Abstract
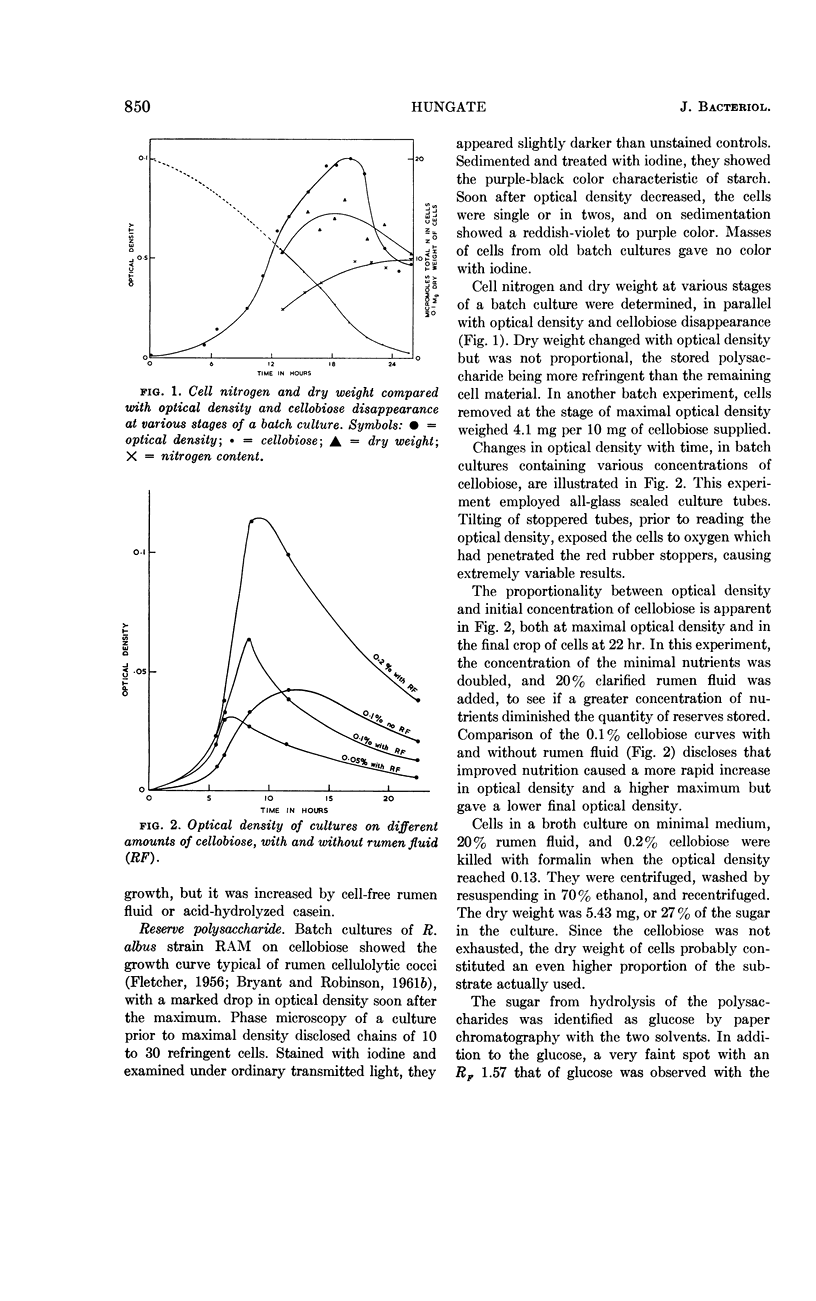
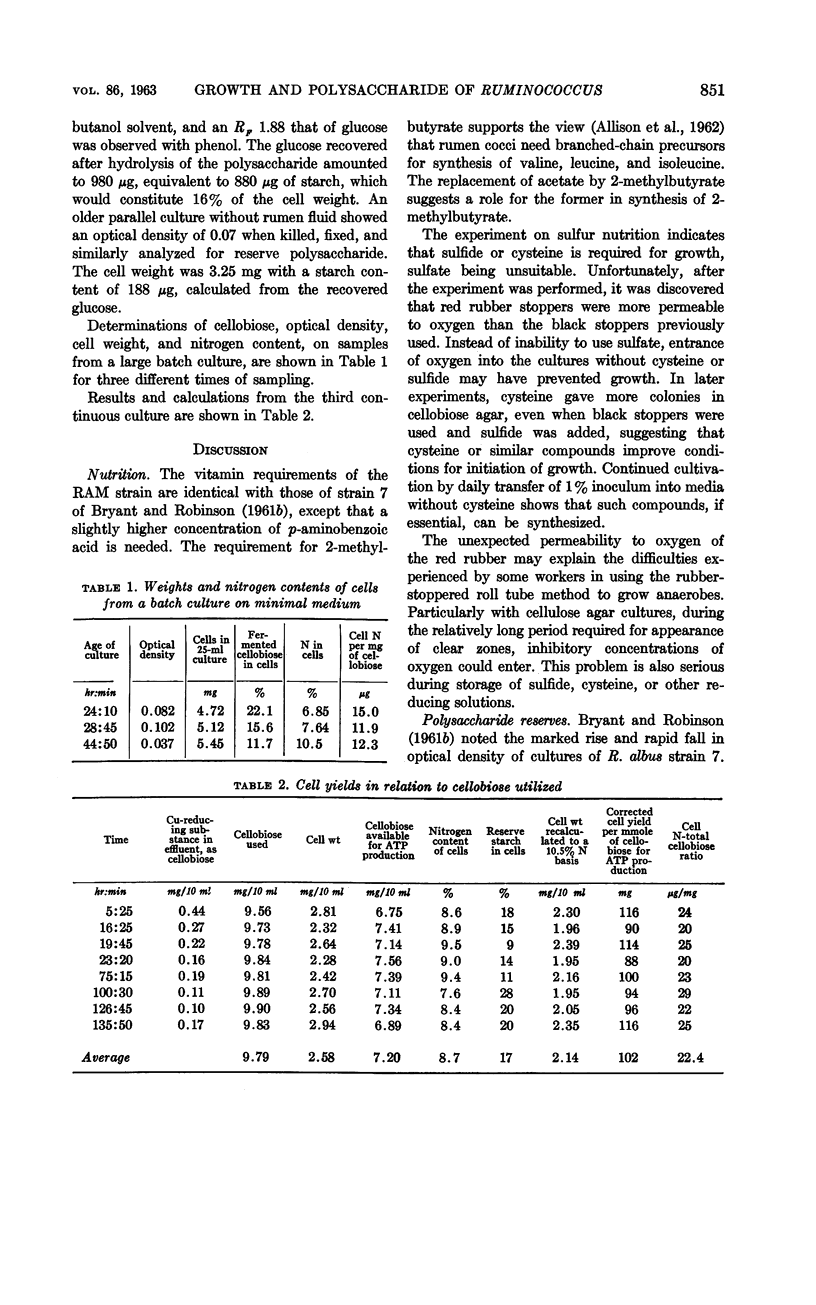
Hungate, R. E. (University of California, Davis). Polysaccharide storage and growth efficiency in Ruminococcus albus. J. Bacteriol. 86:848–854. 1963.—Ruminococcus albus strain RAM requires biotin, p-aminobenzoic acid, pyridoxamine, isovalerate, isobutyrate, 2-methylbutyrate, and either cysteine or sulfide. Rumen fluid and casein hydrolysate improve growth but are not essential. Up to 35% iodophilic polysaccharide is stored in cells from batch cultures and 17% in a continuous culture on a 10-hr cycle. The storage product is a polymer of glucose resembling starch. The yield of cells in continuous culture, corrected for stored starch, averaged 102 mg per mmole of cellobiose fermented to waste products. It is postulated that nine high-energy phosphates are derived from each cellobiose molecule. Conversions providing this number are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P., KATZ I., KEENEY M. Metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. II. Biosynthesis of higher branched-chain fatty acids and aldehydes. J Bacteriol. 1962 May;83:1084–1093. doi: 10.1128/jb.83.5.1084-1093.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- BLOCK R. J., STEKOL J. A., LOOSLI J. K. Synthesis of sulfur amino acids from inorganic sulfate by ruminants. II. Synthesis of cystine and methionine from sodium sulfate by the goat and by the microorganisms of the rumen of the ewe. Arch Biochem Biophys. 1951 Oct;33(3):353–363. doi: 10.1016/0003-9861(51)90123-3. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Some Nutritional Requirements of the Genus Ruminococcus. Appl Microbiol. 1961 Mar;9(2):91–95. doi: 10.1128/am.9.2.91-95.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. Microorganisms in the rumen of cattle fed a constant ration. Can J Microbiol. 1957 Mar;3(2):289–311. doi: 10.1139/m57-034. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES A. T., MARTIN A. J. P. Gas-liquid partition chromatography; the separation and micro-estimation of volatile fatty acids from formic acid to dodecanoic acid. Biochem J. 1952 Mar;50(5):679–690. doi: 10.1042/bj0500679. [DOI] [PMC free article] [PubMed] [Google Scholar]



