Abstract
Arabidopsis possesses a superfamily of ATP-binding cassette (ABC) transporters. Among these, the multidrug resistance-associated protein AtMRP5/AtABCC5 regulates stomatal aperture and controls plasma membrane anion channels of guard cells. Remarkably, despite the prominent role of AtMRP5 in conferring partial drought insensitivity upon Arabidopsis, we know little of the biochemical function of AtMRP5. Our phylogenetic analysis showed that AtMRP5 is closely related to maize MRP4, mutation of which confers a low inositol hexakisphosphate kernel phenotype. We now show that insertion mutants of AtMRP5 display a low inositol hexakisphosphate phenotype in seed tissue and that this phenotype is associated with alterations of mineral cation and phosphate status. By heterologous expression in yeast, we demonstrate that AtMRP5 encodes a specific and high affinity ATP-dependent inositol hexakisphosphate transporter that is sensitive to inhibitors of ABC transporters. Moreover, complementation of the mrp5-1 insertion mutants of Arabidopsis with the AtMRP5 cDNA driven from a guard cell-specific promoter restores the sensitivity of the mutant to abscisic acid-mediated inhibition of stomatal opening. Additionally, we show that mutation of residues of the Walker B motif prevents restoring the multiple phenotypes associated with mrp5-1. Our findings highlight a novel function of plant ABC transporters that may be relevant to other kingdoms. They also extend the signaling repertoire of this ubiquitous inositol polyphosphate signaling molecule.
Introduction
Guard cells form pairs of cells, which are conjoined at their ends, in the epidermis of the aerial tissues of plants. The cells surround a central pore, the stoma, through which gas exchange occurs. The principal gases exchanged are CO2 and water vapor, and the function of the stomatal complex may be considered as the maximization of CO2 assimilation by photosynthesis for the minimization of water loss.
Guard cells and hence the aperture of the central pore are sensitive to environmental factors including light, temperature, CO2, and ozone (1). Stomatal closure is initiated by the drought stress hormone abscisic acid (ABA).3 The closure of stomata is a result of a loss of turgor of the delimiting guard cells as a consequence of ion efflux, predominantly Cl− and K+, and metabolic conversion of organic acids into starch (2).
Although the molecular identity of genes encoding the outward and inward K+ conductances are known for Arabidopsis thaliana (3), it remains to be demonstrated whether the recently identified SLAC protein (4, 5) encodes the S-type anion channel or is a subunit thereof.
The ATP-binding cassette family of membrane proteins is among the most ubiquitous and variable group of membrane proteins and is most commonly associated with membrane transport phenomena. The substrates transported are especially diverse, and consequently a major obstacle to the interpretation of ABC transporter function, particularly pertinent in the context of guard cell function, is the identification of the substrate transported.
A recent study identified the ABC transporter AtABCB14 as an apoplast to symplast malate importer capable of modulating stomatal response to CO2 (6), whereas plants bearing mutations in the MRP type ABC transporter AtMRP5 show partial inhibition of ABA-induced stomatal closure (7); impairment of activation of S-type anion channels by both ABA and cytosolic Ca2+ (8); and, additionally, impairment of the activation of plasma membrane Ca2+-permeable channel activity by ABA (8). A number of hypotheses have been proposed to explain the phenotype of atmrp5 mutants; these hypotheses include that AtMRP5 encodes an anion channel or that AtMRP5 directly regulates a guard cell anion channel (8). Both of these possibilities have precedent in the cystic fibrosis transmembrane conductance regulator, a mammalian ABC protein that has ion (Cl−) channel activity and that also modulates the activity of associated ion channels (9).
The activity of other Cl− conductances, but not cystic fibrosis transmembrane conductance regulator, have been shown to be influenced by inositol polyphosphate signaling molecules (10, 11). Most recently, inositol 3,4,5,6-tetrakisphosphate was identified as a physiological regulator of a specific chloride conductance, that of the CIC-3 channel of mammalian secretory epithelia (12).
Inositol 3,4,5,6-tetrakisphosphate and inositol 1,4,5-trisphosphate are not the only inositol phosphates that directly regulate ion channels. In guard cells, inositol hexakisphosphate mobilizes calcium from endomembrane stores (13) and inhibits the inward rectifying K+ conductance (14).
We were fascinated by a recent report that disruption of a gene encoding the ATP-binding cassette protein ZmMRP4 reduced inositol hexakisphosphate accumulation in maize kernels (15). One interpretation of this report is that ZmMRP4 is responsible for transport of inositol hexakisphosphate or a precursor across the vacuole membrane of inositol hexakisphosphate storage tissues of maize kernels.
Our experiments have addressed the function of AtMRP5, an orthologue of ZmMRP4, in planta. By a combination of biochemical, physiological, and genetic analysis, we identify a hitherto undescribed inositol polyphosphate transport function in Arabidopsis for this example of the most ubiquitous class of membrane transport proteins. Our work links inositol hexakisphosphate transport to the regulation of stomatal response to ABA.
EXPERIMENTAL PROCEDURES
Chemical Analysis of AtMRP5 Loss-of-function Mutants
Fifty milligrams of dried seed material was incinerated for 8 h at 550 °C, and subsequently the ash was solubilized in 2 ml of 6 n HCl, briefly heated up to 100 °C, purified through Whatman No. 40 ashless filter paper, and transferred to double-distilled water to a total volume of 50 ml. Element contents were measured by inductively coupled plasma emission-spectroscopy analysis with a Varian Liberty 220 (Varian Inc., Palo Alto, CA) equipped with an ultrasonic nebulizer (CETAC U-5000 AT+) according to standard procedures. Inositol hexakisphosphate and inorganic phosphate were determined by suppressed ion conductivity HPLC of acid extracts of Arabidopsis seeds (16). Seeds, 2–4 mg, were boiled in 0.8 ml of 0.6 m-HCl for 30 min, and the supernatant was diluted 10 times in water. The samples (50 μl) were analyzed on a Dionex ICS2000 chromatography system fitted with a 25-cm × 2-mm internal diameter Dionex AS11 anion exchange column, with an associated AG11 guard column. The column was eluted with a gradient of KOH (0 min, 0 mm KOH; 20 min, 100 mm KOH) at a flow rate of 0.25 ml/min. The inositol hexakisphosphate and Pi content of samples was determined from a calibration curve of peak area versus the amount injected in the range 0.2–4 and 0.2–2 nmol, respectively. In our hands, leaf inositol hexakisphosphate levels were below the limit of detection of this method.
Functional Analysis in Yeast
The ycf1 yeast mutant was transformed with the expression vector pNEV harboring no insert (pNEV) or AtMRP5 cDNA (pNEV-MRP5). Microsomal vesicles were isolated as described previously (17). For the transport of inositol hexakisphosphate, Ins(1,[33P]2,3,4,5,6)P6 prepared according to (18) was added to the reaction mix consisting of transport buffer, 1 mm dithiothreitol, 5 mm ATP, 10 mm MgCl2, 10 mm creatine phosphate, and 100 μg/ml creatine kinase. Yeast microsomes were added to start the transport assay. The assays were performed at room temperature. At intervals, inositol hexakisphosphate uptake was terminated by the transfer of three aliquots, equivalent to 18.9 nCi (700 Bq) of starting material, onto 0.45-μm-diameter pore size nitrocellulose filters. Under these conditions, the rate of net transport was linear with time during the first 5.5 min. The values are corrected for corresponding controls with vesicles isolated from ycf1 yeasts transformed with the empty pNEV vector. ATP-independent inositol hexakisphosphate uptake was assessed in the absence of ATP. Transport assays for all treatments were performed with the same vesicle preparation. The experiments were repeated three times, with independent vesicle preparations. Labeled material recovered on the filter was analyzed by Partisphere SAX HPLC. For the experiments with the nonhydrolyzable ATP analogue AMPPNP, the 4 mm ATP from the reaction mix was replaced with the equivalent concentration of AMPPNP. For the experiments on ice, the tubes containing the reaction mix and the labeled InsP6 were placed on ice immediately after the addition of the yeast microsomes.
Inhibition Studies
Yeast microsomes were incubated at room temperature in the reaction mix that contained the indicated potential inhibitors (see Table 1). After 8 min, Ins(1,[33P]2,3,4,5,6)P6 was added to the reaction mix, and inositol hexakisphosphate uptake was determined as above. The different inhibitors were always tested with the same vesicle preparation as the Ins(1,[33P]2,3,4,5,6)P6 uptake control. The number of repetitions is indicated in Table 1. For experiments with different inositol polyphosphates (see Table 2), the putative inhibitors/competitors were added to the reaction mix at the concentration indicated in Table 2. The transport assay was started by the addition of microsomes. The assays were performed at room temperature. After 5.5 min, uptake was terminated by the transfer of three replicates onto 0.45-μm-diameter pore size nitrocellulose filters. The number of repetitions of the experiment is indicated in Table 2. ATP-dependent AtMRP5-mediated transport was considered as 100%. The reaction mix contained labeled InsP6 at 1.85 kBq/nitrocellulose filter.
TABLE 1.
The effect of ABC transport inhibitors on AtMRP5-mediated inositol hexakisphosphate transport
Yeast microsomes were incubated in the reaction mix with the potential inhibitors at room temperature. After 8 min inositol hexakisphosphate was added to the reaction mix. For the experiments with the nonhydrolyzable ATP analogue (AMPPNP), the 4 mm ATP from the reaction mix was replaced with the equivalent concentration of AMPPNP. For the experiments on ice, tubes containing the reaction mix and the labeled InsP6 were placed on ice immediately after the addition of the yeast microsomes. The InsP6 uptake was terminated by transfer of three aliquots onto 0.45-μm diameter pore size nitrocellulose filters after 5.5 min. The values are corrected for corresponding controls with vesicles isolated from ycf1 yeasts transformed with the empty pNEV vector. The different inhibitors and competitors were always tested using the same vesicle preparation. The reaction mix contained labeled InsP6 at 1.85 kBq/nitrocellulose filter.
| Condition | Percentage | Repetitions |
|---|---|---|
| + ATP | 100 | 6 |
| + AMPPNP | 0 | 2 |
| + 5 mm NH4Cl | 77.6 ± 15.5 | 3 |
| + 150 μm glibenclamide | 28.8 ± 17.8 | 3 |
| + 1 mm vanadate | 62 ± 11.2 | 3 |
| + 1 mm probenicide | −12.5 ± 22.9 | 3 |
| Ice | 7.3 ± 7.9 | 2 |
TABLE 2.
The effect of inositol polyphosphates on AtMRP5-mediated inositol hexakisphosphate transport
The putative inhibitors and/or competitors were added to the reaction mix consisting of transport buffer, 1 mm dithiothreitol, 5 mm ATP, 10 mm MgCl2, 10 mm creatine-phosphate, and 100 μg/ml creatine kinase at the concentration indicated above. Yeast microsomes were added to start the transport assay. The assays were performed at room temperature. After 5.5 min, inositol hexakisphosphate uptake was terminated by the transfer of three aliquots/repetition onto 0.45-μm-diameter pore size nitrocellulose filters. The putative inhibitors and/or competitors were always tested using the same vesicle preparation. ATP-dependent AtMRP5-mediated transport was considered as 100%. The reaction mix contained labeled inositol hexakisphosphate at 1.85 kBq/nitrocellulose filter.
| Condition | Percentage | Repetitions |
|---|---|---|
| + ATP | 100 | 6 |
| + 600 nm | ||
| Ins(1,4,5)P3 | 111.7 ± 17.6 | 3 |
| Ins(1,3,4,6)P4 | 104.1 ± 18.4 | 3 |
| Ins(1,4,5,6)P4 | 100.6 ± 16.8 | 3 |
| Ins(1,3,4,5,6)P5 | 80.5 ± 6.6 | 2 |
| Scyllo InsP6 | 119.8 ± 12.5 | 3 |
| Ins(1,2,3,4,5,6)P6 | 52.7 ± 1.8 | 2 |
| Estradiol glucuronide | 122.6 ± 16.2 | 3 |
| +1200 nm | ||
| Ins(3,4,6)P3 | 157.3 ± 3.8 | 3 |
| Ins(1,3,4,6)P4 | 100.8 ± 30.3 | 2 |
| Ins(1,4,5,6)P4 | 80.2 ± 20.2 | 3 |
| Ins(1,3,4,5,6)P5 | 61.0 ± 7.1 | 2 |
| Scyllo InsP6 | 57.7 ± 13.6 | 2 |
| Ins(1,2,3,4,5,6)P6 | 0 ± 0 | 2 |
| Estradiol glucuronide | 92.4 ± 4.9 | 3 |
Partisphere SAX HPLC
Extracts of radiolabeled Arabidopsis seedlings, products of transport assays, and preparations of Ins(1,[33P]2,3,4,5,6)P6 were all analyzed by anion exchange HPLC on a 23.5-cm × 4.6-mm internal diameter Partisphere SAX WVS cartridge with guard cartridge (18). The column was eluted at a flow rate of 1 ml/min with a gradient derived by mixing of A, water; B, 1.25 m-(NH4)2HPO4 adjusted to pH 3.8 with H3PO4 according to the following program: 0 min, 0% B; 5 min, 0% B; 65 min, 100% B; 75 min, 100% B. Radioactivity (3H or 33P) in column eluates was estimated by admixture of Optima Flo™ AP (Canberra Packard, Pangbourne, UK) scintillation fluid at 2 ml/min in a Canberra Packard A515 flow detector fitted with a 0.5-ml flow cell. The integration interval was 0.2 min.
Analysis of Stomatal Apertures
Arabidopsis (Ws-2) leaves were transformed with the GUS reporter gene under control of the MYB60 promoter (19) by particle bombardment. For detection of GUS activity, the leaves were incubated for 8 h at 37 °C in 1 mm X-glucuronic acid, 0.5% Triton X-100, 50 mm NaHPO4, pH 7.2, and subsequently cleared in 70% ethanol. The AtMRP5 cDNA under the control of the MYB60 promoter was cloned into Gateway™ compatible plant transformation vectors (20). The resulting constructs were used to transform mrp5-1 mutants by the floral dipping method using Agrobacterium (21). Arabidopsis plants (Ws-2, mrp5-1, and MYB60::MRP5, independent homozygous transformed lines from the T3 generation) were grown in soil in a phytotron (8-h light/16-h dark cycle; 21 °C, 70% relative humidity). For stomatal aperture measurements, the leaves of 6-week-old plants were harvested and incubated for 3 h in the light in a solution, 30 mm KCl, 1 mm CaCl2, 5 mm MES-KOH, pH 5.8, with or without 5 μm ABA. Epidermal strips, prepared from the abaxial surface, were analyzed by light microscopy.
Radiolabeling of A. thaliana
Seeds of Ws-2 and mrp5-1 were germinated and grown on a lab prepared half-strength Murashige and Skoog medium containing 2% agar. The medium was prepared to match the Duchefa (Haarlem, The Netherlands) M0221 product. The inorganic phosphate (KH2PO4) content of the medium was 0.625 mm. The seedlings were grown for 12 days at 22 °C under long day conditions (16-h light/8-h dark cycle) in a Sanyo (Biomedical Europe BV) MLR-351 light cabinet at a fluence rate of 120 μmol/m2/s. The seedlings were subsequently transplanted to agar medium containing either 0.625 mm or 5 μm phosphate and grown for a further 3 days, before transfer to liquid medium with the same phosphate concentration. The seedlings were labeled in 0.5 ml of liquid medium containing 1.125 MBq myo-[2-3H]inositol (PerkinElmer Life Sciences; specific activity, 752 GBq/mmol) in the wells of a multiwell plate for the 5-day duration of growth on liquid medium. The medium was supplemented with 0.2 ml of medium lacking radiolabel after 2 days.
RESULTS
Ablation of AtMRP5 Reduces Inositol Hexakisphosphate Level
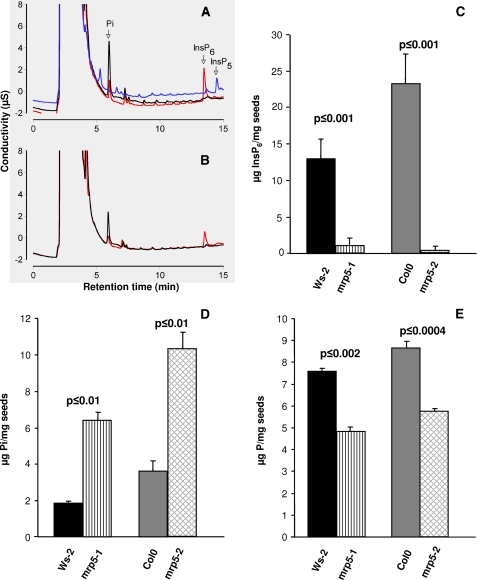
In a recent paper it was shown for maize that the absence of an ATP-binding cassette protein, ZmMRP4, reduced inositol hexakisphosphate accumulation in kernels (15). Phylogenetic studies of ZmMRP4 revealed that AtMRP5 is a very close homologue. Because AtMRP5 is strongly expressed in seeds, we were interested whether knock-out mutants of this ABC transporter exhibit the same low inositol hexakisphosphate phenotype. To this end, we used two independent T-DNA insertion mutants of AtMRP5 in their different genetic backgrounds to determine their corresponding inositol hexakisphosphate contents. Inositol hexakisphosphate accumulates as mixed salts of mineral cations in the globoids of specialized vacuoles in Arabidopsis (22) as in other seeds (23). Seed inositol hexakisphosphate was measured by suppressed ion conductivity (16, 24). Example profiles are shown in Fig. 1A, (note that the identity of the inositol hexakisphosphate peak was confirmed for all genotypes by spiking extracts from these genotypes with an inositol hexakisphosphate standard). Fig. 1A includes a profile of the biosynthetic mutant ipk1-1. On the Dionex AS11 column used, inositol 1,3,4,5,6-pentakis phosphate and inositol 3,4,5,6-tetrakisphosphate, both of which accumulate in ipk1-1 elute after inositol hexakisphosphate (24). Our analysis showed that mrp5-1 and mrp5-2 mutants do not accumulate inositol hexakisphosphate in the seed; nor do they accumulate lower inositol phosphates, in contrast to ipk1-1, which accumulates inositol 1,3,4,5,6-pentakisphosphate and inositol 3,4,5,6-tetrakisphosphate predominantly among tetrakisphosphate species (25). Our measurements of seed inositol hexakisphosphate in the Col0 genetic background of mrp5-2, 24.8 ± 4.1 nmol/mg of dry weight, agrees favorably with the data in Ref. 25 (22.3 ± 0.4 nmol/mg). Seed inositol hexakisphosphate was reduced to 0.3 ± 0.6 nmol/mg in the mrp5-2 mutant (Fig. 1C). This value and that of the mrp5-1 mutant (1.1 ± 1.1 nmol/mg) as compared with its Ws-2 parent (14 ± 3.1 nmol/mg) are significantly greater depletions than that reported for the biosynthesis mutant ipk1-1, 3.9 ± 0.4 nmol/mg (25), for which we obtained a value of 4.1 ± 2.9 nmol/mg (data not shown). These data provide genetic evidence that AtMRP5 is required to accumulate inositol hexakisphosphate in seeds. Measurement of inorganic phosphate levels in mrp5 mutants and their parents (Fig. 1, A, B, and D) revealed that depletions of inositol phosphates in the mutants are accompanied by accumulation of inorganic phosphate. The values obtained for seeds of Col0 (35.9 ± 5.4 nmol/mg of dry weight), mrp5-2 (103.9 ± 9.4 nmol/mg), Ws-2 (18.5 ± 1.0 nmol/mg), and mrp5-1 (65.0 ± 4.3 nmol/mg), when compared with atipk1-1 (30.2 ± 1.2 nmol/mg), indicate that inositol hexakisphosphate transporter mutants show a seed inorganic phosphate accumulation phenotype that is more severe than that of the biosynthetic mutant atipk1. The measurements of total phosphorus (Fig. 1E) revealed a reduction, relative to wild type, respectively, of 37% in the mrp5-1 mutant and of 34% in mrp5-2. We also attempted to measure inositol hexakisphosphate levels in leaf tissue of Arabidopsis. In our hands, the levels were below the level of detection by suppressed ion conductivity, a consequence in part of a number of unidentified anions eluting in the region of inositol hexakisphosphate.
FIGURE 1.
Ablation of AtMRP5 disturbs inositol hexakisphosphate, phosphate, and phosphorus levels in seeds. The conductivity profiles of seed extracts is shown. A, traces for Col0 (red line), mrp5-2 (black line), and ipk1-1 (blue line). B, traces for Ws-2 (red line) and mrp5-1 (black line). Individual traces are offset on the y axis to aid visualization. C, seed InsP6. D, inorganic phosphate (Pi). E, total phosphorus (P) content of two independent AtMRP5 mutants and their corresponding wild types. The data represent the means and standard deviations of four to eight independent measurements.
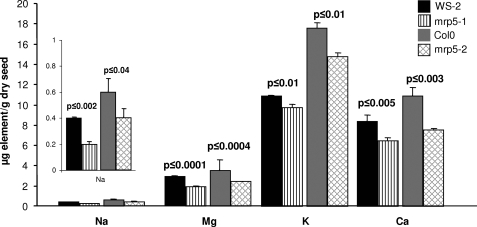
It has already been extensively reported that mrp5 mutants have pronounced phenotypes, some of which manifest in guard cells (7, 8, 26). Determinations of cations (Fig. 2) that form chelates with inositol hexakisphosphate (phytin) revealed concurrent reductions in mineral composition of seeds of mrp5-1 and mrp5-2. Those minerals that differed significantly between wild type parent and mutant included sodium, which was present only in very low amounts and which was reduced by 41 and 32%. Magnesium was reduced by 34 and 31%, whereas calcium was reduced by 23 and 31%. A small reduction was observed for potassium (17%), whereas no significant reduction was determined for iron, manganese, zinc, nickel, and copper. It is known that several divalent cations strongly bind to phytate, but the species can differ from plant to plant. Our data suggest that iron is not associated with phytate in Arabidopsis. Additional data for other minerals that were not found to differ significantly between mutant and wild type genotypes is provided in supplemental Fig. S1.
FIGURE 2.
Analysis of cation reserves in seeds of Arabidopsis. Seed content of cations that differ significantly in the two independent AtMRP5 mutants (mrp5-1 and mrp5-2) as compared with their corresponding wild types. The data represent the means and standard deviations of four independent measurements. Na, sodium; Mg, magnesium; K, potassium; Ca, calcium.
AtMRP5 Encodes a High Affinity Inositol Hexakisphosphate Transporter
The absence of lower inositol phosphates in the conductivity profiles (Fig. 1, A and B) contrasts with the biosynthetic mutant ipk1-1 and with biosynthetic mutants, for example, of barley (27, 28) and maize (29, 30). To test whether AtMRP5 is an inositol hexakisphosphate transporter or a membrane protein participating, but not directly catalyzing inositol hexakisphosphate transport, we expressed AtMRP5 in yeast deficient in the YCF1 ABC transporter. Preliminary transport experiments with different mutants of MRP-type ABC transporters revealed that ycf1 exhibited only 10–20% of the MgATP- dependent transport activity compared with the corresponding wild type strain (W303). This residual activity might be due to one of the MRP-like genes still present in ycf1. We were unsuccessful in our attempts to transform AtMRP5 into double and triple MRP knock-out mutants, so we used AtMRP5-transformed ycf1 for further analysis. Microsomes were prepared and used to perform studies of [33P]inositol hexakisphosphate uptake. Inositol hexakisphosphate transport was dependent on AtMRP5 and ATP and was linear with respect to time up to 5.5 min (Fig. 3A). In the absence of AtMRP5, a low level of ATP-dependent inositol hexakisphosphate uptake was observed. Expression of AtMRP5 induced a slight increase in inositol hexakisphosphate transport in the absence of ATP, whereas the addition of ATP, to microsomes isolated from AtMRP5-transformed ycf1 cells, resulted in a 6-fold increase of the ATP-dependent transport activity.
FIGURE 3.
AtMRP5 is a high affinity inositol hexakisphosphate transporter. A, inositol hexakisphosphate uptake into microsomes isolated from yeast ycf1 mutant cells transformed with an empty vector (pNEV) or with vector harboring AtMRP5 cDNA (pNEV-MRP5). For non-ATP-dependent uptake, the reaction mix lacked ATP. Transport under all conditions was performed with the same vesicle preparation. Open square, pNEV-ATP; filled square, pNEV+ATP; open circle, pNEV-AtMRP5-ATP; filled circles, pNEV-AtMRP5+ATP. The InsP6 concentration used for the time-dependent uptake was 80 nm. B, partisphere SAX HPLC analysis of the 33P recovered from filtered and washed microsomes showing the integrity of inositol hexakisphosphate after transport. C, inositol hexakisphosphate uptake by microsomes isolated from yeast cells harboring AtMRP5. Uptake velocities were measured at different inositol hexakisphosphate concentrations, as indicated. D, a double reciprocal plot was used to determine the Km value of AtMRP5.
Replacement of ATP by the nonhydrolyzable ATP analogue AMPPNP abolished transport (Table 1). This result further confirms that transport of inositol hexakisphosphate is strictly ATP-dependent.
HPLC analysis of the 33P recovered from filtered and washed microsomes revealed that, within the timeframe of our uptake experiments, there was no discernible metabolism of Ins(1,[33P]2,3,4,5,6)P6 to lower inositol phosphate species, nor to inorganic phosphate (Fig. 3B). Thus, the phosphate in the 2-position of inositol hexakisphosphate, and the whole molecule, was metabolically stable. We did not observe phosphorylation of inositol hexakisphosphate, as catalyzed by the KCS1 (31) or VIP1 (32) proteins of Saccharomyces cerevisiae. Inclusion of a range of ABC transport inhibitors revealed that the sufonylureas glibenclamide and probenicid had a very strong inhibitory effect on AtMRP5-mediated inositol hexakisphosphate uptake (Table 1). Vanadate, which is an efficient inhibitor for most ABC-type protein-mediated transport processes, had only a partial inhibitory effect. NH4Cl, which abolishes pH gradients had a very minor inhibitory effect, confirming that the ΔpH plays no or only a minor role in ABC transporter-mediated processes.
Transport experiments performed on ice showed that transport was abolished under these conditions, discounting the possibility that the transport activity reflected binding of inositol hexakisphosphate only, or that transport was a facet of nonspecific permeability of yeast microsomes. A kinetic analysis of inositol hexakisphosphate uptake was undertaken at room temperature. Uptake followed saturation kinetics. A double reciprocal plot of uptake against inositol hexakisphosphate concentration yielded a linear relationship and estimations of Km of 310 nm in one experiment and 263 nm in another (Fig. 3C and supplemental Fig. S2). Vmax values reflecting the expression level of AtMRP5 were estimated at 1.6–2.5 μmol min−1 mg−1 microsome protein (Fig. 3C). The ATP-dependent inositol hexakisphosphate transport activity from nontransformed yeast microsomes corresponded only to 10–20% of that observed with AtMRP5 expressing yeast. Therefore, the obtained Km values should reflect closely the characteristics of AtMRP5-mediated transport.
To characterize the substrate specificity of AtMRP5, we performed competition experiments with a range of inositol polyphosphates identified in plants (Table 2). At a concentration corresponding to a 2-fold Km of inositol hexakisphosphate (600 nm), no significant inhibition was observed for Ins(1,4,5)P3, Ins(1,3,4,6)P4, and Ins(1,4,5,6)P4. With Ins(1,3,4,5,6)P5, transport was reduced by 20%. Scyllo-inositol hexakisphosphate did not inhibit transport at 600 nm, suggesting that substrate binding exhibits stereospecificity and is not exclusively a consequence of high density of negative charge on the substrate. Estradiol glucuronide, a compound shown to be transported by AtMRP5 (26), had a slight stimulatory effect. Increasing the concentration of the inhibitors to 1200 nm resulted in an ∼40% inhibition of myo-inositol hexakisphosphate uptake by both Ins(1,3,4,5,6)P5 and scyllo-inositol hexakisphosphate. Interestingly, at this concentration Ins(1,4,5)P3 exhibited a stimulatory effect of about 50%. From this result it is tempting to speculate that Ins(1,4,5)P3 at a higher concentration can accelerate the depletion of the cytosolic InsP6 and thus the termination of the InsP6 signaling pathway. In summary, these data ascribe novel function to AtMRP5, that of a specific high affinity myo-inositol hexakisphosphate transporter.
The Inositol Phosphate Metabolism of atmrp5 Mutants Is Sensitive to External Phosphate
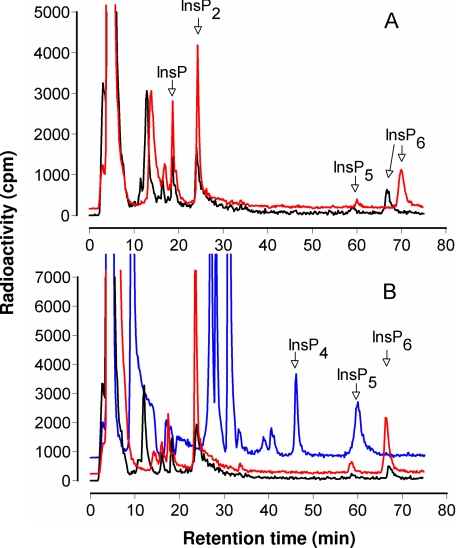
In the context of reduced vacuolar deposition of inositol hexakisphosphate and accumulation of inorganic phosphate, the atmrp5 transporter mutants potentially afford the opportunity to assess the interplay, previously untested, of intracellular inositol phosphate transport and inositol phosphate metabolism. Inositol hexakisphosphate synthesis is responsive to phosphate supply in suspension culture of Arabidopsis (33). We labeled Ws-2 and mrp5-1 seedlings with inositol and analyzed the responsiveness of inositol hexakisphosphate synthesis to phosphate concentration in the growth and labeling media. Our analysis (Fig. 4) revealed that the profile of 3H-labeled peaks of increasing phosphorylation, from inositol up to inositol hexakisphosphate, were similar for Ws-2 and mrp5-1 mutants treated and labeled in low phosphate. The absence of major peaks of label in the inositol 1,4,5- trisphosphate to 1,3,4,5,6-pentakisphosphate region is typical of plant tissues. Material eluting before the InsP peak includes inositol and, likely, cell wall sugars derived from inositol. In a typical experiment, at 5 μm phosphate, the label recovered in inositol pentakisphosphate and inositol hexakisphosphate peaks were, respectively, 0.25 and 0.96% for Ws-2 and 0.07 and 0.60% for mrp5-1. These results and the seed inositol phosphate data (Fig. 1) suggest that the control exerted by AtMRP5 on inositol hexakisphosphate accumulation in seeds or whole seedlings is not manifest as an accumulation of metabolic precursors. Moreover, seedlings of Ws-2 and mrp5-1 grown and labeled on high phosphate accumulated ∼2-fold more label in inositol hexakisphosphate than those transferred to and labeled in low phosphate media. Similar conclusions have been drawn from measurements of inositol hexakisphosphate in suspension cultures of Catharantheus roseus and Arabidopsis grown in low and high phosphate, with Arabidopsis showing weaker responses to phosphate than C. roseus (33). In our experiments, the proportions of label recovered in inositol pentakisphosphate and inositol hexakisphosphate were 0.15 and 1.89% for Ws-2 and 0.25% and 1.46% for mrp5-1 grown and labeled on high phosphate. These data show that, in respect to metabolic flux from inositol to inositol hexakisphosphate, the mrp5-1 mutant is responsive to environmental phosphate status like its wild type Ws-2 parent. We conclude that, unlike the ipk1-1 mutant, which is deficient in recognition of external phosphate (25), mrp5 mutants are not defective in sensing environmental phosphate. The data presented above indicate that AtMRP5 makes a dominant contribution to accumulation of inositol hexakisphosphate in seeds of Arabidopsis. Although we are not aware of gene expression studies of inositol hexakisphosphate accumulation in Arabidopsis, of the biosynthetic gene products, IPK1 has a dominant contribution to inositol hexakisphosphate accumulation (25). We undertook expression analysis of the effect of mutation of AtMRP5 on transcript levels of AtIPK1 and of four members of the inositol tris/tetrakisphosphate kinase family whose contribution to inositol hexakisphosphate synthesis, while undefined in Arabidopsis (34), is anticipated from studies of maize (30). The data presented in the supplementary information (supplemental Fig. S3) indicate that mutation of AtMRP5 was without effect on IPK1 or ITPK1 (At5G16760) in either background, whereas a doubling of the other transcripts was evident in mrp5-1 only.
FIGURE 4.
Profiles of inositol phosphates from [3H]inositol-labeled seedlings. A, traces for Ws-2 labeled with low phosphate (black line) and high phosphate (red line). B, traces for mrp5-1 labeled with low phosphate (black line) and high phosphate (red line) and for ipk1-l labeled with high phosphate (blue line). Note that the retention time of inositol phosphates can vary; InsP6 was confirmed for all genotypes by spiking parallel samples with an InsP6 standard. The traces for mrp5-1 and Ws-2 labeled with high phosphate and the ipk1-1 trace have been offset on the y axis to aid visualization.
Atmrp5 Mutants Show Defective Stomatal Responses That Are Partially Relieved by Guard Cell-targeted Expression of AtMRP5
The foregoing data point not only to a novel inositol hexakisphosphate transport function for AtMRP5 but also to a dominant contribution to inositol hexakisphosphate accumulation. ABC transport proteins have not been extensively characterized in plants, but among them AtMRP5 and AtABCB14 have been shown to exert control over a number of cellular targets, particularly ion channels whose regulation underlies the control of stomatal function. Because we have shown that inositol hexakisphosphate is a physiological regulator of vacuolar (Ca2+-permeable) and plasma membrane (inward rectifying K+) conductances (13, 14), we sought to determine a role for AtMRP5 in guard cell function. We tested the effectiveness of ABA as an inhibitor of stomatal opening in response to light, because this response is a well documented physiological indicator of MRP5 function, one to which anion channel activity contributes markedly. The data of Fig. 5C were obtained from epidermal peels of wild type (Ws-2), the mrp5-1 mutant, and a series of lines of mrp5-1 that had been independently transformed with an AtMRP5 construct driven from a promoter, MYB60, that is strongly expressed in guard cells but not in epidermal cells (Fig. 5, A and B). Stomatal opening was strongly inhibited by ABA in Ws-2 but not in the mrp5-1 mutant (Fig. 5C). Significantly, transgenic expression of AtMRP5 restored ABA responsiveness to the mrp5-1 mutant, a result that was confirmed for several independent transgenic lines. Detailed physiological analyses of the above mentioned transgenic lines revealed that guard cell-targeted expression of AtMRP5 did not restore wild type seed InsP6 content (Fig. 5D) and did not restore the root phenotype (supplemental Fig. S4).
FIGURE 5.
Guard cell-targeted expression of AtMRP5 restores stomatal phenotype in AtMRP5 mutant. A and B, the MYB60 promoter targets GUS exclusively to guard cells. C, the mrp5-1 mutant is insensitive to ABA (ABA inhibits opening of stomata in response to light). The MYB60::AtMRP5 transgene restores ABA sensitivity to mrp5-1. The results for Ws-2, mrp5-1, and three independent transgenic T3 lines are shown. At least 200 stomates of abaxial epidermal strips were measured for each genotype. Two independent experiments were performed, and three additional T3 lines of the transgenic gave the same results (data not shown). The error bars represent the S.E. D, the MYB60::AtMRP5 transgene does not complement the seed inositol hexakisphosphate content of mrp5-1. The data represent the means and standard deviations of three independent measurements. For C, the p value indicates the confidence of significance between light treatment and light+ABA treatment within the same genotype. For D, the p value indicates the confidence of significance between wild type and the four other genotypes.
It remains a matter of debate whether the observed stomatal phenotype of mrp5-1 is the result of an interaction between AtMRP5 and unknown proteins and/or the AtMRP5-mediated transport of a specific molecule. Therefore, to get further insights as to whether a functional transporter is required, site-directed mutagenesis of AtMRP5 was undertaken, and the mutated cDNAs driven by the CaMV35S promoter were transformed into mrp5-1 (supplemental Fig. S5A). The mrp5-1 related phenotypes (seed InsP6 content, root length, drought resistance, and stomatal aperture) were analyzed for all constructs in the T3 generation. Most of mutants showed an intermediate phenotype as compared with Ws-2 and mrp5-1 for all traits. Only those plants with either a D771A or a D1429A exchange in the Walker B domain of NBD1 or NBD2 behaved like mrp5-1 (supplemental Fig. S5, b–e). Because the Walker B domain is known to be essential for the transport activity of most ABC transporters (35), this result suggests that AtMRP5-mediated InsP6 uptake and the stomatal phenotype are both dependent on a functional AtMRP5.
DISCUSSION
Inositol polyphosphates and inositol pyrophosphates contribute to diverse cell biological and developmental phenomena including activated exocytosis (36), mRNA export (37, 38), cell cycle activities (39), and establishment of left-right asymmetry of organ development in zebra fish (40). In plants, inositol hexakisphosphate mobilizes calcium and inhibits the inward rectifying K+ conductance of guard cells (13, 14).
Despite all of these elaborations of higher inositol polyphosphate function, remarkably little is known of the compartmentation of inositol polyphosphate synthesis or of the contribution of compartmentation to the roles identified above. Interestingly, inositol hexakisphosphate is a component of basal resistance to fungal, bacterial, and viral pathogens in Arabidopsis, with an indication that a discrete subcellular pool of inositol hexakisphosphate contributes to these phenomena (41). In higher plants, inositol hexakisphosphate accumulates as mixed salts of alkali and other metal cations in the globoids of specialized vacuoles of storage tissues (23). By corollary, inositol hexakisphosphate is a major component of the laminal layer of hydatid cysts, the reproductive stage of the animal parasite Echinococcus granulosus (24). Both of these examples imply vectorial transport of inositol hexakisphosphate or a precursor across cellular membranes, noncytosolic synthesis of inositol hexakisphosphate, and/or trafficking of membrane-bound inositol hexakisphosphate. These possibilities are all highlighted by the apparent enigma that human multiple inositol polyphosphate phosphatase localizes within the lumen of the endoplasmic reticulum, whereas its inositol phosphate substrates are considered to be exclusively cytosolic (42).
Our elaboration of AtMRP5 as an inositol hexakisphosphate transporter, taken with the analysis of ZmMRP4 mutants (15), provides a mechanistic explanation of the accumulation of inositol hexakisphosphate in storage tissues of plants that differs from established conventions. Previous explanations of inositol hexakisphosphate accumulation in membrane-bound protein bodies, including the aleurone bodies of cereals, are tightly linked to morphological examination of vesicle trafficking phenomena as ontogenetic explanations of the origins of protein bodies themselves (43, 44). We are reminded of analysis of natural variation of inositol hexakisphosphate accumulation in Arabidopsis, which mapped a trait in inositol hexakisphosphate accumulation to a 99-kb region that harbored a tonoplast membrane ATPase G-subunit (16). Although the exact locus of the trait was not identified, one possible explanation of the variation in inositol hexakisphosphate is that accumulation of inositol hexakisphosphate is dependent on vacuolar transport processes.
We now show that two distinct insertion mutants of AtMRP5 (Col0 and Ws-2) in different, genetic backgrounds both display a low phytic acid phenotype in seed tissue. We show that the low inositol hexakisphosphate seed phenotype is associated with alterations of mineral cation and phosphate status in both genetic backgrounds. The low seed phytate phenotype is a direct consequence of the missing transport activity. Our data demonstrate that AtMRP5 is a specific, high affinity inositol hexakisphosphate transporter.
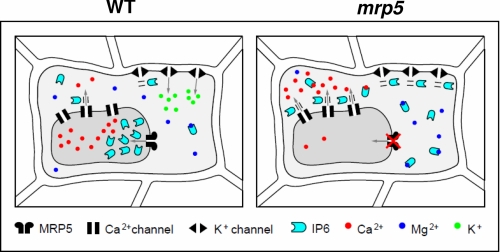
Beyond our identification of the molecular identity of an inositol phosphate transporter, our analysis reveals, in the context of existing literature, that disruption of a gene encoding an inositol hexakisphosphate transporter contributes to stomatal biology. Because we show that MRP5 expression, exclusively, in guard cells of the mrp5-1 mutant restores sensitivity of these cells to ABA, we hypothesize that AtMRP5 is involved in stomatal regulation because of its capacity to transport inositol hexakisphosphate. The physiology of guard cells is dominated by the multitude of ion channels and transporters whose regulation is integrated in this specialized epithelial cell, with tonoplast transport playing a central role (2). It may be significant that the fast and slow Ca2+-permeable conductances of the tonoplast are both targets of inositol hexakisphosphate (13). For the meantime, in the absence of direct studies of electrophysiological targets of inositol hexakisphosphate in our transgenics of mrp5-1, it remains plausible that perturbation of cytosolic and/or vacuolar inositol hexakisphosphate concentration because of the absence of its transporter will necessarily deregulate the transport processes that underlie guard cell physiology (Fig. 6).
FIGURE 6.
Hypothetical model that links AtMRP5, InsP6 signaling and guard cell movements. In wild type plants, inositol hexakisphosphate stimulates the release of Ca2+ by specific channels and inhibits the K+ inward channel. To avoid continuous InsP6 signaling, inositol hexakisphosphate has to be transported into the vacuole by AtMRP5. AtMRP5 mutant plants are impaired in the export of InsP6 into the vacuole. Increased concentrations of cytosolic InsP6 might complex divalent cations or induce continuous Ca2+ release, thus disturbing Ca2+-dependent signaling pathways. Furthermore, during the light period, increased InsP6 levels may reduce K+ uptake into guard cells by inhibiting K+ inward rectifying channels.
In consideration of tonoplast function, we suggested in a first report (8) that Ca MV35S promoter-driven AtMRP5-GFP was localized to the plasma membrane of root cells. It is possible that plasma membrane localization is an ectopic consequence of MRP5 overexpression. Indeed, proteomic analysis predicts AtMRP5 to reside in the vacuolar membrane (45). The latter interpretation is consistent with the low seed inositol hexakisphosphate phenotype of mrp5-1 and mrp5-2 mutants; InsP6 is recognized to accumulate in membrane-bound protein bodies of vacuolar nature. To further address the physiological location of AtMRP5, we have examined the location of AtMRP5 expressed under the control of its native promoter. No GFP signal was detected when plants were cultivated under control conditions. Because transcriptomic studies indicated that AtMRP5 transcript levels are increased upon drought stress (46), we subjected mrp5-1 plants expressing AtMRP5 under the control of its native promoter to 14 days of drought stress. Under such conditions, a weak fluorescence signal associated with the vacuolar membrane was detected (supplemental Fig. S6).
These localization studies raise the intriguing question of how a vacuolar inositol hexakisphosphate transporter could affect guard cell signaling and the plasma membrane anion channel activity. A hypothetical model linking the phenomena mentioned above is presented in Fig. 6. The impaired AtMRP5-dependent transport of inositol hexakisphosphate into the vacuole could lead to an increase in cytosolic InsP6, which could act either by complexing divalent cations such as Mg2+ and Ca2+ or triggering a continuous efflux of Ca2+ into the cytosol by an InsP6-regulated channel. This could lead in both cases to a disturbance of Ca2+-dependent signaling pathways. This hypothesis could explain the impaired ABA and Ca2+ signaling observed in mrp5 mutants. However, it cannot be excluded that the impaired stomatal aperture observed in the mrp5 mutants during the light phase is not additionally the result of increased InsP6 binding to unidentified targets or Ca2+-dependent inhibition of the K+ inward channel (14).
In conclusion, our identification of a high affinity inositol hexakisphosphate transport associated with the tonoplast membrane adds to the diversity of functions assigned to the ABC transporter class of membrane proteins and also answers a long standing question of how inositol phosphates traverse membranes. Given the ubiquity of this class of proteins, we can anticipate that inositol phosphate transport will contribute not only to the regulation of diverse cellular signaling phenomena, perhaps to human disease, but also potentially to the acquisition of environmental inositol hexakisphosphate, which comprises one of the most abundant sources of organic phosphate in the environment (47).
Supplementary Material
Acknowledgments
We are grateful to Thomas Flura (Eidgenössische Technische Hochschule, Zürich, Switzerland) for the inductively coupled plasma measurements and to Maik Hadorn for drawing the model.
This work was funded by Swiss National Foundation Grant 3100AO-117790 (to E. M.), by funds from the Forschungskredit der Universität Zürich (to R. N.), by European Union Project Public Health Impact on Long Term, Low Level Mixed Element Exposure (PHIME) Contract FOOD-CT-2006-0016253 (to J. K. S.), and by Biotechnology and Biological Sciences Research Council Grant BB/C514090/1 (to C. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental text and Figs. S1–S6.
- ABA
- abscisic acid
- ABC
- ATP-binding cassette
- HPLC
- high pressure liquid chromatography
- AMPPNP
- adenyl-5′-yl imidodiphosphate
- MES
- 4-morpholineethanesulfonic acid
- insP6
- inositol hexakisphosphate.
REFERENCES
- 1.Hetherington A. M. (2001) Cell 107, 711–714 [DOI] [PubMed] [Google Scholar]
- 2.MacRobbie E. A. (1998) Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1475–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebaudy A., Vavasseur A., Hosy E., Dreyer I., Leonhardt N., Thibaud J. B., Véry A. A., Simonneau T., Sentenac H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5271–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vahisalu T., Kollist H., Wang Y. F., Nishimura N., Chan W. Y., Valerio G., Lamminmäki A., Brosché M., Moldau H., Desikan R., Schroeder J. I., Kangasjärvi J. (2008) Nature 452, 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negi J., Matsuda O., Nagasawa T., Oba Y., Takahashi H., Kawai-Yamada M., Uchimiya H., Hashimoto M., Iba K. (2008) Nature 452, 483–486 [DOI] [PubMed] [Google Scholar]
- 6.Lee M., Choi Y., Burla B., Kim Y. Y., Jeon B., Maeshima M., Yoo J. Y., Martinoia E., Lee Y. (2008) Nat. Cell Biol. 10, 1217–1223 [DOI] [PubMed] [Google Scholar]
- 7.Klein M., Perfus-Barbeoch L., Frelet A., Gaedeke N., Reinhardt D., Mueller-Roeber B., Martinoia E., Forestier C. (2003) Plant J. 33, 119–129 [DOI] [PubMed] [Google Scholar]
- 8.Suh S. J., Wang Y. F., Frelet A., Leonhardt N., Klein M., Forestier C., Mueller-Roeber B., Cho M. H., Martinoia E., Schroeder J. I. (2007) J. Biol. Chem. 282, 1916–1924 [DOI] [PubMed] [Google Scholar]
- 9.Schwiebert E. M., Egan M. E., Hwang T. H., Fulmer S. B., Allen S. S., Cutting G. R. (1995) Cell 81, 1063–1073 [DOI] [PubMed] [Google Scholar]
- 10.Yang L., Reece J., Gabriel S. E., Shears S. B. (2006) J. Cell Sci. 119, 1320–1328 [DOI] [PubMed] [Google Scholar]
- 11.Vajanaphanich M., Schultz C., Rudolf M. T., Wasserman M., Enyedi P., Craxton A., Shears S. B., Tsien R. Y., Barrett K. E., Traynor-Kaplan A. (1994) Nature 371, 711–714 [DOI] [PubMed] [Google Scholar]
- 12.Mitchell J., Wang X., Zhang G., Gentzsch M., Nelson D. J., Shears S. B. (2008) Curr. Biol. 18, 1600–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemtiri-Chlieh F., MacRobbie E. A., Webb A. A., Manison N. F., Brownlee C., Skepper J. N., Chen J., Prestwich G. D., Brearley C. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10091–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemtiri-Chlieh F., MacRobbie E. A., Brearley C. A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8687–8692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi J., Wang H., Schellin K., Li B., Faller M., Stoop J. M., Meeley R. B., Ertl D. S., Ranch J. P., Glassman K. (2007) Nat. Biotechnol. 25, 930–937 [DOI] [PubMed] [Google Scholar]
- 16.Bentsink L., Yuan K., Koornneef M., Vreugdenhil D. (2003) Theor. Appl. Genet. 106, 1234–1243 [DOI] [PubMed] [Google Scholar]
- 17.Tommasini R., Evers R., Vogt E., Mornet C., Zaman G. J., Schinkel A. H., Borst P., Martinoia E. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 6743–6748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweetman D., Johnson S., Caddick S. E., Hanke D. E., Brearley C. A. (2006) Biochem. J. 394, 95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cominelli E., Galbiati M., Vavasseur A., Conti L., Sala T., Vuylsteke M., Leonhardt N., Dellaporta S. L., Tonelli C. (2005) Curr. Biol. 15, 1196–1200 [DOI] [PubMed] [Google Scholar]
- 20.Curtis M. D., Grossniklaus U. (2003) Plant Physiol. 133, 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clough S. J., Bent A. F. (1998) Plant J. 16, 735–743 [DOI] [PubMed] [Google Scholar]
- 22.Otegui M. S., Capp R., Staehelin L. A. (2002) Plant Cell 14, 1311–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lott J. N. (1984) in Seed Physiology (Murray D. R. ed) Vol. 1, pp. 139–166, Academic Press, New York [Google Scholar]
- 24.Casaravilla C., Brearley C., Soulé S., Fontana C., Veiga N., Bessio M. I., Ferreira F., Kremer C., Díaz A. (2006) FEBS J. 273, 3192–3203 [DOI] [PubMed] [Google Scholar]
- 25.Stevenson-Paulik J., Bastidas R. J., Chiou S. T., Frye R. A., York J. D. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 12612–12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaedeke N., Klein M., Kolukisaoglu U., Forestier C., Müller A., Ansorge M., Becker D., Mamnun Y., Kuchler K., Schulz B., Mueller-Roeber B., Martinoia E. (2001) EMBO J. 20, 1875–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatzack F., Hübel F, Zhang W., Hansen P. E., Rasmussen S. K. (2001) Biochem. J. 354, 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorsch J. A., Cook A., Young K. A., Anderson J. M., Bauman A. T., Volkmann C. J., Murthy P. P., Raboy V. (2003) Phytochemistry 62, 691–706 [DOI] [PubMed] [Google Scholar]
- 29.Raboy V., Gerbasi P. F., Young K. A., Stoneberg S. D., Pickett S. G., Bauman A. T., Murthy P. P., Sheridan W. F., Ertl D. S. (2000) Plant Physiol. 124, 355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi J., Wang H., Wu Y., Hazebroek J., Meeley R. B., Ertl D. S. (2003) Plant Physiol. 131, 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saiardi A., Erdjument-Bromage H., Snowman A. M., Tempst P., Snyder S. H. (1999) Curr. Biol. 9, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 32.Mulugu S., Bai W., Fridy P. C., Bastidas R. J., Otto J. C., Dollins D. E., Haystead T. A., Ribeiro A. A., York J. D. (2007) Science 316, 106–109 [DOI] [PubMed] [Google Scholar]
- 33.Mitsuhashi N., Ohnishi M., Sekiguchi Y., Kwon Y. U., Chang Y. T., Chung S. K., Inoue Y., Reid R. J., Yagisawa H., Mimura T. (2005) Plant Physiol. 138, 1607–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweetman D., Stavridou I., Johnson S., Green P., Caddick S. E., Brearley C. A. (2007) FEBS Lett. 581, 4165–4171 [DOI] [PubMed] [Google Scholar]
- 35.Frelet A., Klein M. (2006) FEBS Lett. 580, 1064–1084 [DOI] [PubMed] [Google Scholar]
- 36.Illies C., Gromada J., Fiume R., Leibiger B., Yu J., Juhl K., Yang S. N., Barma D. K., Falck J. R., Saiardi A., Barker C. J., Berggren P. O. (2007) Science 318, 1299–1302 [DOI] [PubMed] [Google Scholar]
- 37.York J. D., Odom A. R., Murphy R., Ives E. B., Wente S. R. (1999) Science 285, 96–100 [DOI] [PubMed] [Google Scholar]
- 38.Odom A. R., Stahlberg A., Wente S. R., York J. D. (2000) Science 287, 2026–2029 [DOI] [PubMed] [Google Scholar]
- 39.Lee Y. S., Mulugu S., York J. D., O'Shea E. K. (2007) Science 316, 109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarmah B., Latimer A. J., Appel B., Wente S. R. (2005) Dev. Cell 9, 133–145 [DOI] [PubMed] [Google Scholar]
- 41.Murphy A. M., Otto B., Brearley C. A., Carr J. P., Hanke D. E. (2008) Plant J 56, 638–652 [DOI] [PubMed] [Google Scholar]
- 42.Craxton A., Caffrey J. J., Burkhart W., Safrany S. T., Shears S. B. (1997) Biochem. J. 328, 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams C., Norby S. W., Rinne R. W. (1985) Crop Sci. 25, 255–262 [Google Scholar]
- 44.Marty F. (1997) in Advances in Botanical Research: The Plant Vacuole (Leigh R. A., Sanders D. eds) Vol. 25, pp. 1–42, Academic Press, London [Google Scholar]
- 45.Jaquinod M., Villiers F., Kieffer-Jaquinod S., Hugouvieux V., Bruley C., Garin J., Bourguignon J. (2007) Mol. Cell. Proteomics 6, 394–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmermann P., Hirsch-Hoffmann M., Hennig L., Gruissem W. (2004) Plant Physiol. 136, 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner B. L., Papházy M. J., Haygarth P. M., McKelvie I. D. (2002) Philos. Trans. R. Soc. London B Biol. Sci. 357, 449–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.