Abstract
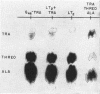
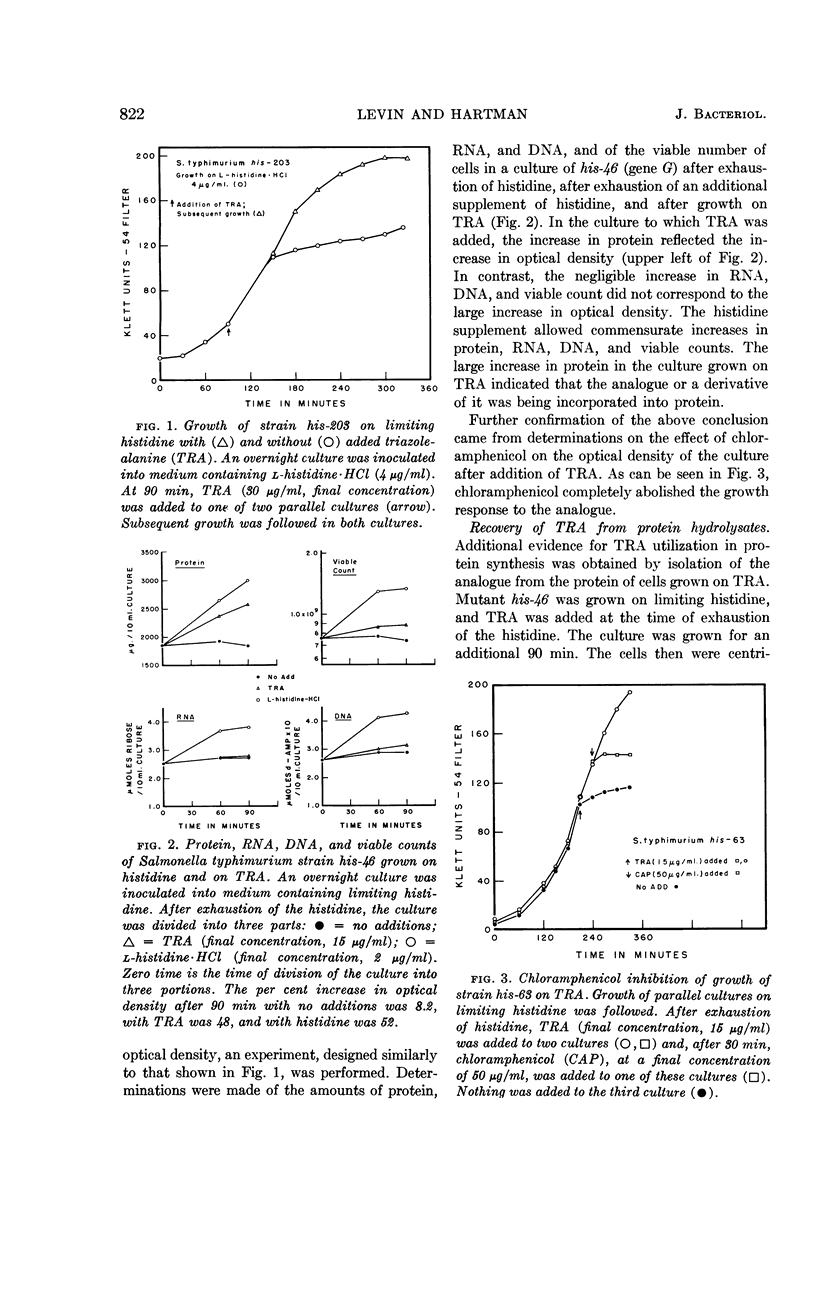
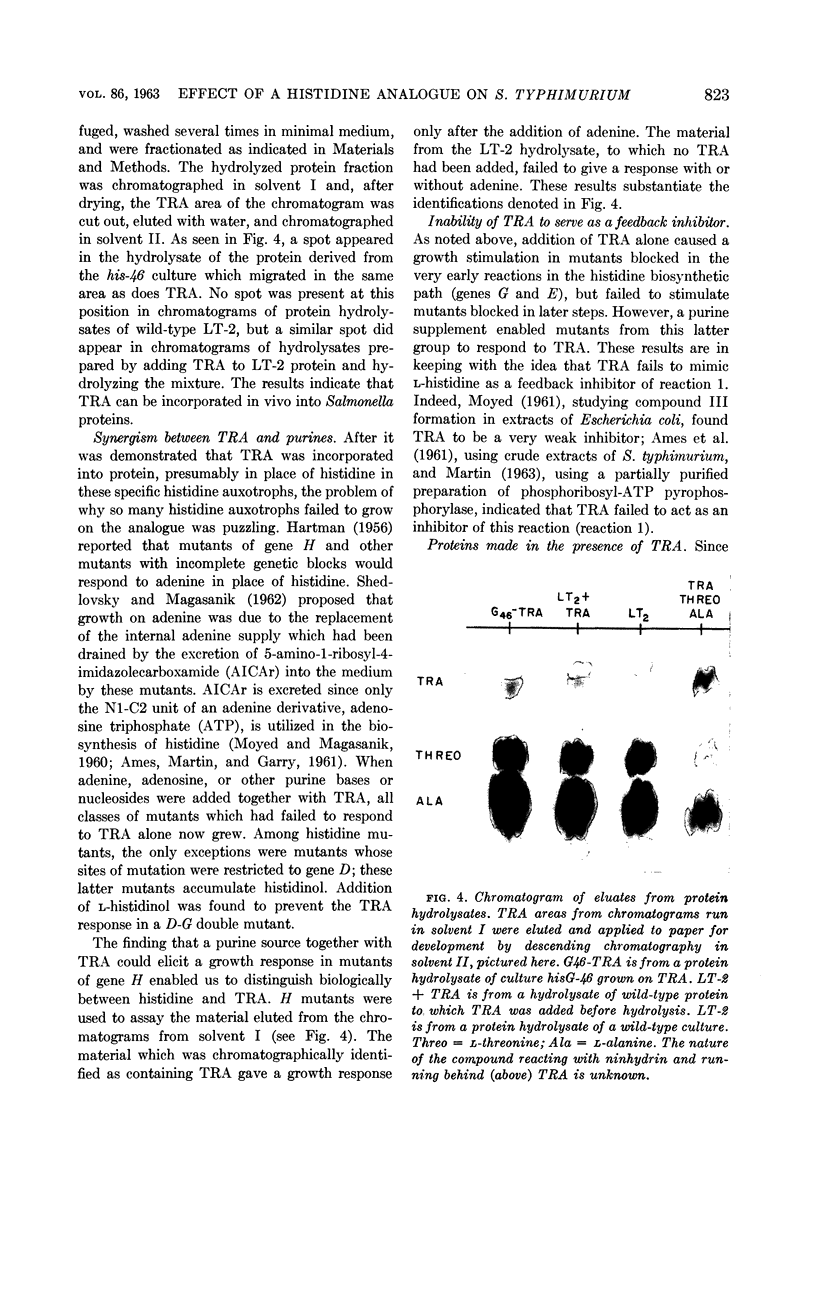
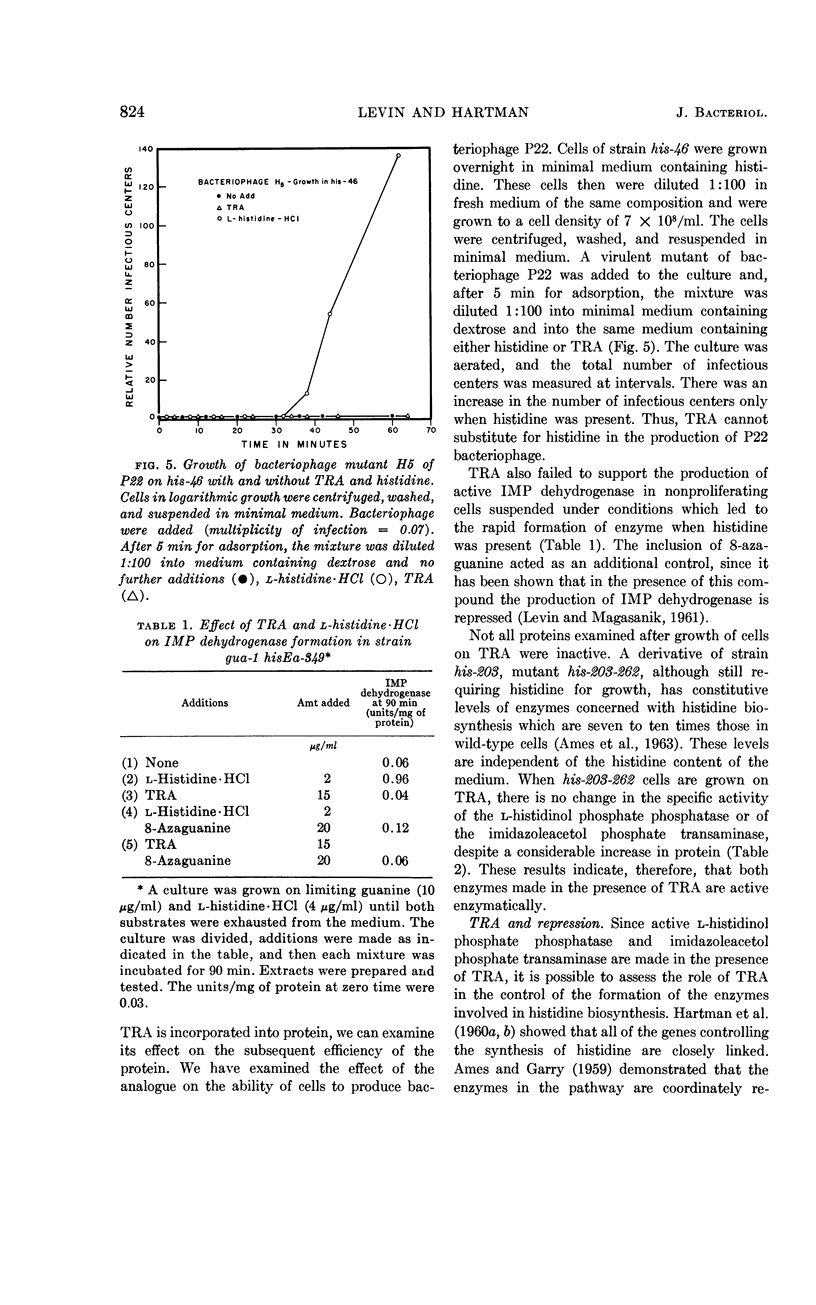
Levin, Alfred P. (The Johns Hopkins University, Baltimore, Md.), and Philip E. Hartman. Action of a histidine analogue, 1,2,4-triazole-3-alanine, in Salmonella typhimurium. J. Bacteriol. 86:820–828. 1963.—The effect of the histidine analogue, 1,2,4-triazole-3-alanine (TRA), on growth and enzyme synthesis in histidine auxotrophs of Salmonella typhimurium has been studied. TRA allows an increase of approximately 50% in the amount of protein in a culture but does not allow concomitant synthesis of ribonucleic acid and deoxyribonucleic acid. Although the analogue prevents the formation of active bacteriophage and of enzymatically active inosine 5′-phosphate dehydrogenase, it does not prevent the formation of enzymatically active l-histidinol phosphate phosphatase or of imidazoleacetol phosphate transaminase, two enzymes involved in the biosynthesis of histidine. Of the three known functions of histidine in the cell, TRA mimics two: it is incorporated into protein, and it acts as a repressor material for synthesis of enzymes involved in the formation of histidine. TRA fails to act as a feedback inhibitor of the first step in the formation of histidine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., GARRY B., HERZENBERG L. A. The genetic control of the enzymes of histidine biosynthesis in Salmonella typhimurium. J Gen Microbiol. 1960 Apr;22:369–378. doi: 10.1099/00221287-22-2-369. [DOI] [PubMed] [Google Scholar]
- AMES B. N., HARTMAN P. E., JACOB F. Chromosomal alterations affecting the regulation of histidine biosynthetic enzymes in Salmonella. J Mol Biol. 1963 Jul;7:23–42. doi: 10.1016/s0022-2836(63)80016-9. [DOI] [PubMed] [Google Scholar]
- AMES B. N., HORECKER B. L. The biosynthesis of histidine: imidazoleacetol phosphate transaminase. J Biol Chem. 1956 May;220(1):113–128. [PubMed] [Google Scholar]
- AMES B. N., MARTIN R. G., GARRY B. J. The first step of histidine biosynthesis. J Biol Chem. 1961 Jul;236:2019–2026. [PubMed] [Google Scholar]
- Ames B. N., Garry B. COORDINATE REPRESSION OF THE SYNTHESIS OF FOUR HISTIDINE BIOSYNTHETIC ENZYMES BY HISTIDINE. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1453–1461. doi: 10.1073/pnas.45.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., GROS F. Protein biosynthesis. Annu Rev Biochem. 1960;29:525–546. doi: 10.1146/annurev.bi.29.070160.002521. [DOI] [PubMed] [Google Scholar]
- COWIE D. B., COHEN G. N., BOLTON E. T., DE ROBICHON-SZULMAJSTER H. Amino acid analog incorporation into bacterial proteins. Biochim Biophys Acta. 1959 Jul;34:39–46. doi: 10.1016/0006-3002(59)90230-6. [DOI] [PubMed] [Google Scholar]
- COWIE D. B., COHEN G. N. Biosynthesis by Escherichia coli of active altered proteins containing selenium instead of sulfur. Biochim Biophys Acta. 1957 Nov;26(2):252–261. doi: 10.1016/0006-3002(57)90003-3. [DOI] [PubMed] [Google Scholar]
- HARTMAN P. E., HARTMAN Z., SERMAN D. Complementation mapping by abortive transduction of histidine requiring Salmonella mutants. J Gen Microbiol. 1960 Apr;22:354–368. doi: 10.1099/00221287-22-2-354. [DOI] [PubMed] [Google Scholar]
- HARTMAN P. E., LOPER J. C., SERMAN D. Fine structure mapping by complete transduction between histidine-requiring Salmonella mutants. J Gen Microbiol. 1960 Apr;22:323–353. doi: 10.1099/00221287-22-2-323. [DOI] [PubMed] [Google Scholar]
- JACOB F., SUSSMAN R., MONOD J. [On the nature of the repressor ensuring the immunity of lysogenic bacteria]. C R Hebd Seances Acad Sci. 1962 Jun 13;254:4214–4216. [PubMed] [Google Scholar]
- LESTER G., YANOFSKY C. Influence of 3-methylanthranilic and anthranilic acids on the formation of tryptophan synthetase in Escherichia coli. J Bacteriol. 1961 Jan;81:81–90. doi: 10.1128/jb.81.1.81-90.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN A. P., MAGASANIK B. Enzyme synthesis in guanine-starved cells. J Biol Chem. 1961 Jun;236:1810–1815. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., MOYED H. S., GEHRING L. B. Enzymes essential for the biosynthesis of nucleic acid guanine; inosine 5'-phosphate dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1957 May;226(1):339–350. [PubMed] [Google Scholar]
- MANDELSTAM J. The free amino acids in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):103–110. doi: 10.1042/bj0690103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYED H. S. Interference with the feed-back control of histidine biosynthesis. J Biol Chem. 1961 Aug;236:2261–2267. [PubMed] [Google Scholar]
- MOYED H. S., MAGASANIK B. The biosynthesis of the imidazole ring of histidine. J Biol Chem. 1960 Jan;235:149–153. [PubMed] [Google Scholar]
- MUNIER R. L. Substitution totale de la phénylalanine par l'oou la m-fluorophénylalanine dans les protéines d'Escherichia coli. C R Hebd Seances Acad Sci. 1959 Mar 23;248(12):1870–1873. [PubMed] [Google Scholar]
- MUNIER R., COHEN G. N. Incorporation d'analogues structuraux d'aminoacides dans les protéines bactériennes au cours de leur synthèse in vivo. Biochim Biophys Acta. 1959 Feb;31(2):378–391. doi: 10.1016/0006-3002(59)90011-3. [DOI] [PubMed] [Google Scholar]
- Morse M. L., Carter C. E. THE SYNTHESIS OF NUCLEIC ACIDS IN CULTURES OF ESCHERICHIA COLI, STRAINS B AND B/R. J Bacteriol. 1949 Sep;58(3):317–326. doi: 10.1128/jb.58.3.317-326.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISMAN B., HIRSCH M. L. Etude de l'activation et de l'incorporation des acides aminées par des fractions enzymatiques d'E. coli. Ann Inst Pasteur (Paris) 1958 Nov;95(5):615–636. [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Effects of azatryptophan on bacterial enzymes and bacteriophage. Biochim Biophys Acta. 1958 Feb;27(2):330–344. doi: 10.1016/0006-3002(58)90340-8. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The dependence of nucleic acid synthesis on the presence of amino acids in Escherichia coli. J Bacteriol. 1956 Jun;71(6):677–683. doi: 10.1128/jb.71.6.677-683.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. Random replacement of phenylalanine by p-fluorophenylalanine in alkaline phosphatase(s) formed during biosynthesis by E. coli. J Mol Biol. 1963 Apr;6:284–294. doi: 10.1016/s0022-2836(63)80089-3. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. The effect of amino acid analogues on growth and protein synthesis in microorganisms. Bacteriol Rev. 1962 Dec;26:398–420. doi: 10.1128/br.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWBURY R. J., WOODS D. D. Further studies on the repression of methionine synthesis in Escherichia coli. J Gen Microbiol. 1961 Jan;24:129–144. doi: 10.1099/00221287-24-1-129. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. Incorporation of canavanine by Staphylococcus aureus 524 SC. Biochem J. 1959 Oct;73(2):261–264. doi: 10.1042/bj0730261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H. Incorporation of dl-beta-(p-fluorophenyl)[beta-C]alanine into exopenicillinase by Bacillus cereus 569/H. Biochem J. 1960 Oct;77(1):121–135. doi: 10.1042/bj0770121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARON N., LIPMANN F. Reactivity of analogs with pancreatic tryptophan-activating enzyme. Arch Biochem Biophys. 1957 Jul;69:219–227. doi: 10.1016/0003-9861(57)90488-5. [DOI] [PubMed] [Google Scholar]
- SHEDLOVSKY A. E., MAGASANIK B. A defect in histidine biosynthesis causing an adenine deficiency. J Biol Chem. 1962 Dec;237:3725–3730. [PubMed] [Google Scholar]
- SHIVE W., SKINNER C. G. Metabolic antagonists. Annu Rev Biochem. 1958;27(3):643–678. doi: 10.1146/annurev.bi.27.070158.003235. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WHEELER G. P., SCHABEL F. M., Jr, SKIPPER H. E. Potentiated inhibition of Escherichia coli by certain combinations of agents. Proc Soc Exp Biol Med. 1956 Jun;92(2):396–399. doi: 10.3181/00379727-92-22490. [DOI] [PubMed] [Google Scholar]
- YOSHIDA A., YAMASAKI M. Studies on the mechanism of protein synthesis; incorporation of ethionine into alpha-amylase of Bacillus subtilis. Biochim Biophys Acta. 1959 Jul;34:158–165. doi: 10.1016/0006-3002(59)90243-4. [DOI] [PubMed] [Google Scholar]