyec J., Gilot D., Cannie I., Gripon P., Hibner U., Guguen-Guillouzo C. (2003) J. Biol. Chem. 278, 18256–18264 [DOI] [PubMed] [Google Scholar]
- 29.Gerber M. A. (1997) Clinics Liver Dis. 1, 529–541 [DOI] [PubMed] [Google Scholar]
- 30.Dai C. Y., Chuang W. L., Ho C. K., Hsieh M. Y., Huang J. F., Lee L. P., Hou N. J., Lin Z. Y., Chen S. C., Hsieh M. Y., Wang L. Y., Tsai J. F., Chang W. Y., Yu M. L. (2008) J. Hepatol. 49, 9–16 [DOI] [PubMed] [Google Scholar]
- 31.Jackel-Cram C., Babiuk L. A., Liu Q. (2007) J. Hepatol. 46, 999–1008 [DOI] [PubMed] [Google Scholar]
- 32.Yang W., Hood B. L., Chadwick S. L., Liu S., Watkins S. C., Luo G., Conrads T. P., Wang T. (2008) Hepatology 48, 1396–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horton J. D., Goldstein J. L., Brown M. S. (2002) J. Clin. Invest. 109, 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waris G., Felmlee D. J., Negro F., Siddiqui A. (2007) J. Virol. 81, 8122–8130 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.McPherson S., Jonsson J. R., Barrie H. D., O'Rourke P., Clouston A. D., Powell E. E. (2008) J. Hepatol. 49, 1046–1054 [DOI] [PubMed] [Google Scholar]
- 36.Kim K. H., Hong S. P., Kim K., Park M. J., Kim K. J., Cheong J. (2007) Biochem. Biophys. Res. Commun. 355, 883–888 [DOI] [PubMed] [Google Scholar]
- 37.Oem J. K., Jackel-Cram C., Li Y. P., Zhou Y., Zhong J., Shimano H., Babiuk L. A., Liu Q. (2008) J. Gen. Virol. 89, 1225–1230 [DOI] [PubMed] [Google Scholar]
- 38.Ishii S., Iizuka K., Miller B. C., Uyeda K. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15597–15602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato R., Miyamoto W., Inoue J., Terada T., Imanaka T., Maeda M. (1999) J. Biol. Chem. 274, 24714–24720 [DOI] [PubMed] [Google Scholar]
- 40.Mirandola S., Realdon S., Iqbal J., Gerotto M., Dal Pero F., Bortoletto G., Marcolongo M., Vario A., Datz C., Hussain M. M., Alberti A. (2006) Gastroenterology 130, 1661–1669 [DOI] [PubMed] [Google Scholar]
- 41.Domitrovich A. M., Felmlee D. J., Siddiqui A. (2005) J. Biol. Chem. 280, 39802–39808 [DOI] [PubMed] [Google Scholar]
- 42.Choukhi A., Ung S., Wychowski C., Dubuisson J. (1998) J. Virol. 72, 3851–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tardif K. D., Mori K., Siddiqui A. (2002) J. Virol. 76, 7453–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavio N., Romano P. R., Graczyk T. M., Feinstone S. M., Taylor D. R. (2003) J. Virol. 77, 3578–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.