Abstract
When small particles (e.g., flour, pollen, etc.) come in contact with a liquid surface, they immediately disperse. The dispersion can occur so quickly that it appears explosive, especially for small particles on the surface of mobile liquids like water. This explosive dispersion is the consequence of capillary force pulling particles into the interface causing them to accelerate to a relatively large velocity. The maximum velocity increases with decreasing particle size; for nanometer-sized particles (e.g., viruses and proteins), the velocity on an air-water interface can be as large as ≈47 m/s. We also show that particles oscillate at a relatively high frequency about their floating equilibrium before coming to stop under viscous drag. The observed dispersion is a result of strong repulsive hydrodynamic forces that arise because of these oscillations.
Keywords: adsorption, capillarity, fluid-fluid interfaces, monolayers
The following experiment can be easily performed in any reasonably well-equipped kitchen. Fill a dish partially with water, wait for a few minutes for the water to become quiescent, and then sprinkle a small amount of wheat or corn flour onto the water surface. The moment the flour comes in contact with the surface it quickly disperses into an approximately circular shaped region, forming a monolayer of dispersed flour particles on the surface (Fig. 1A). The interfacial forces that cause this sudden dispersion of flour particles are, in fact, so strong that a few milligrams of flour sprinkled onto the surface almost instantaneously covers the entire surface of the water contained in the dish.
Fig. 1.
Sudden dispersion of particles sprinkled onto water in a Petri dish. (A) Streak lines formed due to the radially outward motion of the flour particles emanating from the location where it was sprinkled. Approximately 1 s later, almost the entire water surface was covered by a layer of dispersed flour particles (see Movie S1). The size of flour particles was between ≈2–100 μm. (B) Sudden dispersion of colored sand sprinkled onto the water surface (see Movie S2). The size of sand particles was ≈200 μm. The velocity with which sand particles dispersed was smaller than that for the flour particles.
The above experiment can be performed using other finely granulated powders (e.g., corn flour, salt, sugar, sand, etc.) or even small seeds, such as mustard and sesame seeds and pollen (Fig. 1B). The tendency of powders to disperse, however, varies. The fact that salt and sugar dissolve in water is not important in this experiment, because the dispersion occurs at a time scale that is much smaller than the time taken by particles to dissolve. Also, the speeds with which particles disperse increases with decreasing size.
In 2003, we did experiments on the migration of small particles sprinkled onto a liquid surface. When sand was sprinkled on water in a Petri dish, it first dispersed violently at large speeds, which was followed by a phase that was dominated by attractive lateral capillary forces during which particles slowly came back to form monolayer clusters. The same dynamics were observed for more viscous liquids except that the dispersion speeds were smaller. The fluid dynamics of the attractive phase are well understood (1–7), but to our knowledge, there is no mention in the past studies of the initial violent dispersion despite the fact that this dispersion is ubiquitous, and occurs for many common liquids and particles.
Results
Vertical Acceleration of a Particle.
In this article, our focus is on the first (dispersive) phase. We show that when a particle comes in contact with a liquid surface, it experiences a strong vertical force due to capillarity which acts to bring the particle to its equilibrium height within the interface (Fig. 2). The equilibrium height is determined by a balance of the buoyant weight and the vertical interfacial force, and the contact angle. Therefore, for example, if the equilibrium height of a spherical particle is zero (i.e., in equilibrium, its center is at the undeformed surface), the particle must travel downwards a distance equal to its radius to reach equilibrium (Fig. 2). The vertical capillary force that moves the particle toward the equilibrium height can accelerate the particle to a relatively large velocity (for details, see SI Appendix). The maximum velocity attained by a spherical particle of radius R is given by
 |
Here, γ12 is the interfacial tension, μ is the fluid viscosity, ρc is the effective fluid density, and ρp is the particle density. Therefore, a particle of diameter 200 μm (which is approximately the size of a sand particle) on the air-water interface can accelerate to a velocity of the order of 1 m/s, and a 10-nm-sized particle, such as a virus or a protein molecule, may accelerate to a velocity of ≈40 m/s.
Fig. 2.
Trapping (or adsorption) of particles at an interface. (A) The particles come in contact with the interface and are pulled downwards by the interfacial force (γ). (B) The particles oscillate about the equilibrium height within the interface. These oscillations of the particles in close proximity create repulsive hydrodynamic forces, which cause them to move away from each other. The amplitude of oscillation decreases with time.
The above equation implies that the maximum velocity attained by a particle increases with decreasing particle radius and decreases with increasing fluid viscosity. In the limit R approaching zero, the velocity is given by
This is the maximum velocity that can be attained by a particle under the action of the vertical capillary force. The maximum velocity a particle can accelerate to on the air-water interface is ≈46.67 m/s (168.1 km/hr)!
Vertical Oscillations of a Particle.
The motion of the particle is dominated by inertia, and may overshoot equilibrium. Viscous drag causes the particle to slow down, but for a small particle, the drag is not large enough to stop the particle, because its momentum carries it below the equilibrium height. When the particle moves below its equilibrium height, the capillary force reverses direction and acts in the same direction as the drag. Hence, after moving down some additional distance, the particle reverses its direction. This behavior of the particle is similar to that of underdamped spring-dashpot systems which oscillate about the equilibrium height a few times before coming to rest (Figs. 2 and 3). Such underdamped oscillations of a particle were observed in experiments, as well as in direct numerical simulations (DNS) where particles are moved according to the fundamental equations of motion of fluids and solid particles without the use of models (7). In our experiments, the frequency of oscillation for a ≈1.2-mm-sized mustard seed was ≈83 Hz.
Fig. 3.
DNS of the adsorption of two particles released above their equilibrium height. The velocity distribution on the midplane of the computational domain and the deformed interface around the particles are shown. The initial distance between centers and the undeformed fluid interface was 0.95R; the lower 0.05R of the particles were immersed. The contact angle was maintained at 85°. The other parameters are the same as in Fig. S18 in SI Appendix. (A) t = 0.02 s. The particles, and the fluid around them, are moving downwards. (B) t = 0.1 s. The particles are moving upwards and apart. As time increases, the particles continue to oscillate about their equilibrium height and move further apart (see Movie S3).
The equation of motion for a particle in the direction normal to the interface can be linearized used to show that if
the particle oscillates about the equilibrium position before coming to rest (for details, see SI Appendix). The frequency of oscillation is given by
 |
The above equation implies that the frequency of oscillation increases with decreasing particle size, which is in agreement with the trend observed in experiments. Also, according to this equation, the frequency of oscillation for a particle of 1.2-mm diameter with the density of 1,100 kg/m3 on the air-water interface is 106.1 Hz, which is of the same order as the experimental value noted above.
Interfacial Flow and Dispersion Due to Oscillations.
The underdamped oscillatory motion of a single particle about the equilibrium height creates waves leading to a net interfacial flow, which moves small particles on the interface (tracers placed on the interface for flow visualization) away (SI Appendix). The fluid velocity increased with decreasing distance from the particle and decayed to zero a short time after the particle came in contact with the interface (in <1 s on the air water interface).
In our experiments, we found that when two identical particles were dropped together near each other onto a liquid, the surface velocity (measured using tracer particles) was larger than when only one particle was dropped. When four particles were dropped onto the liquid surface, the velocity was even larger. This increase in the surface velocity with increasing number of particles occurs because each particle creates its own radially outward flow, resulting in a net flow that can be approximated as the sum of the flows caused individually by the dropped particles.
When two particles were dropped together near each other, they moved apart along the line joining their centers. The particles moved apart because of the repulsive hydrodynamic forces that arise due to the motion of particles in the direction normal to the interface. Specifically, each of the particles is pulled into the surface by the vertical capillary force and oscillates about its equilibrium height before coming to rest (Figs. 2 and 3). The repulsive hydrodynamic forces arise because particles are near each other and the direction of their motion is normal to the lines joining their centers.
The average velocity with which particles moved apart increased with increasing number of particles. This increase in the dispersion velocity with increasing number of particles was also seen in our DNSs (SI Appendix). For example, when four particles were simultaneously dropped onto a liquid, the maximum average velocity with which they moved apart was approximately two times larger than that for two particles (Fig. 3; Figure S20 in SI Appendix). Also, the velocity with which a small amount of powder sprinkled onto a liquid surface dispersed increased with increasing amount.
Our experiments also showed that clusters of particles disperse radially outward from the center (Fig. 1A, which shows the streak lines of the particle motion). The velocity of the particles increased with increasing distance from the cluster center. Also, when the cluster size was larger, the velocity with which it expanded and the final radius it attained were larger.
The maximum velocity with which particles dispersed was reached shortly after they came into contact with the liquid surface and then slowly decreased with time. Also, for most of our experiments, except those involving micrometer-sized particles for which the attractive capillary forces are negligible, particles reversed direction after some time and clustered. Clusters formed by particles of intermediate size (≈10–50 μm) were rather porous, because the attractive capillary forces for them are relatively weaker. Our analysis shows that nano particles (e.g., viruses, proteins, etc.) are expected to disperse even more violently, because they can accelerate to larger velocities under the action of vertical capillary forces, and then remain dispersed, because for them attractive capillary forces are negligible. However, because their dispersion is expected to be over in less than a fraction of a second, it will be difficult to observe.
Discussion
An alternate way to think of the problem of dispersion of particles is the following. When a particle is adsorbed, a part of the released interfacial energy is acquired by the particle in the form of kinetic energy and the remaining part by the fluids. The particle dissipates this kinetic energy by oscillating about its equilibrium height in the interface. These oscillations give rise to repulsive hydrodynamic forces that cause particles to disperse.
Also, small particles (e.g., flour, pollen, etc.) disperse so violently that the images of dispersion appear strikingly similar to that of an explosion (Fig. 1), except that this “explosion” occurs in two dimensions, because the particles remain trapped at the interface. Small particles disperse explosively because the released interfacial energy scales as the second power of the radius (12), whereas the kinetic energy as the third power of the radius. Therefore, the maximum velocity attained increases with decreasing particle size. To our knowledge, this dispersion, or explosion, in two dimensions is a unique physical phenomenon.
We did experiments (similar to those described in Fig. 1) that show that, when a tiny amount (<1 mg in 1 L) of dish washing detergent is added to the water, the flour no longer disperses. This experiment shows that the presence of surfactants at the surface can significantly inhibit the lateral migration of particles. In this regard, we wish to note that it is known that the presence of the surfactant causes a decrease in the interfacial tension coefficient of the liquid (13).
The results presented in this article show that the adsorption of particles onto a fluid-fluid interface can be a rather violent process. The newly adsorbed particles not only create interfacial waves, but also cause particles already adsorbed on the interface to move away. For example, Fig. 4 shows that a newly adsorbed particle creates a circular particle-free region around itself, the radius of which can be several times larger than its own radius.
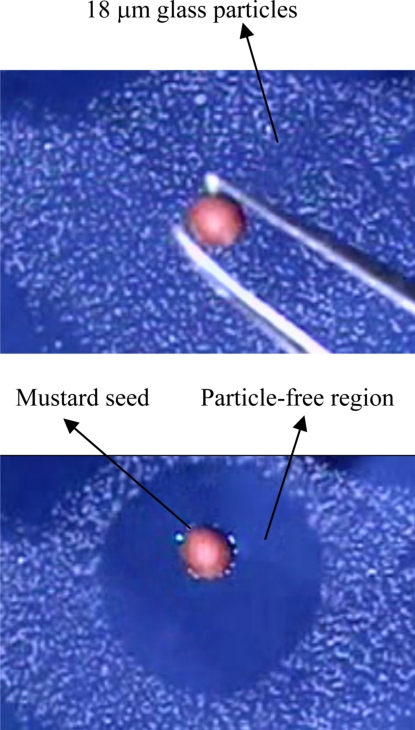
Fig. 4.
Dispersion of particles trapped on the surface due to a newly adsorbed particle. (Upper) A mustard seed of diameter 1.1 mm being dropped onto a monolayer of 18-μm glass particles on the surface of a 60% glycerin in water. (Lower) The mustard seed caused all of the nearby glass particles to move away, and thus created an approximately circular particle-free region.
The sudden dispersion of particles may also have an important role in some biological and chemical processes involving fluid-fluid interfaces. For example, an important first step in the formation of porous pollen structures called “pollen rafts” is the initial dispersion of pollen occurring after it comes in contact with the water surface (14). Cox and Knox (14) did not give a reason for the initial dispersion of pollen, but in our experiments, it disperses like glass particles of the same approximate size. After this initial dispersion, the pollen particles (usually, form a single anther) cluster to form a pollen raft. It was shown in refs. 14 and 15 that the formation of porous pollen rafts increases the probability of pollination, because the surface area of the raft is much greater than that of a single pollen grain. In this regard, we also wish to note that there have been sharp declines in seagrasses of some polluted coastal regions (16, 17) that may be associated with surface contamination, which, even when the concentration of contaminants is very small, can influence the porous structure of pollen rafts.
The results presented may also explain why a female of some mosquito species (Culex) has to hold onto the egg raft with its hind legs to prevent it from drifting away while she attaches new eggs. The eggs are laid one at a time and stuck together to form a raft that enables them to float together on the water. If she did not hold onto the raft, it would move away. The eggs of some other mosquitoes (Anopheles) are laid individually onto the water surface, they aggregate under the action of lateral capillary forces with the ends of the eggs touching each other. The spacing between the eggs in this case is relatively larger (which is perhaps advantageous for this species) as they dispersed initially. Lateral capillary forces cause the eggs to cluster and keep them together while the cluster moves around on the water surface. Traveling in large numbers helps ensure survival of the species, because some of the eggs are eaten by other insects before they hatch.
Materials and Methods
Experiments.
The experimental apparatus consisted of a chamber containing a liquid or two immiscible liquids placed on top of each other and separated by an interface. In the former case, the interface is simply a free-surface (this was the case for most of our experiments). Particles were sprinkled at the free-surface or placed in the upper or the lower liquid through which they fell or rose to the interface. The particle positions were recorded using a digital video camera mounted at the top of the chamber. The distance between the particles was measured by analyzing the movies frame-by-frame with a calibrated digital ruler. The fluid velocity at the interface was measured by tracking small tracer particles trapped on the interface.
DNS.
A finite element code was used to study the physics of particles adsorption at fluid-fluid interfaces (7). In these simulations, particles were assumed to be in contact with the interface, but released at a height of 0.95R above the undeformed interface; the initial height of particles above the interface can be varied. The trajectories of the particles were analyzed to obtain the frequency of oscillation and the velocity with which they moved apart.
Equation of Motion of a Particle.
An equation for the motion of a particle trapped at the interface and subjected to the interfacial, gravity, and viscous forces is derived. The equation is integrated to obtain the particle velocity when it reaches the equilibrium height for the first time and to describe its underdamped oscillatory motion.
Supplementary Material
Acknowledgments.
D.D.J. was supported in part by the Applied Math Division of the National Science Foundation (NSF) under the American Recovery and Reinvestment Act. P.S. was supported by the NSF Grant 0626123.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910343106/DCSupplemental.
References
- 1.Chan DYC, Henry JD, Jr, White LR. The interaction of colloidal particles collected at the fluid interface. J Colloid Interf Sci. 1981;79:410–418. [Google Scholar]
- 2.Fortes MA. Attraction and repulsion of floating particles. Can J Chem. 1982;60:2889–2895. [Google Scholar]
- 3.Katoh K, Fujita H, Imazu E. Motion of a particle floating on a liquid meniscus surface. J Fluids Engrg. 1992;114:411–417. [Google Scholar]
- 4.Nicolson MM. The interaction between floating particles. Proc Camb Philos Soc. 1949;45:288–295. [Google Scholar]
- 5.Princen HM. Equilibrium shape of interfaces, drops and bubbles. Rigid and deformable particles at interfaces. In: Matijevie E, editor. Surface and Colloid Science. Vol. 2. New York: Interscience; 1969. pp. 1–84. [Google Scholar]
- 6.Rapacchietta AV, Neumann AW. Force and free-energy analyses of small particles at fluid interfaces: II. Spheres. J Colloid Interf Sci. 1977;59:555–567. [Google Scholar]
- 7.Singh P, Joseph DD. Fluid dynamics of Floating particles. J Fluid Mech. 2005;530:31–80. [Google Scholar]
- 8.Osher S, Sethian JA. Fronts propagating with curvature-dependent speed: Algorithms based on Hamilton-Jacobi formulations. J Comput Phys. 1988;83:12–49. [Google Scholar]
- 9.Pillapakkam SB, Singh P. A Level Set Method for computing solutions to viscoelastic two-phase flow. J Comput Phys. 2001;174:552–578. [Google Scholar]
- 10.Singh P, Hesla TI, Joseph DD. A Modified Distributed Lagrange Multiplier/Fictitious Domain Method for Particulate Flows with Collisions. Int J Multiphas Flow. 2003;29:495–509. [Google Scholar]
- 11.Singh P, Joseph DD, Hesla TI, Glowinski R, Pan TW. A distributed Lagrange multiplier/fictitious domain method for viscoelastic particulate flows. J Non-Newton Fluid. 2000;91:165–188. [Google Scholar]
- 12.Binder WH. Supramolecular assembly of nanoparticles at liquid-liquid interfaces. Angew Chem Int Ed. 2005;44:5172–5175. doi: 10.1002/anie.200501220. [DOI] [PubMed] [Google Scholar]
- 13.Tsujii K. Surface Activity: Principles, Phenomena, and Applications. San Diego, CA: Academic; 1998. [Google Scholar]
- 14.Cox PA, Knox RB. Two-dimensional pollination in hydrophilous plants: Convergent evolution in the genera Halodule (Cymodoceaceae), Halophila (Hydrocharitaceae), Ruppia (Ruppiaceae), and Lepilaena (Zannichelliaceae) Amer J Bot. 1989;76:164–175. [Google Scholar]
- 15.Cox PA. Hydrophilous pollination. Annu Rev Ecol Syst. 1988;19:261–280. [Google Scholar]
- 16.Short FT, Wyllie-Eciieverria S. Natural and human-induced disturbance of seagrasses. Environ Conserv. 1996;23:17–27. [Google Scholar]
- 17.Hughes AR, Williams SL, Duarte CM, Heck KL, Jr, Waycott M. Associations of concern: Declining seagrasses and threatened dependent species. Front Ecol Environ. 2009;7:242–246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.