Abstract
Sleep loss is known to potently suppress adult hippocampal cell proliferation and neurogenesis. Whether sleep suppression following acute administration of stimulant drugs also decreases hippocampal cell proliferation is not known. The present study examined the effect of three mechanistically distinct stimulants (caffeine, methamphetamine, and modafinil) on cell proliferation. To maximize sleep suppression, these drugs were administered to rats (three i.p. injections, once every 4 hours) during their sleep period (i.e., 12-h light phase). At the end of the light phase, 5-bromo-2′-deoxyuridine (200 mg/kg, i.p.) was injected and animals were sacrificed 2 hours later. Polygraphic recordings and locomotor activity measurements confirmed the wake-promoting/sleep-suppressing action of each treatment. Results indicate that caffeine (20 mg/kg), methamphetamine (1.5 mg/kg) and modafinil (300 mg/kg) differentially suppressed sleep (45%-91%) and selectively reduced cell proliferation in the hilus (12%-44%), which was significant for both caffeine and modafinil. When the same experiment was repeated in the dark (active) phase, the suppressant effect on hippocampal cell proliferation was either absent or greatly attenuated. In a further experiment, the effect of acute modafinil treatment in the light phase was shown to persist three weeks after BrdU administration. We hypothesize that the differential effect of the stimulant drugs in the light versus dark phase is attributable primarily to sleep suppression in the light. Since abuse of stimulant drugs invariably leads to disrupted sleep in humans, our results suggest that they may, at least in part, decrease hippocampal neurogenesis via sleep loss, and thereby adversely affect hippocampal-dependent processes.
Keywords: caffeine, methamphetamine, modafinil, sleep suppression, neurogenesis
Introduction
Loss of sleep in humans can arise from many sources and is known to compromise health, cognition, mood, and vigilance state. Recently, a number of studies have shown that sleep deprivation in adult rats produces profound deficits in hippocampal cell proliferation and neurogenesis (Guzman-Marin et al., 2005; Mirescu et al., 2006; Mueller et al., 2008; Roman et al., 2005; Tung et al., 2005). These neural deficits have the potential for affecting not only the processes of learning and memory (Shors et al., 2001; Snyder et al., 2005; Winocur et al., 2006), but also contributing to psychopathologies, such as clinical depression (Jacobs et al., 2000; Malberg & Duman, 2003; Kempermann et al., 2008).
To date, all of these studies have utilized environmental or mechanical means to induce sleep loss. For example, animals have been placed on treadmills, or on small platforms or movable disks suspended over water. Administration of stimulant drugs represents another approach for increasing waking/suppressing sleep. Drugs such as caffeine and modafinil are widely used for this purpose (e.g., military personnel, long-distance truckers, healthcare providers, college students, and patients with sleep disorders). Whether or not acute administration of CNS stimulants, by promoting wakefulness and suppressing sleep, also decreases cell proliferation is not known.
The present study examined three, mechanistically distinct stimulants (caffeine, methamphetamine, and modafinil). The drugs were intentionally administered in high doses in order to maximize sleep suppression over a 14 hour period that included the entire 12 hours of the adult rat’s normal inactive or sleep phase of the daily (12:12) light-dark cycle. Because these agents can ameliorate the adverse effects of sleep deprivation on mood, cognitive performance, and vigilance state (Baranski & Pigeau, 1997; Beaumont et al., 2001; Bonnet et al., 2005), the examination of these drugs is particularly relevant.
Of the three stimulant drugs, modafinil is perhaps the most interesting since it induces prolonged wakefulness without excessive behavioral stimulation or subsequent sleep rebound in rats and cats (Edgar & Seidel, 1997; Lin et al., 2000; Touret et al., 1995). The finding that modafinil also reduces sleep debt when administered following sleep deprivation in both animals and humans (Chapotot et al., 2003; Lin et al., 2000) suggest that it may attenuate the need for sleep. Therefore, while our primary hypothesis was that modafinil, caffeine, and methamphetamine would produce a decrease in cell proliferation due to their sleep suppressant actions in rats, we also considered the possibility that the drugs themselves might influence the impact of sleep loss on hippocampal cell proliferation.
Materials and methods
Animals
Adult, male Sprague-Dawley rats (8 wks, approx. 300 g) obtained from Taconic Farm (Germantown, NY, USA) were used in all experiments. The rats were housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved animal facility under controlled conditions (standard or reversed 12-12 h light/dark cycle, lights on at 0900 h or 2100 h; ambient temperature 21 ± 1°C; relative humidity 45-55%) and maintained on commercial rat chow and water ad libitum. Upon arrival, rats were group-housed in hanging metal cages (3 or 4 per cage) for 7 days, to habituate to the new environment. One week prior to the experiment, the rats were singly housed in standard polycarbonate cages. All animal procedures were in accordance with the National Institutes of Health animal care guidelines and were approved by The Princeton University Institutional Animal Care and Use Committee. Every effort was made to minimize pain and/or discomfort in the experimental animals.
Stimulant drugs
The three stimulant drugs employed in these studies were caffeine, methamphetamine and modafinil. Caffeine is the most widely consumed CNS stimulant and exerts its vigilance-enhancing properties by antagonizing brain adenosine receptors, which are hypothesized to promote sleep onset (Huang et al., 2005; Nishino & Mignot, 2005). Methamphetamine is the prototypical CNS stimulant and promotes wakefulness by enhancing the synaptic availability of dopamine and norepinephrine in the brain (Nishino & Mignot, 2005). Modafinil is a relatively new wake-promoting agent which has a pharmacological profile distinct from that of amphetamine and related psychostimulant drugs. Its mode of action is currently unknown, but may involve modulation of multiple neurotransmitter systems (reviewed by Minzenberg & Carter, 2008).
Caffeine (anhydrous) and D-methamphetamine hydrochloride were purchased from Sigma-Aldrich Company (St. Louis, MO, USA), whereas modafinil was a gift from Cephalon, Inc. (West Chester, PA, USA). The drugs were dissolved (or suspended in the case of modafinil) in 0.25% methycellulose, prior to i.p. administration. Injections were given in a volume of 2 ml/kg of body weight and doses are expressed as the free base of the drug. Control animals received an equivalent volume of drug vehicle and were run concurrently with the drug-treated animals.
Stimulant drug treatment
Rats were randomly assigned to one of four parallel treatments: 1) caffeine (20 mg/kg); 2) methamphetamine (1.5 mg/kg); 3) modafinil (300 mg/kg); and 4) vehicle control (0.25% methycellulose). Each treatment was given three times, once every 4 hours.
The stimulant drugs were administered to rats during their normal physiological sleep period (i.e., light phase), with the specific aim of producing nearly continuous wakefulness over the next 12 hours. To control for possible drug effects unrelated to sleep loss per se, the entire experiment was repeated in dark (active) phase, when rats are spontaneously awake most of the time (see Figure 1). Accordingly, the stimulant drug treatment was initiated either at light onset (light-phase experiment) or light offset (dark-phase experiment).
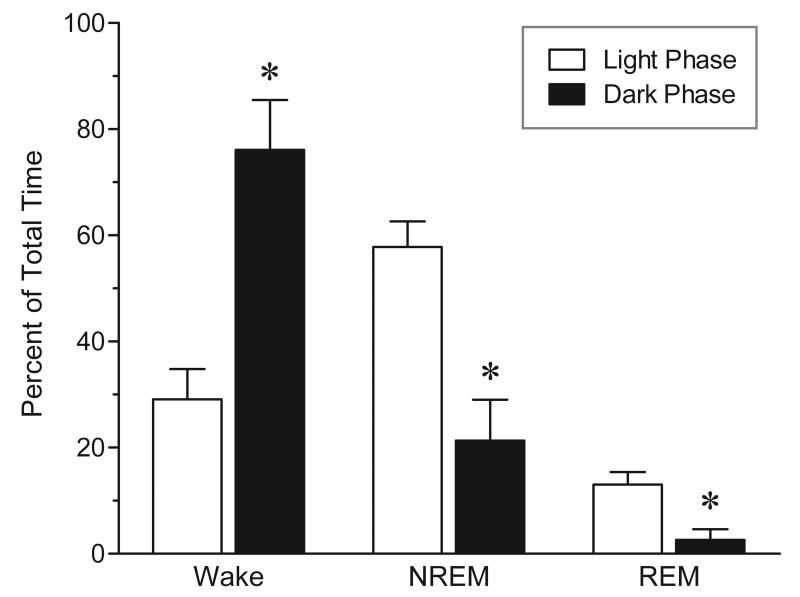
FIG. 1.
Percentage of time spent in sleep and waking during the 12-h light phase versus the 12-h dark phase. Rats spent significantly more time asleep and less time awake during the light phase, as compared to the dark phase. Values are means ± SEM; n = 4. * P < 0.01 vs. respective light phase; by paired (two-tailed) t test.
The doses of caffeine, methamphetamine and modafinil were chosen based on previous literature (Yanik et al., 1987; Schwierin et al., 1996; Edgar & Seidel, 1997) showing that they produce approximately equivalent wake-promoting effects in the rat.
BrdU administration and animal perfusion
At the end of the corresponding light or dark phase, rats received a single i.p. injection of 200 mg/kg of thymidine analog 5-bromo-2′′-deoxyuridine (BrdU), to label proliferating cells in the hippocampus. The BrdU (Sigma-Aldrich) was dissolved in sterile physiological saline (containing 0.007 N NaOH) and given in a volume of 10 ml/kg of body weight. Animals were sacrificed either 2 hours (proliferation study) or 21 days (survival study) after BrdU. On the day following stimulant drug treatment, rats in the survival study were paired-housed (one control and one drug-treated animal) in standard cages, until time of sacrifice. Rats were deeply anesthetized with chloral hydrate (1000 mg/kg, i.p.) and perfused transcardially with cold physiological saline (containing 10 IU heparin/ml), followed by paraformaldehyde (4% in 0.1 M phosphate buffer, pH 7.4). Brains were removed, postfixed in paraformaldehyde, cyroprotected with 30% sucrose (in 0.1 M PBS), and then sectioned on a microtome.
Immunohistochemistry
Frozen coronal sections (40-μm thick) were cut throughout the entire rostral-caudal extent of the hippocampus (bregma − 1.80 mm to − 6.80 mm; Paxinos & Watson, 1986) and every 12th section was then processed for BrdU using a slide-mounted immunoperoxidase technique, as described previously (Fornal et al., 2007).
Briefly, sections were boiled in citric acid, digested with trypsin, denatured with hydrochloric acid, and then incubated with a mouse monoclonal antibody raised against BrdU (Novacastra Laboratories Ltd, Newcastle upon Tyne, UK) for 48 h at 4° C. Following the primary incubation, sections were incubated with a biotinylated horse anti-mouse IgG and with avidin-biotin complex (Vector Laboratories, Burlingame, CA, USA), and then reacted with 3,3′-diaminobenzidine (DAB) to visualize BrdU-labeled cells. Sections were then counterstained with cresyl violet (Sigma-Aldrich), dehydrated and coverslipped with DPX (Fluka Biochemika, Buchs, Switzerland).
All slides were analyzed blind with respect to treatment using an Olympus BX-60 light microscope. BrdU+ cells (stained brown) were counted bilaterally in the DG at 400× magnification. The cell counts for each animal were summed across all sections and then multiplied by 12 to obtain an estimate of the total number of proliferating cells in the DG. In addition, BrdU+ cells were counted separately in the subgranular zone (SGZ) and in the hilus. Cells located within two cell-body widths of the granule cell layer (GCL) were considered to be in the SGZ; cells found more distally were considered to be in the hilus.
Implantation of sleep recording electrodes
The efficacy of each stimulant drug treatment in promoting waking and suppressing sleep was evaluated in a separate group of rats surgically prepared for chronic sleep-wake recordings. Briefly, under deep anesthesia (ketamine 80 mg/kg, i.p. + xylazine 10 mg/kg, i.p.), two stainless-steel screw electrodes were implanted unilaterally over the frontal (2 mm anterior to bregma, 2 mm lateral to midline) and parietal (2 mm anterior to lambda, 2 mm lateral to midline) cortex to record the electroencephalogram (EEG). In addition, two Telfon-coated stainless-steel wires were inserted into the dorsal neck muscles to record the electromyogram (EMG). All electrode leads were soldered to a miniature connector (Microtech Inc., Boothwyn, PA, USA), which was then fixed to the skull with dental acrylic. Postoperative care included pain management with buprenorphine (0.05 mg/kg, s.c.) and prophylactic antibiotic treatment (ampicillin: 50 mg/kg, s.c.) for one day. Following surgery, the rats were housed in individual polycarbonate cages and were allowed 7 days to recover from surgery prior to study. During this time, animals were habituated to the recording cable and set up.
Sleep-wake recordings and analysis
Separate groups of rats implanted with EEG/EMG electrodes received caffeine, methamphetamine, modafinil or drug vehicle (according to the same treatment protocol used in the proliferation study) and were recorded continuously for 14 hours, beginning immediately after the first drug injections at light onset (0900 h). In addition, another group of implanted animals was recorded across the 24-h day, in order to quantify basal levels of sleep and wakefulness during the 12-h light and dark phases. All recordings were conducted while rats remained in their home cages, fitted with modified cage tops. Electrical potentials were recorded from each animal via a flexible cable and low-torque commutator, permitting unimpeded movement throughout the cage. EEG and EMG signals were amplified, band-pass filtered (EEG, 1-35 Hz; EMG, 30-1000 Hz), and recorded on a polygraph (Grass Model 7D; Astro-Med, Inc., Warwick, RI, USA) at a chart speed of 5 mm/sec. EEG and EMG data were divided into 10-sec epochs and scored as waking, non-rapid eye movement (non-REM) sleep, or rapid eye movement (REM) sleep. The waking state was characterized by low-voltage, high-frequency EEG and high to moderate neck muscle activity. Non-REM sleep was defined by high-amplitude, low-frequency (delta-wave) EEG with sleep spindles (11–16 Hz) and diminished neck muscle tone. REM sleep was defined by intermediate-amplitude EEG with dominant theta frequency (4–8 Hz), combined with loss of muscle tone. The amount of time each animal spent in sleep and waking was determined over the entire recording period and expressed as a percentage of total time.
Home cage activity
Animal were recorded via videotape throughout the entire experiment and gross locomotor activity was quantified by counting the number of cage crossings each animal made. A vertical stripe of black adhesive tape (1-cm wide), running down the middle of the exterior cage wall, divided the length of the cage (~ 45-cm long) into two equal halves. The number of times the rat crossed this line during each hour was determined blind. A cage crossing was scored whenever two-thirds (or more) of the animal’s body moved from one side of the cage to the other side.
Data analysis
The baseline sleep-wake data across the light and dark phases were analyzed by paired (two-tailed) t test. The overall effects of the stimulant drugs on each sleep-wake state were analyzed by one-way analysis of variance (ANOVA). The cage crossing data were analyzed by two-way repeated-measures ANOVA (treatment as the between-subject factor, time as the within-subject factor). For statistical analysis of the cell proliferation data in each hippocampal region, a one-way ANOVA was used. Post-hoc testing of significant ANOVAs was conducted using Bonferroni’s multiple comparison test. The cell survival data in each hippocampal region were analyzed by unpaired (two-tailed) t test. Comparisons of proliferation responses to each stimulant drug in both the light and dark phases were made by unpaired (two-tailed) t test. All statistical analyses were carried out using GraphPad Prism 4.02 (GraphPad Software, La Jolla, CA, USA). In all cases, a probability value (P) ≤ 0.05 was considered statistically significant. Data are expressed as means ± SEM.
Results
Sleep-wake states
For the purpose of determining the amount of sleep loss due to stimulant drug administration, a baseline study of sleep-waking was conducted in a separate group of animals. As shown in the open bars of Figure 1, the predominant state observed during the light phase was NREM sleep (58 ± 5% of total time) while REM sleep occupied a much smaller proportion of time (13 ± 2% of total time). The remaining time was devoted to waking (29 ± 6% of total time).
Since we also administered the same stimulant drugs during the dark phase, we examined the baseline amounts of sleep-wake states during this 12 hour period. As shown in the black bars of Figure 1, animals are mostly awake during this phase (76 ± 9% of total time) and virtually no REM sleep is seen (3 ± 2% of total time). The remaining time is spent in NREM sleep (21 ± 8% of total time). The amounts of sleep and waking were significantly different between the light and dark phases (paired t tests: wakefulness: t3 = 11.30, P = 0.002; NREM sleep: t3 = 10.26, P = 0.002; REM sleep: t3 = 6.20, P = 0.008).
Administration of the stimulant drugs produced substantial variation in the patterns of sleep and wakefulness over the 14 hour observation period. During the light phase (hours 0-12), vehicle-treated animals spent 56 ± 8% of the time in NREM sleep, and 12 ± 4% in REM sleep. These values are very similar to the baseline recordings (without vehicle injections) described above. As Figure 2 (top, left panel) demonstrates, in vehicle-treated animals, NREM sleep was predominant throughout hours 0-12, with slight declines following injections at hours 0, 4, and 8 (and reciprocal increases in waking). After the onset of the dark period (hour 12), vehicle-treated animals were primarily awake.
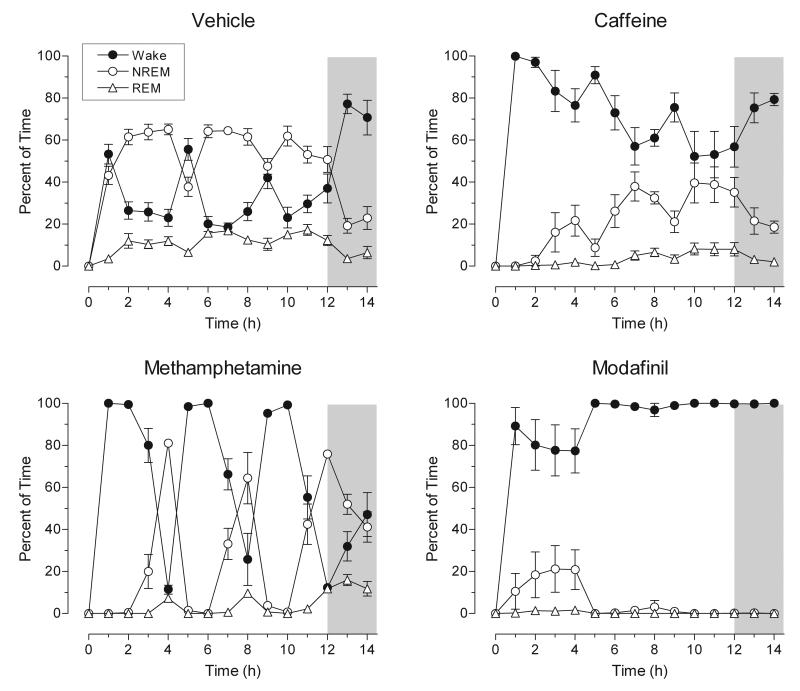
FIG. 2.
Effect of stimulant drugs on the sleep-wake cycle in the rat. Data are expressed as the hourly percentage of time spent in wakefulness, NREM and REM sleep. Caffeine (20 mg/kg), methamphetamine (1.5 mg/kg), modafinil (300 mg/kg) or drug vehicle were administered at hours 0, 4, and 8. The unshaded portion of each graph represents the 12-h light phase, whereas the shaded portion represents the first two hours of the dark phase. Values are means ± SEM; n = 7 for vehicle; n = 6 for caffeine, methamphetamine, and modafinil. All three stimulants markedly altered sleep-wake patterns over the 14-h recording period (see text for details).
In caffeine-treated animals, wakefulness was the predominant state for the first 4 hours following the initial drug injection, but increasing levels of NREM sleep were observed in subsequent hours (Figure 2; top, right panel). A peak in waking can also be seen following each injection of caffeine. The progressive decrease in waking following each drug administration may reflect the development of rapid tolerance to caffeine. REM sleep was almost completely suppressed for the duration of the experiment in caffeine-treated animals.
Animals treated with methamphetamine demonstrated pronounced wakefulness in the hours following drug administration, with almost complete sleep suppression in the 2 hours following each of the three injections (Figure 2; bottom, left panel). At approximately 3 hours post-administration, however, wakefulness rapidly declined, with NREM sleep becoming the predominant state. Notably, after the onset of the dark phase (hours 12-14) methamphetamine-treated animals spent 47 ± 10% of the time in NREM sleep and 14 ± 5% of the time in REM sleep, as compared to 21 ± 7% and 5 ± 4%, respectively, in vehicle-treated animals. This is most likely rebound sleep following the drug-induced sleep suppression.
The most profound wake promoting effect was observed in modafinil-treated animals. As shown in Figure 2 (bottom, right panel), following the second drug injection at hour 4, modafinil-treated animals exhibited almost complete wakefulness, with little variation among animals. There was a nearly complete suppression of REM sleep for the duration of the experiment (hours 0-14), as less than 0.5% of the time was spent in REM sleep. Furthermore, following the second injection, there was also a nearly complete suppression of NREM sleep.
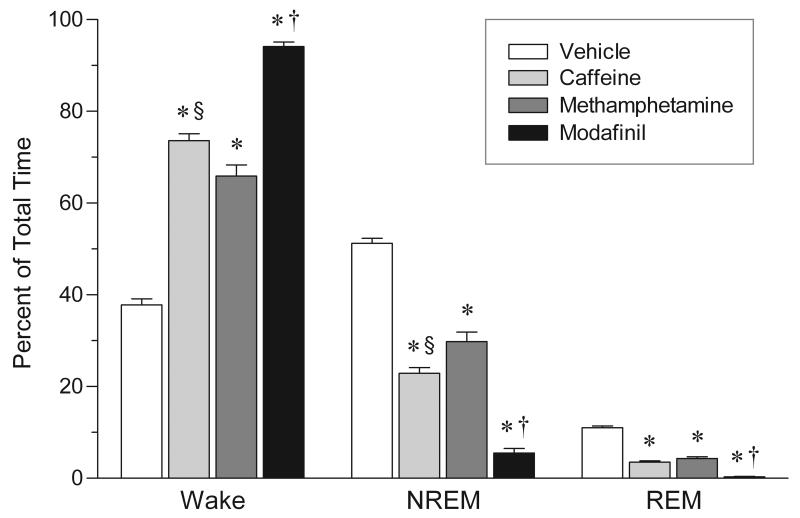
Figure 3 summarizes the effects of the three stimulant drugs on wakefulness, NREM and REM sleep. Significant changes were observed in the amount of time animals spent in sleep and waking (one-way ANOVAs: wakefulness: F3,21 = 218.3, P <0.0001; NREM sleep: F3,21 = 190.6, P < 0.0001; REM sleep: F3,21 = 193.3, P < 0.0001). The overall levels of sleep suppression (NREM + REM sleep) were 45% for methamphetamine, 58% for caffeine, and 91% for modafinil, relative to vehicle-treated animals. The sleep suppression produced by modafinil was significantly greater than that produced by either caffeine (Bonferroni posttests: NREM sleep: t10 = 8.63, P < 0.001; REM sleep: t10 = 6.67, P < 0.001) or methamphetamine (Bonferroni posttests: NREM sleep: t10 = 12.05, P < 0.001; REM sleep: t10 = 8.33, P < 0.001).
FIG. 3.
Overall effects of stimulant drugs on sleep and waking in the rat. Data are expressed as a percentage of total time spent in wakefulness, NREM and REM sleep over the entire 14-h recording experiment. Values are means ± SEM; n = 7 for vehicle; n = 6 for caffeine, methamphetamine, and modafinil. Drug dosages as in figure legend 2. * P < 0.001 vs. vehicle; † P < 0.001 vs. both caffeine and methamphetamine; § P < 0.05 vs. methamphetamine; by one-way ANOVA and post-hoc Bonferroni’s test.
Home cage activity
In addition to polygraph recordings of sleep-wake states, we also examined gross locomotion by counting the number of home cage crossings.
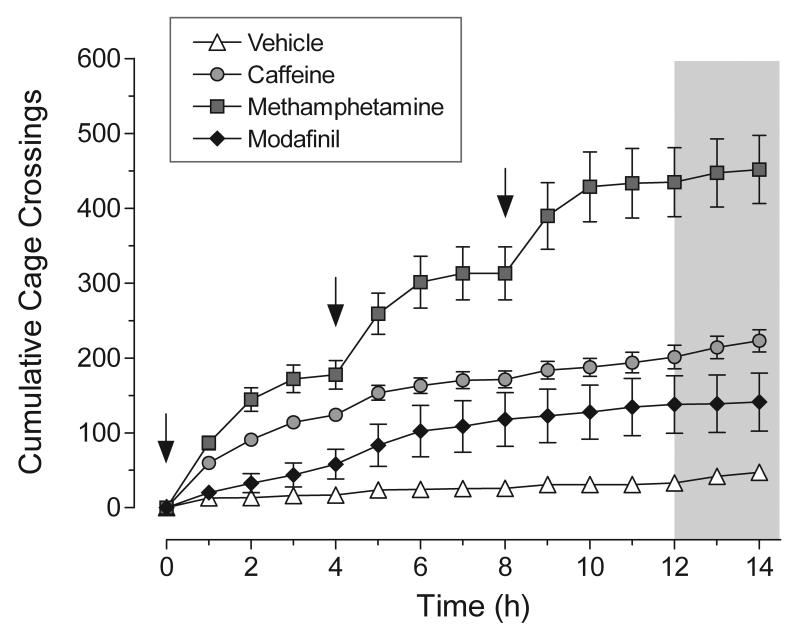
Paralleling the changes observed in sleep/wakefulness following administration of stimulant drugs, significant increases in gross locomotor activity were observed in animals treated with caffeine, methamphetamine, and modafinil (two-way repeated measures ANOVA; main effect of treatment: F3,416 = 31.49, P < 0.0001; Figure 4). Over the 14 h period, methamphetamine produced the most pronounced increase in locomotor activity as compared to control animals (~ 8.5-fold increase in total crossings; Bonferroni posttest: t18 = 12.07, P < 0.001), with each drug injection eliciting a strong locomotor response. Modafinil, on the other hand, produced no significant increase in locomotor activity (Bonferroni posttest: t16 = 2.64, P > 0.05). This was due in part to the fact that animals often remained relatively immobile while engaging in stereotyped head and chewing movements; these behaviors were particularly pronounced following the last drug injections. Caffeine produced an intermediate increase in locomotor activity (~ 3.7-fold increase in total crossings; Bonferroni posttest: t16 = 4.94, P < 0.001). Whereas the initial drug injection elicited a large locomotor response (almost comparable to methamphetamine), subsequent injections were less effective, paralleling caffeine’s diminished wake-promoting effect. The increase in locomotor activity produced by methamphetamine was significantly different from that of either caffeine (Bonferroni posttest: t21 = 6.44, P < 0.001) or modafinil (Bonferroni posttest: t17 = 8.74, P < 0.001).
FIG. 4.
Effect of stimulant drugs on the cumulative number of cage crossings in the rat. Values are means ± SEM; n = 10 for vehicle and methamphetamine; n = 8 for caffeine and modafinil. Drug doses as in figure legend 2. The arrows in the graph denote the times of drug injection. The unshaded portion of each graph represents the 12-h light phase, whereas the shaded portion represents the first two hours of the dark phase. Significance differences (P < 0.05) relative to vehicle control were observed for caffeine (from 4 to 14 h), methamphetamine (from 2 to 14 h) and modafinil (12 h time interval only). The effect of caffeine was significantly different from that of methamphetamine (from 5 to 14 h) but not modafinil. In contrast, the effect of methamphetamine was significantly different from that of modafinil (from 2 to 14 h). Data were analyzed by two-way repeated measures ANOVA and post-hoc Bonferroni’s test.
The weak locomotor-stimulating action of modafinil contrasts with the drug’s strong wake-promoting action observed on the sleep/wake cycle (see Figures 2 and 3). Thus, modafinil-treated animals exhibited a behavioral profile characterized by prolonged waking in the absence of excessive locomotor stimulation (approximately 0.18 crossings per minute of waking vs. 0.15 crossings per minute of waking for control animals, or 20% increase). Conversely, methamphetamine, while eliciting the largest increase in locomotor activity, produced the smallest increase in total waking time. Thus, methamphetamine-treated animals exhibited enhanced waking characterized by intense locomotor stimulation (approximately 0.82 crossings per minute of waking, or 450% increase above control levels). Caffeine-treated animals also exhibited enhanced locomotor activity during waking (approximately 0.36 crossings per minute of waking, or 140% increase above control levels).
Light-phase cell proliferation
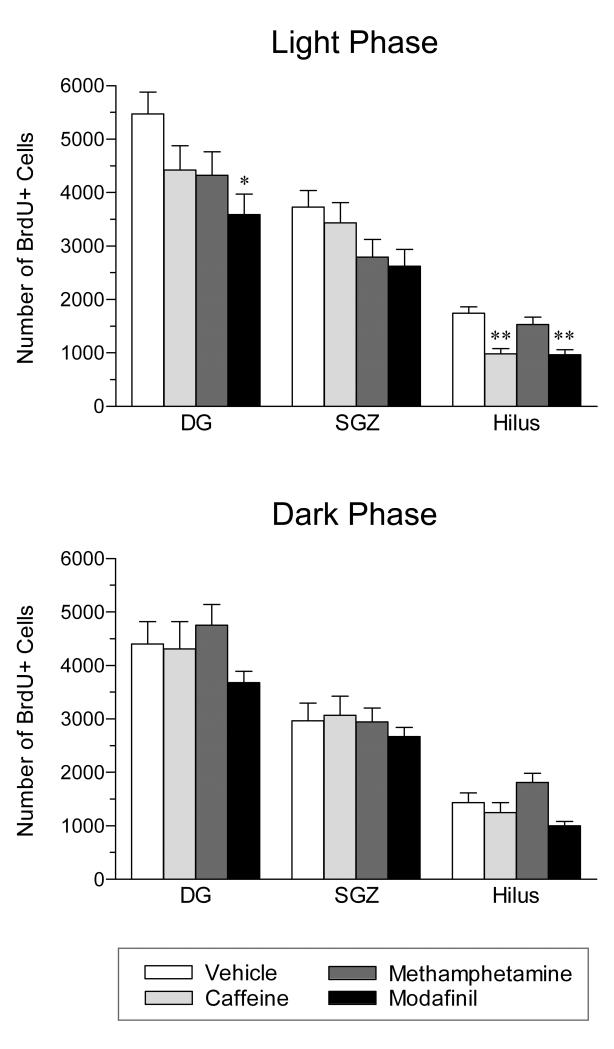
Proliferating cells in the DG were identified by staining for the exogenous marker, BrdU (see Figure 5, top panel). As shown in Figure 6 (top panel), acute administration of stimulant drugs during the light phase of the daily cycle resulted in a significant overall suppression of cell proliferation in the DG (one-way ANOVA: F3,36 = 3.03, P = 0.042). This effect was statistically significant only for modafinil (− 34%; Bonferroni posttest: t17 = 2.87, P < 0.05).
FIG. 5.
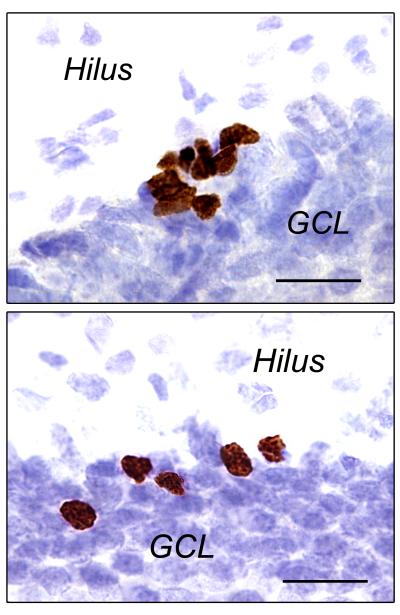
Photomicrographs (1000× magnification) showing BrdU labeling in the dentate gyrus 2 hours (top panel, cell proliferation) or 21 days (bottom panel, cell survival) after BrdU administration (200 mg/kg, i.p.) in the adult rat. Immunoreactive cells appear brown in cresyl violet-stained sections. The top panel depicts a large cluster of newly-generated BrdU+ cells between the granule cell layer (GCL) and the hilus, in the subgranular zone. The bottom panel depicts several mature BrdU+ cells mitigating into the GCL. Scale bar = 20 μm
FIG. 6.
Effect of stimulant drugs administered during either the light phase (top) or the dark phase (bottom) on hippocampal cell proliferation in the rat. Cell proliferation was measured in the dentate gyrus (DG) and this total is subdivided into counts in the subgranular zone (SGZ) and hilus. Caffeine (CAF, 20 mg/kg), methamphetamine (MET, 1.5 mg/kg), modafinil (MOD, 300 mg/kg) or drug vehicle (VEH) were administered every 4 hours (3 injections total), starting at the onset of either the 12-h light phase or the 12-h dark phase. Animals were sacrificed 2 hours after BrdU injection. Values are means ± SEM. For the light phase: n = 12 for vehicle, 11 for caffeine, 10 for methamphetamine, and 7 for modafinil. For the dark phase: n = 7 for vehicle, 5 for caffeine, 6 for methamphetamine, and 10 for modafinil. * P < 0.05 vs. vehicle; by one-way ANOVA and post-hoc Bonferroni’s test. When administered during the light phase, all three stimulants reduced cell proliferation relative to vehicle control and this effect reached statistical significance for modafinil. In contrast, administration of each stimulant during the dark phase had little, if any, effect on cell proliferation.
When the level of proliferation was examined specifically in the hilus, there was an overall decrease in proliferation (one-way ANOVA: F3,36 = 10.60, P < 0.0001) (vehicle = 1741 ± 121; caffeine = 982 ± 101; methamphetamine = 1530 ± 144; and modafinil = 967 ± 93). This effect reached statistical significance for only caffeine (− 44%; Bonferroni posttest: t21 = 4.74, P < 0.001) and modafinil (− 44%; Bonferroni posttest: t17 = 4.25, P < 0.001). In addition, the effects of both drugs were significantly different from methamphetamine (Bonferroni posttests: caffeine: t19 = 3.27, P < 0.05; modafinil: t15 = 2.98, P < 0.05).
When the SGZ was examined separately, there were no statistically significant differences in proliferation in response to any drug (one-way ANOVA: F3,36 = 2.27, P = 0.097) (vehicle = 3729 ± 313; caffeine = 3439 ± 373; methamphetamine = 2794 ± 326; and modafinil = 2623 ± 314).
Dark-phase cell proliferation
Because rats are spontaneously active and awake in the dark (see data above) we hypothesized that if the results found in the light phase were due to sleep loss, the same experiment conducted during the dark phase would yield smaller decrements in cell proliferation. Consistent with this, no significant differences in overall cell proliferation were found in the DG when stimulant drugs were administered during the dark phase (one-way ANOVA: F3,24 = 1.86, P = 0.163; Figure 6, bottom panel).
Similarly, there was no overall change in cell proliferation relative to vehicle control specifically in the hilus (vehicle = 1431 ± 187; caffeine = 1246 ± 186; methamphetamine = 1810 ± 172; and modafinil = 1004 ± 78) or SGZ (vehicle = 2969 ± 330; caffeine = 3065 ± 361; methamphetamine = 2948 ± 253; and modafinil = 2674 ± 169) when stimulant drugs were administered in the dark phase (one-way ANOVAs: hilus: F3,24 = 5.66, P = 0.004; SGZ: F3,24 = 0.46, P = 0.710).
Comparison of light-phase versus dark-phase cell proliferation
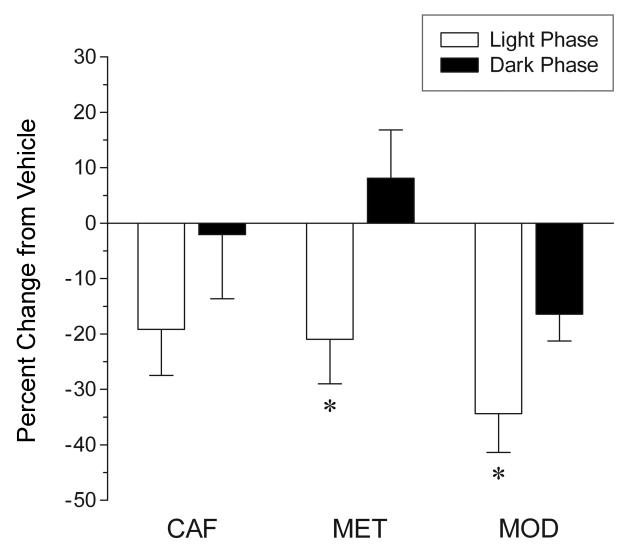
The proliferation responses to each of the three stimulant drugs in both the light phase and the dark phase were compared in the DG. As shown in Figure 7, all three stimulant drugs produced a greater suppression of proliferation when administered in the light phase, as compared to the dark phase (caffeine: − 19 ± 8% vs. − 2 ± 12%; methamphetamine: − 21 ± 8% vs. 8 ± 9%; modafinil: − 34 ± 7% vs. − 16 ± 5%, respectively). This effect was significant for both methamphetamine (unpaired t test: t14 = 2.34, P = 0.034) and modafinil (unpaired t test: t15 = 2.18, P = 0.046), but not caffeine (unpaired t test: t14 = 1.17, P = 0.261).
FIG. 7.
Comparison of the effects of stimulant drugs administered during either the light or dark phase on hippocampal cell proliferation in the dentate gyrus of the rat. Data are expressed as a percentage of change from the respective vehicle control group. Values are means ± SEM. Drug abbreviations and number of animals per experimental group as in figure legend 5. * P < 0.05 vs. respective dark phase; by unpaired (two-tailed) t test. All three stimulants induced a larger suppression of cell proliferation when administered during the light phase, as compared to the dark phase.
Cell survival (modafinil)
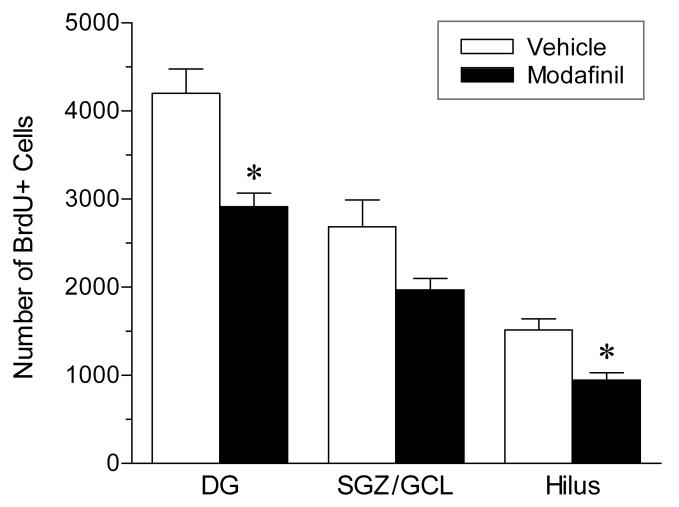
To determine whether stimulant-induced changes in proliferation persist over time, we measured the number of surviving BrdU+ cells in the DG 21 days after BrdU administration (see figure 5, bottom panel). Because modafinil produced the most robust changes in proliferation of the three stimulant drugs tested, it was selected for further study in this experiment. The drug was administered during the light phase, using the same treatment protocol as in the proliferation experiment. As shown in Figure 8, administration of modafinil resulted in a significant overall reduction (− 31%; unpaired t test: t9 = 3.84, P = 0.004) in the number of BrdU-labeled cells in the DG after 3 weeks, suggesting that there was no appreciable compensation for the initial decrease in cell proliferation. Similarly, there was a significant decrease in BrdU+ cell counts (− 38%; unpaired t test: t9 = 3.51, P = 0.007) in the hilus (1514 ± 128 vs. 946 ± 86), whereas no significant change in BrdU labeling was seen in the SGZ/GCL (2688 ± 302 vs. 1970 ± 131; unpaired t test: t9 = 2.03, P = 0.073). Overall, these data demonstrate that stimulant drugs can produce long-lasting effects on the generation and incorporation of new cells in the hippocampus.
FIG. 8.
Effect of modafinil administered during the light phase on hippocampal cell survival in the rat. Cell survival was measured in the dentate gyrus (DG) and this total is subdivided into counts in the subgranular zone/ granule cell layer (SGZ/GCL) and hilus. Modafinil (300 mg/kg) or drug vehicle were administered every 4 hours (3 injections total), starting at the onset of the 12-h light phase. Animals were sacrificed 21 days after BrdU injection. Values are means ± SEM; n = 6 for vehicle; n = 5 for modafinil. * P < 0.01 vs. vehicle; by unpaired (two-tailed) t test.
Discussion
The two most significant findings in this study are: 1) acute administration of stimulant drugs during the quiescent/sleep phase of the light-dark cycle produces a reduction in cell proliferation in the hippocampus of adult rats; and 2) this effect is abolished or markedly reduced when the stimulant drugs are administered during the active/waking phase.
Along with increased wakefulness during the light phase of the light-dark cycle, suppression of the number of proliferating cells in the DG was observed in all three drug-treated groups, with modafinil-treated animals showing the largest and statistically significant reduction. These data demonstrate that modafinil at high doses (300 mg/kg × 3, i.p.), which produces almost complete wakefulness, also produces a 34% decrease in overall cell proliferation in the DG as compared to vehicle-treated animals. Caffeine and methamphetamine produced approximately equivalent waking effects, 74% and 66% of the 14 h period of the study, respectively, and produced approximately the same degree of suppression of hippocampal cell proliferation, 19% and 21%, respectively.
We hypothesize that the suppression of cell proliferation following high doses of stimulant drugs administered during the light phase is attributable, primarily to sleep deprivation. This is consistent with previous studies, using other methods of sleep deprivation, lasting for longer periods of time, which have reported effects similar to these, but generally of a larger magnitude (Guzman-Marin et al., 2005; Mirescu et al., 2006; Tung et al., 2005). The data demonstrating small and non-statistically significant, decreases in cell proliferation when these same drug doses were administered during the active/waking phase of the light-dark cycle support this hypothesis. In the light phase, control animals spent only 29% of the time awake, whereas in the dark phase control animals were awake for 76% of the time. Thus, in the dark phase, the opportunity for drug-induced sleep deprivation is markedly diminished.
Following both caffeine and modafinil administration during the light period, significant decreases in cell proliferation in the hilus were also observed compared to control. It is thought that proliferating cells in the hilus primarily give rise to glia, while cells in the SGZ of the DG tend to differentiate into new neurons (Cameron et al., 1993; Bondolfi et al., 2004; Steiner et al., 2004). Thus the decreases in proliferation in the hilus may demonstrate a suppression in the number of proliferating glial cells. The functional significance of this decrease in gliogenesis is unknown.
These results cannot be attributed to simple increases in gross motor activity. Methamphetamine produced the largest increase in cage crossings but had a smaller effect on cell proliferation than modafinil, which had the largest suppressant effect on cell proliferation, while producing the smallest increase in cage crossings. However, modafinil did produce strong motor stereotypy, which could account, at least in part, for these effects.
How do the effects of the stimulant drugs on hippocampal cell proliferation compare with those of sleep deprivation? A number of studies have reported changes in cell proliferation in the DG of rodents following sleep deprivation (reviewed by Meerlo et al., 2008). In the majority of these studies, prolonged periods of sleep deprivation (48 hours or more) were necessary to produce robust decreases in cell proliferation and neurogenesis. The effects of shorter, more clinically-relevant sleep deprivation periods (less than 24 hours) are less clear. In several recent studies, short–term sleep deprivation has been found to produce inconsistent and even contradictory results. In rats, Roman et al. (2005) reported a significant decrease in cell proliferation following 24 hours of sleep deprivation by forced activity, while Mirescu et al. (2006) observed no change in cell proliferation after the same period of sleep deprivation using the small-platform method. In contrast, a third study in rats (Grassi Zucconi et al., 2006) reported an increase in cell proliferation after 12 hours of sleep deprivation by gentle handling, although sleep was not measured in this study. A similar study carried out in mice found no change in cell proliferation after 10-12 hours of sleep deprivation (van der Borght et al., 2006). To our knowledge, these latter two studies are the only published reports that have examined the acute effects of short-term sleep deprivation of less than one day and, therefore, come closest to our drug study, in terms of duration.
Recently, we have reexamined the effects of short-term (14 hour) sleep deprivation using the gentle handling method in adult rats (Kochman, 2007). Unlike the two previous studies, we employed polygraphic recordings to verify sleep suppression. Rats received brief tactile stimulation (small nudges) to induce arousal whenever they were about to fall asleep, as indicated by the appearance of high-amplitude EEG slow waves. This procedure resulted in an overall level of sleep suppression comparable to that seen with modafinil in the present study (98% vs. 91%, respectively). Compared to undisturbed controls, the sleep-deprived animals showed no significant change in cell proliferation in either the DG as a whole (− 16%, P > 0.05) or the SGZ (− 12%, P > 0.05). However, a significant decrease (− 25%, P < 0.05) in cell proliferation was found in the hilus, as seen with both caffeine and modafinil, albeit this effect was somewhat smaller than that of the stimulant drugs.
The similarities between short-term sleep deprivation and stimulant-induced sleep suppression noted in our studies support the hypothesis that sleep loss primarily affects cell proliferation (and gliogenesis) in the hilus, as previously suggested by Roman and colleagues (2005). These investigators reported a specific reduction of hippocampal cell proliferation in the hilus after 24 hours of sleep deprivation by forced activity, whereas cell proliferation in both the SGZ and hilus was significantly reduced after 8 days of repeated partial sleep deprivation (20 h/day). Collectively, these data suggest that an early manifestation of sleep loss is a selective suppression of hippocampal cell proliferation in the region of the hilus.
Besides being more sensitive to sleep loss, cell proliferation in the hilus may be influenced to a greater extent than cell proliferation in the SGZ. For example, although differential effects of prolonged sleep deprivation on BrdU labeling in the hilus and SGZ have not always been observed (e.g., Mueller et al., 2008), in our previous sleep deprivation study (Tung et al., 2005), we observed a much stronger suppression of cell proliferation in the hilus versus the SGZ (− 60% vs. − 29%) following 56 hours of total sleep deprivation by the disk-over-water method (data not reported).
The effects of sleep deprivation may be related to the cellular consequences of prolonged waking (e.g., depletion of brain energy substrates, increased adenosine concentrations, reduced protein synthesis, etc.). Sleep deprivation also invariably leads to a homeostatic increase in sleep pressure, which opposes waking mechanisms. Thus, during deprivation, sleep-deprived animals spent a considerable amount of time in a drowsy, semi-awake state. Administration of stimulant drugs, on the other hand, strongly suppresses sleep while activating the EEG. Because these agents intensify brain activity, they may enhance or facilitate the processes underlying the effects of sustained waking. Consequently, these agents may be expected to have a greater impact on hippocampal cell proliferation. While this appears to be the case in the present study for both caffeine and modafinil, these stimulant drugs could have actions that affect proliferation other than those produced by sleep suppression.
These findings provide the first report of the impact of acute caffeine and modafinil administration on adult hippocampal cell proliferation. Furthermore, these data extend the reports of Teuchert-Noodt and colleagues (2000) on the effects of methamphetamine on hippocampal cell proliferation in the adult gerbil to a more traditional research species, the rat. In the gerbil, administration of a single high dose of methamphetamine (25 mg/kg, i.p.) significantly reduced granule cell proliferation by 28% when measured 7 days post BrdU labeling. In contrast to these findings, only non-significant changes from control were observed following methamphetamine administration in the rat.
The absence of a significant drug effect on cell proliferation or survival in the SGZ of the DG in this study suggests that these stimulants do not affect hippocampal neurogenesis, at least when administered over a single sleep period, in accordance with previous sleep deprivation studies. However, with longer treatment durations (2 or more days), these drugs may eventually inhibit hippocampal neurogenesis, as is the case with sleep deprivation. In support of this, chronic administration of caffeine (via drinking water) has been shown to suppress hippocampal SGZ cell proliferation and neurogenesis in rats (Han et al., 2007), although it is unclear whether this effect is related to sleep disruption or other actions of the drug.
Our results may be directly relevant to other pharmacological studies on adult neurogenesis. For example, several drugs of abuse such as cocaine and 3,4-methylenedioxymethamphetamine (MDMA), which act as CNS stimulants, have been shown to decrease the production and/or survival of newborn cell in the adult hippocampus (Cho et al., 2007; Domínguez-Escribá et al., 2006; Yamaguchi et al., 2004). Interestingly, in those studies, the drugs were repeatedly administered to rodents during the inactive/sleep phase of the light-dark cycle (personal communications) which most likely resulted in a disruption of normal sleep patterns in the animals. Thus, sleep suppression might have contributed to the deficits in hippocampal neurogenesis seen with these drugs. Since a wide variety of pharmacological agents (in addition to CNS stimulants) can disrupt sleep, especially REM sleep, it may be important in future investigations to control for the influence of drugs on the sleep/wake cycle, when evaluating their effects on hippocampal neurogenesis. Finally, psychoactive substance abuse in humans often leads to a disruption of the normal sleep-wake cycle and this, in and of itself, may have a negative impact on adult hippocampal neurogenesis.
Overall, these data demonstrate that acute administration of specific CNS stimulant drugs that suppress sleep can result in significant decreases in cell proliferation in the adult rat hippocampus. However, it remains for further investigation to determine whether chronic or repeated administration of these drugs exerts persistent effects. For at least one of these agents (modafinil), the effects appear to be long-lasting, and therefore may have implications for the ultimate incorporation of new cells in the hippocampus. Furthermore, attribution of any cognitive effects of alterations in cell proliferation and neurogenesis or gliogenesis could only be assessed in longer-term studies.
Acknowledgements
This work was supported by NIMH Grant MH 023433. We thank Cephalon, Inc. for kindly providing the modafinil for this study. We also thank Matthew Elias, Anne-Lise Maag, Nitya Prabhakar, Joanne Stevens, Jessica Barson, and Drs. Ranata Sanders and Luiz Takase for their excellent technical assistance.
Abbreviations
- AAALAC
Association for Assessment and Accreditation of Laboratory Animal Care
- ANOVA
analysis of variance
- BrdU
bromodeoxyurdine
- CNS
central nervous system
- DAB
3,3′-diaminobenzidine
- DG
dentate gyrus
- EEG
electroencephalogram
- EMG
electromyogram
- GCL
granule cell layer
- NREM
non-rapid eye movement
- REM
rapid eye movement
- SGZ
subgranular zone
References
- Baranski JV, Pigeau RA. Self-monitoring cognitive performance during sleep deprivation: effects of modafinil, d-amphetamine and placebo. J. Sleep Res. 1997;6:84–91. doi: 10.1111/j.1365-2869.1997.00032.x. [DOI] [PubMed] [Google Scholar]
- Bastuji H, Jouvet M. Successful treatment of idiopathic hypersomnia and narcolepsy with modafinil. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1988;12:695–700. doi: 10.1016/0278-5846(88)90014-0. [DOI] [PubMed] [Google Scholar]
- Beaumont M, Batejat D, Pierard C, Coste O, Doireau P, Van Beers P, Chauffard F, Chassard D, Enslen M, Denis JB, Lagarde D. Slow release caffeine and prolonged (64-h) continuous wakefulness: effects on vigilance and cognitive performance. J. Sleep Res. 2001;10:265–276. doi: 10.1046/j.1365-2869.2001.00266.x. [DOI] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol. Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Balkin TJ, Dinges DF, Roehrs T, Rogers NL, Wesensten NJ. The use of stimulants to modify performance during sleep loss: a review by the Sleep Deprivation and Stimulant Task Force of the American Academy of Sleep Medicine. Sleep. 2005;28:1163–1187. doi: 10.1093/sleep/28.9.1163. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Chapotot F, Pigeau R, Canini F, Bourdon L, Buguet A. Distinctive effects of modafinil and d-amphetamine on the homeostatic and circadian modulation of the human waking EEG. Psychopharmacology (Berl.) 2003;166:127–138. doi: 10.1007/s00213-002-1315-8. [DOI] [PubMed] [Google Scholar]
- Cho KO, Kim SK, Rhee GS, Kwack SJ, Cho DH, Sung KW, Kim SY. Chronic 3,4-methylenedioxymethamphetamine treatment suppresses cell proliferation in the adult mouse dentate gyrus. Eur. J. Pharmacol. 2007;566:120–123. doi: 10.1016/j.ejphar.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Domínguez-Escribá L, Hernández-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, García-Verdugo JM, Canales JJ. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur. J. Neurosci. 2006;24:586–594. doi: 10.1111/j.1460-9568.2006.04924.x. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J. Pharmacol. Exp. Ther. 1997;283:757–769. [PubMed] [Google Scholar]
- Fornal CA, Stevens J, Barson JR, Blakley GG, Patterson-Buckendahl P, Jacobs BL. Delayed suppression of hippocampal cell proliferation in rats following inescapable shocks. Brain Res. 2007;1130:48–53. doi: 10.1016/j.brainres.2006.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ME, Park KH, Baek SY, Kim BS, Kim JB, Kim HJ, Oh SO. Inhibitory effects of caffeine on hippocampal neurogenesis and function. Biochem. Biophys. Res. Commun. 2007;356:976–980. doi: 10.1016/j.bbrc.2007.03.086. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol. Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn. Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Grassi Zucconi G, Cipriani S, Balgkouranidou I, Scattoni R. ‘One night’ sleep deprivation stimulates hippocampal neurogenesis. Brain Res. Bull. 2006;69:375–381. doi: 10.1016/j.brainresbull.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur. J. Neurosci. 2005;22:2111–2116. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr. Opin. Psychiatry. 2008;21:290–295. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- Kochman LJ. Ph.D. dissertation. Princeton University; 2007. The Effects of Sleep Deprivation and Stimulant Drugs on Hippocampal Cell Genesis; p. 108. [Google Scholar]
- Lin JS, Gervasoni D, Hou Y, Vanni-Mercier G, Rambert F, Frydman A, Jouvet M. Effects of amphetamine and modafinil on the sleep/wake cycle during experimental hypersomnia induced by sleep deprivation in the cat. J. Sleep Res. 2000;9:89–96. doi: 10.1046/j.1365-2869.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med. Rev. 2008 doi: 10.1016/j.smrv.2008.07.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc. Natl. Acad. Sci. U.S.A. 2006;103:19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A, Pollock MS, Lieblich SE, Epp JR, Galea LA, Mistlberger RE. Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1693–R1703. doi: 10.1152/ajpregu.00858.2007. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mignot E. Wake-promoting medications: Basic mechanisms and pharmacology. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier/Saunders; Philadelphia: 2005. pp. 468–483. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Roman V, Van der Borght K, Leemburg SA, Van der Zee EA, Meerlo P. Sleep restriction by forced activity reduces hippocampal cell proliferation. Brain Res. 2005;1065:53–59. doi: 10.1016/j.brainres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Schwierin B, Borbely AA, Tobler I. Effects of N6-cyclopentyl-adenosine and caffeine on sleep regulation in the rat. Eur. J. Pharmacol. 1996;300:163–171. doi: 10.1016/0014-2999(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Shelton J, Nishino S, Vaught J, Dement WC, Mignot E. Comparative effects of modafinil and amphetamine on daytime sleepiness and cataplexy of narcoleptic dogs. Sleep. 1995;18:817–826. [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46:41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- Yanik G, Glaum S, Radulovacki M. The dose-response effects of caffeine on sleep in rats. Brain Res. 1987;403:177–180. doi: 10.1016/0006-8993(87)90141-7. [DOI] [PubMed] [Google Scholar]
- Teuchert-Noodt G, Dawirs RR, Hildebrandt K. Adult treatment with methamphetamine transiently decreases dentate granule cell proliferation in the gerbil hippocampus. J. Neural Transm. 2000;107:133–143. doi: 10.1007/s007020050012. [DOI] [PubMed] [Google Scholar]
- Touret M, Sallanon-Moulin M, Jouvet M. Awakening properties of modafinil without paradoxical sleep rebound: comparative study with amphetamine in the rat. Neurosci. Lett. 1995;189:43–46. doi: 10.1016/0304-3940(95)11448-6. [DOI] [PubMed] [Google Scholar]
- Tung A, Takase L, Fornal C, Jacobs B. Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neuroscience. 2005;134:721–723. doi: 10.1016/j.neuroscience.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Ferrari F, Klauke K, Roman V, Havekes R, Sgoifo A, van der Zee EA, Meerlo P. Hippocampal cell proliferation across the day: increase by running wheel activity, but no effect of sleep and wakefulness. Behav. Brain Res. 2006;167:36–41. doi: 10.1016/j.bbr.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann. N.Y. Acad. Sci. 2004;1025:351–362. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]