Abstract
Taurine α-ketoglutarate dioxygenase (tauD) is one of the best-studied α-ketoglutarate (αKG)-dependent nonheme iron oxygenases. As with all oxygenases, a fine balance must be struck between generating a species sufficiently reactive for the required chemistry and controlling that species to prevent undesirable side reactions [Klinman JP (2007) Accts Chem Res 40:325–333]. In the case of tauD, the substrate oxidizing species has been shown to be a ferryl-oxo, and the introduction of deuterium at the reactive position of substrate results in an enormous kinetic isotope effect together with a partial uncoupling of oxygen activation from substrate oxidation [Price JC, Barr EW, Glass TE, Krebs C, Bollinger JM (2003) J Am Chem Soc 125:13008–13009]. We have generated a series of site-specific variants at a position that resides directly behind bound substrate (F159 to L, V, A, and G). Decreasing side-chain bulk diminishes the coupling of oxygen activation to C-H cleavage, which is further reduced by substrate deuteration. Despite this impact, oxygen activation remains completely coupled to the oxidative decarboxylation of αKG. The concentration of bis-Tris buffer impacts the extent of coupling of oxygen activation to C-H cleavage, implicating the buffer in the uncoupling pathway. These data indicate a critical role for residue 159 in substrate positioning and reaction in tauD and show that minor active-site perturbations in these enzymes could allow for changes in substrate reactivity while maintaining substrate triggering and oxygen binding/activation.
Keywords: enzyme modularity, hydrogen transfer, oxygen activation
Taurine α-ketoglutarate dioxygenase (tauD) or its orthologs are critical in microorganisms, including Escherichia coli (1), for aerobic growth in the absence of cysteine or sulfate (2). TauD catalyzes the hydroxylation of taurine (2-aminoethanesulfonic acid) alpha to the sulfonate, leading to the spontaneous release of sulfite to be used as a sulfur source. E. coli tauD has been extensively studied and represents a canonical member of the large, ubiquitous family of α-ketoglutarate (αKG)-dependent nonheme iron oxygenases (3, 4). These enzymes oxidatively decarboxylate αKG to succinate and carbon dioxide to generate a reactive O2-derived species that oxidizes a third substrate, often referred to as the prime substrate (5). The biologic pathways and oxidative reactions catalyzed by the various members of the family are astoundingly diverse (5–9): for example, clavaminate synthase (CAS) catalyzes 3 different reactions in the same active site during the biosynthesis of clavaminate, a precursor to clavulanic acid antibiotics (10). In the case of CAS, protein structural factors influencing the different reactions catalyzed with structurally similar substrates have been discussed (11), but the origins of these structural differences and the overall broad substrate specificity of these enzymes are incompletely understood.
This diverse array of reactions and pathways can be accommodated by a consensus mechanism, notably involving kinetically ordered binding. This requires αKG to bind before prime substrate; subsequently, prime substrate binds off-metal, and an iron coordination site becomes vacant, allowing the binding and activation of dioxygen at the metal center (12). This “substrate triggering” mechanism is one of many strategies used by oxygenase enzymes to avoid a buildup of highly oxidizing intermediates in the absence of substrate (13). The highly oxidizing species generated by tauD, and the αKG-dependent oxygenases as a family, is an Fe(IV)-oxo (4, 14–17). In the case of tauD, this intermediate abstracts a hydrogen atom from C1 of taurine in a fully coupled manner. Subsequent oxygen rebound onto the substrate radical generates hydroxylated product, which then decomposes to sulfite and aminoacetaldehyde.
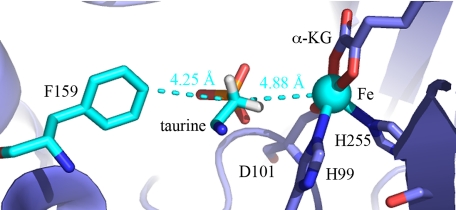
The aforementioned chemical mechanism and reactions require a careful coupling of the highly oxidizing Fe(IV)-oxo and the cleavage of a specific C-H bond. Uncoupled turnover in tauD (reduction of oxygen without oxidation of prime substrate) has been observed under various conditions (18). It has also been observed that related enzymes undergo slow inactivation under physiologic conditions, raising the possibility of a small degree of uncoupled turnover in the presence of prime substrate (19, 20). This inactive species is likely a ferric form of the enzyme, which can be reduced by ascorbic acid to generate the catalytically active ferrous form (19, 20). Understanding the coupling between oxygen activation and C-H cleavage is central to our ability to describe and predict the structure/reactivity relationships in these enzymes and to elucidate the determinants responsible for their diverse substrate specificity. Previous mechanistic studies have shown tauD to exhibit a kinetic isotope effect of ca. 35, which is inferred to be a lower limit of the intrinsic value due to an uncoupling reaction when deuterated substrate is used (21). These results illustrated the importance of the coupling of oxygen activation and C-H cleavage but did not elucidate any structural or mechanistic determinants. The orientation of the C-H bond with respect to the ferryl-oxo species is likely to be critical to the coupling of the ferryl-oxo to C-H cleavage. Spectroscopic studies have implied very short distances between the transferred hydrogen and the ferryl-oxo (22), and crystallographic and kinetic data for tauD indicate a wealth of interactions pertinent to substrate binding and reactivity (23–25). In particular, F159 stands out because of its relative position, with 4.25 Å separating C4 of F159 and the reactive C1 of taurine (Fig. 1). The angle formed by these 2 carbon atoms and the active site iron is ≈180°, indicating that F159 is optimally positioned to guide the C-H bond to be cleaved toward the hydrogen abstracting ferryl-oxo. The placement of F159 in the active site may also play a role in influencing substrate selectivity due to steric effects.
Fig. 1.
Active-site architecture of wild-type tauD, from pdb 1OS7 (23). F159, taurine, and the active-site iron are in cyan.
Herein, we report the combined impact of substrate deuteration and site-specific mutagenesis at position 159 (designed to create a progressively enlarged active-site cavity: F159L, -V, -A, and -G), observing a trend in uncoupling of the Fe(IV)-oxo away from productive C-H cleavage. Because the uncoupling pathway requires the input of electrons, assay components capable of donating electrons were investigated. Somewhat surprisingly, partitioning of the ferryl intermediate shows no dependence on the reducing agent ascorbate but does depend on the concentration of bis-Tris buffer. In addition, the kinetic effect on uncoupling, caused by the introduction of deuterium and resultant slowing of C-H cleavage, is augmented by the enlargement of the active site cavity, which allows greater access to the uncoupling buffer. Perhaps of greatest importance, the breakdown of αKG to succinate is found to be fully coupled to O2 uptake under all conditions, in marked contrast to the significant disparity between O2 uptake and C-H cleavage for the F159 variants. These findings implicate a modularity of oxygen activation and C-H cleavage in tauD that is likely linked both to the ability of this family of enzymes to perform similar chemistries on an extraordinarily diverse range of substrates and to the evolution of novel substrate specificities and reactivities.
Results
Stoichiometry of Succinate Production to Dioxygen Consumption.
The consensus mechanism of the αKG-dependent oxygenases requires succinate to be produced in a one-to-one stoichiometry to oxygen consumed during the formation of the Fe(IV)-oxo, even if prime substrate is not subsequently oxidized. If an alternate dioxygen activation pathway were to become active under conditions suboptimal for catalysis, deviations from this stoichiometry could be observed. These alternate oxygen activation pathways would likely lead to diffusion of either superoxide or hydrogen peroxide from the active site. Assays using peroxidase or peroxidase/superoxide dismutase and a chromogenic peroxidase substrate failed to detect hydrogen peroxide or superoxide ion under any conditions. Alternatively, mutations could slow the reactivity of the superoxo species with αKG, allowing the superoxo to persist, and possibly react with either the substrate or an adjacent protein side-chain. In any of these cases, more oxygen would be consumed than succinate produced. To assess the molar ratio of oxygen uptake to succinate production, assays were performed in which oxygen consumption was monitored continuously and subsequently acid quenched. Once oxygen consumption had ceased, the assay was neutralized and succinate concentration measured. Regardless of conditions, the molar ratio of oxygen consumption to succinate production remains within experimental error of unity (Table 1), strongly indicating that all oxygen bound and activated at the iron center is rapidly and irreversibly consumed in the oxidative decarboxylation of αKG, leading to the production of the Fe(IV)-oxo. Consistent with full coupling of oxygen activation to αKG decarboxylation, only a small impact (≤10-fold) was observed for the rate constant describing oxygen activation among the F159 variants.
Table 1.
Stoichiometry of succinate production to oxygen consumption for tauD and its variants with N-methyltaurine as substrate
| Enzyme | Substrate | Stoichiometry succinate:dioxygen† |
|---|---|---|
| Wild-type | H | 1.07 ± 0.14 |
| D | 1.01 ± 0.12 | |
| F159L | H | 1.07 ± 0.08 |
| D | 1.02 ± 0.14 | |
| F159V | H | 0.95 ± 0.36 |
| D | 1.03 ± 0.20 | |
| F159A | H | 0.98 ± 0.10 |
| D | 1.12 ± 0.12 |
H, 1,1-[1H2]; D, 1,1-[2H2]-2-methylaminoethane-1-sulfonic acid. All assays were performed at 30 °C under ambient O2 in 50 mM bis-Tris pH 6.2 in the presence of the indicated substrate and 200 μM ascorbate.
†Errors reported are 2σ determined from replicate experiments (n = 3–5).
Stoichiometry of Sulfite Production to Dioxygen Consumption.
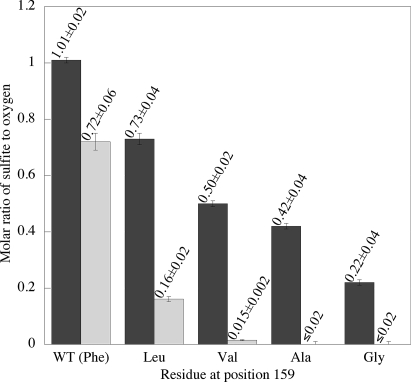
Although the ability of tauD to activate oxygen was not significantly perturbed by mutagenesis or substrate deuteration (above), it was anticipated that the ability of the enzyme to couple the Fe(IV)-oxo species to C-H cleavage could be radically impacted under similar conditions, owing to the strong distance and orientational dependence of hydrogen transfer reactions (26, 27). The formation of sulfite reports directly on the ability of the Fe(IV)-oxo species to abstract a hydrogen atom from C1 of the prime substrate. Under all conditions in this study, aldehydic product is only observed upon formation of sulfite, implying no alternative site of oxidation within the substrate. It has been observed previously that the introduction of deuterium in the reactive position causes dioxygen activation and C-H cleavage to be decoupled in wild-type tauD (21). When phenylalanine 159 is mutated to successively smaller residues (L, V, A, and G), cavities of increasing size are expected to result. Upon introduction of these active site defects, the stoichiometry of sulfite formed from protio substrate to oxygen consumed decreases from unity for wild-type enzyme to ≈0.2 for F159G (Fig. 2). When using deuterio substrate, the H atom abstraction reactions become, within experimental limits of detection, completely uncoupled from oxygen activation for F159A and -G (Fig. 2). In the context of tunneling mechanisms for hydrogen transfer in enzymatic reactions (26, 27), the impact of mutagenesis (A and G variants) is most reasonably attributed to an increase in active site distance that impacts deuterium wave function overlap between the C-D donor and the Fe(IV)-oxo acceptor atoms. We further note that taurine analogs, in which substituents were added to the amino-bearing methylene (CH3-H2N+-CHR-CH2-SO3−), led exclusively to uncoupled turnover.
Fig. 2.
Molar ratio of sulfite to oxygen for wild-type tauD and F159 variants, determined in 50 mM bis-Tris pH 6.2 containing 200 μM ascorbate. Darker bars represent values obtained using 1,1-[1H]2-N-methyltaurine, and lighter bars represent values obtained using 1,1-[2H]2-N-methyltaurine. Error bars represent 2σ deviations determined from replicate determinations (n = 3–5).
Ascorbate is commonly used in assays of the αKG-dependent oxygenases to reduce any inactive Fe(III) enzyme back to catalytically active Fe(II) (28). However, the presence of a good reducing agent raised the possibility of a competitive quenching of the Fe(IV)-oxo intermediate in tauD. Thus, increasing concentrations of ascorbate could have led to reduced stoichiometries of sulfite to dioxygen, but increasing the ascorbate level to 1 mM had no impact on the molar ratio of sulfite to oxygen (Table 2). By contrast, when the stoichiometry of sulfite to oxygen was determined at varying concentrations of bis-Tris buffer, the ratio was found to be impacted, with the coupling of the reaction showing an inverse dependence on buffer concentration (Table 2). Critically, the impact of buffer concentration on coupling becomes more pronounced as the bulk at position 159 decreases, indicating that the extent of side-reactivity may be related to the size of the cavity. This suggested that the buffer itself was accessing the active site and being oxidized during uncoupled turnover. When the extent of coupling measured at 50 mM buffer was compared with the lifetime of the Fe(IV)-oxo species under pre-steady-state conditions at the same buffer concentration (Table 3), an interesting phenomenon was observed. In general, the extent of uncoupling is elevated when the lifetime of the ferryl-oxo is increased. However, the observed lifetimes of the ferryl intermediate for F159L and F149V with protio substrate are shorter than that for wild-type enzyme with deuterio substrate; yet the extent of uncoupling for the variants is equal to or greater than that for wild-type. This suggests that it is not merely the extended lifetime of the Fe(IV)-oxo that allows uncoupling; in addition, an enlarged cavity resulting from the mutation of F159 is proposed to facilitate an interaction between the Fe(IV)-oxo and buffer.
Table 2.
Impact of putative uncoupling agents on sulfite to oxygen stoichiometry
| Enzyme | Substrate | SO32−:O2 ratio*† |
|||||
|---|---|---|---|---|---|---|---|
| 0 ascorbate | 20 μM ascorbate | 1 mM ascorbate | Average‡ | 20 mM buffer | 200 mM buffer | ||
| Wild-type | H | 0.97 ± 0.04 | 0.97 ± 0.04 | 1.00 ± 0.04 | 0.99 ± 0.07 | ≈ 1§ | ≈ 1§ |
| D | 0.61 ± 0.04 | 0.74 ± 0.06 | 0.70 ± 0.04 | 0.70 ± 0.10 | 0.91 ± 0.06 | 0.72 ± 0.05 | |
| F159L | H | 0.67 ± 0.08 | 0.73 ± 0.04 | 0.74 ± 0.01 | 0.72 ± 0.10 | 0.88 ± 0.02 | 0.65 ± 0.03 |
| D | ID¶ | ID¶ | 0.17 ± 0.01 | 0.17 ± 0.02 | 0.22 ± 0.02 | 0.13 ± 0.01 | |
| F159V | H | — | — | — | — | 0.79 ± 0.02 | 0.38 ± 0.02 |
| F159A | H | — | — | — | — | 0.55 ± 0.04 | 0.29 ± 0.02 |
| F159G | H | ID¶ | ID¶ | 0.21 ± 0.02 | 0.22 ± 0.03 | 0.52 ± 0.04 | 0.12 ± 0.05 |
H, 1,1-[1H2]; D, 1,1-[2H2]-2-methylaminoethane-1-sulfonic acid.
*All experiments were performed at ambient O2 and 30 °C. Experiments in which ascorbate was varied were performed in 50 mM bis-Tris pH 6.2 at the indicated concentration of ascorbate. Experiments in which buffer was varied were performed in the indicated concentration of bis-Tris pH 6.2 with 200 μM ascorbate. The stoichiometries at 50 mM buffer and 200 μM ascorbate are shown in Fig. 2.
†Errors reported are 2σ determined from multiple experiments (n = 3–5).
‡The values reported are the averages of the values at all ascorbate concentrations.
§These values were not determined in triplicate, but single measurements were within experimental error of unity. In light of an absence of an effect of the 10-fold increase in buffer concentration on the wild-type behavior, the decision was made to allow the ionic strength to vary in subsequent experiments.
¶Indeterminate: the enzymes did not undergo sufficient turnovers to allow reproducible measurements of sulfite.
Table 3.
Comparison of stopped-flow parameters for wild-type and variant tauD to sulfite to O2 molar ratios*
| Enzyme | Substrate | k (s−1)† | τ (s)‡ | SO32−:O2§ |
|---|---|---|---|---|
| Wild-type | D | 0.48 ± 0.08 | 2.1 ± 0.3 | 0.72 ± 0.06 |
| F159L | H | 1.80 ± 0.11 | 0.56 ± 0.03 | 0.73 ± 0.04 |
| F159V | H | 0.81 ± 0.04 | 1.23 ± 0.06 | 0.50 ± 0.02 |
H, 1,1-[1H2]; D, 1,1-[2H2]-2-methylaminoethane-1-sulfonic acid.
*Assays performed in 50 mM bis-Tris pH 6.2 at 10 °C; concentrations of substrates after mixing were as follows: 5 mM αKG, 0.6 mM O2, and 5 mM of isotopically labeled N-methyltaurine.
†The rate of decay of the ferryl-oxo species was determined using a monoexponential fit of the kinetic traces as described in SI Materials and Methods; representative traces (Figs. S2–S4) are also shown.
‡The mean lifetime of the Fe(IV)-oxo intermediate, calculated as the inverse of the rate of decay of the same species.
§Stoichiometry of sulfite formation to oxygen consumption determined under steady-state conditions as described in SI Materials and Methods.
Number of Enzyme Turnovers Under Steady-State Conditions.
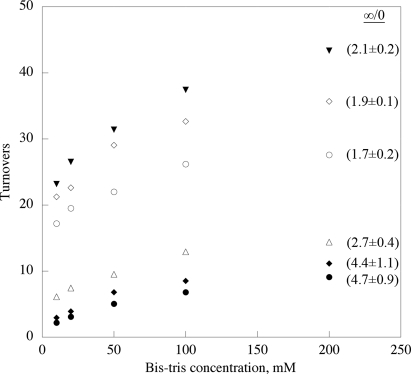
One possible outcome of uncoupled enzymatic turnover is the progressive inactivation of tauD. The oxidation of tyrosine 73 to dihydroxyphenylalanine (DOPA) in tauD has been reported; however, this has not been observed under conditions replete with αKG and taurine, nor has stoichiometric formation of DOPA been seen (18, 29, 30). Both the tyrosyl radical intermediate in the formation of DOPA and DOPA itself display characteristic spectroscopic signals (18, 29, 30), none of which were observed under pre-steady-state conditions herein. The fact that enzyme inactivation has been reported to occur concomitant with DOPA formation (18) led us to look for enzyme inactivation under various conditions. As shown above (Table 2), ascorbate does not support an uncoupled reduction of the ferryl-oxo, whereas buffer does. Thus, the number of turnovers undergone per enzyme active site was determined at varied bis-Tris levels (10–200 mM) for tauD and variants with protiated and deuterated substrates (Fig. 3). Aside from the completely coupled case of wild-type enzyme and protio substrate that undergoes in excess of 300 turnovers independent of buffer concentration, the number of turnovers is found to increase in the presence of higher buffer levels. The ratio of turnovers undergone in the limit of infinite buffer concentration to those in the limit of zero buffer concentration provides an indication of the extent to which buffer “rescues” enzyme from inactivation and leads to the regeneration of ferrous enzyme. The wild-type, buffer-independent reaction with protio substrate provides a baseline ratio of unity. Ratios for other enzyme–substrate pairs are indicated in Fig. 3. In the completely uncoupled case, the presence of “saturating” buffer allows the enzyme to turn over ca. 5 times more than in the limit of zero buffer. The fact that injection of 200 μM ascorbate after cessation of enzymatic oxygen uptake leads to a slow resumption of oxygen uptake implies that some proportion of the inactive enzyme is capable of being reduced to Fe(II) and reactivated. The aggregate data indicate clearly that as the enzymatic reaction becomes more uncoupled, the ability of the enzyme to turn over becomes more dependent on buffer concentration, confirming a role for buffer in the uncoupling pathway. Furthermore, the fact that enzyme can turn over more that once under the least favorable conditions (F159V with deuterated substrate) shows that a single uncoupled turnover does not result in stoichiometric enzyme inactivation.
Fig. 3.
Turnovers undergone by tauD and variants as a function of buffer concentration. inverted filled triangles, Wild-type with 1,1-[2H]2-N-methylamine (d2 substrate); open diamonds, F159L with 1,1-[1H]2-N-methylamine (h2 substrate); filled diamonds, F159L with d2 substrate; open circles, F159V with h2 substrate; filled circles, F159V with d2 substrate; open triangles, F159A with h2 substrate. ∞/0 represents the ratio of turnovers undergone in the limit of infinite buffer to the number of turnovers undergone in the limit of zero buffer, described in SI Materials and Methods.
Discussion
Oxygen uptake measurements in conjunction with product analysis studies have allowed a quantitative assessment of oxygen reactivity vs. C-H bond cleavage in wild-type and site-specific variants of tauD. The results are interpreted in a framework of the decoupling of oxygen activation and C-H bond cleavage that is impacted by both substrate deuteration and the reduction of steric bulk at a position critical to the positioning of substrate relative to the reactive Fe(IV)-oxo species. These results were initially unexpected, given the degree of exposure of the tauD active site to solvent water (23, 24). The data implicate a modularity of oxygen binding/activation and C-H bond cleavage within the tauD active site that may be directly relevant to the divergent specificities observed across the nonheme iron enzymes.
Dioxygen Activation Pathway.
It seems that the canonical oxygen activation pathway is largely unperturbed by mutation of F159 and substrate deuteration. That the stoichiometry of dioxygen to succinate remains very nearly unity irrespective of conditions, along with an inability to detect 1 or 2 electron reduced oxygen species, indicates that virtually every oxygen activation event leads to the production of succinate and the formation of the Fe(IV)-oxo intermediate. This observation implies that the large family of αKG-dependent oxygenases are tuned to ensure that the incipient Fe(III)-superoxo intermediate is rapidly trapped by αKG regardless of the chemistry subsequent to formation of the Fe(IV)-oxo, a notion supported by recently reported 18O kinetic isotope effects (31). The conserved role for “substrate triggering” dictates that substrate must be bound in a catalytically relevant orientation before oxygen binding/activation, with a rapid and irreversible formation of the Fe(IV)-oxo intermediate following, irrespective of subsequent substrate chemistry. The generation of the active oxidant in the presence of substrate-like molecules that can undergo alternate oxidative transformations may have been critical to the evolution of a wide range of substrate specificities.
Substrate C-H Cleavage.
The foregoing indicates that all oxygen activation proceeds rapidly to the Fe(IV)-oxo intermediate; however, the final disposition of that intermediate and the extent to which oxygen activation and C-H cleavage can be decoupled shows a drastic dependence on both substrate deuteration and the steric bulk at position 159. Modeling the variants (L, V, A, and G) into position 159 (32) reveals the extent of possible increase in distance between C1 of taurine and the nearest carbon atom on the residue at position 159 (4.25 Å in wild-type, 8.25 and 8.75 Å in F159A and -G, respectively). The size of the mutated cavity is expected to increase correspondingly, with the consequence that the active site will no longer be organized to support optimal hydrogenic wavefunction overlap from the substrate to the Fe(IV)-oxo center. This is, in fact, what occurs: as residue size decreases, the molar ratio of sulfite produced to dioxygen consumed decreases. Substrate deuteration also impacts the ability of the enzyme to use the Fe(IV)-oxo to perform hydrogen transfer chemistry owing to the increased sensitivity of deuterium transfer to donor–acceptor distance (26). The lack of product formation from deuterated substrate observed in the case of the enlarged active site cavities in F159A and -G implies significantly diminished wave function overlap between the substrate C-D donor and the Fe(IV)-oxo acceptor that allows other pathways to predominate.
With respect to the uncoupling pathway, the results presented here support the canonical role of ascorbate in these reactions as an occasional 1 electron reductant capable of allowing oxidized ferric enzyme to reenter the catalytic cycle; by contrast, they do not support the notion that ascorbate can act as a “substrate,” by quenching the ferryl-oxo intermediate. That the concentration of buffer impacts the coupling of oxygen consumption to product formation suggests that the buffer itself is being oxidized by the Fe(IV)-oxo intermediate.* Impairing the coupled pathway clearly makes it possible for the oxidizing equivalents of the Fe(IV)-oxo to leak via multiple pathways and serves to underscore the importance of hydrophobic bulk at position 159 to guide the C-H cleavage reaction for the preferred substrate.
The impact of deuteration on the behavior of these enzymes can be viewed as a kinetic effect, leading to uncoupling by increasing the lifetime of the reactive species and providing sufficient time for side reactions to occur. Mutation might be expected to be interpretable in the same way; the active site substitution distorts the geometry of the reacting moieties from some optimum, slowing the reaction and leading to uncoupling. This may be partially true, but the observation that enzymes with shorter Fe(IV)-oxo lifetimes relative to a reference reaction exhibit greater extents of uncoupling with respect to the same reference (Table 3) indicates that a kinetic argument alone is insufficient. A reasonable conclusion is that the accessibility of the active site to buffer is enhanced by mutation. The combination of these 2 factors provides a satisfactory explanation of the origins of the uncoupling behavior exhibited by tauD upon substrate deuteration and active site mutation and a model from which to perform future experiments.
The data presented herein further our understanding of oxygen activation and C-H cleavage by tauD and indicate a crucial role for F159 in coupling the Fe(IV)-oxo to C-H cleavage. The unexpected observation that the extent of uncoupling shows a dependence on the concentration of buffer, together with a dependence of buffer-uncoupled chemistry on the size of the cavity introduced by mutagenesis at position 159, raises intriguing questions with respect to the origins of substrate specificity. The observed modularity of oxygen activation and substrate chemistry clearly allows the formation of the Fe(IV)-oxo intermediate even when subsequent C-H cleavage does not proceed easily. The ability of nonheme iron enzymes to change substrate specificity, while maintaining the same oxygen activation pathway and chemistry, allows them to take full advantage of the reactivity of the common Fe(IV)-oxo intermediate. This overall flexibility with respect to substrate specificity and the reactivity of the Fe(IV)-oxo no doubt has contributed to the evolution of this family of enzymes. The present results elucidate the structural determinants and mechanism of C-H cleavage by these enzymes. In particular, they reveal how the reactive iron core can be preserved while possibly allowing for the evolution of a remarkably wide range of substrate specificities (5–9), as well as how the geometry and distance dependences of hydrogen transfer (26, 27) can influence the chemistry catalyzed.
Materials and Methods
Cloning, Mutagenesis, and Enzyme Purification.
TauD was cloned, overexpressed, and purified as in ref. 17, with minor modifications. Mutagenesis was performed by standard methods; mutagenic primer sequences are given in Table S1. Protein overexpression and purification were accomplished similarly for variants as for wild-type. Further details are available in SI Materials and Methods.
Enzyme Assays.
Initial rates of enzymatic oxygen uptake were measured with a YSI model 5300 biologic oxygen electrode. Sulfite concentrations were determined as in ref. 17, with minor modifications. Succinate concentration was determined using a succinic acid assay kit from Megazyme International Ireland, according to the manufacturer's protocol. 1,1-[1H2] and 1,1-[2H2]-2-methylaminoethane-1-sulfonic acid were synthesized as previously described (33). Stopped-flow assays were performed as in ref. 34, with minor modifications. The number of turnovers undergone by an enzyme was taken as the ratio of the concentration of oxygen consumed enzymatically before inactivation to the concentration of enzyme present in the assay. Further details are available in SI Materials and Methods.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grant GM025765 (to J.P.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910660106/DCSupplemental.
The current study has been restricted to bis-Tris due to technical limitations. Phosphate and carboxylate buffers lead either to iron precipitation or enzyme inhibition, respectively while Tris at basic pHs causes a very large background rate of oxygen consumption in the absence of enzyme. Additionally, sulfonate containing buffers are generally tauD substrates. The precise pathway for bis-Tris oxidation will be the subject of further investigation.
References
- 1.vanderPloeg JR, et al. Identification of sulfate starvation-regulated genes in Escherichia coli: A gene cluster involved in the utilization of taurine as a sulfur source. J Bacteriol. 1996;178:5438–5446. doi: 10.1128/jb.178.18.5438-5446.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichhorn E, vanderPloeg JR, Kertesz MA, Leisinger T. Characterization of alpha-ketoglutarate-dependent taurine dioxygenase from Escherichia coli. J Biol Chem. 1997;272:23031–23036. doi: 10.1074/jbc.272.37.23031. [DOI] [PubMed] [Google Scholar]
- 3.Bollinger JM, Price JC, Hoffart LM, Barr EW, Krebs C. Mechanism of taurine: Alpha-ketoglutarate dioxygenase (TauD) from Escherichia coli. Eur J Inorg Chem. 2005;2005:4245–4254. doi: 10.1021/bi050227c. [DOI] [PubMed] [Google Scholar]
- 4.Bollinger JM, Krebs C. Stalking intermediates in oxygen activation by iron enzymes: Motivation and method. J Inorg Biochem. 2006;100:586–605. doi: 10.1016/j.jinorgbio.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Costas M, Mehn MP, Jensen MP, Que L. Dioxygen activation at mononuclear non-heme iron active sites: Enzymes, models, and intermediates. Chem Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 6.Falnes PO, Johansen RF, Seeberg E. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature. 2002;419:178–182. doi: 10.1038/nature01048. [DOI] [PubMed] [Google Scholar]
- 7.Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature. 2002;419:174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 8.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O-2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 9.Fukumori F, Hausinger RP. Purification and characterization of 2,4-dicholorophenoxyacetate alpha-ketoglutarate dioxygenase. J Biol Chem. 1993;268:24311–24317. [PubMed] [Google Scholar]
- 10.Salowe SP, Marsh EN, Townsend CA. Purification and characterization of clavaminate synthase from Streptomyces clavuligerus—an unusual oxidative enzyme in natural product biosynthesis. Biochemistry. 1990;29:6499–6508. doi: 10.1021/bi00479a023. [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZH, et al. Structural origins of the selectivity of the trifunctional oxygenase clavaminic acid synthase. Nat Struct Biol. 2000;7:127–133. doi: 10.1038/72398. [DOI] [PubMed] [Google Scholar]
- 12.Solomon EI, Decker A, Lehnert N. Non-heme iron enzymes: Contrasts to heme catalysis. Proc Natl Acad Sci USA. 2003;100:3589–3594. doi: 10.1073/pnas.0336792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinman JP. Life as aerobes: Are there simple rules for activation of dioxygen by enzymes? J Biol Inorg Chem. 2001;6:1–13. doi: 10.1007/s007750000172. [DOI] [PubMed] [Google Scholar]
- 14.Que L, Ho RYN. Dioxygen activation by enzymes with mononuclear non-heme iron active sites. Chem Rev. 1996;96:2607–2624. doi: 10.1021/cr960039f. [DOI] [PubMed] [Google Scholar]
- 15.Solomon EI, et al. Geometric and electronic structure/function correlations in non-heme iron enzymes. Chem Rev. 2000;100:235–349. doi: 10.1021/cr9900275. [DOI] [PubMed] [Google Scholar]
- 16.Hanauskeabel HM, Gunzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase—applicability to classification and design of inhibitors. J Theor Biol. 1982;94:421–455. doi: 10.1016/0022-5193(82)90320-4. [DOI] [PubMed] [Google Scholar]
- 17.Price JC, Barr EW, Tirupati B, Bollinger JM, Krebs C. The first direct characterization of a high-valent iron intermediate in the reaction of an alpha-ketoglutarate-dependent dioxygenase: A high-spin Fe(IV) complex in taurine/alpha-ketoglutarate dioxygenase (TauD) from Escherichia coli. Biochemistry. 2003;42:7497–7508. doi: 10.1021/bi030011f. [DOI] [PubMed] [Google Scholar]
- 18.Ryle MJ, et al. O-2 and alpha-ketoglutarate-dependent tyrosyl radical formation in TauD, an alpha-keto acid-dependent non-heme iron dioxygenase. Biochemistry. 2003;42:1854–1862. doi: 10.1021/bi026832m. [DOI] [PubMed] [Google Scholar]
- 19.Puistola U, Turpeenniemihujanen TM, Myllyla R, Kivirikko KI. Studies on the lysyl hydroxylase reaction 1. Initial velocity kinetics and related aspects. Biochim Biophys Acta. 1980;611:40–50. doi: 10.1016/0005-2744(80)90040-6. [DOI] [PubMed] [Google Scholar]
- 20.Myllyla R, Kuuttisavolainen ER, Kivirikko KI. Role of ascorbate in prolyl hydroxylase reaction. Biochem Biophys Res Commun. 1978;83:441–448. doi: 10.1016/0006-291x(78)91010-0. [DOI] [PubMed] [Google Scholar]
- 21.Price JC, Barr EW, Glass TE, Krebs C, Bollinger JM. Evidence for hydrogen abstraction from C1 of taurine by the high-spin Fe(IV) intermediate detected during oxygen activation by taurine:alpha-ketoglutarate dioxygenase (TauD) J Am Chem Soc. 2003;125:13008–13009. doi: 10.1021/ja037400h. [DOI] [PubMed] [Google Scholar]
- 22.Muthukumaran RB, Grzyska PK, Hausinger RP, McCracken J. Probing the iron-substrate orientation for taurine/alpha-ketoglutarate dioxygenase using deuterium electron spin echo envelope modulation spectroscopy. Biochemistry. 2007;46:5951–5959. doi: 10.1021/bi700562t. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien JR, Schuller DJ, Yang VS, Dillard BD, Lanzilotta WN. Substrate-induced conformational changes in Escherichia coli taurine/alpha-ketoglutarate dioxygenase and insight into the oligomeric structure. Biochemistry. 2003;42:5547–5554. doi: 10.1021/bi0341096. [DOI] [PubMed] [Google Scholar]
- 24.Elkins JM, et al. X-ray crystal structure of Escherichia coli taurine/alpha-ketoglutarate dioxygenase complexed to ferrous iron and substrates. Biochemistry. 2002;41:5185–5192. doi: 10.1021/bi016014e. [DOI] [PubMed] [Google Scholar]
- 25.Grzyska PK, et al. Steady-state and transient kinetic analyses of taurine/alpha-ketoglutarate dioxygenase: Effects of oxygen concentration, alternative sulfonates, and active-site variants on the Fe(IV)-oxo intermediate. Biochemistry. 2005;44:3845–3855. doi: 10.1021/bi048746n. [DOI] [PubMed] [Google Scholar]
- 26.Klinman JP. An integrated model for enzyme catalysis emerges from studies of hydrogen tunneling. Chem Phys Lett. 2009;471:179–193. doi: 10.1016/j.cplett.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagel ZD, Klinman JP. A 21st century revisionist's view at a turning point in enzymology. Nat Chem Biol. 2009;5:543–550. doi: 10.1038/nchembio.204. [DOI] [PubMed] [Google Scholar]
- 28.Loenarz C, Schofield CJ. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol. 2008;4:152–156. doi: 10.1038/nchembio0308-152. [DOI] [PubMed] [Google Scholar]
- 29.Koehntop KD, Marimanikkuppam S, Ryle MJ, Hausinger RP, Que L. Self-hydroxylation of taurine/alpha-ketoglutarate dioxygenase: Evidence for more than one oxygen activation mechanism. J Biol Inorg Chem. 2006;11:63–72. doi: 10.1007/s00775-005-0059-4. [DOI] [PubMed] [Google Scholar]
- 30.Ryle MJ, Koehntop KD, Liu AM, Que L, Hausinger RP. Interconversion of two oxidized forms of taurine/alpha-ketoglutarate dioxygenase, a non-heme iron hydroxylase: Evidence for bicarbonate binding. Proc Natl Acad Sci USA. 2003;100:3790–3795. doi: 10.1073/pnas.0636740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirica LM, McCusker KP, Munos JW, Liu HW, Klinman JP. O-18 kinetic isotope effects in non-heme iron enzymes: Probing the nature of Fe/O-2 intermediates. J Am Chem Soc. 2008;130:8122–8123. doi: 10.1021/ja800265s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLano WL. The Pymol Molecular Graphics System, 1.1r1. Palo Alto, CA: DeLano Scientific; 2008. [Google Scholar]
- 33.McCusker KP, Klinman JP. Facile synthesis of 1,1-[2H2]-2-methylaminoethane-1-sulfonic acid as a substrate for taurine alpha-ketoglutarate dioxygenase (TauD) Tet Lett. 2009;50:611–613. [Google Scholar]
- 34.Price JC, Barr EW, Hoffart LM, Krebs C, Bollinger JM. Kinetic dissection of the catalytic mechanism of taurine: Alpha-ketoglutarate dioxygenase (TauD) from Escherichia coli. Biochemistry. 2005;44:8138–8147. doi: 10.1021/bi050227c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.