Abstract
Trichomonas vaginalis is a protozoan parasite of humans that is able to synthesize cysteine de novo using cysteine synthase but does not produce glutathione. In this study, high pressure liquid chromatography analysis confirmed that cysteine is the major intracellular redox buffer by showing that T. vaginalis contains high levels of cysteine (∼600 μm) comprising more than 70% of the total thiols detected. To investigate possible mechanisms for the regulation of cysteine levels in T. vaginalis, we have characterized enzymes of the mercaptopyruvate pathway. This consists of an aspartate aminotransferase (TvAspAT1), which transaminates cysteine to form 3-mercaptopyruvate (3-MP), and mercaptopyruvate sulfurtransferase (TvMST), which transfers the sulfur of 3-MP to a nucleophilic acceptor, generating pyruvate. TvMST has high activity with 3-MP as a sulfur donor and can use several thiol compounds as sulfur acceptor substrates. Our analysis indicated that TvMST has a kcat/Km for reduced thioredoxin of 6.2 × 107 m−1 s−1, more than 100-fold higher than that observed for β-mercaptoethanol and cysteine, suggesting that thioredoxin is a preferred substrate for TvMST. Thiol trapping and mass spectrometry provided direct evidence for the formation of thioredoxin persulfide as a product of this reaction. The thioredoxin persulfide could serve a biological function such as the transfer of the persulfide to a target protein or the sequestered release of sulfide for biosynthesis. Changes in MST activity of T. vaginalis in response to variation in the supply of exogenous cysteine are suggestive of a role for the mercaptopyruvate pathway in the removal of excess intracellular cysteine, redox homeostasis, and antioxidant defense.
INTRODUCTION
Trichomoniasis is a common sexually transmitted disease caused by Trichomonas vaginalis, a protozoan parasite that infects the human urogenital tract. T. vaginalis causes vaginitis in about half of women infected and, less frequently, urethritis in men. Symptoms are generally mild, but T. vaginalis infection has been associated with problems in pregnancy, premature birth, and low birth weight (1, 2). Most alarmingly, several independent studies have identified T. vaginalis as a cofactor in increasing the rate of transmission of human immunodeficiency virus (3–7). The infection can be treated effectively with metronidazole and other nitroimidazole drugs such as tinidazole. However, there is growing concern over the incidence and the level of drug resistance, and there is a need for new drugs with a different mode of action (2, 8). Sulfur amino acid metabolism is one of a number of areas under investigation with a view to identifying targets for drug development (9–11).
Cysteine is vitally important for all living organisms as an amino acid for protein synthesis, as a precursor for glutathione and biomolecules such as coenzyme A, and as a source of sulfide for synthesis of iron-sulfur clusters. The strongly nucleophilic character of the sulfur atom and the unique redox properties of the thiol group make cysteine a key residue for enzyme catalysis, protein folding, and redox signaling and regulation. However, the highly reactive thiol group also makes cysteine rather toxic to the cell (12–14). In most organisms, cysteine is converted to glutathione, which serves as an intracellular pool of cysteine and the main intracellular redox buffer. Cysteine itself is maintained at relatively low levels, sufficient for protein synthesis and production of essential metabolites but below the threshold of toxicity (14). T. vaginalis does not synthesize glutathione, and cysteine is believed to be the main intracellular antioxidant in this organism (9, 10, 15). This raises questions as to how T. vaginalis regulates intracellular cysteine levels so that it functions effectively as a redox buffer without inducing a toxic effect on the cell.
T. vaginalis appears to be able to obtain cysteine from its environment but also has the ability to synthesize cysteine de novo from phosphoserine and sulfide with cysteine synthase (TvCS)2 (Ref. 10 and Fig. 1). Up-regulation of cysteine synthase expression allows T. vaginalis to maintain intracellular cysteine levels when exogenous cysteine is limiting. Pathways for glutathione biosynthesis and oxidative catabolism of cysteine play a major role in cysteine metabolism in mammals (14, 16) but are not found in T. vaginalis. The parasite is known to produce two enzymes with cysteine desulfurase activity (Fig. 1). The cysteine desulfurase of T. vaginalis, an ortholog of bacterial and eukaryotic Isc proteins, is localized to the hydrogenosome and produces sulfide from cysteine for iron sulfur cluster formation (17). The enzyme methionine γ-lyase, which is absent from mammals, degrades cysteine to form pyruvate, ammonia, and sulfide, although its main function is thought to be catabolism of homocysteine (18). We have analyzed the genome content of T. vaginalis to identify additional enzymes potentially involved in cysteine catabolism and to gain understanding on how cysteine metabolism in T. vaginalis is coordinated.
FIGURE 1.
Sulfur amino acid metabolism in T. vaginalis. MGL, methionine γ-lyase; MAT, methionine adenosyltransferase; SAM-MT, S-adenosylmethionine-dependent methyltransferases; SAHH, S-adenosylhomocysteine hydrolase; PGDH, phosphoglycerate dehydrogenase; PSAT, phosphoserine aminotransferase; CD, cysteine desulfurase; R-SH, thiol compound; R-S-SH, persulfide compound.
In this study, we show that T. vaginalis encodes homologs of the transaminative or mercaptopyruvate pathway for cysteine catabolism. This comprises an aspartate aminotransferase (AspAT) that is able to convert cysteine to 3-mercaptopyruvate (3-MP) and a mercaptopyruvate sulfurtransferase (MST) that degrades 3-MP to pyruvate (Fig. 1). MST is a rhodanese family protein catalyzing the transfer of sulfur from 3-MP to a sulfur acceptor. The enzyme is found in most organisms, but its physiological significance is not well understood. The observation that the mammalian MST is subject to redox regulation in vitro supports a role for the mercaptopyruvate pathway in cysteine homeostasis and antioxidant defense (19, 20). However, there have been conflicting reports concerning the quantitative importance of this pathway to cysteine metabolism in mammals (21, 22). MST is thought to use a thiol as a sulfur acceptor substrate (Fig. 1). The enzyme reaction generates “sulfane” or persulfidic sulfur, an activated sulfur species required for synthesis of iron-sulfur clusters, cofactors such as biotin, thiamin, molybdopterin, and lipoic acid, and for thiolation of tRNAs (23–26). It has also been proposed that persulfide formation represents a reversible modification of protein thiol groups involved in the regulation of enzymes and receptors (23, 24). Our analyses provide more insight into the possible functioning of MST and suggest that the mercaptopyruvate pathway may have a number of roles in T. vaginalis.
EXPERIMENTAL PROCEDURES
Cloning and Expression
T. vaginalis genomic DNA was prepared as described previously (10). The putative MST gene, TVAG_129830, was amplified from genomic DNA using primers NT386 (5′-CAGCCATGGTTGAGACAATTGTAACACC-3′) and NT387 (5′-CACTCGAGTTCCTGGTATGGATTTGGTCC-3′) and Hi-Fidelity polymerase under the following conditions: 94 °C for 2 min; 25 cycles of 94 °C for 15 s, 55 °C for 30 s, and 72 °C for 1 min; and then a further 7 min at 72 °C. The 878-bp product was purified, cloned into pGEM-T EASY, and sequenced before subcloning the NcoI and XhoI fragment encoding MST into pET28a+ digested with the same enzymes to produce pBP351.
BL21/DE3 Escherichia coli harboring pBP351 was grown at 37 °C in LB medium with 25 μg ml−1 kanamycin and induced with 1 mm isopropyl β-d-thiogalactopyranoside for 4 h at 37 °C. T. vaginalis MST (TvMST) was expressed as a soluble protein with a C-terminal His tag and purified on a 1-ml nickel-nitrilotriacetic acid-agarose (Qiagen) column, as described by the manufacturer. The recombinant protein (rTvMST) was dialyzed at 4 °C against 20 mm Tris-HCl, 1 mm sodium thiosulfate, pH 7.9. The enzyme was stored at −20 °C in the same buffer containing 2 mm β-mercaptoethanol and 50% glycerol (v/v).
Using a similar procedure, the putative aspartate aminotransferase gene, TVAG_020800 was amplified using primers NT395 (5′-CCCATATGTCTGTTTTCAAGAACATTCCAGAATGTCC-3′) and NT396 (5′-CCCTCGAGTTATTCATTCCTTAAAGCGACAACGTTGGC-3′), cloned into GEM-T Easy, and subcloned into pET28a+ using NdeI and XhoI to produce the expression construct pBP350. The T. vaginalis aspartate aminotransferase (designated TvAspAT1) was expressed as a soluble protein with an N-terminal His tag in E. coli BL21/DE3 (pLysS). The cells were grown in LB medium with 34 μg ml−1 chloramphenicol, 25 μg ml−1 kanamycin and induced with 2 mm isopropyl β-d-thiogalactopyranoside for 4 h. The recombinant protein (rTvAspAT1) was purified using nickel-nitrilotriacetic acid-agarose and stored at 4 °C in elution buffer (50 mm NaH2PO4, 250 mm imidazole, 300 mm NaCl, pH 7.8) containing 0.2 mm pyridoxyl phosphate. The enzyme was stable for more than 1 month.
Enzyme Assays
MST activities were determined at 30 °C in 100 mm Tris-HCl, 0.3 mm 3-MP, 10 mm β-mercaptoethanol, 0.15 mm lead nitrate, 0.2 nm rTvMST, pH 8.4. The change in absorbance at 360 nm was measured for 5 min. In this assay, the sulfane sulfur is transferred enzymatically to mercaptoethanol and is released spontaneously as hydrogen sulfide. The formation of lead sulfide is quantified using the extinction coefficient of 5205 m−1 cm−1. The Km for 3-MP was calculated by measuring the MST activities with a fixed concentration of β-mercaptoethanol (10 mm) and various concentrations of 3-MP (5–400 μm). The Km for β-mercaptoethanol was determined from reactions containing 0.3 mm 3-MP and from 0.8–20 mm β-mercaptoethanol. The Km for cysteine was determined in reactions containing 0.3 mm 3-MP and 0.5–50 mm cysteine. Thiosulfate sulfurtransferase activities were determined in the same way in reactions containing 100 mm Tris-HCl, 100 mm sodium thiosulfate, 10 mm β-mercaptoethanol, 0.15 mm lead nitrate, 7 nm rTvMST, pH 8.4. The Km for thiosulfate was determined using 10 mm β-mercaptoethanol and 40–600 mm sodium thiosulfate.
The MST activity with thioredoxin as a sulfur acceptor was determined by quantifying the rate of thioredoxin oxidation and the rate of hydrogen sulfide production. The rate of thioredoxin oxidation was determined by measuring the rate of oxidation of NADPH in 100 mm potassium phosphate, 200 μm NADPH, 0.1 μm rTvTrxR (27), 12.5 μm rTvTrx (27), 0.3 mm 3-MP, 7.5 nm rTvMST, pH 7.0. The absorbance at 340 nm was measured for 5 min at 37 °C, and the rate of NADPH oxidation was quantified using the extinction coefficient 6220 m−1 cm−1. Hydrogen sulfide formation was measured in 100 mm potassium phosphate, 1 mm DTT, 12.5 μm rTvTrx, 0.3 mm 3-MP, 0.3 mm lead acetate, 1.5 nm rTvMST, pH 8.0. The absorbance at 360 nm was measured for 5 min at 30 °C, and lead sulfide was quantified using the extinction coefficient of 5205 m−1 cm−1. The sulfide production assay was used to determine the Km of rTvMST for rTvTrx with a fixed concentration of 3-MP (0.3 mm) and various concentrations of rTvTrx (0.5–15 μm).
Aspartate aminotransferase activities were measured at 37 °C in a coupled assay using malate dehydrogenase (from porcine heart; Sigma). The reactions contained 200 mm Tris-HCl, 10 mm aspartate, 5 mm α-ketoglutarate, 0.2 mm NADH, 10 units ml−1 malate dehydrogenase, and 40 nm rTvAspAT1, pH 7.5. The absorbance at 340 nm was measured for 5 min at 37 °C. In this assay the oxaloacetate, a product of the transamination reaction, is reduced by malate dehydrogenase. The rate of NADH oxidation was quantified using the extinction coefficient 6220 m−1 cm−1.
Cysteine aminotransferase activities were measured using lactate dehydrogenase (LDH) (from rabbit muscle; Sigma) as the coupling enzyme. Initial experiments showed that 3-MP, the product of the cysteine aminotransferase reaction, was a substrate for LDH with a Vmax of 3.35 μmol min−1 mg of protein−1 and a Km for 3-MP of 6 mm. Cysteine aminotransferase reactions contained 200 mm Tris-HCl, 20 mm l-cysteine, 5 mm α-ketoglutarate, 0.2 mm NADH, 100 units ml−1 LDH, 1 μm rTvAspAT1. 1 unit of LDH reduces 1 μmol of pyruvate in 1 min at 37 °C, and the enzyme was 50-fold less active toward 3-MP. The change in absorbance at 37 °C was measured for 5 min, and under these conditions the rate was proportional to TvAspAT1 concentration for the range 0.4–1.8 μm. A standard curve for 3-MP was determined using the LDH assay. The change in absorbance at 340 nm was 1.6 times lower than that expected from the extinction coefficient for NADH of 6220 m−1 cm−1. This was most likely due to the absorbance of mercaptolactate at 340 nm. A correction factor of 1.6 was therefore used to convert the apparent rate of NADH oxidation calculated from the absorbance at 340 nm to the rate of 3-MP production. The Km for cysteine was determined in reactions containing 5 mm α-ketoglutarate and 1–50 mm cysteine.
Identification of Thioredoxin Persulfide by Thiol Trapping
Mutagenesis of pBP1 was carried out with primers NT461 (5′-GGTGTGGTCCAGCCCAACGCCTTGG-3′) and NT462 (5′-CCAAGGCGTTGGGCTGGACCACACC-3′) using a QuikChange II site-Directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The mutation was confirmed by nucleotide sequencing, and the resulting plasmid was used to express mutated thioredoxin in which Cys-38 had been converted to alanine, rTvTrx (C38A).
The rTvTrx(C38A) mutant was expressed and purified as described previously for rTvTrx (27). The purified protein was treated with DTT to reduce any dimers formed during expression or purification. A 700-μl reaction containing 100 mm potassium phosphate, pH 7.0, 5 mm EDTA, 30 μm rTvTrx(C38A), and 10 mm DTT was incubated for 30 min at 37 °C. The protein was purified using a ZebaTM desalt spin column (Thermo Scientific) and used directly as a substrate in an MST reaction. For this, the 700-μl reaction contained 100 mm potassium phosphate, 5 mm EDTA, 0.3 mm 3-MP, 15 μm rTvTrx(C38A), and 10 nm MST. The reaction was started by the addition of rTvMST and incubated for 7 min at 30 °C. The reaction was passed through a ZebaTM desalt spin column to remove mercaptopyruvate, and the purified protein was treated with I-AEDANS. The reaction contained 100 mm potassium phosphate, 250 μm I-AEDANS, 15 μm rTvTrx(C38A), pH 7.0, and was incubated at room temperature for 30 min. The protein was purified by an additional desalting step and concentrated using a C18 Zip tip. The mass of molecules in the reaction mix was determined by quadrupole time of flight mass spectrometry.
Growth of T. vaginalis
T. vaginalis G3 was grown in 25 ml of modified Diamond's medium with little gas phase (10). The cultures were initiated with 105 cells ml−1 and harvested by centrifugation after 18 h of growth at 37 °C.
Preparation of T. vaginalis Extracts for Measurement of MST Activity
107 T. vaginalis cells were washed three times by centrifugation and resuspension in phosphate-buffered saline and lysed in 100 μl of ice-cold lysis buffer containing peptidase inhibitors (0.25 m sucrose, 0.25% Triton X-100, 10 μm N-trans-epoxysuccinyl-l-leucine-4-guanidinobutylamide, 2 μm 1,10-phenanthroline, 4 μm pepstatin A, and 1 μm phenylmethanesulfonyl fluoride) by repeated aspiration using a 1-ml micropipette. Cell debris was removed by centrifugation at 12,000 × g for 10 min at 4 °C, and soluble protein in the supernatant was quantified using the Bio-Rad protein assay with bovine serum albumin as standard.
HPLC Analysis of Intracellular Thiols
107 T. vaginalis cells were washed three times by centrifugation and resuspension in phosphate-buffered saline and resuspended in 50 μl of 40 mm N-[2-hydroxyethyl]-piperazine-N′[3-propanesulfonic acid], 4 mm diethylenetriamine pentaacetic acid, pH 8.0, containing 1.4 mm tris(2-carboxyethyl)phosphine and incubated at room temperature for 45 min. Monobromobimane was added to give a final concentration of 2 mm, and the mixture was heated for 3 min at 70 °C. The extracts were deproteinized by the addition of an equal volume of 4 m methanesulfonic acid, pH 1.6, and incubation on ice for 30 min. Denatured cell protein was removed by centrifugation for 7 min at 13,000 × g and 4 °C. The supernatants were stored at −20 °C until analysis by HPLC. Separation was performed at a flow rate of 0.55 ml min−1 by application of the following gradient (% of solvent B): 0 min, 0%; 10 min, 0%; 40 min, 8%; 100 min, 15%; 110 min, 50%; 111 min 0%; and 121 min, 0%. Thiols were detected by a fluorescence spectrophotometer (excitation, 365 nm; emission, 480 nm). The mobile phases consisted of 0.25% acetic acid (solvent A) and 100% acetonitrile (solvent B). Thiol levels were calculated by using peak area and comparing with standard curves in the linear range 0–350 pmol. Thiol compounds were identified by comparison of their retention times with those of standard compounds (glutathione, cysteine, homocysteine, and trypanathione). Standard curves were used to convert peak areas into thiol concentrations (nmol (108 cells)−1).
RESULTS
Analysis of T. vaginalis MST Sequence
Identification of a putative MST gene (TVAG_129830) by blastP search of the T. vaginalis genome data base was reported previously (10). The 879-bp reading frame encodes a 292-residue protein with a predicted size of 32.7 kDa. The protein has the N-terminal rhodanese domain and the structurally similar catalytic domain found in both thiosulfate sulfurtransferase and MST. Further analysis of the predicted amino acid sequence indicated that the T. vaginalis protein was probably a MST and hence was designated TvMST. TvMST has 25% identity with the Leishmania major MST but lacked the C-terminal extension found in trypanosomatid MSTs (28). Indeed, the T. vaginalis protein appears more similar to the mammalian and bacterial MSTs, showing 30 and 28% identity with rat MST and E. coli SseA, respectively.
Multiple alignments identified in TvMST conserved domains containing catalytically important residues including the active site cysteine, Cys-244 (supplemental Fig. S1). The amino acid residues adjacent to the active site cysteine determine the substrate specificity of sulfurtransferases. MSTs have the consensus Cys-Gly-Ser-Gly-Val-(Thr/Ser), distinct from the rhodanese motif (Cys-Arg-Xaa-Gly-Xaa-(Arg/Thr)) found in thiosulfate sulfurtransferase (29). The active site motif of the T. vaginalis protein has an MST consensus (residues 244–249), differing only in the conservative substitution of isoleucine for valine. A serine peptidase-like triad (His, Ser, and Asp), identified in L. major MST (30), is a common feature of the MST family that distinguishes it from the thiosulfate sulfurtransferase enzymes. His-75 and Ser-255 of L. major MST are conserved in the T. vaginalis enzyme (His-71 and Ser-246), and although Asp-61 of L. major MST is replaced by His-54 in TvMST, the catalytic triad could be completed by an adjacent aspartate (Asp-56). Two surface-exposed cysteine residues implicated in redox regulation of rat MST activity (20) are not conserved in TvMST (supplemental Fig. S1).
Expression and Purification of T. vaginalis MST
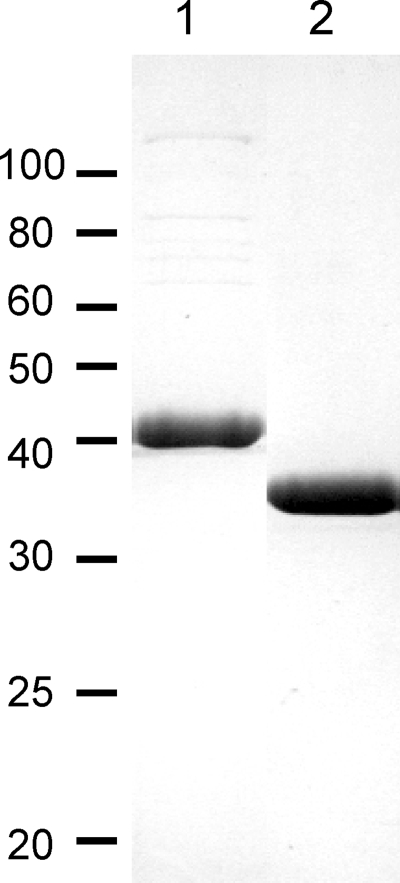
TvMST was expressed in E. coli as a soluble recombinant protein (rTvMST) with a C-terminal His tag. The purified protein, rTvMST, had a size of ∼33 kDa in SDS-PAGE, in agreement with the predicted size of 33.7 kDa, and the protein showed a high level of purity (Fig. 2). The yield was ∼19 mg of rTvMST from a liter of culture.
FIGURE 2.
Recombinant proteins used in activity analyses. Lane 1, 5 μg of rTvAspAT1; lane 2, 5 μg of rTvMST.
Kinetic Analysis of rTvMST
MST transfers sulfur from 3-MP to mercaptoethanol, generating a persulfide product that breaks down spontaneously to release sulfide (Fig. 3a). In this study, MST activity was determined by measuring sulfide production. The rTvMST showed high level of sulfurtransferase activity with 3-MP as a sulfur donor and β-mercaptoethanol as a sulfur acceptor. A much lower activity was detected with thiosulfate as a sulfur donor, representing less than 1% of the activity toward 3-MP. Substrate saturation curves and double-reciprocal plots used to determine kinetic parameters of the rTvMST reaction with 3-MP and thiosulfate are shown in supplemental Fig. S2. The kcat/Km with 3-MP was orders of magnitude higher than that obtained with thiosulfate (Table 1), indicating that 3-MP is the preferred sulfur donor substrate. Cysteine could substitute for β-mercaptoethanol as a sulfur acceptor (Table 1).
FIGURE 3.
Proposed mechanisms of the reactions catalyzed by MST. a, MST activity. Part i, MST catalyzes the transfer of sulfur from 3-MP to a nucleophilic acceptor such as a thiol (R-SH), generating activated (sulfane) sulfur in the form of a persulfide (R-S-SH). Part ii, in the presence of an excess of thiol, the persulfide is reduced spontaneously generating H2S and a disulfide (R-S-S-R). b, thioredoxin oxidation with 3-MP as a sulfur donor. Part i, thioredoxin acts as a sulfur acceptor in the sulfurtransferase reaction catalyzed by MST, and one of the thioredoxin active site cysteines is converted to cysteine persulfide. Part ii, this is followed by a spontaneous reaction in which sulfide is released and thioredoxin is oxidized. c, thioredoxin oxidation without a sulfur donor. Part i, oxidation of active site cysteine forms a sulfenate (-S-OH). Part ii, reduction of sulfenate by thioredoxin regenerates active site cysteine and thioredoxin is oxidized.
TABLE 1.
Kinetic parameters of T. vaginalis MST
Kinetic parameters of rTvMST with different sulfur donors and acceptors. Km values for the sulfur donors mercaptopyruvate and thiosulfate were determined with 10 mm β-mercaptoethanol as sulfur acceptor; the Km values for the sulfur acceptors β-mercaptoethanol, cysteine, and thioredoxin were determined with 0.3 mm mercaptopyruvate as sulfur donor. The values for Vmax and Km are the means ± S.D. from three independent substrate saturation curves. kcat and kcat/Km were calculated from the mean Vmax and Km values.
| Vmax | Km | kcat | kcat/Km | |
|---|---|---|---|---|
| mmol min−1mg−1 | mm | s−1 | m−1s−1 | |
| Mercaptopyruvate | 4.0 ± 1.0 | 5.3 × 10−2 ± 2.0 × 10−3 | 2260 | 3.5 × 107 |
| Thiosulfate | 0.02 ± 0.01 | 266 ± 64 | 11.3 | 42.7 |
| β-Mercaptoethanol | 9.5 ± 3.7 | 19 ± 12 | 5310 | 3.9 × 105 |
| Cysteine | 4.1 ± 2.1 | 8.2 ± 6.0 | 2240 | 2.7 × 105 |
| Thioredoxin | 0.31 ± 0.03 | 2.8 × 10−3 ± 0.4 × 10−3 | 172 | 6.2 × 107 |
Reduced Thioredoxin Is a Sulfur Acceptor for rTvMST
The production of recombinant T. vaginalis thioredoxin reductase (rTvTrxR) and recombinant T. vaginalis thioredoxin (rTvTrx) was described previously (27). A thioredoxin-reducing system consisting of 0.1 μm rTvTrxR, 12.5 μm rTvTrx, and 0.2 mm NADPH was incubated at 37 °C, and the rate of NADPH oxidation was quantified by measuring absorbance at 340 nm. The low background rate in this assay is due to the NADPH oxidase activity of the rTvTrxR (27). The addition of 10 nm rTvMST had no effect, but the rate increased more than 10-fold when 0.3 mm 3-MP was subsequently added (Fig. 4a). The reaction scheme for oxidation of thioredoxin in two half-reactions shown in Fig. 3b was originally proposed for bovine rhodanese (31). MST catalyzes the transfer of sulfur from 3-MP to thioredoxin to generate a persulfide derivative in the first reaction. The oxidized thioredoxin is formed spontaneously in the second reaction. The production of H2S as predicted by this scheme was confirmed for rTvMST by the addition of lead acetate at the end of the reaction (data not shown). The MST activity with thioredoxin as sulfur acceptor was determined by calculating the rate of NADPH oxidation and subtracting the spontaneous rate caused by the activity of the thioredoxin-reducing system itself. The specific activity was determined to be 81.7 ± 7.8 μmol min−1 mg of protein−1. No activity was detected without 3-MP as the sulfur donor or reduced thioredoxin as the sulfur acceptor. Increasing the concentration of rTvTrxR from 0.1 to 0.2 μm resulted in a 2-fold increase in the background rate of NADPH oxidation, but there was no change in MST activity. The MST activity was proportional to rTvMST concentration for enzyme concentrations in the range 2.5–10 nm. When the rTvTrx concentration was varied in the range 0.8–12.5 μm, the coupled reaction showed apparent Michaelis-Menten kinetics with a half-maximal rate with 1.5 μm rTvTrx (Fig. 4b). However, the relationship between this value and the Km of rTvMST for rTvTrx is not clear. The Km of rTvTrxR is 0.5 μm, so the effect of reduced saturation of rTvTrxR on the rate of reaction has to be considered. Because of the complexity of this two enzyme system, the kinetic parameters of the reaction of rTvMST with rTvTrx were determined with an alternative assay in which DTT was used to reduce rTvTrx, and rTvMST activity was quantified by measuring sulfide production.
FIGURE 4.
Sulfurtransferase activity with reduced thioredoxin as sulfur acceptor, determined by quantification of thioredoxin oxidation. a, change in the rate of NADPH oxidation following the addition of rTvMST and 3-MP to a thioredoxin-reducing system. The initial reaction contained 0.1 μm rTvTrxR, 12.5 μm rTvTrx, and 0.2 mm NADPH. NADPH oxidation was followed by measuring absorbance at 340 nm. rTvMST (E) and 3-MP (S) were added at the times indicated to give final concentrations of 10 nm and 0.3 mm, respectively. ▴, reaction with rTvMST; ○, reaction without rTvMST. b, variation of sulfurtransferase activity of rTvMST with thioredoxin concentration. Inset, double-reciprocal plot. The reactions contained 0.3 mm 3-MP with 0.8–12.5 μm rTvTrx.
The low background rate observed when 1.5 nm rTvMST was incubated with 1 mm DTT and 0.3 mm 3-MP in the absence of rTvTrx (Fig. 5a) is due to MST activity with DTT as the sulfur acceptor substrate. There was a marked increase in rate when 15 μm Trx was added, and the reaction was linear for more than 5 min (Fig. 5a). The rate was proportional to rTvMST concentration in the range 0.75 to 3 nm, and the specific activity was 215 ± 25 μmol min−1 mg of protein−1. Kinetic analysis showed that the Km of rTvMST for rTvTrx was 2.8 ± 0.4 μm, in agreement with previous experiments using rTvTrxR and NADPH as the thioredoxin-reducing system. The kcat/Km with thioredoxin was 100-fold higher than that observed with other sulfur acceptor substrates such as β-mercaptoethanol and cysteine (Table 1) and was similar to that obtained for mercaptopyruvate, the sulfur donor substrate.
FIGURE 5.
Sulfurtransferase activity with reduced thioredoxin as sulfur acceptor, determined by quantification of sulfide production. a, change in the rate of sulfide production following the addition of 1.5 nm rTvMST to a reaction containing 1 mm DTT, 12.5 μm rTVTrx, and 0.3 mm 3-MP. Sulfide production was quantified by measuring the change in absorbance at 360 nm. ▴, reaction with rTvMST; ○, reaction without rTvMST. b, variation of sulfurtransferase activity of rTvMST with thioredoxin concentration. Inset, double-reciprocal plot. The reactions contained 0.3 mm 3-MP with 0.5–15 μm rTvTrx.
rTvMST Catalyzes the Formation of Thioredoxin Persulfide
Like most thioredoxins, the active site of TvTrx has the consensus Cys-Gly-Pro-Cys (residues 35–38) containing a pair of cysteine residues that form a redox-active disulfide. Our model for the reaction of MST with thioredoxin predicts that MST transfers sulfane sulfur to Cys-35 of reduced TvTrx to form a persulfide that is reduced by Cys-38 in a thiol-disulfide exchange reaction that results in release of sulfide and production of oxidized TvTrx. Evidence of the formation of thioredoxin persulfide was obtained by adapting a method used to detect persulfide intermediates in thiouridine biosynthesis (32). To increase the stability of thioredoxin persulfide, Cys-38 of TvTrx was mutated to alanine. In addition, I-AEDANS, which reacts with thiol groups to form a covalent adduct, was used as thiol trapping agent.
A control reaction containing 0.3 mm mercaptopyruvate and 15 μm rTvTrx(C38A), but no rTvMST enzyme, was treated with I-AEDANS and analyzed by quadrupole time of flight mass spectrometry. A single major peak was identified as the AEDANS derivative of rTvTrx(C38A) (observed mass, 13,473.00; predicted mass, 13,472.70) (supplemental Fig. S3a). The unmodified rTvTrx(C38A) was also detected as a minor peak (observed mass, 13,167.00; predicted mass, 13,166.36). In the complete reaction containing 10 nm rTvMST, the major peak was identified as the AEDANS derivative of thioredoxin persulfide (observed mass, 13,505.80; predicted mass, 13,504.77) (supplemental Fig. S3b). Other species present were the AEDANS-modified thioredoxin (observed mass, 13,474.00; predicted mass, 13,472.70) and a molecule identified as a mixed disulfide of thioredoxin and mercaptopyruvate (observed mass, 13,285.80; predicted mass, 13,284.49). The mixed disulfide is formed when sulfur of mercaptopyruvate acts as a nucleophile to attack the first, bridging sulfur of the persulfide to expel the second, terminal sulfur as bisulfide. Minor peaks representing the unmodified forms of thioredoxin and thioredoxin persulfide were also detected. The observed masses of the different species were in good agreement with the theoretical values calculated on the basis of the sequence of rTvTrx, deviating by ≤0.01%. These results provide direct evidence for the formation of thioredoxin persulfide in the reaction of TvMST with thioredoxin as sulfur acceptor substrate.
rTvMST Has Thioredoxin Oxidase Activity
When the amount of rTvMST added to the thioredoxin recycling system was increased from 10 nm to 1 μm, thioredoxin oxidation could be quantified by measuring NADPH oxidation without the addition of 3-MP as a sulfur donor. The reaction did not require the addition of an exogenous oxidizing agent and presumably involves reactive oxygen species present in the buffer under air-saturated conditions, according to the scheme shown in Fig. 3c originally proposed for bovine rhodanese (33). The thioredoxin oxidase specific activity was 36.2 ± 6.9 nmol min−1 mg of protein−1. The rate was proportional to rTvMST concentration in the range 1–10 μm. The rate was increased only slightly by the addition of 20 μm hydrogen peroxide.
TvMST Expression Is Regulated by Exogenous Cysteine Levels
T. vaginalis was grown under conditions previously shown to modulate cysteine synthase expression; the addition of 10 mm cysteine to the medium resulted in marked down-regulation of TvCS, whereas the addition of 5 μm propargylglycine (PAG) resulted in 3-fold up-regulation of cysteine synthase protein levels and enzyme activity (10). In this study we determined the effect of the same growth conditions on the levels of MST activity. Cells grown under standard conditions had an MST activity of 14.1 ± 7.8 nmol min−1 mg of protein−1. The MST activity was increased 3–4-fold when the cells were grown in the presence of 10 mm cysteine (p = 0.001), whereas there was no change in the level of MST activity when the cells were grown in medium containing 5 μm PAG (p = 0.96) (Fig. 6).
FIGURE 6.
Changes in MST enzymatic activity under different conditions. Shown are the MST activities in nmol min−1 mg of protein−1. The values are the means ± S.D. from three replicate assays for each extract. The different growth conditions were: control (lane 1), with added 10 mm cysteine (lane 2), and with 5 μm propargylglycine (lane 3).
Analysis of Intracellular Thiol Levels in T. vaginalis Using HPLC
Analysis of extracts of T. vaginalis revealed a major peak representing 76% of the total thiols detected and with a retention time of 43.2 min; use of standards showed that this was cysteine. A second peak, representing 9% of the total peak area, had a retention time of 33.1 min that did not correspond to any of the standards used. There were also minor peaks with areas ranging from 1.7 to 3.5% of the total area. Two of these were identified as homocysteine and glutathione from their retention times of 51.5 and 71.8 min, respectively. The remaining small peaks were not identified. Quantitation of the peaks through the use of standards revealed that the cysteine concentration was 31.8 ± 3.1 nmol (108 cells)−1, whereas the homocysteine and glutathione concentrations were 0.5 ± 0.1 and 1.0 ± 0.2 nmol (108 cells)−1, respectively. The volume of T. vaginalis has been reported as 60 or 47 μl/108 cells (34, 35). Applying these values to this study gives an intracellular cysteine concentration of 530–676 μm, with homocysteine and glutathione concentrations of 8.1–10.6 and 16.2–21.2 μm, respectively. The thiol content of modified Diamond's medium, which contains no added cysteine, was determined to be 4.7 ± 1.2 μm cysteine and 19.9 ± 1.4 μm glutathione. Thus, taking into account the absence from the T. vaginalis genome of genes clearly encoding the necessary biosynthetic enzymes, it was concluded that the glutathione detected in the T. vaginalis samples was a contaminant from the medium.
The thiol content of T. vaginalis grown in modified Diamond's medium and modified Diamond's medium plus 10 mm cysteine or 5 μm PAG were compared (Fig. 7). Growth in media containing 10 mm cysteine resulted in a 1.5-fold increase in cysteine concentration (p = 0.0014), whereas the concentration of homocysteine did not change. Treatment with PAG resulted in a 3-fold decrease in cysteine concentration (p = 0.0001) and a >10-fold increase in homocysteine concentration (p = 0.02).
FIGURE 7.
Thiol content of T. vaginalis grown under different conditions. a, cysteine (nmol (108 cells)−1). b, homocysteine (nmol (108 cells)−1). The results are the means ± S.D. from three extracts. The different growth conditions were: control (lane 1), with added 10 mm cysteine (lane 2), and with 5 μm propargylglycine (lane 3).
3-MP Is Synthesized from Cysteine by T. vaginalis Aspartate Aminotransferase
In mammals, transamination of cysteine to form 3-MP is carried out in the liver by aspartate aminotransferase (36). BlastP search of the T. vaginalis genomic data base with the amino acid sequence of rat liver aspartate aminotransferase identified a homologous sequence (TVAG-020800) designated TvAspAT1. The gene consists of 1200 bp and encodes a protein of 400 amino acids and a predicted molecular mass of 44.6 kDa. Two additional sequences were identified, designated TvAspAT2 and TvAspAT3, with 62.1 and 63.1% identity to TvAspAT1. Sequence motifs that are characteristic of rat AspAT and other aspartate aminotransferases belonging to subgroup 1a of the aminotransferase superfamily (37) were highly conserved in the T. vaginalis proteins (data not shown). TvAspAT1 showed the highest similarity to rat cytosolic AspAT and was selected for further study. The TvAspAT1 was expressed in E. coli as a soluble protein with an N-terminal His tag and purified to apparent homogeneity (Fig. 4) with a yield of ∼68 mg of protein/liter of culture. The purified protein had both aspartate aminotransferase and cysteine aminotransferase activities. The substrate saturation curves showed Michaelis-Menten kinetics (supplemental Fig. S4). The Km values for aspartate and cysteine were similar, but the turnover number with cysteine as a substrate was 300-fold lower (Table 2). The activity of rat AspAT with cysteine was reported to be 20-fold lower than its activity with aspartate (36). The results show that aspartate aminotransferase can form 3-MP by oxidative deamination of cysteine, although the data suggest that TvAspAT1 is less efficient as a cysteine aminotransferase than the homologous mammalian enzyme.
TABLE 2.
Kinetic parameters of T. vaginalis AspAT1
The Km values for aspartate and cysteine were determined with 1 mm α -ketoglutarate. The values for Vmax and Km are the means ± S.D. from three independent substrate saturation curves. kcat and kcat/Km were calculated from the mean Vmax and Km values.
| Vmax | Km | kcat | kcat/Km | |
|---|---|---|---|---|
| μmol min−1mg−1 | mm | s−1 | m−1s−1 | |
| Aspartate | 39.5 ± 11.2 | 14.6 ± 6.6 | 30.6 | 2100 |
| Cysteine | 0.15 ± 0.03 | 9.0 ± 1.2 | 0.12 | 13.3 |
DISCUSSION
T. vaginalis encodes a functional mercaptopyruvate sulfurtransferase (TvMST) that potentially is linked to cysteine catabolism by an aspartate aminotransferase (TvAspAT1), which has been shown to be able to catalyze the transamination of cysteine to form 3-MP. Changes in MST activity in response to variation in the supply of exogenous cysteine are suggestive of a role for the pathway in redox homeostasis and antioxidant defense.
T. vaginalis lacks mitochondria and has a fermentative energy metabolism involving specialized organelles called hydrogenosomes. The parasite is not a strict anaerobe and is adapted to growth under microaerophilic conditions. T. vaginalis is exposed to oxygen in its environment, and mechanisms for defense against the resulting oxidative stress have been identified (27). Cysteine has been considered to be the major intracellular thiol (see Refs.9, 10, and 15); our data confirm this, because at ∼600 μm it comprised more than 70% of the total thiols detected. Similar results have been reported for the other amitochondrial parasites, Giardia lamblia and Entamoeba histolytica, in which cysteine is thought to replace glutathione as the main intracellular antioxidant and redox buffer (38–40). T. vaginalis does not encode genes for glutathione biosynthesis or enzymes that use glutathione as a cofactor; the glutathione detected in our analyses (the very low concentration of 20 μm) is likely to have been derived from the glutathione present in the medium. The volatile thiols produced by T. vaginalis such as methanethiol, propanethiol, and H2S have been postulated to have antioxidant roles (41) but were not detectable using the methods applied in this study. Additional defense mechanisms include cytoplasmic oxidases (42) and superoxide dismutase (43), which scavenge molecular oxygen and superoxide anion radical, respectively. Moreover, thioredoxin peroxidase forms a redox couple with thioredoxin and thioredoxin reductase to detoxify hydrogen peroxide and other harmful oxidants (27).
For most organisms, free cysteine is toxic, and intracellular cysteine is maintained at relatively low levels (0.1–0.2 mm) compared with the less reactive glutathione (0.5–4 mm). Supraphysiological levels of cysteine can increase oxidative stress by generating reactive oxygen species. Cysteine chelates transition metals such as Fe3+ and Cu2+ and is rapidly oxidized, and the metal ions reduced in this reaction are reoxidized by H2O2 in the Fenton reaction, resulting in highly toxic hydroxyl radicals (44). High levels of intracellular cysteine in this way promote oxidative DNA damage in E. coli (12) and membrane damage in liver hepatocyte cells (45). The thiol group of glutathione is less easily oxidized by transition metals than cysteine and can be maintained in millimolar concentrations without initiating Fenton chemistry, hence its advantages as an intracellular buffer. The use of cysteine rather than glutathione as a redox buffer by T. vaginalis could be an adaptation to a microaerophilic lifestyle. The organism is exposed to low levels of oxygen in its environment and does not produce reactive oxygen species by aerobic respiration, and this may facilitate its tolerance of high levels of intracellular levels of cysteine. Moreover, the increased reactivity of cysteine in transition metal catalyzed autoxidation reactions could be important in scavenging oxygen, thus protecting the highly sensitive hydrogenosomal enzymes from exposure to oxygen. Our aim is to determine how T. vaginalis is able to regulate its intracellular cysteine levels to provide sufficient for redox homeostasis without exceeding its own toxic threshold.
In this study, we have looked for enzymes that could be involved in the regulation of intracellular cysteine levels. Trichomonas can obtain cysteine by protein degradation and potentially by uptake from the environment. The parasite is capable of producing cysteine when required by de novo biosynthesis involving TvCS (10). In addition, phagocytosis of bacterial cells, macrophages, or vaginal epithelial cells could expose the parasite to a transient increase in intracellular cysteine concentration. An increase in cysteine level could be reversed by cysteine catabolism. T. vaginalis enzymes known to degrade cysteine are methionine γ-lyase (18) and cysteine desulfurase (17). Enzymes important in the regulation of intracellular cysteine in mammals and other organisms were searched for by genome mining of T. vaginalis, but the parasite apparently lacks genes encoding γ-glutamyl cysteine synthase, cysteine dioxygenase, or cystathionine γ-lyase. However, we identified enzymes of the transaminative or mercaptopyruvate pathway for cysteine catabolism; in this pathway, transamination of cysteine to 3-MP is followed by desulfuration to form pyruvate. We have now shown that the proteins encoded by these genes are indeed functionally active as postulated.
In mammals, cysteine aminotransferase was found to be identical to aspartate aminotransferase. We have shown that T. vaginalis encodes a homolog of the rat aspartate aminotransferase that is able to produce mercaptopyruvate by transamination of cysteine. Although the rate of the cysteine transamination is low, aspartate aminotransferase is an abundant cytosolic enzyme (46), and the Km for cysteine is 9 mm, so TvAspAT1 could contribute to cysteine metabolism in vivo. The efficiency of the TvAspAT1 in cysteine transamination appears to be considerably lower (about 10-fold) than that reported for the rat AspAT. However, this could still be consistent with a role in cysteine homeostasis because T. vaginalis maintains a higher level of intracellular cysteine.
Kinetic analysis of rTvMST confirmed that 3-MP is the strongly preferred sulfur donor substrate. The enzyme is a highly active mercaptopyruvate sulfurtransferase with a kcat/Km(3-MP) of 3.5 × 107 m−1s−1. This compares with a kcat/Km of 1.4 × 107 m−1s−1 for L. major MST in the same reaction (28). The sulfur acceptor in vivo is not known, but it is believed to be a low molecular mass thiol. A cysteine persulfide is an intermediate of the thiosulfate sulfurtransferase reaction, but this is not thought to be the case for MST (47). A reaction mechanism has been proposed in which the active site cysteine of MST forms a mixed disulfide with 3-MP (48). Tautomerization to thiosulfoxide is favored thermodynamically because of the carbonyl group of 3-MP (23) and the proximity of active site residues (48). This generates the sulfane sulfur, which can be transferred to a nucleophile such as a cyanide ion or a thiol group.
In common with the L. major MST (28) and bovine rhodanese (31), rTvMST is able to use thioredoxin as a sulfur acceptor in vitro. In this study we found that kcat/Km for rTvMST with thioredoxin was 100-fold higher than the values obtained with alternative sulfur acceptors such as β-mercaptoethanol or cysteine. Notably, rTvMST gave a higher kcat/Km value with thioredoxin than that reported for rTvTrxR (27). Thioredoxin is therefore a possible in vivo substrate for TvMST, and it is relevant to consider the biological significance of the reaction. Our data agree with an earlier model for the reaction mechanism in which the transfer of sulfane sulfur from 3-MP to the exposed cysteine residue of the thioredoxin active site (Cys-35 in TvTrx) generated a thioredoxin persulfide product. The buried cysteine (Cys-38 in TvTrx) then acts as a nucleophile to attack the persulfide, resulting in release of hydrogen sulfide and oxidation of the thioredoxin active site. We show that the reaction can be followed by measuring thioredoxin oxidation or release of sulfide. Importantly we have been able to demonstrate the formation of the persulfide product using thiol-trapping and mass spectrometry. Although the thioredoxin persulfide is unstable under in vitro reaction conditions, its half-life in vivo could be long enough to serve a biological function such as transfer of the terminal sulfur to the cysteine residue of a target protein to generate a new persulfide group or to a subcellular location for sequestered release of sulfide for biosynthesis (25, 26, 31). Alternatively, the formation of thioredoxin persulfide could be important in antioxidant defense because the perthiol group is more reactive than the thiol group in scavenging free radicals (24). This study confirms that thioredoxin is a high affinity substrate for diverse rhodanese family sulfurtransferases (28, 31). However, there is currently no data available for mammalian MSTs. The rat MST is known to interact with thioredoxin, which is thought to function in redox regulation of enzyme activity (19, 20), but the possibility that thioredoxin is also used as a substrate has not been reported.
rTvMST had a low thioredoxin oxidase activity in the absence of a sulfur donor. This activity appears to be a general characteristic of rhodanese family proteins, although differences in their sensitivity to oxidation have been reported. The rat MST requires the addition of stoichiometric concentrations of hydrogen peroxide (19), whereas the TvMST resembles the bovine rhodanese, which was readily oxidized by concentrations of hydrogen peroxide present in air-saturated buffers (33). The reaction of rat MST with hydrogen peroxide was shown to involve reversible oxidation of the active site cysteine to form a cysteine sulfenate (19), confirming the reaction scheme proposed for bovine rhodanese (33). In view of the very low specific activity of the rTvMST thioredoxin oxidase reaction, the enzyme is unlikely to function in detoxification of hydrogen peroxide in vivo. However, the ability of rTvMST to undergo reversible oxidation under air-saturated conditions could be significant with respect to regulation of enzyme activity, as proposed for rat MST.
The rat MST has a low rhodanese activity, with thiosulfate as sulfur donor and cyanide as sulfur acceptor that is inhibited reversibly by oxidation (19, 20). Making the assumption that its mercaptopyruvate sulfurtransferase activity, which can only be measured under reducing conditions, is similarly inhibited by oxidation, it was proposed that rat MST is a thioredoxin-regulated enzyme (20). Inhibition of the rhodanese activity of rat MST was shown to be the result of oxidation of the active site cysteine to sulfenate and dimerization of the enzyme caused by oxidation of two redox-active cysteine residues on the surface of the enzyme (20). It was postulated that under reducing conditions, reduced thioredoxin activates MST, resulting in the removal of excess cysteine through the mercaptopyruvate pathway (20). Conversely, under oxidizing conditions MST is inactivated, conserving cysteine, whereas biosynthetic pathways act to restore cysteine levels. The thioredoxin oxidase activity shows that the active site cysteine of TvMST can undergo reversible oxidation in a similar manner to the rat MST. However, the exposed redox-active cysteine residues involved in thioredoxin-dependent activation of rat MST are not conserved in TvMST.
Importantly our results show that an increase in cysteine availability leads to an up-regulation of TvMST in T. vaginalis. Growth of cells in 10 mm cysteine resulted in a 1.5-fold increase in the intracellular cysteine level, so the observed increase in TvMST activity together with an increased rate of cysteine aminotransferase activity resulting from higher substrate saturation would produce increased flux through the mercaptopyruvate pathway. TvCS expression is down-regulated under the same conditions (10), suggesting that the biosynthetic and catabolic pathways are regulated to maintain cysteine homeostasis and that TvMST has a role in removing excess intracellular cysteine, as proposed for the mammalian enzyme. Treatment of T. vaginalis cells with PAG results in up-regulation of TvCS (10). In this study, the PAG-treated cells showed a decrease in intracellular cysteine levels, but there was no change in the expression of TvMST. However, as discussed above, low cysteine levels may lead to inhibition of MST activity caused by oxidation of the enzyme. Growth in PAG resulted in a 10-fold increase in the level of homocysteine, a substrate for methionine γ-lyase that is strongly inhibited by PAG (18). Cysteine levels showed a 3-fold decrease under the same conditions, even though TvCS was up-regulated (10). These results support our hypothesis that catabolism of homocysteine by methionine γ-lyase is a major source of sulfide for cysteine biosynthesis (10).
MST is likely to serve other functions in the cell in addition to its role in cysteine homeostasis. Mercaptolactate-cysteine disulfiduria is an in-born error of metabolism caused by a defect in MST that is diagnosed by the appearance of mercaptolactate in urine as a mixed disulfide with cysteine (49). Patients also show an increased risk of neurotoxicity following exposure to cyanide, indicating that MST is important for cyanide detoxification. The mercaptopyruvate pathway is an important source of persulfidic sulfur. Overexpression of MST in HEK 293-F cells resulted in an increase in the level of “bound” sulfane sulfur (50), possibly in the form of protein-bound cysteine persulfide. This is a storage form of sulfur that releases hydrogen sulfide when the intracellular conditions become more reducing (51). Persulfide sulfur is the likely source of sulfide for many biosynthetic pathways in vivo, including cofactor biosynthesis, iron-sulfur cluster biosynthesis, lipoic acid biosynthesis, and tRNA sulfuration (23–26, 32, 52). A regulatory role is also suggested by the observation that sulfane sulfur-generating pathways, including the mercaptopyruvate pathway, are down-regulated in some types of cancer (53). In view of the fact that thioredoxin is a sulfur acceptor substrate for TvMST and L. major MST (28), it is possible that thioredoxin persulfide could play a role in mediating the effects associated with the mercaptopyruvate pathway. Which of these functions are relevant to MST in T. vaginalis remains to be determined; however, this study has opened up the possibility of investigating further the importance of MST to the parasite and its role in maintaining cysteine homeostasis.
Supplementary Material
Acknowledgments
We thank Roderick Williams and Saskia Decuypere (Strathclyde University) and Sylke Müller and Lesley McCaig (Glasgow University) for advice and assistance with aspects of the study. We thank Richard Burchmore (Sir Henry Wellcome Functional Genomics Facility, Glasgow University) for performing the mass spectrometry.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- Tv
- T. vaginalis
- CS
- cysteine synthase
- 3-MP
- 3-mercaptopyruvate
- LDH
- lactate dehydrogenase
- MST
- mercaptopyruvate sulfurtransferase
- PAG
- propargylglycine
- Trx
- thioredoxin
- TrxR
- thioredoxin reductase
- HPLC
- high pressure liquid chromatography
- AspAT
- aspartate aminotransferase
- I-AEDANS
- 5-(2-((2-iodoacetyl)amino)ethylamino)naphthalene-1-sulfonic acid
- DTT
- dithiothreitol.
REFERENCES
- 1.Cotch M. F., Pastorek J. G., 2nd, Nugent R. P., Hillier S. L., Gibbs R. S., Martin D. H., Eschenbach D. A., Edelman R., Carey J. C., Regan J. A., Krohn M. A., Klebanoff M. A., Rao A. V., Rhoads G. G. (1997) Sex. Transm. Dis. 24, 353–360 [DOI] [PubMed] [Google Scholar]
- 2.Schwebke J. R., Burgess D. (2004) Clin. Microbiol. Rev. 17, 794–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorvillo F., Smith L., Kerndt P., Ash L. (2001) Emerg. Infect. Dis. 7, 927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClelland R. S., Sangare L., Hassan W. M., Lavreys L., Mandaliya K., Kiarie J., Ndinya-Achola J., Jaoko W., Baeten J. M. (2007) J. Infect. Dis. 195, 698–702 [DOI] [PubMed] [Google Scholar]
- 5.Van der Pol B., Kwok C., Pierre-Louis B., Rinaldi A., Salata R. A., Chen P. L., van de Wijgert J., Mmiro F., Mugerwa R., Chipato T., Morrison C. S. (2008) J. Infect. Dis. 197, 548–554 [DOI] [PubMed] [Google Scholar]
- 6.Kissinger P., Amedee A., Clark R. A., Dumestre J., Theall K. P., Myers L., Hagensee M. E., Farley T. A., Martin D. H. (2009) Sex. Transm. Dis. 36, 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shafir S. C., Sorvillo F. J., Smith L. (2009) Clin. Microbiol. Rev. 22, 37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunne R. L., Dunn L. A., Upcroft P., O'Donoghue P. J., Upcroft J. A. (2003) Cell Res. 13, 239–249 [DOI] [PubMed] [Google Scholar]
- 9.Müller S., Liebau E., Walter R. D., Krauth-Siegel R. L. (2003) Trends Parasitol. 19, 320–328 [DOI] [PubMed] [Google Scholar]
- 10.Westrop G. D., Goodall G., Mottram J. C., Coombs G. H. (2006) J. Biol. Chem. 281, 25062–25075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali V., Nozaki T. (2007) Clin. Microbiol. Rev. 20, 164–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park S., Imlay J. A. (2003) J. Bacteriol. 185, 1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A., John L., Alam M. M., Gupta A., Sharma G., Pillai B., Sengupta S. (2006) Biochem. J. 396, 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stipanuk M. H., Dominy J. E., Jr., Lee J. I., Coloso R. M. (2006) J. Nutr. 136, 1652S–1659S [DOI] [PubMed] [Google Scholar]
- 15.Walker J., Barrett J. (1997) Int. J. Parasitol. 27, 883–897 [DOI] [PubMed] [Google Scholar]
- 16.Dominy J. E., Jr., Hwang J., Stipanuk M. H. (2007) Am. J. Physiol. Endocrinol. Metab. 293, E62–E69 [DOI] [PubMed] [Google Scholar]
- 17.Sutak R., Dolezal P., Fiumera H. L., Hrdy I., Dancis A., Delgadillo-Correa M., Johnson P. J., Müller M., Tachezy J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10368–10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKie A. E., Edlind T., Walker J., Mottram J. C., Coombs G. H. (1998) J. Biol. Chem. 273, 5549–5556 [DOI] [PubMed] [Google Scholar]
- 19.Nagahara N., Katayama A. (2005) J. Biol. Chem. 280, 34569–34576 [DOI] [PubMed] [Google Scholar]
- 20.Nagahara N., Yoshii T., Abe Y., Matsumura T. (2007) J. Biol. Chem. 282, 1561–1569 [DOI] [PubMed] [Google Scholar]
- 21.Mikami H., Hosaki Y., Ubuka T. (1984) Acta Med. Okayama 38, 415–421 [DOI] [PubMed] [Google Scholar]
- 22.Drake M. R., De La Rosa J., Stipanuk M. H. (1987) Biochem. J. 244, 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toohey J. I. (1989) Biochem. J. 264, 625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iciek M., Wlodek L. (2001) Pol. J. Pharmacol. 53, 215–225 [PubMed] [Google Scholar]
- 25.Kessler D. (2006) FEMS Microbiol. Rev. 30, 825–840 [DOI] [PubMed] [Google Scholar]
- 26.Mueller E. G. (2006) Nat. Chem. Biol. 2, 185–194 [DOI] [PubMed] [Google Scholar]
- 27.Coombs G. H., Westrop G. D., Suchan P., Puzova G., Hirt R. P., Embley T. M., Mottram J. C., Müller S. (2004) J. Biol. Chem. 279, 5249–5256 [DOI] [PubMed] [Google Scholar]
- 28.Williams R. A., Kelly S. M., Mottram J. C., Coombs G. H. (2003) J. Biol. Chem. 278, 1480–1486 [DOI] [PubMed] [Google Scholar]
- 29.Nagahara N., Okazaki T., Nishino T. (1995) J. Biol. Chem. 270, 16230–16235 [DOI] [PubMed] [Google Scholar]
- 30.Alphey M. S., Williams R. A., Mottram J. C., Coombs G. H., Hunter W. N. (2003) J. Biol. Chem. 278, 48219–48227 [DOI] [PubMed] [Google Scholar]
- 31.Nandi D. L., Westley J. (1998) Int. J. Biochem. Cell Biol. 30, 973–977 [DOI] [PubMed] [Google Scholar]
- 32.Wright C. M., Christman G. D., Snellinger A. M., Johnston M. V., Mueller E. G. (2006) Chem. Commun. 3104–3106 [DOI] [PubMed] [Google Scholar]
- 33.Nandi D. L., Horowitz P. M., Westley J. (2000) Int. J. Biochem. Cell Biol. 32, 465–973 [DOI] [PubMed] [Google Scholar]
- 34.Ter Kuile B. H., Müller M. (1993) FEMS Microbiol. Lett. 110, 27–31 [DOI] [PubMed] [Google Scholar]
- 35.Knodler L. A., Edwards M. R., Schofield P. J. (1994) Exp. Parasitol. 79, 117–125 [DOI] [PubMed] [Google Scholar]
- 36.Akagi R. (1982) Acta Med. Okayama 36, 187–197 [DOI] [PubMed] [Google Scholar]
- 37.Jensen R. A., Gu W. (1996) J. Bacteriol. 178, 2161–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown D. M., Upcroft J. A., Upcroft P. (1993) Mol. Biochem. Parasitol. 61, 155–158 [DOI] [PubMed] [Google Scholar]
- 39.Fahey R. C., Newton G. L., Arrick B., Overdank-Bogart T., Aley S. B. (1984) Science 224, 70–72 [DOI] [PubMed] [Google Scholar]
- 40.Ariyanayagam M. R., Fairlamb A. H. (1999) Mol. Biochem. Parasitol. 103, 61–69 [DOI] [PubMed] [Google Scholar]
- 41.Ellis J. E., Yarlett N., Cole D., Humphreys M. J., Lloyd D. (1994) Microbiol. 140, 2489–2494 [DOI] [PubMed] [Google Scholar]
- 42.Linstead D. J., Bradley S. (1988) Mol. Biochem. Parasitol. 27, 125–133 [DOI] [PubMed] [Google Scholar]
- 43.Lindmark D. G., Müller M. (1974) J. Biol. Chem. 249, 4634–4637 [PubMed] [Google Scholar]
- 44.Meyer A. J., Hell R. (2005) Photosynth. Res. 86, 435–457 [DOI] [PubMed] [Google Scholar]
- 45.Viña J., Saez G. T., Wiggins D., Roberts A. F., Hems R., Krebs H. A. (1983) Biochem. J. 212, 39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lowe P. N., Rowe A. F. (1986) Mol. Biochem. Parasitol. 21, 65–74 [DOI] [PubMed] [Google Scholar]
- 47.Westley J., Adler H., Westley L., Nishida C. (1983) Fundam. Appl. Toxicol. 3, 377–382 [DOI] [PubMed] [Google Scholar]
- 48.Spallarosa A., Forlani F., Carpen A., Armirotti A., Pagani S., Bolognesi M., Bordo D. (2004) J. Mol. Biol. 335, 583–593 [DOI] [PubMed] [Google Scholar]
- 49.Billaut-Laden I., Rat E., Allorge D., Crunelle-Thibaut A., Cauffiez C., Chevalier D., LoGuidice J., Broly F. (2006) Toxicol. Lett. 165, 101–111 [DOI] [PubMed] [Google Scholar]
- 50.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K., Kimura H. (2009) Antioxid. Redox Signal. 11, 703–714 [DOI] [PubMed] [Google Scholar]
- 51.Ishigami M., Hiraki K., Umemura K., Ogasawara Y., Ishii K., Kimura H. (2009) Antioxid. Redox Signal. 11, 205–214 [DOI] [PubMed] [Google Scholar]
- 52.Beinert H. (2000) Eur. J. Biochem. 267, 5657–5664 [DOI] [PubMed] [Google Scholar]
- 53.Iciek M., Marcinek J., Mleczko U., Wlodek L. (2007) Eur. J. Pharmacol. 569, 1–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.