Abstract
Heme nitric oxide/oxygen (H-NOX) proteins are found in eukaryotes where they are typically part of a larger protein such as soluble guanylate cyclase and in prokaryotes where they are often found in operons with a histidine kinase, suggesting that H-NOX proteins serve as sensors for NO and O2 in signaling pathways. The Fe(II)–NO complex of the H-NOX protein from Shewanella oneidensis inhibits the autophosphorylation of the operon-associated histidine kinase, whereas the ligand-free H-NOX has no effect on the kinase. NMR spectroscopy was used to determine the structures of the Fe(II)–CO complex of the S. oneidensis H-NOX and the Fe(II)–CO complex of the H103G H-NOX mutant as a mimic of the ligand-free and kinase-inhibitory Fe(II)–NO H-NOX, respectively. The results provide a molecular glimpse into the ligand-induced conformational changes that may underlie kinase inhibition and the subsequent control of downstream signaling.
Keywords: hemoprotein, nitric oxide, signaling, NMR, phosphorylation
Gas-sensing proteins play vital roles in mediating biological events. For example, soluble guanylate cyclases (sGCs) coordinate nitric oxide (NO) or oxygen (O2), leading to the control of specific signal transduction pathways (1). A number of diatomic gas sensor proteins consist of two distinct domains: a heme-containing sensor domain that binds diatomic ligands and an effector domain that generates an output signal (2). The cofactor coordination properties are precisely tuned by the active site of the protein to discriminately bind a specific ligand. For example, most sGCs selectively bind NO at nanomolar concentrations even in the presence of micromolar concentrations of oxygen (3). It is this unique property of sGC that enables the enzyme to act as a specific NO sensor in an aerobic environment. Ligand selectivity is largely dictated by residues in the heme distal pocket, and the prediction and characterization of O2-regulated sGCs has supported that understanding (4).
The heme domain in sGC belongs to a larger family of proteins termed the H-NOX (heme nitric oxide/oxygen) proteins (5, 6). H-NOX proteins share similar spectroscopic properties, sequence homology, and key conserved residues with sGC and, therefore, serve as models of the heme domain of sGC (6, 7). Although H-NOX domains are present in the genomes of prokaryotes and eukaryotes, the biological functions of these signaling modules have diverged considerably along with their ligand binding properties (3, 8). For example, SO2144 from Shewanella oneidensis and VCA0720 from Vibrio cholerae have nearly identical ligand-binding characteristics to those of sGC (i.e., bind NO but not O2) and may, therefore, act as NO sensors in their respective cellular settings (6, 9). In contrast, GCY-88E from Drosophila melanogaster and GCY-35 from Caenorhabditis elegans both bind O2 and have ligand-binding properties similar to those of hemoglobin (4, 10). In vivo, GCY-35 regulates aerotaxis in C. elegans, supporting the hypothesis that some H-NOX proteins act as O2 sensors (10). Orphan Drosophila sGCs were shown to bind O2 and be regulated by that ligand (4).
The crystal structure of Tar4H, an O2-binding H-NOX domain from Thermoanaerobacter tengcongensis, was solved at 1.77-Å resolution (11). In that structure, a hydrogen bond between the bound O2 and a distal pocket tyrosine residue (Y140) was observed and subsequently shown to play an important role in O2 affinity (3). Although this observation suggests that hydrogen bonding between the protein and oxygen ligand plays a crucial role in modulating ligand affinity, it does not provide insight into the conformational transitions that H-NOX proteins, such as sGC, make during the course of cell signaling. How do H-NOX proteins bind diatomic gases at their heme iron cofactors and transduce ligand binding into cell signaling pathways? To address this question, high-resolution structures of H-NOX protein in different states of activity are required. Herein we report solution NMR spectroscopy studies of the H-NOX protein SO2144 from S. oneidensis to accomplish this goal.
Results
Identification of Active and Inactive Diamagnetic States of the SO2144 H-NOX for NMR Studies.
The SO2144 H-NOX and SO2145 histidine kinase form a complex, and the autophosphorylation activity of SO2145 is suppressed by the Fe(II)–NO form of the H-NOX SO2144 (defined as an active H-NOX state), but is not affected by the unliganded Fe(II) H-NOX (defined as an inactive H-NOX state) (9). In terms of activity, the unliganded and NO-bound forms of SO2144 would be the targets for structural studies to correlate activity with ligand binding. However, the heme is paramagnetic in both the Fe(II) WT (S = 2) and the Fe(II)–NO WT (S = 1/2) complexes that limit observation in the vicinity of the heme because electron-nuclear spin relaxation broadens resonance lines beyond the limit of detection for protons within a ≈10-Å radius around the heme iron (12).
Six coordinate Fe(II)–CO complexes are diamagnetic (13), allowing for detailed NMR analysis, so efforts were focused on diamagnetic CO derivatives of SO2144. The Fe(II)–CO WT H-NOX weakly inhibits the kinase activity of SO2145, IC50 = 84 ± 5 μM, which is intermediate in activity (10-fold less potent than the NO complex, IC50 = 9 ± 2 μM) (Table 1). In proteins containing H-NOX domains, such as sGC or SO2144, displacement of the axial histidine from the heme iron caused by NO binding has been correlated with a significant change in activity (9, 14). To mimic histidine displacement from the heme, the H103G mutant was made, thereby disconnecting the helix that makes contact to the iron through that histidine side chain. Mutation of the distal histidine, a ligand to Fe in the heme, often leads to apoprotein; however, heme binding may be “rescued” by expression and purification in the presence of imidazole (Fig. 1) (15). This mutant binds CO and forms a stable diamagnetic complex [Fe(II)–CO H103G] similar to other mutants of this type; furthermore, the inhibitory activity of this protein against the SO2145 kinase (IC50 = 22 ± 5 μM) approaches that of the WT-Fe(II)–NO complex (Fig. 1C) (Table 1). The unliganded, paramagnetic complex of Fe(II) H103G has inhibitory activity; however, its relative instability precluded accurate measurement.
Table 1.
H-NOX inhibition of histidine kinase SO2145 autophosphorylation
Complexes were prepared as described in Materials and Methods. Autophosphorylation assays and the determination of the IC50 values are described in Materials and Methods.
*No inhibition was observed at the highest concentration tested.
†Assays with the H103G mutant were carried out in the presence of 10 mM imidazole to stabilize heme binding.
Fig. 1.
SO2144 H-NOX constructs for NMR studies. (A) Full-length SO2144 H-NOX is 181 residues as shown. The histidine kinase SO2145 is 311 residues including a dimerization and histidine phosphotransfer domain (DHp) and a catalytic and ATP-binding domain (CA). (B) WT SO2144 Fe(II)heme diatomic ligand complexes and the H103G SO2144 H-NOX mutant Fe(II)–CO complex. (C) Inhibition of the autophosphorylation reaction catalyzed by the SO2145 histidine kinase by Fe(II)–CO H103G.
Autophosphorylation assays of H103G were carried out in the presence of 10 mM imidazole to stabilize heme binding. Although imidazole was not needed for the assays with WT protein, it was included in the assays. The 1H-15N HSQC spectra of the paramagnetic WT Fe(II)—NO and H103G Fe(II)–NO complexes were superimposable, consistent with the conclusion that the mutation and addition of imidazole did not drastically alter the structure (Fig. S1). Given the activity results described above, NMR structures of Fe(II)–CO WT and Fe(II)–CO H103G were solved as a first step toward understanding the structural basis for signaling in this family.
NMR Structure Determinations of Fe(II)–CO WT and Fe(II)–CO H103G.
Fe(II)–CO WT and Fe(II)–CO H103G H-NOXs yielded 1H-15N HSQC spectra with high cross-peak dispersion, indicating that both states were stably folded (Fig. S2). Chemical shift assignments for both Fe(II)–CO WT and Fe(II)–CO H103G were made by using standard triple-resonance coherence transfer and NOESY experiments (16) as described in Materials and Methods. Some assignments were additionally confirmed by amino acid specific labeling. A majority of resonances were assigned, the exceptions being residues 2, 20, 35, 48, 110–112, and 114. There was no significant difference in linewidths between WT and H103G, indicating no substantial difference in fast dynamics between them. A few cross-peaks in the 1H-15N HSQC could not be assigned because of lack of connectivities, likely arising from some of the unassigned residues. Many of the unassigned residues are in loops and may be broadened beyond detection because of conformational exchange. Temperature- and pH-dependent broadening was seen for some residues, including 101, 104, 105, and 106, which are near H103, the proximal heme ligand. Peak shifts with pH and temperature occurred without broadening in H103G, indicating faster exchange in the mutant protein. Ionizable groups in the region were affected, but no specific source of the effect could be identified. Additionally, heme insertion isomerism about the α-γ meso axis gave rise to two distinct cross-peaks for some residues within the immediate vicinity of the heme (17), with one set of peaks considerably weaker than the other. The weak set of peaks was ignored in the structural analysis.
The structures of both proteins were determined by using NOE-derived distances, chemical shift-derived dihedral angle restraints, and global orientation restraints from protein and heme-derived residual dipolar couplings (RDCs). A summary of structure statistics is provided in Table S1. The 20 structures agreeing best with the data (with no NOE violations >0.3 Å and no dihedral angle violations >5°) from 200 random starting structures were chosen to represent the solution structure ensembles of Fe(II)–CO WT and Fe(II)–CO H103G (Fig. 2). Average rms differences from the mean for this group of 20 lowest-energy structures were 0.33 Å for backbone and 0.94 Å for heavy atoms for Fe(II)–CO WT and 0.37 and 0.99 Å for Fe(II)–CO H103G. The overall topology of the H-NOX domain, with seven α-helices and four β-strands previously observed in X-ray studies of the H-NOX domains from T. tengcongensis (Tt) and Nostoc sp PCC 7120 (Ns), is preserved in the SO2144 H-NOX domain structure (11, 18, 19).
Fig. 2.
Solution structures of the Fe(II)–CO WT and Fe(II)–CO H103G states. (A) Fe(II)–CO WT with the protein backbone and the side chain of proximal heme ligand H103 (blue); the heme cofactor with a distally bound CO ligand (yellow). (B) Fe(II)–CO H103G with the protein backbone (green) and the heme cofactor, the distal CO ligand, and proximal imidazole (purple). Residues are numbered according to their positions in the primary sequence.
Heme Environment Structure Refinement.
The distance between the iron atom and the axial histidine nitrogen was fixed at 2.2 Å and that of the CO carbon was fixed at 1.9 Å. These values are based on extended x-ray absorption fine structure data from Fe(II)–CO myoglobin and X-ray crystallographic data for the Fe(II)-CO complex of the Ns H-NOX domain (18, 20). In the calculated structures, the Fe–C–O bonding was modeled as linear based on structures of small-molecule heme models, X-ray crystallographic data derived from the Fe(II)–CO H-NOX from Ns, and X-ray crystallographic and spectroscopic data from Fe(II)–CO myoglobin (18, 21–24). Because there were no unambiguous NOE distance restraints identified between the propionates of the heme cofactor and the protein, loose (5 Å) distance restraints were used to restrain the position of the heme propionate groups so as to preserve their expected electrostatic contacts with the strictly conserved YxSxR motif. The use of these restraints is justified by the observations that (i) the YxSxR motif is strictly conserved in the H-NOX family, (ii) crystallographic data display heme-YxSxR hydrogen bonds in two different prokaryotic H-NOX domains, and (iii) they do not cause violation of any of the experimental NMR geometric restraints (11, 18).
Myoglobin samples in solution and crystals have very similar spectroscopic features and binding kinetics parameters, suggesting that the protein is conformationally similar in these states (25). Similarly, X-ray crystallographic and resonance Raman studies have provided evidence that the heme cofactors in H-NOX proteins adopt nonplanar conformations in both crystalline and solution phases (6, 11, 26). The residues that make up the heme-binding scaffold of SO2144, including those that induce nonplanarity of the heme (H103, P116, Y132, S134, and R136) are conserved across the family (Fig. 3) (11). A two-step simulated annealing protocol was used to determine conformations of the SO2144 H-NOX heme cofactor consistent with all of the NMR data. The protocol consisted of (i) a high-temperature step to determine the overall protein fold by annealing the protein from an extended random chain around a heme cofactor constrained to a planar configuration and (ii) a low-temperature annealing step with only a weak planarity restoring potential on the heme cofactor so that it adopts a planar configuration in the absence of protein-derived van der Waals contacts and NMR-derived geometric restraints, but is allowed to sample nonplanar conformations as required to satisfy the protein and heme NMR constraints (27). Although the heme planarity restoring potential does not accurately reflect the quantum mechanical potential energy surface of heme deformations, the focus of the protocol is to allow steric contacts between the heme and local side chains that abut the heme to distort it from planarity. Observing heme distortion by NMR is difficult; however, the differences between the Fe(II)–CO WT and Fe(II)–CO H103G ensembles are significant and reflect changes in the van der Waals contacts between the heme pocket and the porphyrin. A similar strategy has been successfully used to characterize the heme cofactor and heme pocket van der Waals contacts in Fe(II)–CO myoglobin (25).
Fig. 3.
Strictly conserved residues across all members of the H-NOX domain family. The residues in the heme-binding scaffold of the SO2144 H-NOX domain (H103, P116, Y132, S134, and R136) are conserved across the family, including those that induce heme nonplanarity. Residues that are strictly conserved in prokaryotic and eukaryotic H-NOX domains are colored in red.
Conformational Differences Between Fe(II)–CO WT and Fe(II)–CO H103G.
To obtain an initial assessment of the regions with conformational differences between Fe(II)–CO WT and Fe(II)–CO H103G, the differences in backbone amide and side-chain methyl chemical shifts were quantified (Fig. S3) and mapped onto the structure of Fe(II)–CO WT. Affected residues are found primarily in regions of secondary structure that immediately flank the proximal face of the heme cofactor. The largest changes are located in residues 100–115 that comprise helix αF and the turn that connects helix αF to strand β1. Smaller changes were seen in sites more peripheral to the heme, such as residues D86 and K87 in αE and I118 in β1. The heme cofactor makes significant contributions to chemical shifts of residues within a ≈10-Å distance from the heme iron (28). The magnitudes and locations of chemical-shift changes between Fe(II)–CO WT and Fe(II)–CO H103G are expected to reflect a change in the position of the proximal helix αF relative to the heme cofactor of the H-NOX domain. The absence of chemical-shift changes in the distal subdomain suggests that the heme cofactor position does not change relative to this half of the molecule (Fig. S3).
A difference distance matrix was constructed to characterize the changes occurring between CO–H-NOX and CO-H–NOX(H103G) structures to correlate with the changes in activity. Matrix plots of differences in α-carbon distances were calculated with the program DDMP (Center for Structural Biology, Yale University, New Haven, CT). The matrix generated using the single structure closest to the average structure in each ensemble is shown in Fig. S4. The most significant changes were between the subdomains on opposite sides of the heme. This conclusion was reinforced by examining superpositions generated using an individual subdomain. Superimposing the well-ordered residues of the proximal domain in Fe(II)–CO WT (residues 100–110 and 120–178) the pairwise rmsd of backbone atoms was 0.48 Å, and for the Fe(II)–CO H103G set of structures it was 0.58 Å (Fig. 4). These values are typical for well-defined structures determined by NMR. If the comparison is made between proximal domains of the WT and those of the H103G structures the pairwise rmsd increases modestly to 0.72 Å, indicating there is very little structure change within the proximal domain. When the ordered regions of the distal domains (residues 5–30 and 46–99) are compared, within the families of structures aligned using the proximal domain, the rmsd values are 0.88 Å for the WT family and 0.74 Å for the H103G family. However, when the cross comparison of the distal domains of the WT family is done relative to the H103G family, the pairwise rmsd increases substantially to 1.35 Å. This finding reinforces the interpretation of the conformational change between Fe(II)–CO WT and Fe(II)–CO H103G as arising from a rigid body displacement of the distal subdomain relative to the proximal. Comparison of the superimposed structures (Fig. 4) indicates that the change arises from a hinge-like rotation about an axis near αD.
Fig. 4.
Structure overlay with alignment of the proximal subdomain of Fe(II)–CO WT and Fe(II)–CO H103G. Fe(II)–CO WT is colored blue and Fe(II)-H103G is colored green. Backbone atoms of residues 100–110 and 120–178 in the Fe(II)–CO WT and Fe(II)–CO H103G structure ensembles were aligned.
Heme Environment.
The position of the heme cofactor was well-defined in both the Fe(II)–CO WT and Fe(II)–CO H103G structures through 70 heme–protein NOE distance restraints in Fe(II)–CO WT and 48 heme–protein and 2 imidazole–protein NOE restraints in Fe(II)–CO H103G. The 16 (Fe(II)–CO WT) and 15 (Fe(II)–CO H103G) NOE distance restraints from sites in the protein to the methine protons of the heme ring greatly restrict the position of the heme cofactor during the structure calculation process. 13C-1H RDCs were measured by using a specifically methine position 13C-labeled heme to define the orientations in the heme protein molecular frame of the C–H bond vectors that are part of the heme ring (Figs. S5 and S6) (29, 30). Nonplanar heme conformations better represent the solution configuration of the heme cofactor because structures calculated with a planar heme cofactor fit the methine CH RDCs [Fe(II)–CO WT/Fe(II)–CO H103G] with a Rdip, heme methine of 13.2%/17.1%, whereas structures calculated allowing the heme to sample nonplanar configurations reduces Rdip,heme methine to 0.6%/1.7% (31). In contrast, protein backbone Rdip (derived from HN and HαCα RDCs) did not change to a significant extent with the enforcement of heme planarity.
Fig. 5C shows the heme region in the Fe(II)–CO WT and Fe(II)–CO H103G structures. The distal heme pocket is lined with aliphatic and aromatic residues as shown in Fig. 5 A–C (I5, L77, L145). Deviations of the heme from planarity were characterized by using the Normal-Coordinate Structure Decomposition (NSD) algorithm of Shelnutt and coworkers (32). The average amplitudes for specific distortion modes are shown for each ensemble in Fig. 5D. Particularly striking differences between Fe(II)–CO WT and Fe(II)–CO H103G are observed in the ruffling and doming modes. On average there is less heme doming and ruffling in Fe(II)–CO H103G than in Fe(II)–CO WT, that is the heme becomes more planar in the kinase-inhibitory conformation. The plots shown in Fig. 5E and Fig. S6 illustrate changes in the median and width of the distribution of heme distortions for the NMR structure ensembles. Changes in doming are the most significant. The Fe(II)–CO WT ensemble of structures show doming >0.5 Å than in Fe(II)–CO H103G (see Fig. S6 for the complete results of this analysis).
Fig. 5.
Heme site structure. (A–C) (Upper) Fe(II)–CO WT. (Lower) Fe(II)–CO H103G. (A) The backbones of the Fe(II)–CO WT and Fe(II)–CO H103G structural ensembles are shown in blue and green and the heme cofactors are in yellow and purple, respectively. Residues 31–45 and 110–114 are omitted for clarity. Key residues that comprise the heme pocket are displayed in orange (I5, L77, H/G103, L115, P116, and L145). (B) Close-up view of the heme cofactor and the residues that comprise the heme site in the structural ensemble. (C) The heme site from the single structure in the NMR structural ensemble that is closest to the average conformation. (D) Heme deviations from planarity calculated using NSD (32). The average values of each mode across the ensembles are plotted in units of Å. (E) Box plots of selected NSD data.
Structural Consequences of Histidine Displacement from the Heme Iron.
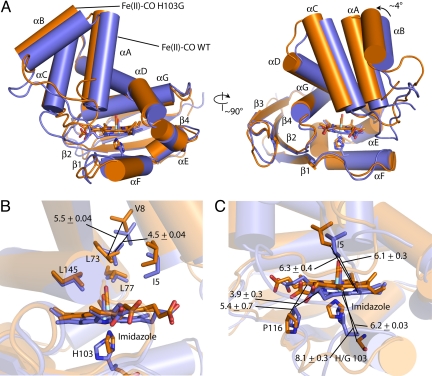
Superimposing the NMR-derived structures of Fe(II)–CO WT and Fe(II)–CO H103G in the regions containing residues 100–110 (αF) and 120–178 (αG and β1–4) reveals a rotation of the distal subdomain of the protein relative to the proximal by ≈4° (Fig. 6A) [the program DyDom was used for domain rotation angle calculations (33)]. Changes in relative positions of residues around the heme in the H-NOX domain are associated with this rotation. For the discussion that follows structural changes are quantified by taking the average distance change between specific atomic positions in the NMR ensembles, reported with the standard deviation for the values in the ensemble. Distal heme pocket residues, including I5, V8, L73, and L145 in helices αA-D and αG, are constrained by a dense network of NOE distance restraints in both Fe(II)–CO WT and Fe(II)–CO H103G. Distal heme pocket conformational changes upon activation are relatively small. For example, as the distal subdomain pivots about αD, the distance between the γ carbon of L73 and the β carbon of V8 changes from 4.5 ± 0.04 Å in Fe(II)–CO WT to 5.5 ± 0.04 Å in Fe(II)–CO H103G (Fig. 6B). The observed pivoting motion is required to accommodate relaxation of the heme cofactor.
Fig. 6.
Conformational differences between the Fe(II)–CO WT and Fe(II)–CO H103G states. (A) Overlay of the Fe(II)–CO WT and Fe(II)–CO H103G solution structures with the proximal subdomain, residues 100–110 (αF) and 120–178 (αG and β1–4), aligned. Fe(II)–CO WT is colored blue and Fe(II)–CO H103G is colored orange. (B) Key distance changes in the heme pocket distal to the heme. (C) Distance changes that underlie the conformational change after rupture of the axial histidine-iron bond. The single structures in each ensemble closest to the average are shown.
Three distance changes in the heme pocket capture the essence of the conformational change after displacement of the axial histidine from the iron (Fig. 6C). First, the proximal helix αF moves away from the heme after rupture of the iron–histidine bond. The distance between the α carbon of H103 and the heme iron is 6.2 ± 0.03 Å in Fe(II)-CO WT and 8.1 ± 0.3 Å in Fe(II)–CO H103G. Second, in the Fe(II)–CO WT structure the heme is held against P116 by the Fe–H103 coordination, resulting in a domed and ruffled heme configuration. The disconnection of the His side chain from the backbone produces a change in Fe(II)–CO H103G, releasing proximal helix αF from coordination to the heme iron, resulting in heme flattening as the van der Waals clash between P116 and the pyrrole A ring is relieved. This change can be quantified by the distance between the γ carbon of P116 and the carbon at the vertex of propionate A and heme pyrrole ring A. This distance is 3.9 ± 0.3 Å in Fe(II)–CO WT and 5.4 ± 0.7 Å in Fe(II)-CO H103G. Third, the distance between the terminal methyl carbon of I5 and methine carbon D of the heme is similar in Fe(II)–CO WT and Fe(II)–CO H103G, 6.1 ± 0.3 and 6.3 ± 0.4 Å, respectively. Thus, the heme and the distal subdomain move in concert away from proximal helix αF.
Discussion
Investigations that were initially focused on understanding the ligand discrimination against O2 by the heme cofactor in the β-subunit of sGC led to the discovery of the H-NOX family. For sGC it is clear that elimination of O2 binding to ferrous heme was essential for this protein to act as a trap for very low concentrations of NO that are generated during signaling. The toxicity of NO dictates function in biology at nM concentrations when involved in signaling, forcing sGC to trap NO at that concentration in the presence of μM concentrations of O2.
H-NOX domains from facultative anaerobes have essentially identical ligand binding characteristics as sGC, namely the Fe(II) oxidation state forms complexes with NO or CO but not O2. Additionally, all facultative anaerobe H-NOXs in the Fe(II) oxidation state are five-coordinate as isolated and again, like sGC, form a five-coordinate complex with NO via severing of the axial His–Fe bond. The H-NOXs from Nostoc sp. and Legionella pneumophila are the only exceptions in forming an NO complex that are a mixtures of five- and six-coordinate (34). Most facultative anaerobe H-NOXs are in a predicted operon with a histidine kinase. H-NOX domain function in prokaryotes has been elusive; however, recently we have made observations that point toward a signaling function for NO in S. oneidensis, a freshwater dwelling organism that has received attention in bioremediation studies (9). The H-NOX-associated histidine kinase in S. oneidensis is constitutively expressed and is inhibited by the Fe(II)–NO H-NOX. In addition, when grown under microaerobic conditions and provided with NO3− as a common environmental electron acceptor, S. oneidensis synthesizes NO, suggesting that the H-NOX is serving as a sensor for responding to changing growth conditions, in this case sensing O2 levels indirectly through the NO complex.
Structures of the inactive Fe(II) H-NOX and the active Fe(II)–NO complex would obviously provide a window into the structural changes that are concomitant with the kinase inhibitory activity, and perhaps insight into sGC activation. Despite much effort, these S. oneidensis H-NOX complexes did not crystallize. Although NMR was an option, the presence of the paramagnetic heme presented a serious technical challenge. Consequently, we chose to solve the diamagnetic Fe(II)–CO complex because it effectively represented an off-state with respect to the kinase activity. While the WT Fe(II)–CO structure determination was underway, we obtained the activity results showing that with the H103G Fe(II)–CO imidazole-rescued mutant has kinase inhibitory activity approaching that of the NO complex and then turned to solve that structure.
Two lines of evidence suggest that the structure of Fe(II)–CO WT is in a conformation close to that of the “inactive” Fe(II) state. First, crystal structures of the Ns H-NOX Fe(II), and Fe(II)–CO complexes revealed a subtle <1-Å pivoting motion of the heme cofactor about an axis perpendicular to the plane of the pyrrole D ring; however, no large-scale conformational changes in the relative positions of the distal and proximal subdomains were observed (18). Second, reminiscent of sGC in eukaryotes, binding of CO to the SO2144 H-NOX domain modulates the kinase activity of SO2145 but to a much lesser extent than NO (9, 14).
Confidence in the relevance of the H103G mutant came from a comparison of the Fe(II)–NO H103G and Fe(II)–NO WT NMR spectra that showed almost identical resonances, indicating that the H103G mutant structure is very similar to the native protein. Furthermore, evidence that H103G is trapped into an “active” conformation of the H-NOX domain, resembling that of Fe(II)–NO as the Fe(II) and Fe(II)–CO states of the H103G mutant, can be seen in the inhibition of the SO2145 histidine kinase by the H103G H-NOX mutant.
In the crystal structure of the T. tengcongensis Fe(II)–O2 H-NOX, two distinct conformations were seen that showed that the degree of heme planarity is coupled to a rotation of the proximal half of the H-NOX domain relative to the distal half (11). A conformational change of a similar nature was observed when comparing NMR structure ensembles of Fe(II)–CO WT to Fe(II)–CO H103G. This is likely because the residue network responsible for transmitting changes in heme planarity into large-scale conformational changes in the rest of the protein is conserved across H-NOX domains (11). Furthermore, key residues that induce heme distortion are strictly conserved between the Tt and So H-NOX domains. For example, P115, L144, and I5 in the Fe(II)–O2 WT Tt protein maintain the distortion of the heme through van der Waals interactions at positions flanking the heme proximal and distal faces, respectively. Within the tertiary structure of the SO2144 H-NOX domain, residues P116, L145, and I5 are positioned equivalently (Fig. 6B). In the Fe(II)–CO WT and Fe(II)–CO H103G structures reported here, the distorted heme cofactor relaxes after the cleavage of the axial–iron bond, providing evidence that changes in heme planarity are an important component of the signaling mechanism of these domains. Concomitantly, the heme cofactor and N-terminal subdomain move as a single body away from helix αF.
Energy minimizations carried out on Tt H-NOX highlighted residue P115 (P116 in S. oneidensis) as a major contributor to heme distortion (11). This proline is strictly conserved across the entire H-NOX protein family. When this residue was mutated to alanine in the Tt H-NOX domain, thereby reducing the steric bulk at this position, the heme adopted a flatter conformation as quantified by rmsd from planarity (26). Within each asymmetric unit cell of the P115A crystal lattice four distinct Fe(II)–O2 complexes (A–D) were observed.
Each of the P115A Tt molecules A–D exhibits a different amount of heme distortion that directly correlated with changes in the overall conformation of the protein (26). When the C termini of the four P115A structures were overlaid together with a representative WT Tt H-NOX structure [Protein Data Bank (PDB) ID code 1U55, Fe(II)-O2 monoclinic, molecule A] it became apparent that the N-terminal subdomain occupies different locations, with displacements generated by bending at a hinge. A similar displacement was also seen in other H-NOX crystal structures. When the C-terminal domains of the Fe(II)–CO WT and Fe(II)–CO H103G S. oneidensis H-NOX solution structures are overlaid an exactly analogous hinge bending displacement of the N-terminal domain is observed.
The hinge bending displacement observed in Tt H-NOX is correlated with heme cofactor planarity. The results of the P115A heme cavity mutation are profound and are mimicked by the H103G mutation. Decreasing the size of a bulky protein residue in close van der Waals contact of the heme significantly reduces the heme distorting force exerted by the protein scaffold. In the WT protein, the invariant P115 compresses the heme at the pyrrole D ring, inducing a pivoting of the attached proprionate group. Upon alanine substitution, the heme pyrrole D ring moves back into the heme plane, reducing the degree of pivoting of the associated proprionate. In the S.oneidensis H-NOX domain, the heme is inserted into the protein in the opposite orientation about the α-γ meso axis relative to the Tt H-NOX domain. Thus, pyrrole ring A is positioned analogously in this H-NOX. In Tt H-NOX, heme flattening triggers a rearrangement in the relative positions of distal and proximal heme pocket residues. In particular, I5 in close contact with pyrrole ring A shifts its position relative to the positions of the other residues in the heme pocket. The corresponding shift in the position of I5 induces a displacement of αA. Shifting of αA propagates a large-scale rotation of the N-terminal subdomain relative to the C-terminal subdomain; the N-terminal subdomain pivots about αD leading to a 3.8-Å rmsd (residues 1–83) with respect to the fixed C terminus (26). Thus, the heme wedged between the N and C termini acts as a pivot point where small deviations away from planarity are amplified into large-scale N- and C-terminal subdomain rotations.
In the S. oneidensis H-NOX domain (Fig. 6) NMR structures, the same hinge bending displacement occurs after axial–iron bond cleavage with the subtle difference that the heme translates away from αF by ≈2 Å. We carried out a similar analysis of the large-scale rotation of the N-terminal subdomain relative to the C-terminal subdomain seen in Fe(II)–O2 Tt WT and P115A crystal structures (11, 26) and Fe(II)–CO WT and H103G S.oneidensis NMR structures. The conformational change was quantified by N-terminal rmsd and rotation angle after alignment of C-terminal subdomains. From this analysis, it becomes clear that the conformational differences between Tt Fe(II)–O2 WT monoclinic molecule A (PDB ID code 1U55) (11) and Tt Fe(II)–O2 P115A molecule A (PDB ID code 3EEE) (26) are most analogous to the conformational differences observed between the Fe(II)–CO WT and Fe(II)–CO H103G S.oneidensis H-NOX domain with respect to the relative orientations of the N- and C-terminal domains.
NMR observables including NOEs, coupling constants, chemical shifts, and RDCs were used to calculate structures de novo. Overall the data support a model in which heme distortion is involved in the conformational change. The methine C-H RDC values reflect the bond orientation in the alignment frame (35, 36), and allow the planar and nonplanar configurations of the meso positions of the heme to be distinguished. Nonplanar heme configurations better fit the RDCs than planar configurations. Residues that are within van der Waals contact of the heme cofactor are well restrained by NMR data derived from RDCs, NOE distance restraints, and chemical-shift dihedral angle restraints.
The Fe(II)–CO WT and Fe(II)–CO H103G structure ensembles were refined with NMR-derived geometric restraints (37). An equivalent number of restraints of similar types (e.g., NOEs, RDCs, etc.) were used to calculate the solution structures and the conformation of the heme. The NMR restraints obtained were used to resolve differences in heme and protein conformation. For example, the global orientations of the N- and C-terminal subdomains of the H-NOX protein are defined by RDC data derived from atoms in the protein backbone (HN and HαCα RDCs). In the structure calculation process the heme is not constrained to be flat, hence the protein NMR restraints influence the conformation of the bound heme cofactor, particularly if they are within van der Waals contact of the heme ring. In analogy to a RDC-based structure ensemble of ubiquitin (38), we found that the width of the ranges of heme distortions observed in the Fe(II)–CO WT and Fe(II)–CO H103G structure ensembles are similar to the width of the ranges of heme distortions observed in X-ray crystal structures of other H-NOX domains (11, 18, 19, 26). Thus, the differences observed between the two S. oneidensis NMR structure ensembles [Fe(II)–CO WT versus Fe(II)–CO H103G] reflect the conformational differences in the H-NOX protein scaffold induced by changes of the heme cofactor. The NMR structures are consistent with the idea that the heme may sample different conformations, as observed in crystal structures of other H-NOX domains.
The conserved proline and axial histidine residues have been suggested as partners that work together to carry out conformational changes associated with signaling. In all H-NOX structures solved to date these two residues are packed together against the proximal heme face (11, 18, 19, 26). As noted above, binding of NO triggers breakage of the Fe-His bond and the H103G mutant was made to mimic this structure. As summarized in Results, this mutation leads to less distorted heme cofactor that initiates the hinge movement of the N-terminal subdomain with respect to the C-terminal subdomain. In these structures, the heme pivots about P116 as it becomes less distorted. Thus, P116 and H103 do appear to work together with the P116 residue acting as a molecular wedge, distorting the heme into a strained conformation poised for conformational change. In the proposed model, a conformational change is initiated when H103 dissociates from the heme iron after the binding of NO. Like a compressed spring, the heme stores energy required to change conformation. In the H103G mutant the spring is effectively sprung.
The finding that the loss of the axial histidine iron bond initiates a rotation of the distal subdomain relative to the proximal suggests a model for the mechanism of conformational transition between the inactive unligated Fe(II) and active Fe(II)–NO H-NOX. In what we term a “heme strain model,” P116 is pushed into the Fe(II) unligated heme. The resulting steric clash between the heme macrocycle and the proline ring distorts the heme away from planarity. When NO binds to the heme, the Fe–H103 bond breaks and the heme then moves away from proximal helix αF and concomitantly relaxes. Finally, the distal subdomain follows the trajectory of the heme, rotating away from proximal helix αF about a pivot point formed by residues G70 of helix αD and G144 of helix αG (Fig. 3). Strong support for the involvement of heme distortion in the conformational transition between the inactive unligated Fe(II) and active Fe(II)–NO conformations derives from previously determined X-ray crystallographic structure of H-NOX domains (11, 18, 19, 26). In those studies, structural comparisons between different crystal forms of one particular H-NOX ligation state highlighted a region of conformational plasticity similar to that observed in the Fe(II)–CO WT and Fe(II)–CO H103G solution structures, thus providing a potential mechanism for how heme flattening leads to a transition in the relative orientation of the distal and proximal halves of the H-NOX domain. Here, we extend those observations by providing both structural and biochemical evidence that correlates release of the axial histidine heme ligand with changes in heme planarity and H-NOX domain structure in solution. How these conformational changes modulate the activity of associated signaling domains awaits further investigation.
Materials and Methods
Protein Expression, Purification, Mutagenesis, Phosphorylation Assays, and NMR Sample Preparations.
Protein expression, purification, and mutagenesis were performed according to a protocol described (9) and modifications as described in SI Text.
Kinase Inhibition.
The kinase assay was carried out as reported (9). To determine the IC50, the data were fit to a three-parameter sigmoidal dose–response curve according to Eq 1.
where x is the log of the μM protein concentrations, y is the relative intensity, and IC50 is the apparent half-maximal inhibitory concentration for the H-NOX on kinase activity. Each measurement was done in duplicate, and the error was calculated by the range of the values.
NMR Spectroscopy: Assignment of Protein Backbone, Side-Chain, and Heme Resonances.
NMR spectra were recorded at 298 K on either Bruker Avance 600-, 800-, or 900-MHz spectrometers, all equipped with cryogenic triple-resonance probes. Samples for NMR measurements typically contained 0.4–0.8 mM protein in buffer containing 90% H2O/10% D2O or 100% D2O and 50 mM K3PO4, 5 mM DTT, 5% glycerol at pH 7.4. H103G NMR samples were prepared in the same manner with the addition of 10 mM imidazole. The Fe(II)–CO and Fe(II)–NO complexes were prepared as described. Chemical-shift changes between Fe(II)–CO WT and Fe(II)–CO H103G were reported as normalized chemical shift differences defined as , where 1H and 15N are the proton and nitrogen chemical shifts, respectively. NMR experiments and analysis are detailed in SI Text.
NMR Spectroscopy: Identification of Distance, Dihedral Angle, and RDC Restraints.
Intramolecular protein distance restraints were obtained from 3D 15N-NOESY-HSQC, 2D NOESY, and 3D 13C-NOESY-HSQC, all collected with 100-ms mixing times. The program TALOS was used to estimate backbone dihedral angles from characteristic chemical shifts. RDCs were obtained by calculating differences in the J couplings from unaligned and pf1 phage-aligned samples (39). Hydrogen bond restraints for the protein were defined from slowly exchanging amide protons and analysis of characteristic NOE patterns indicative of elements of regular secondary structure. Details of the experiments and protocols used to geometric restraints by NMR are described in SI Text.
Structure Calculations.
Structure calculations were performed as described in SI Text.
Supplementary Material
Acknowledgments.
We thank Joey Davis (MIT) for help with protein preparations; Douglas Mitchell (University of Illinois) for help with mass spectrometry; Milton Werner (Rockefeller University, New York) for help with RDC measurements; and James Chou (Harvard Medical School, Boston) for help with RDC measurements and structure calculations. We thank Dr. Jeff Pelton (University of California, Berkeley) and the Central California 900 MHz Facility (supported by NIH-GM68933) for experimental resources and assistance. This work was supported by National Institutes of Health Grant GM070671. W.K.E. was supported in part by NIH Training Grant GM 08295. We also thank the NSF (BBS 01-19304) and the NIH (RR 15756) for funding for the 800 MHz NMR spectrometer.
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2006.
The authors declare no conflict of interest.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2kii, 2kil).
This article contains supporting information online at www.pnas.org/cgi/content/full/0911645106/DCSupplemental.
References
- 1.Derbyshire ER, Marletta MA. Biochemistry of soluble guanylate cyclase. Handb Exp Pharmacol. 2009:17–31. doi: 10.1007/978-3-540-68964-5_2. [DOI] [PubMed] [Google Scholar]
- 2.Chan MK. Recent advances in heme-protein sensors. Curr Opin Chem Biol. 2001;5:216–222. doi: 10.1016/s1367-5931(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 3.Boon EM, Huang SH, Marletta MA. A molecular basis for NO selectivity in soluble guanylate cyclase. Nat Chem Biol. 2005;1:53–59. doi: 10.1038/nchembio704. [DOI] [PubMed] [Google Scholar]
- 4.Huang SH, Rio DC, Marletta MA. Ligand binding and inhibition of an oxygen-sensitive soluble guanylate cyclase, Gyc-88E, from Drosophila. Biochemistry. 2007;46:15115–15122. doi: 10.1021/bi701771r. [DOI] [PubMed] [Google Scholar]
- 5.Iyer LM, Anantharaman V, Aravind L. Ancient conserved domains shared by animal soluble guanylyl cyclases and bacterial signaling proteins. BMC Genomics. 2003;4:5. doi: 10.1186/1471-2164-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karow DS, et al. Spectroscopic characterization of the soluble guanylate cyclase-like heme domains from Vibrio cholerae and Thermoanaerobacter tengcongensis. Biochemistry. 2004;43:10203–10211. doi: 10.1021/bi049374l. [DOI] [PubMed] [Google Scholar]
- 7.Poulos TL. Soluble guanylate cyclase. Curr Opin Struct Biol. 2006;16:736–743. doi: 10.1016/j.sbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Boon EM, Marletta MA. Ligand discrimination in soluble guanylate cyclase and the H-NOX family of heme sensor proteins. Curr Opin Chem Biol. 2005;9:441–446. doi: 10.1016/j.cbpa.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Price MS, Chao LY, Marletta MA. Shewanella oneidensis MR-1 H-NOX regulation of a histidine kinase by nitric oxide. Biochemistry. 2007;46:13677–13683. doi: 10.1021/bi7019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray JM, et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 11.Pellicena P, Karow DS, Boon EM, Marletta MA, Kuriyan J. Crystal structure of an oxygen-binding heme domain related to soluble guanylate cyclases. Proc Natl Acad Sci USA. 2004;101:12854–12859. doi: 10.1073/pnas.0405188101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertini I, Luchinat C, Parigi G, Pierattelli R. NMR spectroscopy of paramagnetic metalloproteins. ChemBioChem. 2005;6:1536–1549. doi: 10.1002/cbic.200500124. [DOI] [PubMed] [Google Scholar]
- 13.Lukin JA, Ho C. Nuclear magnetic resonance of hemoglobins. Methods Mol Med. 2003;82:251–269. doi: 10.1385/1-59259-373-9:251. [DOI] [PubMed] [Google Scholar]
- 14.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: Activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Schelvis JP, Babcock GT, Marletta MA. Identification of histidine 105 in the β1 subunit of soluble guanylate cyclase as the heme proximal ligand. Biochemistry. 1998;37:4502–4509. doi: 10.1021/bi972686m. [DOI] [PubMed] [Google Scholar]
- 16.Ferentz AE, Wagner G. NMR spectroscopy: A multifaceted approach to macromolecular structure. Q Rev Biophys. 2000;33:29–65. doi: 10.1017/s0033583500003589. [DOI] [PubMed] [Google Scholar]
- 17.Lamar GN, Budd DL, Viscio DB, Smith KM, Langry KC. Proton nuclear magnetic-resonance characterization of heme disorder in hemoproteins. Proc Natl Acad Sci USA. 1978;75:5755–5759. doi: 10.1073/pnas.75.12.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X, Sayed N, Beuve A, van den Akker F. NO and CO differentially activate soluble guanylyl cyclase via a heme pivot-bend mechanism. EMBO J. 2007;26:578–588. doi: 10.1038/sj.emboj.7601521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nioche P, et al. Femtomolar sensitivity of a NO sensor from Clostridium botulinum. Science. 2004;306:1550–1553. doi: 10.1126/science.1103596. [DOI] [PubMed] [Google Scholar]
- 20.Powers L, Sessler JL, Woolery GL, Chance B. CO bond angle changes in photolysis of carboxymyoglobin. Biochemistry. 1984;23:5519–5523. doi: 10.1021/bi00318a021. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov D, et al. Determination of CO orientation in myoglobin by single-crystal infrared linear dichroism. J Am Chem Soc. 1994;116:4139–4140. [Google Scholar]
- 22.Peng SM, Ibers JA. Stereochemistry of carbonylmetalloporphyrins. The structure of (pyridine)(carbonyl)(5,10,15,20-tetraphenylprophinato)iron(II) J Am Chem Soc. 1976;98:8032–8036. doi: 10.1021/ja00441a025. [DOI] [PubMed] [Google Scholar]
- 23.Quillin ML, Arduini RM, Olson JS, Phillips GN. High-resolution crystal structures of distal histidine mutants of sperm whale myoglobin. J Mol Biol. 1993;234:140–155. doi: 10.1006/jmbi.1993.1569. [DOI] [PubMed] [Google Scholar]
- 24.Ray GB, Li XY, Ibers JA, Sessler JL, Spiro TG. How far can proteins bend the feco unit: Distal polar and steric effects in heme proteins and models. J Am Chem Soc. 1994;116:162–176. [Google Scholar]
- 25.Osapay K, Theriault Y, Wright PE, Case DA. Solution structure of carbonmonoxy myoglobin determined from nuclear magnetic resonance distance and chemical shift constraints. J Mol Biol. 1994;244:183–197. doi: 10.1006/jmbi.1994.1718. [DOI] [PubMed] [Google Scholar]
- 26.Olea C, Boon EM, Pellicena P, Kuriyan J, Marletta MA. Probing the function of heme distortion in the H-NOX family. ACS Chem Biol. 2008;3:703–710. doi: 10.1021/cb800185h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou JJ, Li S, Bax A. Study of conformational rearrangement and refinement of structural homology models by the use of heteronuclear dipolar couplings. J Biomol NMR. 2000;18:217–227. doi: 10.1023/a:1026563923774. [DOI] [PubMed] [Google Scholar]
- 28.Cross KJ, Wright PE. Calibration of ring-current models for the heme ring. J Magn Reson. 1985;64:220–231. [Google Scholar]
- 29.Alontaga AY, Bunce RA, Wilks A, Rivera M. 13C NMR spectroscopy of core heme carbons as a simple tool to elucidate the coordination state of ferric high-spin heme proteins. Inorg Chem. 2006;45:8876–8881. doi: 10.1021/ic0607484. [DOI] [PubMed] [Google Scholar]
- 30.Rivera M, Walker FA. Biosynthetic preparation of isotopically labeled heme. Anal Biochem. 1995;230:295–302. doi: 10.1006/abio.1995.1477. [DOI] [PubMed] [Google Scholar]
- 31.Clore GM, Garrett DS. R-factor, free R, and complete cross-validation for dipolar coupling refinement of NMR structures. J Am Chem Soc. 1999;121:9008–9012. [Google Scholar]
- 32.Jentzen W, Ma JG, Shelnutt JA. Conservation of the conformation of the porphyrin macrocycle in hemoproteins. Biophys J. 1998;74:753–763. doi: 10.1016/S0006-3495(98)74000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayward S, Berendsen HJ. Systematic analysis of domain motions in proteins from conformational change: New results on citrate synthase and T4 lysozyme. Proteins. 1998;30:144–154. [PubMed] [Google Scholar]
- 34.Boon EM, et al. Nitric oxide binding to prokaryotic homologs of the soluble guanylate cyclase β1 H-NOX domain. J Biol Chem. 2006;281:21892–21902. doi: 10.1074/jbc.M600557200. [DOI] [PubMed] [Google Scholar]
- 35.Tjandra N, Bax A. Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science. 1997;278:1111–1114. doi: 10.1126/science.278.5340.1111. [DOI] [PubMed] [Google Scholar]
- 36.Tolman JR, Flanagan JM, Kennedy MA, Prestegard JH. Nuclear magnetic dipole interactions in field-oriented proteins: Information for structure determination in solution. Proc Natl Acad Sci USA. 1995;92:9279–9283. doi: 10.1073/pnas.92.20.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy EC, Zhurkin VB, Louis JM, Cornilescu G, Clore GM. Structural basis for SRY-dependent 46-X,Y sex reversal: Modulation of DNA bending by a naturally occurring point mutation. J Mol Biol. 2001;312:481–499. doi: 10.1006/jmbi.2001.4977. [DOI] [PubMed] [Google Scholar]
- 38.Lange OF, et al. Recognition dynamics up to microseconds revealed from an RDC-derived ubiquitin ensemble in solution. Science. 2008;320:1471–1475. doi: 10.1126/science.1157092. [DOI] [PubMed] [Google Scholar]
- 39.Hansen MR, Mueller L, Pardi A. Tunable alignment of macromolecules by filamentous phage yields dipolar coupling interactions. Nat Struct Biol. 1998;5:1065–1074. doi: 10.1038/4176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.