Fusion of cellular compartments is a central aspect of cellular homeostasis and intercellular communication in virtually all eukaryotic cells. Although the general principles of the fusion reaction are beginning to be firmly established, it is also becoming clear that several distinct molecular events need to occur first, before the actual fusion reaction can. Coming from different angles, different research groups have developed different ideas about these upstream steps. A new study in a recent issue of PNAS (1), using reconstituted vacuole fusion in yeast, establishes important elements of consensus. One exciting aspect is that a well-known component of fusion reactions, the R-SNARE, appears to be universally dispensable for the initial docking step.
For organelle inheritance in budding yeast, the mother cell projects tubular and vesicular structures into the growing bud, which then fuse to generate a new vacuole in the daughter cell (2). This vacuole fusion reaction has been reproduced in vitro, initially using an isolated vacuole preparation and recently also with purely synthetic components. The vacuole system has been highly instrumental to address the question of whether genes are necessary and sufficient to orchestrate the fusion and upstream reactions, using a combination of genetic studies in intact cells and isolated vacuoles and in vitro fusion in synthetic proteoliposomes. Because vacuole fusion can be arrested experimentally in vitro at different stages, this system has also been exploited to investigate the sequence of events leading up to fusion. This option is particularly valuable given the current state of the field where new proteins are rarely identified, but the way known proteins do the job is a matter of intense debate.
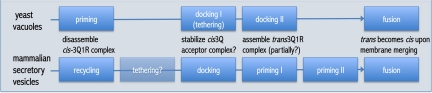
The upstream molecular events are referred to as priming, tethering, and docking steps (see ref. 3 for a review). Here, considerable difference of ideas exists among researchers working on different systems, in addition to vacuole fusion, for instance, mammalian endosome fusion (4, 5) and fusion of different secretory vesicles in mammalian cells (6–9). Adding to the confusion is the fact that the nomenclature describing the different steps in different systems evolved to be dissimilar and confusing (see Fig. 1). All of this has precluded the establishment of a universal model for the initial steps upstream of the membrane fusion reaction.
Fig. 1.
Schematic representation of the proposed upstream steps of the organelle fusion reaction in two different experimental systems. Steps are aligned at the final fusion step and at earlier steps using the proposed configuration of SNARE complexes that drive the fusion reaction as a criterion. The proposed actions on SNARE complexes during the different steps are indicated in the middle. The diagram assumes a linear sequence of events, although some evidence argues for more complex scenarios (see discussion in ref. 3). Docking is proposed to consist of two separate steps in yeast vacuole fusion (see ref. 1). Instead, docking is assumed to be followed by several priming steps in mammalian secretory vesicle fusion. Some of these may be specific for secretory cells, for instance the association of complexins to the SNARE bundle (see refs. 9 and 18). Evidence for a tethering step upstream of secretory vesicle fusion is sketchy and is therefore dimmed in the diagram.
The new study by Stroupe et al. (1) now generates more consensus among fusion and upstream reactions in different systems. First, several already-established conclusions are further strengthened, for instance, the essential role of specific lipids such as sterols and phosphoinositides, the universal role of SNARE proteins, the general configuration of three Q-SNAREs on one side and one R-SNARE on the other together engaging in a four-helical bundle to fuse two membranes (transSNARE bundles; see ref. 10), and the fact that dissociation of SNARE bundles in one membrane (cisSNARE bundles) is actually the first essential step in setting up the fusion reaction. This idea has been initially proposed in the vacuole system (the actions of Sec17p/18p; see ref. 11) and has been long disputed for other systems (expressing the orthologs N-ethylmaleimide sensitive factor/α sensitive factor attachment protein), but is now generally accepted (see Fig. 1).
Second, more consensus is now also reached on the essential role of a Ras-like GTPase, in the case of vacuole fusion Ypt7p. With an improved lipid preparation (the direct method, see ref. 1), both docking and fusion are strictly Ypt7p dependent. With this new preparation, reconstituted vacuole docking/fusion is now consistent with reconstituted mammalian endosome fusion, probably the best-characterized in vitro fusion reaction to date. In this system, the Ras-like GTPase Rab5 was shown to coordinate the establishment of a specific local lipid milieu and the recruitment of a large protein complex that orchestrates homotypic endosome fusion (5).
Third and most importantly, the work by Stroupe et al. (1) strengthens some new and unexpected conclusions about the docking step. Using a proteoliposome clustering assay, they conclude that the R-SNARE Nyv1p is dispensable for vacuole docking, whereas the three Q-SNAREs are required. A very similar conclusion was recently reached for secretory vesicle docking in intact mammalian cells (12). This is an exciting convergence of new findings, which together suggest that establishing/stabilizing a cis3Q-SNARE complex may be a central step for docking to occur (see Fig. 1) and that conditions/factors that favor the existence of such complexes or promote their stability promote docking. Moreover, other factors than the R-SNARE may perform the initial docking reaction and bind to the cis3Q-SNARE complex. In mammalian secretory vesicles this is the vesicular protein synaptotagmin-1 (12). It would be very interesting to see whether the vacuole SM-protein Vps33p promotes the existence/stability of cis3Q-SNARE complexes in a similar manner as the mammalian SM-protein Munc18-1 does for the mammalian cis3Q-SNARE complex (12) and whether artificial stabilization of the 3Q SNARE complex using a short C-terminal R-SNARE peptide (see ref. 13) might bypass the requirement of SM-proteins for docking in the vacuole system as shown for Munc18-1 requirement in secretory vesicle docking (12). Although dispensable for the initial docking step, R-SNAREs remain essential for the downstream fusion reaction in both yeast vacuole and other systems. Furthermore, although conclusions from yeast vacuole and mammalian secretory vesicles are strikingly similar, it was recently concluded that in early endosomes all SNAREs (Q and R) are dispensable for docking (4).
One open issue is how universal the role is of the other important components identified in the vacuole system, i.e., the homotypic fusion and vacuole protein sorting (HOPS) complex, the Ras-like GTPase. The HOPS complex is a six-subunit protein complex including the SM-protein subunit, Vps33, and a GDP-exchange factor, Vps39/Vam6. This complex regulates fusion-competent SNARE complex assembly and is probably required throughout the different steps leading up to fusion. Although such complexes appear to be evolutionarily conserved among endolysosomal trafficking pathways (14), homologs of the HOPS complex subunits have not been identified for docking or fusion for secretory vesicles at the plasma membrane yet. One exception is the SM-protein subunit, Vps33 (see above). An SM protein appears to be required for most, if not all, cellular fusion reactions (see ref. 15). It is uncertain whether the HOPS subunits Vps16p, a presumed ubiquitin ligase, and Vps11 and Vps18 proteins with C-terminal RING domains that cluster at phosphoinositide-rich membrane domains have functional homologs in secretory vesicle docking and fusion. Synaptotagmin-1, the vesicular partner that binds to the cis3Q-SNARE complex in mammalian secretory vesicle docking [instead of the R-SNARE (12)], also binds to phosphoinositides, at least upon Ca2+ elevation, but in a mechanistically very different way (via its C2-domain, not FYVE domains). And synaptotagmin's role in docking is principally Ca2+-independent. Finally, no Ras-like GTPases have been identified that are really essential for the initial steps in secretory vesicle docking. Those Ras-like GTPases implicated in this type of fusion reactions probably act late (after SNARE assembly; see ref. 16). However, for the Ras-like GTPases, secretory vesicle fusion in mammalian cells may be the odd one out, because such genes are central components not only in yeast vacuole fusion (1) but also in yeast plasma membrane fusion (17) and mammalian endosome fusion (5).
The R-SNARE Nyv1p is dispensable for vacuole docking, whereas the three Q-SNAREs are required.
Reconstitution of fusion assays using purely synthetic components is getting more and more sophisticated, without lysis, as previously observed upon overexpression of SNAREs, and with an improved discrimination between hemifusion and full fusion using lumenally oriented lipids or a soluble luminal dye. Nevertheless, diverging outcomes of reconstituted docking and fusion assays are still a matter of concern and ask for even more rigorous standardization. Differences in HOPS complex binding to membranes, vacuole fusion requirements, and the role of Ypt7p in HOPS binding, all have been identified by Stroupe et al. (1) and can now be controlled for in the future. For clustering assays to assess docking, similar fine-tuning may still be ahead. Current clustering assays are nonlinear, suffer from substantial passive (background) clustering and, depending on the experimental conditions, are in fact a mixture of docking and fusion reactions, where the cluster score is determined by a combination of clustered small unfused proteoliposomes and larger fused proteoliposomes. Still, reconstituted proteoliposomes can in principle be generated with more rigorous control of lipid and protein composition than isolated organelles or intact cells and therefore hold great potential to help establish a universal model for the different upstream molecular events leading up to the final fusion reaction.
Footnotes
See companion article on page 17626 in issue 42 of volume 106.
The author declares no conflict of interest.
References
- 1.Stroupe C, Hickey CM, Mima J, Burfeind AS, Wickner W. Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components. Proc Natl Acad Sci USA. 2009;106:17626–17633. doi: 10.1073/pnas.0903801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisman LS, Wickner W. Intervacuole exchange in the yeast zygote: A new pathway in organelle communication. Science. 1988;241:589–591. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- 3.Verhage M, Sorensen JB. Vesicle docking in regulated exocytosis. Traffic. 2008;9:1414–1424. doi: 10.1111/j.1600-0854.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- 4.Geumann U, Barysch SV, Hoopmann P, Jahn R, Rizzoli SO. SNARE function is not involved in early endosome docking. Mol Biol Cell. 2008;19:5327–5337. doi: 10.1091/mbc.E08-05-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohya T, et al. Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459:1091–1097. doi: 10.1038/nature08107. [DOI] [PubMed] [Google Scholar]
- 6.Jahn R, Scheller RH. SNAREs: Engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 7.Neher E. A comparison between exocytic control mechanisms in adrenal chromaffin cells and a glutamatergic synapse. Pflügers Arch. 2006;453:261–268. doi: 10.1007/s00424-006-0143-9. [DOI] [PubMed] [Google Scholar]
- 8.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 9.Sudhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wit H, et al. Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell. 2009;138:935–946. doi: 10.1016/j.cell.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Pobbati AV, Stein A, Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 14.Nickerson DP, Brett CL, Merz AJ. Vps-C complexes: Gatekeepers of endolysosomal traffic. Curr Opin Cell Biol. 2009;21:543–551. doi: 10.1016/j.ceb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toonen RF, Verhage M. Munc18–1 in secretion: Lonely Munc joins SNARE team and takes control. Trends Neurosci. 2007;30:564–572. doi: 10.1016/j.tins.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Schluter OM, Basu J, Sudhof TC, Rosenmund C. Rab3 superprimes synaptic vesicles for release: Implications for short-term synaptic plasticity. J Neurosci. 2006;26:1239–1246. doi: 10.1523/JNEUROSCI.3553-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novick P, Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brose N. For better or for worse: Complexins regulate SNARE function and vesicle fusion. Traffic. 2008;9:1403–1413. doi: 10.1111/j.1600-0854.2008.00758.x. [DOI] [PubMed] [Google Scholar]