Abstract
Wnts regulate important intracellular signaling events, and dysregulation of the Wnt pathway has been linked to human disease. Here, we uncover numerous Wnt canonical effectors in human platelets where Wnts, their receptors, and downstream signaling components have not been previously described. We demonstrate that the Wnt3a ligand inhibits platelet adhesion, activation, dense granule secretion, and aggregation. Wnt3a also altered platelet shape change and inhibited the activation of the small GTPase RhoA. In addition, we found the Wnt-β-catenin signaling pathway to be functional in platelets. Finally, disruption of the Wnt Frizzled 6 receptor in the mouse resulted in a hyperactivatory platelet phenotype and a reduced sensitivity to Wnt3a. Taken together our studies reveal a novel functional role for Wnt signaling in regulating anucleate platelet function and may provide a tractable target for future antiplatelet therapy.
Keywords: Wnt-β-catenin pathway, integrin αIIbβ3, frizzled 6 knockout mice
Anucleate platelets are the principle effectors of haemostasis and are found circulating in a nonadhesive, quiescent state. At sites of vascular damage, platelets adhere to various exposed subendothelial matrix proteins and are activated, converting from a resting, discoid shape into larger, flattened structures with extended pseudopodia (1). Such activated platelets secrete and synthesize further agonists, inflammatory mediators, and vasoactive substances and through conformational changes in their major integrin receptor, αIIbβ3, aggregate to other platelets via fibrinogen (Fb) to form a haemostatic plug (2). Aberrant platelet activation can cause pathological thrombus formation, leading to thrombosis and ultimately vessel occlusion and tissue ischemia, the processes underlying myocardial infarction and stroke. Understanding the regulation of platelet activity is thus fundamental to comprehending thrombotic disorders and developing therapeutic strategies.
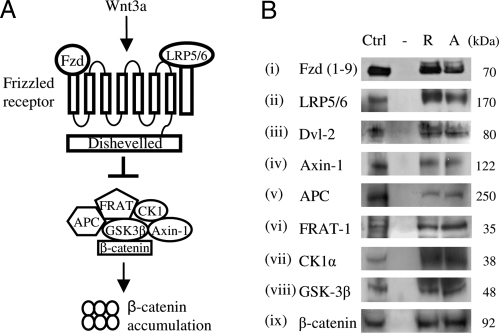
The mammalian Wnt gene family is comprised of 19 secreted Wnt glycoproteins, which play essential roles in cell proliferation, cell-fate determination, and cell-fate differentiation during embryonic development and adult homeostasis (3, 4). These Wnt ligands activate a number of different signaling pathways via distinct receptors and downstream effectors to mediate effects on gene transcription and cell adhesion/migration (5, 6). For the Wnt-β-catenin (β-cat) signaling pathway (Fig. 1A), traditionally referred to as the “canonical” pathway, Wnts bind to a surface receptor complex comprised of a Frizzled (Fzd) receptor and the Lipoprotein Receptor-related Protein 5/6 (LRP5/6) coreceptor (5, 7). The signal is then transduced to the cytoplasmic protein Dishevelled (Dvl) where downstream pathways regulate the stability of β-cat (5, 7). In the absence of Wnt, β-cat is phosphorylated by a destruction complex containing Casein Kinase 1 (CK1), Glycogen Synthase Kinase 3β (GSK3β), Axin-1, FRAT-1, and Adenomatous Polyposis Coli (APC), which targets β-cat for degradation via ubiquitination and subsequent proteosomal degradation (8). In the presence of Wnt, Dvl negatively regulates the phosphorylation of β-cat, preventing its degradation and leading to its cytosolic stabilization (8) (Fig. 1A).
Fig. 1.
The canonical Wnt-β-catenin pathway is present in platelets. (A) Wnt binds to a surface receptor complex comprising of the Fzd and LRP5/6 receptors. In the absence of Wnt, β-cat is phosphorylated by a destruction complex containing CK1, GSK3β, Axin-1, FRAT-1, and APC, which target it for proteosomal degradation. In the presence of Wnt, β-cat is not phosphorylated and accumulates in the cytosol. Activatory signals are denoted by normal arrows, inhibitory signals by flat-headed arrows. (B) Positive control lysates (Ctrl), Resting (R) and 5 μM TRAP-activated (A) platelets were resolved by SDS/PAGE and immunoblotted with antibodies to (i) Fzd isoforms 1–9, (ii) LRP5/6, (iii) Dvl-2, (iv) Axin-1, (v) APC, (vi) FRAT-1, (vii) CK1α, (viii) GSK-3β and (ix) β-cat. Representative blots are shown from 3 replicates.
Here, we demonstrate that components of the canonical Wnt-β-cat signaling pathway are present and functional in anucleate platelets and that the Wnt3a ligand inhibits platelet adhesion, shape change, dense granule secretion, RhoA activation, and aggregation. We also demonstrate that activation of the Wnt pathway through the Fzd6 receptor is functional in limiting platelet activation and is in part responsible for exogenous Wnt3a-mediated platelet inhibition. Our studies define a novel role for the Wnt signaling pathway in regulating platelet function.
Results
Evidence of Wnt Signaling Components in Platelets.
Analysis of in-house human platelet proteomic datasets revealed several Wnt signaling pathway components, including Dvl-2 (Q53XM0), LRP5 (O75197), and Soggy-1 (Q9UK85- Dkk-like 1). To confirm these findings, resting and Thrombin Receptor Activating Peptide (TRAP)-activated (5 μM) platelet lysates were resolved by SDS/PAGE and immunoblotted. Fig. 1B shows (i) Fzd isoforms 1–9, (ii) LRP5/6, (iii) Dvl-2, (iv) Axin-1, (v) APC, (vi) FRAT-1, (vii) CK1α, (viii) GSK3β, and (ix) β-cat in resting and activated platelet lysates, some of which were previously reported to be in platelets (9). We observed no significant changes in protein levels between resting and activated platelets, suggesting that these Wnt-signaling proteins do not undergo release upon platelet activation. We also found that the canonical agonist, Wnt3, and putative antagonist, Soggy-1, were secreted from TRAP-activated platelets (Fig. S1A).
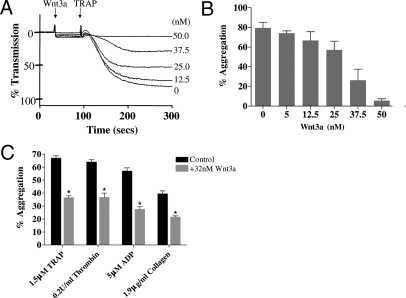
Wnt3a Inhibits Platelet Aggregation.
Pretreatment with recombinant Wnt3a (5–50 nM) dose-dependently inhibited TRAP (SFLLRN; 1.5 μM) induced platelet aggregation (Fig. 2 A and B). Maximal inhibition was observed at 50 nM Wnt3a and the IC50 calculated as 32 nM using GraphPad Prism. Wnt3a 32 nM inhibited platelet aggregation to a range of agonists including 0.2U/ml thrombin (P = 0.009), 5 μM ADP (P = 0.002) and 1.9 μg/ml collagen, (P = 0.011) demonstrating a broad inhibitory effect on distinct signaling pathways (Fig. 2C). The structurally distinct Wnt5a ligand had no effect on control or 1.5 μM TRAP-induced platelet aggregation at concentrations up to 200 nM (Fig. S1B). MTT toxicity assays (SI Text) determined that neither Wnt3a nor Wnt5a were toxic to platelets (≤200 nM) (Fig. S1 C and D).
Fig. 2.
Wnt3a inhibits platelet aggregation. (A) A representative aggregation trace showing Wnt3a to decrease aggregation in a concentration-dependant manner in 1.5 μM TRAP-stimulated platelets. (B) The mean result of 3 independent TRAP (1.5 μM)-induced aggregation experiments in the presence of increasing doses of Wnt3a are shown (IC50 = 32 nM). (C) Aggregation was measured in response to numerous platelet agonists in the absence (black bars) and presence (gray bars) of 32 nM Wnt3a. All data shown are representative of 3 or more independent platelet preparations ± SEM. (*, P < 0.05)
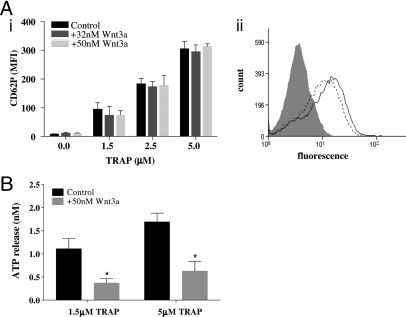
Wnt3a Selectively Inhibits Dense Granule Secretion.
P-selectin (CD62P) surface exposure was used as a marker of platelet α-granule release (Fig. 3Ai). In the absence of Wnt3a, CD62P expression per platelet increased with increasing concentrations of TRAP and was unaffected by 32 or 50 nM Wnt3a. A representative histogram trace is shown in Fig. 3Aii. The effect of Wnt3a on dense granule secretion was determined by measuring ATP release (Fig. 3B) where 50 nM Wnt3a inhibited ATP release in response to 1.5 μM (P = 0.009) and 5 μM TRAP (P = 0.022). Similarly, Wnt3a (50 nM) inhibited ATP release in response to both 1.9 and 3.8 μg/ml collagen (P = 0.025 and P = 0.017 respectively) (Fig. S1E).
Fig. 3.
Effects of Wnt3a on granule secretion. (A) (i) CD62P expression was measured using flow cytometry in the absence (black bars) and presence of increasing concentrations of Wnt3a (32 nM is denoted by dark gray bars and 50 nM by light gray bars). No treatment or EDTA-treated samples served as controls. (ii) A representative histogram trace detailing CD62P expression in resting (gray shaded curve) and TRAP-activated platelets in the absence (black dotted line) and presence (black solid line) of Wnt3a. (B) ATP release from 1.5 μM and 5 μM TRAP stimulated platelets in the absence (black bars) and presence (light gray bars) of 50 nM Wnt3a measured in a luminescent aggregometer. All results represented as a mean value ± SEM from 3 independent platelet preparations (*, P < 0.05).
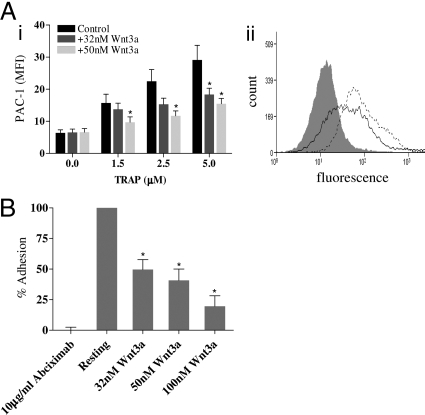
Wnt3a Inhibits Platelet Integrin αIIbβ3 Activation.
We examined by flow cytometry Wnt3a effects on: (a) PAC-1 binding (an antibody specific for the activated conformation of αIIbβ3) (Fig. 4Ai) and (b) soluble Fb binding (SI Text and Fig. S2A). Platelets were activated with 1.5, 2.5, and 5 μM TRAP in the absence or presence of 32 or 50 nM Wnt3a; 32 nM reduced and 50 nM Wnt3a significantly inhibited PAC-1 binding in response to all doses of TRAP [1.5 μM (P = 0.050), 2.5 μM (P = 0.019) and 5 μM TRAP (P = 0.016)], thereby reducing the number of activated integrins per platelet (Fig. 4A). Consistent with these results, Fb binding was reduced under similar conditions (Fig. S2A). Wnt3a had a stronger inhibitory effect on PAC-1 binding to ADP 2.5, 5, and 10 μM (P = 0.004, 0.026, and 0.010, respectively) (Fig. S2B). The different effect of Wnt3a on TRAP and ADP-induced PAC-1 binding is likely to reflect TRAP-induced release of internal αIIbβ3 pools.
Fig. 4.
Wnt3a inhibits integrin αIIbβ3 activation. (A) (i) A FITC conjugated antibody for PAC-1 binding was used in flow cytometry to measure αIIbβ3 activation to increasing concentrations of TRAP in the absence (black bars) and presence of 32 nM (dark gray bars) and 50 nM (light gray bars) Wnt3a. (ii) A representative histogram trace shows resting (solid gray curve) and 2.5 μM TRAP stimulated platelets in the absence (black dotted line) and presence of 50 nM Wnt3a (black solid line). (B) A static adhesion assay was performed to quantify the number of platelets adhering to Fb in the presence of increasing concentration of Wnt3a. Data are expressed as a % of the positive control (platelet-only sample, containing no Wnt3a). Abciximab (10 μg/ml) was used as a negative control. All results represented as a mean value ± SEM from 3 independent platelet preparations (*, P < 0.05).
We also examined the effect of Wnt3a on platelet adhesion to Fb using a static adhesion assay. Platelet adhesion to Fb was inhibited at 32 nM (P = 0.010), 50 nM (P = 0.030), and 100 nM Wnt3a (P = 0.011), where adhesion was reduced by approximately 80% (Fig. 4B). Abciximab (10 μg/ml) was used as a negative control and abrogated static platelet adhesion (Fig. 4B). Wnt3a also reduced phosphorylation of the integrin β3 tail (Y785), essential for outside-in signaling events following αIIbβ3 activation (Fig. S2C). Taken together, these studies implicate Wnt signaling in the regulation of αIIbβ3 activation.
Wnt3a Inhibits RhoA Activation and Blocks Rho-Kinase Dependant Shape Change.
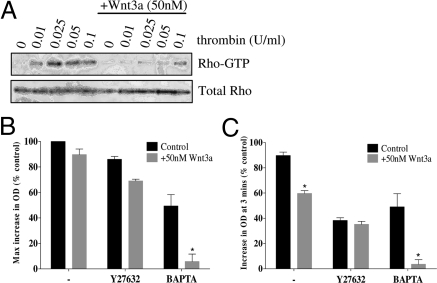
We investigated Wnt3a effects on the activation of the small GTPase RhoA using a Rhotekin binding assay (Fig. 5A). In control samples, precipitated Rho-GTP was found at all concentrations of thrombin (0.01–0.1U/ml) with lowest levels at 0.01U/ml and maximal levels at 0.025U/ml. In the presence of 50 nM Wnt3a, only basal levels of Rho-GTP were precipitated up to concentrations of 0.05U/ml thrombin, with low levels precipitated at 0.1U/ml. Thus, preincubation with 50 nM Wnt3a inhibited thrombin-induced platelet RhoA activation (Fig. 5A).
Fig. 5.
Wnt3a inhibits RhoA activation and the rho-kinase dependant pathway of platelet shape change. (A) Inhibition of RhoA activation when 50 nM Wnt3a was added to platelets before thrombin activation (0–0.1U/ml). Anti-RhoA blot shows total RhoA in lysed samples. 5 μM ADP-induced platelet shape change in response to a variety of conditions was monitored by Optical Density (OD) in a ChronoLog aggregometer by measuring (B) maximum % increase in OD and (C) % increase in OD 3 min after stimulation, both versus vehicle-treated only. Data shown are representative of 3 separate donors and are presented as mean ± SEM (*, P < 0.05).
We further compared the effects of Wnt3a on platelet shape change to the calcium chelator (BAPTA-AM), which inhibits the “early” calcium-dependent shape change response and also to a Rho kinase inhibitor (Y27632) which inhibits the “sustained” Rho-kinase-dependent response. Wnt3a 50 nM had little effect on the extent of platelet shape change induced by 5 μM ADP, but when Wnt3a was combined with BAPTA, shape change to ADP was blocked (Fig. 5B). Although Wnt3a did not influence the maximum platelet shape change, the response was less sustained at 3 min an effect that was also seen with Y27632 alone (Fig. 5C). Furthermore, when Wnt3a was combined with BAPTA, there was essentially no optical shape change to sustain (Fig. 5C). These results are consistent with Wnt3a inhibiting the Rho-kinase-dependent component of ADP-induced platelet shape change. Similar results were found for TRAP-induced shape change although as TRAP induces greater intracellular calcium mobilization, Y27632 had less of an inhibitory effect (Fig. S2 D and E and SI Text).
Modification of Canonical Wnt Signaling Events in Platelets.
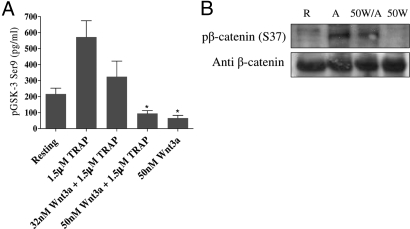
To demonstrate that Wnt/β-cat signaling is active in platelets, we assessed the effects of Wnt3a on the phosphorylation status of two pivotal downstream effectors, GSK3β and β-cat. Using an ELISA plate assay specific for the phospho-Ser 9 (S9) residue of GSK3β (Fig. 6A), we found that GSK3β was phosphorylated on S9 in resting platelets and phosphorylated S9 levels increased upon TRAP (1.5 μM) activation, as reported previously (10, 11). Wnt3a 32 nM decreased TRAP-induced phosphorylated S9 levels, while pretreatment with 50 nM Wnt3a reduced the levels of phosphorylated GSK3β below baseline in both resting and TRAP-activated platelets. The effect seen is consistent with studies showing absence of GSK3β phosphorylation at S9 upon Wnt signaling (12, 13).
Fig. 6.
Modification of Wnt canonical pathway events in platelets. (A) A phospho-GSK3β (S9) ELISA was carried out using resting and 1.5 μM TRAP-activated washed platelet samples in the presence/absence of Wnt3a. The phospho-GSK3 concentration (pg/ml) of each sample is represented as a mean value ± SEM from at least 3 separate platelet preparations. (B) Resting (R) and 1.5 μM TRAP-activated (A) platelet lysates, pretreated with 50 nM Wnt3a (50W and 50W/A respectively) were immunoblotted for phosphorylated (S37) β-cat. Blots were stripped and reprobed with anti-β-cat to demonstrate equal loading. Data shown are representative of 3 replicate experiments.
Activation of the Wnt/β-cat pathway leads to a decreased β-cat Ser-37 (S37) phosphorylation (8, 14). S37 phosphorylation of β-cat was slightly evident in resting (R) platelets (Fig. 6B) and these low levels were confirmed using Rho-GTP negative resting platelets (as in Fig. 5A). β-cat S37 phosphorylation increased upon TRAP (1.5 μM) activation (A) and this was attenuated by 50 nM Wnt3a (50W/A). 50 nM Wnt3a (50W) also abolished S37 phosphorylation in resting platelets.
Wnt3a Induced No Significant Change in the Extent of Ca2+ Signaling.
Wnt signaling via the Wnt/Ca2+ pathway results in a rise in intracellular Ca2+ release and subsequent activation of calcium calmodulin-dependent kinase 2 (CamK2) and protein kinase C (PKC) (15). We found no change in the extent of Ca2+ signaling at TRAP concentrations that were otherwise affected by Wnt3a (i.e., up to 5 μM TRAP), suggesting that the Wnt3a-mediated platelet effects were likely independent of altered Ca2+ (Fig. S2F and SI Text).
Fzd6 Is Present and Functional in Platelets.
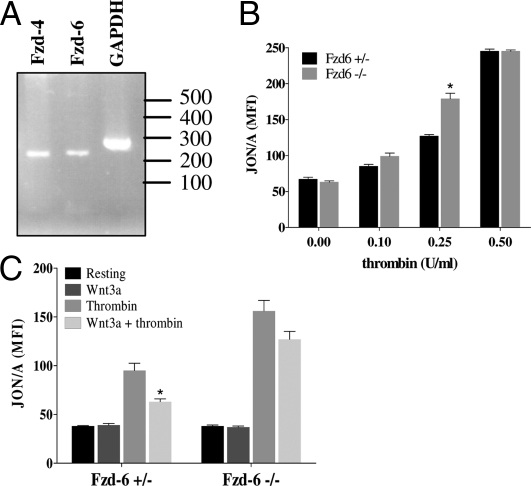
A recent megakaryocyte (MK) transcriptome study (16) reported that Fzd4 and 6 were the most abundantly expressed Fzd isoforms in MKs, suggesting that these may also present in platelets. Indeed, we detected Fzd4 and 6 transcripts (Fig. 7A) and the Fzd6 protein in platelets (Fig. S3A).
Fig. 7.
Fzd4 and 6 are present in platelets and Fzd6 −/− platelets have an increased activatory phenotype. (A) RT-PCR of platelet RNA using primers specific for Fzd4, 6 and GAPDH demonstrated their expression in platelets (predicted product sizes 211, 222, and 288 bp, respectively. (B) Fzd6−/− mice have increased platelet activation. Platelets were isolated from Fzd6+/− and Fzd6−/− mice and activated with thrombin (n = 4± SD.; P < 0.01 vs. Fz6+/−). (C) Wnt3a does not antagonize Fzd6−/− platelet activation. Fzd6+/− (Left) and Fzd6−/− (Right) platelets were incubated in the absence/presence of Wnt3a and stimulated with thrombin (0.25 U/ml).
To further examine the functional role of Wnt signaling in platelets, we isolated washed platelets from Fzd6−/− and Fzd6+/− mice, and measured αIIbβ3 activation to thrombin using JON/A antibody binding. Fzd6+/− and Fzd6−/− platelets had similar JON/A binding at low and high concentrations of thrombin stimulation (Fig. 7B). However, Fzd6−/− platelets demonstrated significantly higher JON/A binding at moderate agonist stimulation (Fig. 7B, 0.25U/ml thrombin). Wnt3a reduced platelet activation in Fzd6+/− (over 30%), (P = 0.016) in response to 0.25U/ml thrombin (Fig. 7C, Left) but had no significant effect on platelets from Fzd6−/− mice (P = 0.073) (Fig. 7C, Right). Taken together these data demonstrate that Fzd6 signaling blunts platelet activation and is responsible in part for Wnt-mediated inhibition of the platelet response to thrombin. However, as the response to Wnt3a was not abolished, other Fzd receptors may also be implicated.
Discussion
Our study shows the platelet expression of Frizzled receptors (including Fzd4 and 6), Dvl-2, LRP5/6, Axin-1, Frat-1, CK1α, GSK3β, and β-cat and that Wnt3 and Soggy-1 are released upon platelet activation. In nucleated cells, the canonical Wnt pathway is widely regarded as a key regulator of gene expression through the stabilization and subsequent nuclear translocation of β-cat, which operates as a transcriptional coregulator (5, 7). The presence of such canonical Wnt signaling components in the anucleate and transcriptionally silent platelet was somewhat surprising. Yet our studies suggest a functional role for canonical Wnt signaling on platelet activity based on an array of functional assays of platelet activation, secretion, aggregation, αIIbβ3 activation, and adhesion.
We demonstrated that the Wnt ligand, Wnt3a, has a dose-dependent inhibitory effect (IC50:32 nM) on human platelet aggregation in response to a variety of platelet agonists, suggesting that Wnt3a is having a general effect on platelet signaling, not limited to a specific surface receptor. There was no effect on calcium influx or α-granule secretion, but we did observe a decrease in dense granule ATP release in response to agonist stimulation, which may attenuate the secondary wave of platelet aggregation.
Furthermore, Wnt3a, which lacks an RGD sequence, modulated the activation of the major platelet integrin receptor αIIbβ3 measured as the extent of Fb binding; the conformational change in the receptor that occurs with activation, which we detected as expression as the PAC-1 binding site; and the phosphorylation status of the intracellular tail of integrin β3 subunit. Pretreatment with Wnt3a, therefore, inhibits the agonist-dependent transition of this integrin from a low- to a high-affinity state and thus reduces the extent of Fb binding across the platelet surface and subsequent platelet aggregation. A reduced static adhesion to Fb without prior agonist stimulation was also found as reported previously in platelets for certain other inhibitors, for example the Syk inhibitor piceannatol (17). Furthermore, although modulation of integrin activation is not a described response to canonical Wnt signaling, this was further confirmed in platelets lacking the Fzd6 receptor, where αIIbβ3 activation by thrombin was significantly attenuated.
Wnt3a also inhibited thrombin-induced RhoA activation and blocked ADP- and TRAP-induced Rho-kinase-dependent shape change. RhoA is a small GTPase, upstream of Rho-kinase, which plays an important role in cytoskeleton reorganization, αIIbβ3 activation, and granule exocytosis and can do so in a calcium-independent manner (18). Interestingly, the noncanonical Wnt signaling pathway modulates cell migration via activation of the small G proteins RhoA and Rac (6, 19). Therefore, inactivation of RhoA could be due to Wnt3a-mediated cross-suppression of the noncanonical Wnt pathway (20, 21).
As platelets are anucleate and devoid of transcription, they do not lend themselves to analysis using reporter gene assays for canonical Wnt signaling. Furthermore, the time scale for platelet activation, and the observed inhibitory effect of Wnt (0–60 seconds), is relatively brief when compared to Wnt responses in nucleated cells. On the other hand, platelets have an extensive and dynamic phosphoproteome, which is central to the rapid reactivity of these cells and we found that Wnt3a induces a global reduction in tyrosine phosphorylation (Fig. S3B). Phosphorylation of two key components of the Wnt signaling pathway, GSK3β and β-cat was specifically assessed. GSK3β is a negative regulator of platelet function (11). Pretreatment with Wnt3a in both resting and TRAP-activated platelets significantly inhibited the S9 phosphorylation of GSK3β which is in agreement with other Wnt signaling studies (12, 13) and suggests that Wnt3a may influence the Akt-dependent platelet pathway leading to the phosphorylation of GSK3β (S9) (11).
In the absence of Wnt, GSK3β phosphorylates β-cat at multiple sites including Ser-33 and S37 (8, 22), targeting this protein for degradation. In the presence of Wnt, the GSK3β-associated destruction complex is inhibited, allowing cytosolic accumulation of β-cat and its subsequent nuclear translocation (8). In the presence of Wnt3a, the phosphorylation of platelet β-cat at S37 was inhibited even in resting platelets. However, as platelets are anucleate, unphosphorylated and thus stabilized β-catenin cannot have a nuclear transcriptional role. Other roles, however, have been described. For example, studies in human monocytes and THP-1 monocyte cells show that Wnt3a activation of the canonical Wnt signaling pathway did not result in TCF-promoter activity or the expression of classical Wnt target genes (23, 24). Yet, induction of cytoplasmic β-cat upon exposure of human monocytes to Wnt3a resulted in a decrease in direct monocyte adherence to endothelial cells and subsequent transendothelial migration (24). A key role for cytosolic β-cat is to link adhesion receptors of the cadherin family to the actin cytoskeleton (25, 26). While adhesion through cadherins has not been observed in platelets, cadherins are expressed (9), and we find a number of cadherin/cadherin-like proteins present in our in-house human platelet proteomics datasets, which we are currently investigating.
In summary, we have demonstrated the expression of the major components of the canonical Wnt-β-cat signaling pathway in anucleate platelets and suppression of platelet activity by its ligand, Wnt3a. The Wnt3a effect on platelets is mediated in part through the Fzd6 receptor, suggesting that other Wnt signaling receptor pathways are probably involved. Our research, combined with other findings that include Wnt agonists found in plasma (27) and the Wnt pathway antagonist Dkk-1 present in and released from platelet α-granules (28), uncovers a new pathway of platelet regulation. Moreover, there is already evidence that circulating Wnt ligands levels may be modified in disease states (28, 29). There are many potential sources of circulating Wnt ligands including activated platelets, endothelial cells or possibly other circulating cells (30, 31). Further studies will be necessary to understand how the interplay of various Wnt ligands can modulate platelet function but one could hypothesize based on our genetic studies that Wnt agonists acting through Fzd6 may dampen platelet reactivity, akin to other endogenous regulators such as prostacyclin and nitric oxide. The canonical Wnt signaling pathway may thus represent a novel endogenous mechanism for regulating platelet activity.
Materials and Methods
Platelet Isolation.
Human platelets were obtained from adult volunteers in accordance with approved guidelines from the University College Dublin research ethics committee. All animal procedures/protocols were approved by The John Hopkins Animal Care and Use Committee. Fzd6−/− mice and Fzd6+/− littermate controls were kindly provided by Dr. Jeremy Nathans and Dr. Yanshu Wang (The Johns Hopkins University) (32). Isolation of human and murine platelets was performed as described (33, 34). Platelet releasate was prepared as before (35).
Platelet Aggregation/ATP Release.
Aggregation was measured using a Chrono-log 700 luminescent aggregometer (Chrono-log Co.). Aggregation experiments were carried out as described (35) with recombinant Wnt3a/Wnt5a (R&D Systems) added 30 seconds before agonist loading. For measurement of ATP release, 25 μl of Chrono-Lume reagent was added before agonist loading and the maximum ATP release calculated according to the manufacturers' instructions.
SDS PAGE and Immunoblotting.
Equal amounts of protein were analyzed by Western blotting and visualized as described (35). Antibodies used were as follows: Santa Cruz: Dvl-2, Fzd1–9, Wnt-3, LRP5/6, CK1α, APC, RhoA, Fzd6; Abcam: Anti GSK-3β, FRAT-1, Soggy-1; R&D Systems: Anti Axin-1, phospho-β-Catenin (S33/S37), phospho-GSK-3 (S9/S21), Anti β-Catenin; Invitrogen: Phospho-Integrin β3 (Y785); Millipore: 4G10. Lysates used as positive controls were as per manufacturers' instructions.
Flow Cytometry.
Washed human platelets (1 × 108/ml) were analyzed using a CyanADP flow cytometer by acquiring 15000 events of the gated platelet population (36). Isolated mouse platelets were prepared as described previously (34). All data are recorded as median fluorescent intensity (MFI) alongside representative histogram traces for each experiment. Antibodies used: BD Bioscience: conjugated FITC/PAC-1 and PE mouse antihuman CD62P; Dako: rabbit antihuman Fb-FITC; Emfret Analytics: JON/A antibody.
Static Platelet Adhesion Assay.
Static adhesion was measured as described (37); 2 × 108/ml gel filtered platelets were adhered to static fibrinogen (Fb) and lysed with Buffer A containing a substrate for acid phosphatases (0.1M sodium acetate, 0.1% Triton-X-100 and 10 mM p-nitrophenol phosphate). The reaction was stopped on addition of 1M NaOH and read at 405 nm in a plate reader (EL808, Bio-Tek Instruments).
Shape Change Assay.
Indomethacin-treated (10 μM) washed platelets (2 × 108/ml) were treated with the Rho kinase inhibitor, Y27632 (10 μM) (Sigma), and/or the intracellular Ca2+ chelator, BAPTA-AM (20 μM) (Sigma), or vehicle control, for 10 min as before (38). Wnt3a (50 nM) or its carrier was added for 1 min before platelet stimulation with 5 μM ADP or 1.5 μM TRAP in a Chrono-log aggregometer under stirring in the presence of 1 mM EGTA. Optical shape change was recorded as the increase in optical density (OD)/height above basal. The maximum increase in OD and the increase in OD 3min after stimulation were measured and expressed as a percentage of control (vehicle-treated) platelets.
RhoA Activation Assay.
Washed platelets (1 × 109/ml) preincubated with 50 nM Wnt3a (30 seconds) were activated with thrombin for 1 min. Activation was terminated by addition of 500 μl ice cold 2x lysis buffer (1% Nonidet P-40, 10% glycerol, 50 mM Tris-HCl (pH 7.4), 200 mM NaCl, 2.5 mM MgCl2, 1 mM PMSF, 1X protease inhibitors, 1X phosphatase inhibitors). 20 μl supernatant was kept for analysis of total Rho expression and Rho-GTP was precipitated using Rhotekin-RBD protein agarose beads (Cytoskeleton Inc.) according to the manufacturer's instructions. Total RhoA and precipitated Rho-GTP was measured by immunoblotting with anti-RhoA (Santa Cruz).
Measurement of GSK3β Phosphorylation.
Washed platelet samples, stimulated under described conditions, were prepared in an aggregometer; 3 × 108 plts/ml were lysed as above and the phospho-GSK3β (S9) levels determined by ELISA (R&D Systems) in accordance with the manufacturer's instructions.
For additional methodology see SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. Jeremy Nathans and Dr. Yanshu Wang (Johns Hopkins Medical School, Baltimore) for the kind gift of the Fzd6+/− and Fzd6−/− mice and Dr. Alfonso Blanco (University College Dublin Conway flow cytometry core facility) for helpful advice. This work is supported by the Health Research Board of Ireland (P.B.M, RP/2006/286); the Irish Research Council for Science, Engineering, and Technology (I.C.M., PD/2008/33); and the Programme for Research in Third-Level Institutions, administered by the Higher Education Authority of Ireland (D.J.F. and P.B.M.). A.W.P. is supported by the British Heart Foundation (RG/05/015, PG/07/118/24152, and PG/08/049/25130) and also holds a Biotechnology and Biological Sciences Research Council Research Development Fellowship. C.N.M. is supported by National Institutes of Health Grant 1R01HL093179. R.H. is supported by March of Dimes, NSF Grant #0544061 and National Institutes of Health Grant GM078172.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906268106/DCSupplemental.
References
- 1.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 2.Ma YQ, Qin J, Plow EF. Platelet integrin αIIbβ3: Activation mechanisms. J Thromb Haemost. 2007;5:1345–1352. doi: 10.1111/j.1538-7836.2007.02537.x. [DOI] [PubMed] [Google Scholar]
- 3.Logan C, Nusse R. The Wnt signalling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald BT, Semenov MV, He X. Snap-shot: Wnt/β-catenin signalling. Cell. 2007;134:1204e1–1204e2. [Google Scholar]
- 6.Semenov MV, Habas R, Macdonald BT, He X. Snap-Shot: Noncanonical Wnt signalling pathways. Cell. 2007;131:1378.e1–1378.e2. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Haung H, He X. Wnt/β-catenin signalling: New (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 9.Elrod JW, et al. Expression of junctional proteins in human platelets. Platelets. 2003;14:247–251. doi: 10.1080/0953710031000118894. [DOI] [PubMed] [Google Scholar]
- 10.Barry FA, Graham GJ, Fry MJ, Gibbins JM. Regulation of glycogen synthase kinase 3 in human platelets: A possible role in platelet function? Febs Lett. 2003;553:173–178. doi: 10.1016/s0014-5793(03)01015-9. [DOI] [PubMed] [Google Scholar]
- 11.Li D, August S, Woulfe DS. GSK3β is a negative regulator of platelet function and thrombosis. Blood. 2008;111:3522–3530. doi: 10.1182/blood-2007-09-111518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruel L, Stambolic V, Ali A, Manoukian AS, Woodgett JR. Regulation of the protein kinase activity of Shaggy (Zeste-white3) by components of the wingless pathway in Drosophila cells and embryos. J Biol Chem. 1999;274:21790–21796. doi: 10.1074/jbc.274.31.21790. [DOI] [PubMed] [Google Scholar]
- 13.Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signalling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 14.Van Noort M, et al. Wnt signalling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 15.Kohn AD, Moon RT. Wnt and calcium signalling: β-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Watkins, et al. A HaemAtlas: characterizing gene expression in differentiated human blood cells. Blood. 2009;113:e1–9. doi: 10.1182/blood-2008-06-162958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law DA, et al. Genetic and pharmacological analyses of Syk function in IIbIIIa signalling in platelets. Blood. 1999;8:2645–2652. [PubMed] [Google Scholar]
- 18.Bauer M, et al. Dichotomous regulation of myosin phosphorylation and shape change by Rho-kinase and calcium in intact human platelets. Blood. 1999;94:1665–1672. [PubMed] [Google Scholar]
- 19.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/frizzled signalling is required for vertebrate gastrulation. Genes Dev. 2003;17:395–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topol L, et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westfall TA, et al. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel S, Doble B, Woodgett JR. Glycogen synthase kinase-3 in insulin and Wnt signalling: A double-edged sword? Biochem Soc Trans. 2004;32:803–808. doi: 10.1042/BST0320803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiele A, Wasner M, Muller C, Engeland K, Hauschildt S. Regulation and possible function of β-catenin in human monocytes. J Immunol. 2001;167:6786–6793. doi: 10.4049/jimmunol.167.12.6786. [DOI] [PubMed] [Google Scholar]
- 24.Tickenbrock L, et al. Wnt signalling regulates transendothelial migration of monocytes. J Leukocyte Biol. 2006;79:1306–1313. doi: 10.1189/jlb.0905539. [DOI] [PubMed] [Google Scholar]
- 25.Nelson WJ, Nusse R. Convergence of Wnt, β-catenin and cadherin pathways. Science. 2004;5:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Ze'ev A, Shtutman M, Zhurinsky J. The integration of cell adhesion with gene expression: The role of beta-catenin. Exp Cell Res. 2000;261:75–82. doi: 10.1006/excr.2000.5045. [DOI] [PubMed] [Google Scholar]
- 27.Omenn GS, et al. Overview of the HUPO Plasma Proteome Project: Results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and a publicly-available database. Proteomics. 2005;5:3226–3245. doi: 10.1002/pmic.200500358. [DOI] [PubMed] [Google Scholar]
- 28.Voorzanger-Rousselot, et al. Platelet is a major contributor to circulating levels of Dickkopf-1: clinical implications in patients with multiple myeloma. Br J Haematol. 2009;145:255–267. doi: 10.1111/j.1365-2141.2009.07587.x. [DOI] [PubMed] [Google Scholar]
- 29.Weber, et al. The prognostic value of the Wnt antagonist Dickkopf-1 (dkk-1) in patients presenting with an acute coronary syndrome. Circulation. 2007;116:II_579–II_580. [Google Scholar]
- 30.Goodwin AM, Sullivan KM, D'Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signalling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signalling. Dev Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 31.Qiang YW, Rudikoff S. Wnt signalling in B and T lymphocytes. Front Biosci. 2004;9:1000–1010. doi: 10.2741/1309. [DOI] [PubMed] [Google Scholar]
- 32.Guo N, Hawkins C, Nathans J. Frizzled-6 controls hair patterning in mice. Proc Natl Acad Sci USA. 2004;101:9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maguire PB, et al. Identification of the phosphotyrosine proteome from thrombin activated platelets. Proteomics. 2002;2:642–648. doi: 10.1002/1615-9861(200206)2:6<642::AID-PROT642>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 34.Morrell C, et al. Glutamate mediates platelet activation through the AMPA receptor. J Exp Med. 2008;205:575–584. doi: 10.1084/jem.20071474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppinger JA, et al. Proteomic characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 36.Jones CI, et al. Mapping the platelet profile for functional genomic studies and demonstration of the effect size of the GP6 locus. J Thromb Haemost. 2007;5:1756–1765. doi: 10.1111/j.1538-7836.2007.02632.x. [DOI] [PubMed] [Google Scholar]
- 37.Kerrigan S, et al. A role for glycoprotein Ib in Streptococcus sanguis-induced platelet aggregation. Blood. 2002;100:509–516. doi: 10.1182/blood.v100.2.509. [DOI] [PubMed] [Google Scholar]
- 38.Hardy AR, Hill DJ, Poole AW. Evidence that the purinergic receptor P2Y12 potentiates platelet shape change by a Rho kinase-dependent mechanism. Platelets. 2005;16:415–429. doi: 10.1080/09537100500163424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.