Abstract
In metastatic prostate cancer (PCa) cells, imbalance between cell survival and death signals such as constitutive activation of phosphatidylinositol 3-kinase (PI3K)-Akt and inactivation of apoptosis-stimulated kinase (ASK1)-JNK pathways is often detected. Here, we show that DAB2IP protein, often down-regulated in PCa, is a potent growth inhibitor by inducing G0/G1 cell cycle arrest and is proapoptotic in response to stress. Gain of function study showed that DAB2IP can suppress the PI3K-Akt pathway and enhance ASK1 activation leading to cell apoptosis, whereas loss of DAB2IP expression resulted in PI3K-Akt activation and ASK1-JNK inactivation leading to accelerated PCa growth in vivo. Moreover, glandular epithelia from DAB2IP−/− animal exhibited hyperplasia and apoptotic defect. Structural functional analyses of DAB2IP protein indicate that both proline-rich (PR) and PERIOD-like (PER) domains, in addition to the critical role of C2 domain in ASK1 activity, are important for modulating PI3K-Akt activity. Thus, DAB2IP is a scaffold protein capable of bridging both survival and death signal molecules, which implies its role in maintaining cell homeostasis.
Keywords: cell apoptosis, prostate cancer, signal transduction
Homeostatic balance among cell proliferation, survival, and apoptosis is essential for ontogenesis. Any imbalance in these processes often leads to pathologic changes in cells. Many cancer cells often exhibit accelerated cell proliferation, increased cell survival, and resistance toward apoptosis. Dissecting homeostatic machinery operative in normal cells will provide important clues for its alteration in malignant cells.
The PI3-Kinase (PI3K)-Akt signaling pathway plays a central role in modulating cell proliferation, survival, and motility. Class IA PI3K is a heterodimer consisting of a 110-kDa catalytic subunit (p110) and an 85-kDa regulatory subunit (p85). Mammalian cells contain three different genes for p85 (α, β, and γ) and three different genes for p110 (α, β, and σ) (1, 2). The p85 stabilizes p110 in the steady state (3). Activation of PI3K occurs via the binding of p85 to phosphotyrosine proteins, which relieves the inhibitory effect of p85 on the p110, and then PI3K translocates from cytosol to plasma membrane where its substrate PI-4,5-P2 resides (4, 5). After PI3K activation, Akt, phosphorylated at T308 and S473, becomes activated (6). The role of the PI3K-Akt pathway in carcinogenesis has been extensively investigated, and altered expression or mutation of many components in this pathway has been implicated in human cancer (1). The mechanisms leading to PI3K-Akt overactivation including gene activating mutation (7, 8), protein overexpression (9), mutations of Ras oncogenes (10), or absence of inhibitor such as phosphatase and tensin homolog (PTEN) (11, 12) have been shown in many cancer types. In addition to promoting cell proliferation and survival, the PI3K-Akt pathway can also influence apoptotic cell death machinery (1). For example, Akt can inactivate apoptosis signal-regulating kinase 1 (ASK1) by phosphorylating S83 residue (13). ASK1, a member of the MAPK kinase kinase (MAP3K) family, is an upstream activator of c-Jun kinase (JNK) and p38 MAPK signaling cascades (14). The activation of ASK1 triggers apoptosis in response to diverse stress and apoptotic stimuli such as TNF-α or reactive oxygen species (15).
We previously identified DAB2IP/AIP1 (DOC-2/DAB2 interactive protein, or ASK1 interacting protein) (16, 17) as a member of the RAS-GTPase activating protein (RAS-GAP) family with growth inhibitory activity in prostate cancer (PCa). Loss of DAB2IP expression, mainly due to epigenetic regulation of its promoter, is often detected in androgen-independent PCa (18, 19) as well as in other cancer types (20–23). Furthermore, a single nucleotide polymorphism in the DAB2IP gene has been associated with the risk of aggressive PCa from a study using two Genome-Wide Association databases (24). DAB2IP can inhibit H-RAS, R-RAS, and TC21 but not RAP1A activities associated with suppressing epidermal growth factor-elicited PCa growth (16). In addition to its GAP activity, DAB2IP is also involved in TNF-α-mediated cell apoptosis in endothelial cells by facilitating dissociation of ASK1 from its inhibitor 14-3-3 via its pleckstrin homology (PH) and C2 domains (25, 26). Obviously, DAB2IP is involved in multiple biologic functions in various cell types.
Here, we further reveal that DAB2IP can coordinate PI3K-Akt inactivation with ASK1 activation pathways. In addition to the critical role of its N-terminal C2 domain in ASK1 activity (17), structural functional analyses indicate that the C-terminal proline-rich (PR) and PERIOD-like (PER) domains are critical for modulating PI3K and Akt activity, respectively. Therefore, DAB2IP protein is a unique scaffold protein to balance pathways of cell proliferation, survival, and death.
Results
DAB2IP Induces G0/G1 Cell Cycle Arrest and Promotes Apoptosis.
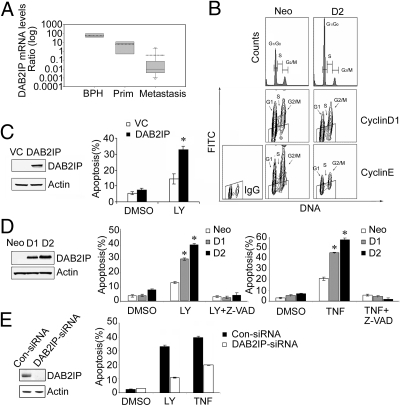
Our previous data showed that loss of DAB2IP is associated with metastatic PCa cell lines (16). Using human prostate specimens with different pathologic status such as benign prostatic hyperplasia (BPH), primary cancer, and bone metastases to determine the relative DAB2IP expression pattern, we further showed that DAB2IP mRNA levels in metastatic PCa were significantly lower than that in BPH or primary cancer specimens (Fig. 1A), indicating that decreased DAB2IP is associated with PCa progression. In addition to its GAP activity (16), the other function of DAB2IP in PCa is largely unknown. Thus, we first examined whether DAB2IP might affect cell cycle progression. Using a stable DAB2IP-transfected subline (i.e., D2) generated from C4-2 cell line, a tumorigenic PCa line without endogenous DAB2IP expression, and a control subline (i.e., Neo) (16), we compared their cell cycle distributions. The percentage of G0/G1 phase cells in D2 was ≈20% more than that in Neo subline; in contrast, S or G2/M phase cells in D2 subline was less than that in Neo subline [Fig. 1B and supporting information (SI) Fig. S1A, indicating that DAB2IP could elicit G0/G1 phase cell cycle arrest. Bivariate analysis of cellular DNA with cyclin D1 and E using flow cytometry (27) showed “unscheduled” expression pattern of both cyclins (28) in the Neo subline (Fig. 1B). In the D2 subline, cyclin D1 and E protein levels decreased in either G0 phase or G1 phase (Fig. 1B), indicating that DAB2IP can reduce G1 phase cyclin levels resulting in G0 or early G1 phase cell cycle arrest.
Fig. 1.
The expression and functional role of DAB2IP in PCa. (A) Box plot of relative DAB2IP mRNA levels from human specimens of BPH (n = 12, left bar), primary cancer (n = 12, center bar), and bone metastases (n = 12, right bar). Thin line = median; dotted line = mean; P < 0.001 by Kruskal–Wallis ANOVA on RANKS. (B) DAB2IP induced G0/G1 cell cycle arrest. Cell cycle distribution was detected by flow cytometry. For cyclins/DNA multiparameter flow cytometry, the trapezoidal “window” represented the level of fluorescence of the isotype IgG control, indicating the range within which cyclin-negative cells were located. (C) Transient expression of DAB2IP promoted apoptosis in C4-2 cells under LY treatment. Apoptosis was determined by Annexin V/PI flow cytometry. Asterisk indicates statistical significance in cells transfected with DAB2IP vs. VC (P < 0.01). (D) DAB2IP promoted apoptosis in D1 and D2 cells treated with LY or TNF-α. Asterisk indicates statistical significance between DAB2IP-expressing cells and neo cells (P < 0.01). (E) Knockdown of DAB2IP reduced the apoptotic response of PZ-HPV-7 cells. Asterisk indicates statistical significance in cells transfected with DAB2IP-siRNA vs. control siRNA (P < 0.01). All of the data (mean ± SEM) were generated from three independent experiments.
Because DAB2IP can facilitate TNF-α-elicited apoptosis via ASK1 in endothelia (17), we decided to determine whether DAB2IP could affect apoptosis in PCa cells (e.g., transient and stable DAB2IP-transfected C4-2 cells) in the presence of PI3K inhibitor LY294002 (LY) or TNF-α capable of activating mitochondrial or death receptor pathways, respectively (29). In the presence of LY, fewer viable cells were detected in transient DAB2IP-transfected cells (Fig. S1B). Annexin V/PI analysis indicated that under LY treatment, apoptotic cell death increased about 3-fold in transient DAB2IP-transfected cells compared with vector control (VC) cells (Fig. 1C). In stable transfectants (i.e., D1 or D2), apoptosis induced by either LY or TNF-α was significantly higher than that in Neo cells, which was correlated with DAP2IP expression levels (Fig. 1D). This DAB2IP-enhanced cell death is caspase-dependent, because the presence of pan-caspase inhibitor (Z-VAD) can abolish DAB2IP effect (Fig. 1D).
To further determine the involvement of DAB2IP in LY or TNF-α-induced apoptosis pathway, siRNA was used to knock down the endogenous DAB2IP in an immortalized normal prostate epithelium (PZ-HPV-7) cells. Reduced DAB2IP diminished cell apoptosis ≈3-fold in LY- or 2-fold in TNF-α-treated cells compared with the control siRNA (Fig. 1E). Collectively, these findings indicate that DAB2IP can cause cell cycle arrest and is also a proapoptotic factor capable of potentiating intrinsic or extrinsic apoptotic pathways.
DAB2IP Inhibits Akt and Activates ASK1 Activity.
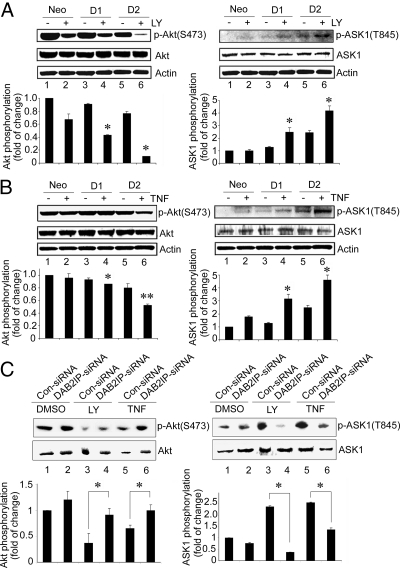
To reveal the mechanism of DAB2IP function in these events, the activation status of Akt (S473) or ASK1 (T845) was determined based on the specific phosphorylation site of each protein. Compared with neo cells, a dramatic inhibition of p-Akt (S473) was detected in D1 and D2 cells under LY treatment (Fig. 2A Left); concurrently, p-ASK1 (T845) was significantly elevated in both D1 and D2 cells (Fig. 2A Right). Similar findings were observed in D1 and D2 cells under TNF-α treatment (Fig. 2B). These data demonstrated that DAB2IP could suppress Akt and facilitate ASK1 activation under stress condition. Moreover, in PZ-HPV-7 cells under the same treatment, both increased Akt and decreased ASK1 activation were detected in the presence of DAB2IP siRNA to knock down endogenous DAB2IP levels (Fig. 2C). Thus, DAB2IP appears to be a modulator to bridge both Akt and ASK1 pathways.
Fig. 2.
DAB2IP inhibited PI3K-Akt and activated ASK1. (A and B) Cell lysates were prepared from neo, D1, and D2 cells after LY (10 μM) (A) or TNF-α (100 ng/mL) (B) treatment for 30 min, and Akt or ASK1 activation was determined by Western blot analysis. The same membrane was stripped and reprobed with total Akt or ASK1 antibody, by which the quantification of relative kinase activation was normalized. Data (mean ± SEM) were generated from two independent experiments. Asterisk indicates statistical significance between DAB2IP-expressing cells and neo cells, (*, P < 0.1; **, P < 0.05). (C) PZ-HPV-7 cells were transfected with DAB2IP- or control-siRNA then treated with LY or TNF-α as described in A or B, and relative Akt and ASK1 kinase activity was quantified. Asterisks indicate statistically significant in cells transfected with DAB2IP-siRNA cell vs. Control siRNA (P < 0.05).
DAB2IP Binds to PI3K-p85 via the PR Domain.
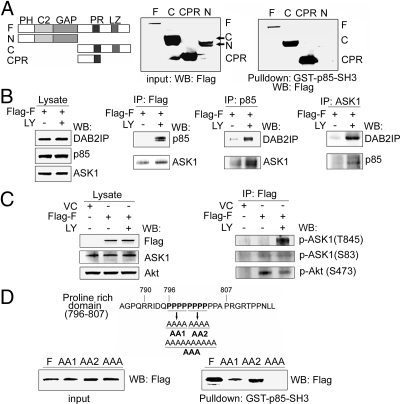
Structurally, DAB2IP contains several potential functional domains (Fig. 3A Left) including the PH, C2, GAP, PER, PR, and leucine zipper (LZ) domains. Because the PI3K regulatory subunit (i.e., p85) contains Src homology 3 (SH3), we predicted that the PR domain could bind to p85-SH3 domain. The pull-down data (Fig. 3A Right and Fig. S2A) indicated that DAB2IP-F, -C, and -CPR but not -N could bind to GST-p85-SH3, indicating the PR is a binding domain to p85-SH3. Using coimmunoprecipitation (co-IP), a weak interaction of DAB2IP with either p85 or ASK1 was detected; however, a dramatic increase of complex formation was detected under either LY (Fig. 3B) or TNF-α treatment (Fig. S2B). In the ASK1-DAB2IP-PI3K complex, the active form of ASK1 (T845) became more predominant than its inactive form (S83). Also, a decreased p-Akt (S473) was detected in this complex (Fig. 3C), suggesting that DAB2IP might inhibit PI3K via a direct protein interaction with both PI3K and Akt. Because this PR domain contains an unusual sequence of ten repeats of proline, we further mapped the binding site for p85 using three different PR mutants (Fig. 3D). Pull-down assay indicated that the first four proline residues were critical for p85 binding and the AAA mutant completely abolished the binding to p85 (Fig. 3D). Co-IP data further confirmed that the AAA mutant not only failed to interact with p85 (Fig. S2B) but also impaired Akt inhibition and ASK1 activation in this complex (Fig. S2C). All these data confirm that the PR domain is the binding site to p85.
Fig. 3.
Interaction of DAB2IP with p85 via PR domain. (A) In vitro analysis of the binding domain in DAB2IP to p85. After transfecting with various cDNA constructs, 293 cells were subjected to pull-down assay using GST-p85-SH3 fusion protein. Five percent of each input lysate was used as a loading control. (B) In vivo analysis of DAB2IP complex. Cell lysates of 293 cells under the same transfection and treatment condition were subjected to co-IP probed with Flag, p85, and ASK1 antibody. (C) DAB2IP complex associated with active ASK1 and inactive Akt. 293 cells were transfected with DAB2IP-F or VC, and cell lysates were subjected to co-IP with Flag antibody then probed with p-ASK1 (T845 or S83) or p-Akt (S473) antibody. (D) In vitro analysis of the key proline residues in DAB2IP for p85 binding. The cluster of four prolines in DAB2IP is underlined and replaced with alanine to generate AA1, AA2, and AAA. Cell lysates were pulled down with GST-p85-SH3 fusion protein and probed with Flag antibody.
PR Domain in DAB2IP Dictates PI3K-Akt Inhibition and ASK1 Activation.
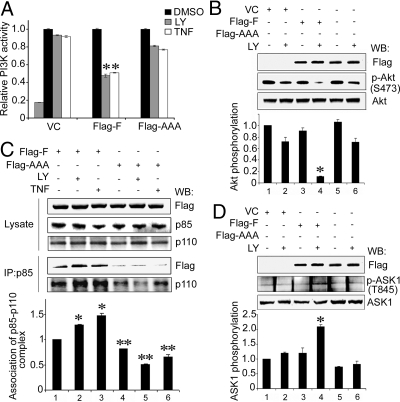
We next examined whether the formation of DAB2IP and p85 complex had any effect on the PI3K activity and ASK1 activation. DAB2IP-F (full length) could potentiate PI3K-Akt inactivation elicited by LY or TNF-α; however, the AAA mutant lost such activity (Fig. 4 A and B), indicating that PR domain is critical for PI3K-Akt inhibition. Moreover, under LY or TNF-α treatment, DAB2IP-F sequestered p85 and stabilized PI3K complex (p85 and p110), in contrast, AAA mutant interrupted such interaction (Fig. 4C). In this event, DAB2IP did not impact on membrane translocation of p85 based on the status of p85 (Y458) phosphorylation (Fig. S2D). Meanwhile, in the presence of DAB2IP-F, ASK1 activity (T845) was increased significantly in cells under LY treatment but not detected with AAA mutant (Fig. 4D). These data indicate that the PR domain is the key motif for both PI3K-Akt inhibition and ASK1 activation.
Fig. 4.
PR domain dictated PI3K-Akt inhibition and ASK1 activation. In all experiments, 293 cells were transfected with DAB2IP-F, AAA mutant or VC after LY or TNF-α treatment as described in Fig. 2. (A) DAB2IP inhibited PI3K activity. Cell lysates were IP by using p110 antibody and PI3K activity was measured by an ELISA-based assay. Relative PI3K activity was normalized with VC cells without treatment. VC cells treated with 80 μM LY was used as a positive control. All of the data (mean ± SEM) were carried out in triplicate. Asterisk indicated statistical significance in cells transfected with VC or AAA vs. DAB2IP-F (P < 0.01). (B) Functional PR domain dictated Akt inhibition. Relative Akt activity was quantified as previously. Data (mean ± SEM) were generated from three independent experiments. Asterisk indicates statistical significance in cells transfected with VC or AAA vs. DAB2IP-F (P < 0.01). (C) DAB2IP sequestered PI3K complex under stress signal. Cell lysates were IP with p85 antibody and then probed with p110 antibody. In DAB2IP-F cells without treatment, p110 level was considered as a basal line and the fold of induction from each sample was calculated. One asterisk indicated statistical significance in cells treated with LY or TNF-α (*, P < 0.01). Two asterisks indicated statistical significance in cells transfected with DAB2IP-F vs. AAA (**, P < 0.01). (D) DAB2IP dictated ASK1 activation. Relative ASK1 activity was quantified as previously. Data (mean ± SEM) were generated from three independent experiments. Asterisk indicates statistical significance in cells transfected with VC or AAA vs. DAB2IP (P < 0.01).
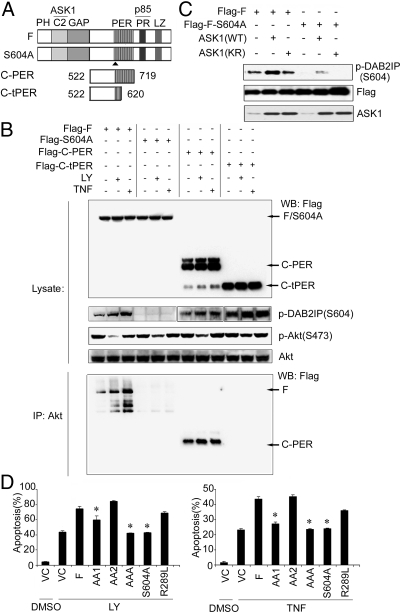
S604 Phosphorylation Is Critical for DAB2IP Activity.
From our previous study, the phosphorylation of S604 in DAB2IP is critical for changing its conformation (26). Here, we showed that DAB2IP-F was phosphorylated at S604 under either LY or TNF-α treatment, which resulted in more complex formation with p85; in contrast, S604A mutant failed to be phosphorylated and interact with p85 (Fig. S4A). These data suggest that S604 phosphorylation is critical for changing DAB2IP structure to sequester PI3K complex.
Considering that S604 is located in the PER domain and the function of this domain is not well characterized, the PER protein sequence was scanned by using the Scansite program, and the data unveiled Akt as a potential binding protein (Fig. S3). To confirm this, two deletion mutants of DAB2IP-PER domain [C-PER (amino acids 522–719), C-tPER (amino acids 522–620), Fig. 5A] were generated. Co-IP data indicated that the Akt-binding site was located between amino acids 620–719 in DAB2IP (Fig. 5B). In addition, the interaction between DAB2IP and Akt was substantially reduced in cells expressing S604A mutant protein (Fig. 5B and S4B). Furthermore, we found the degree of DAB2IP on Akt inhibition as F > C-PER ≈S604A > C-tPER (Fig. 5B), indicating the critical role of S604 in the PER domain for modulating Akt activity.
Fig. 5.
The role of S604 phosphorylation in DAB2IP activity. (A) Schematic depiction of PER mutant constructs. (B) The 293 cells were transfected with DAB2IP-F, S604A, C-PER, and C-tPER constructs after LY or TNF-α treatment. Cell lysates were subjected to Western blot analysis or co-IP with Akt and probed with Flag antibody. (C) Increased S604 phosphorylation by ASK1. The 293 cells were cotransfected with DAB2IP-F or S604A mutant with either ASK1 wild type (WT) or kinase inactive (KR; Lys 709 was replaced by Arg), then cell lysates were detected with p-DAB2IP, DAB2IP, or ASK1 antibody. (D) The role of each functional domain in DAB2IP-mediated apoptosis. C4-2 cells were transfected with various DAB2IP mutants after LY or TNF-α treatment, and apoptosis was determined by flow cytometry.
To further define the candidate kinase for S604 phosphorylation, ASK1 was examined because its association with DAB2IP was increased under LY (Fig. 3) or TNF-α treatment (26). ASK1-WT, not ASK1-KR, significantly increased S604 phosphorylation (Fig. 5C). Taken together, S604 phosphorylation is a prerequisite for DAB2IP activation by forming a complex with PI3K-Akt in which ASK1 is one of the key initiator(s).
PR Domain Is Critical for DAB2IP-Mediated Apoptosis.
To correlate the structure–functional relationship of DAB2IP in PCa, C4-2 cells were transfected with various DAB2IP constructs, and cell apoptosis was assessed. Both AAA and S604A mutants diminished LY- or TNF-α-induced apoptosis, whereas the AA1 mutant exhibited less effect and the AA2 mutant had no effect (Fig. 5D), which is consistent with the functional role of PR domain (Fig. 3D). In addition, DAB2IP-R289L, a GAP mutant (16), also had no effect (Fig. 5D), indicating that GAP domain is not involved in DAB2IP-mediated apoptosis. Apparently, in response to various exogenous or endogenous stress signals, the change of DAB2IP conformation is critical to modulate key effector molecules involved in either cell survival or apoptosis (Fig. 6E).
Fig. 6.
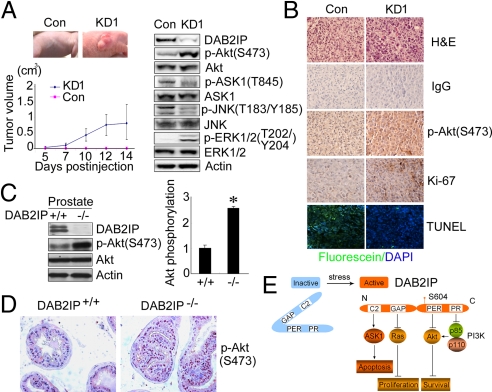
Physiologic role of DAB2IP in prostate. (A) The in vivo tumor growth of KD1 and Con cells. Western blot analyses of DAB2IP, p-Akt(S473), p-ASK1(T845), p-JNK(T183/Y185), p-ERK1/2(T202/Y204) in KD1 and Con cells. (B) Elevated p-Akt, proliferation marker and decreased apoptosis in KD1 vs. Con tumors. IHC from the paraffin sections were determined by p-Akt (S473) and Ki-67 antibodies. (Magnification: 200×.) TUNEL assay were determined from paraffin sections. (Magnification: 200×.) (C) Increased Akt activation in the prostate of DAB2IP−/− mice. Tissue lysates were analyzed by Western blot using DAB2IP and p-Akt (S473) antibodies. β-Actin was used as a loading control. Relative Akt activity was quantified as previously. Asterisk indicated statistical significance in DAB2IP−/− vs. DAB2IP+/+ mice (P < 0.01). (D) Increased Akt activation in the prostate epithelia of DAB2IP−/− mice. IHC from the paraffin sections were determined by p-Akt (S473) antibody (Magnification: 400×.) (E) A model of DAB2IP function. In response to stress signals, S604 phosphorylated DAB2IP, as a scaffold protein, sequesters ASK1, Ras, Akt, and PI3K (p85-p110) via different domains, which elicits various distinct biologic activities in a coordinated manner.
Down-Regulation of DAB2IP Leads to Akt Activation and ASK1 Inactivation Associated with Accelerated Tumor Growth or Prostate Hyperplasia.
To determine any impact of DAB2IP on tumor growth in vivo, stable DAB2IP knockdown (i.e., KD1) and control (i.e., Con) cells were generated by using PC-3 cells. From an s.c. mouse model, significantly higher tumor take and growth rate were observed in KD1 cells (12 of 12) compared with control cells (3 of 12) within 2 weeks after injection (Fig. 6A). In contrast, all control tumors became palpable 6–8 weeks after injection. Both Western blot analysis and immunohistochemistry (IHC) showed that Akt activity was highly elevated, and ASK1-JNK activity was diminished in KD1 tumors (Fig. 6A) where tumor cells showed active cell proliferation (i.e., Ki-67 staining) and reduced apoptosis (i.e., TUNEL staining) (Fig. 6B). To further determine the physiological role of DAB2IP in prostate, knockout (KO, DAB2IP−/−) mice were used (30). DAB2IP−/− mice developed prostate hyperplasia in epithelial compartment at 6 months of age (Fig. 6D), where Akt was hyperactivated (Fig. 6 C and D) and ASK1 was suppressed (Fig. S5A). Similarly, Ki-67 staining was also elevated in these hyperplastic lesions (Fig. S5B). Taken together, loss of DAB2IP increases the survival advantage of prostate epithelial cells with hyperactivated Akt, which is often detected with aggressive PCa.
Discussion
Altered homeostatic control in cell proliferation, survival, and apoptosis underlies many diseases particularly cancer malignancy. In many cancer types, including PCa, the PI3K-Akt signaling axis has been shown as a dominant survival pathway (1, 31). In contrast, insufficient activation of ASK1, an important mediator of apoptotic signaling responses to many stress stimuli, and/or its downstream MKK4-JNK-c-Jun pathway has been correlated with the development of aggressive PCa (32). Although there is cross-talk between the Akt and ASK1 pathways (13, 33), the mechanism of how these signal pathways are coordinated to maintain cell homeostasis in response to diverse stimulus remains undetermined. Our data (Figs. 2 and 3) have unveiled that DAB2IP as a scaffold protein sequesters both Akt and ASK1 and counterbalances the activity of each distinct kinase by modulating their phosphorylation status in response to different signals. Mechanistically, there are several components in the cell-death machinery that could be common target(s) for Akt or ASK1. For example, Bad is a proapoptotic member of the Bcl-2 family that promotes cell death by forming a nonfunctional heterodimer with the survival factor Bcl-XL. Phosphorylation of Bad by Akt prevents this interaction, restoring Bcl-XL's antiapoptotic function (34). In addition, Akt inhibits the catalytic activity of a prodeath protease, caspase-9, through phosphorylation (35). On the other hand, ASK1 can activate JNK or p38, which can activate several proapoptotic targets in the Bcl-2 family such as Bim and Bax, respectively (36). Thus, the presence of DAB2IP is expected to augment the apoptotic effect via inactivating Akt and activating ASK1.
The p85, a regulatory subunit of PI3K, contains SH3, BCR, two SH2 domains, and an inter-SH2 domain that binds constitutively to the p110 catalytic subunit. In general, the SH3 and BCR domains have a negative role in modulating PI3K kinase activity (3), which is due to the conformational changes in the p85–p110 complex (1). Thus, the SH3 or BCR domain is considered as an intrinsic inhibitor toward the catalytic activity of p110 subunit (1, 2). Clearly, our results (Fig. 3 and Fig. S2) indicate that the first four prolines in the DAB2IP-PR domain is a critical binding site for the p85-SH3 domain. Despite that, this interaction has no effect on p85 phosphorylation, and DAB2IP is able to inhibit PI3K activity by sequestering and stabilizing p85-p110 complex and then further inactivating Akt (Fig. 4). Because DAB2IP is able to relocate from membrane to cytosol in the presence of TNF-α (25), we believe that the formation of DAB2IP-p85-p110 complex may prevent them from translocating to the cell membrane, which provides additional evidence for the inhibitory effect of the p85-SH3 domain on PI3K activity. Taken together, DAB2IP has a similar function with PTEN as a negative regulator in PI3K-Akt pathway. Nevertheless, DAB2IP is a scaffold protein associated with various effector molecules rather than a phosphatase.
Noticeably, DAB2IP functions as a key ”platform” to modulate various signals by recruiting PI3K, Akt, and ASK1 proteins via its individual binding domain (Fig. 6E). For example, LY- or TNF-α-mediated different pathways eventually merge into the same downstream effect—Akt inactivation and ASK1 activation. It is known that TNF-α-elicited ASK1 activation requires several sequential steps (37–39), and we have shown that DAB2IP dissociates ASK1 inhibitor 14-3-3 from its C terminus and then facilitates ASK1 activation (17). In this study, we further show that the DAB2IP-mediated binding and inhibition of PI3K-Akt also contributes to ASK1 activation (Fig. 4D). In a feedback loop, ASK1 increases S604 phosphorylation in DAB2IP, which significantly enhances the binding ability of DAB2IP to Akt and PI3K complex (Fig. 5 and Fig. S4), implying that this phosphorylation site relates to the conformational changes of DAB2IP from inactive to active status. Thus, the ASK1–DAB2IP–PI3K complex is a “signal platform” to counterbalance between cell survival and apoptosis, which exists not only in PCa or normal prostate epithelial cells but also in other cell type as well (Fig. S6). Based on these outcomes, DAB2IP is capable of enhancing the effect of targeted therapy with pharmacological inhibitors of PI3K or TNF-α. Given the possible off-target effect of these agents at higher dosage, we believe that restoring DAB2IP expression in target tissue by either genetic or inductive approach is expected to increase the therapeutic index of these agents.
Overall, as illustrated in Fig. 6E, DAB2IP is a unique scaffold protein that can modulate cell growth, survival, and death via several signaling pathways such as Ras-Raf-ERK, PI3K-Akt and ASK1-JNK. Thus, loss of DAB2IP leads to imbalance of cell survival and apoptosis pathways, which plays a significant role in tumor development (Fig. 6).
Materials and Methods
For plasmid constructs and transfection, antibodies, immunoprecipitation, pull-down assay, and Western blot analysis, see SI Text.
Cell Culture and Clinical Specimens.
C4-2 (WT, Neo, D1, D2) and PC-3 cells were maintained in T medium supplemented with 5% FBS (16). PZ-HPV-7, an immortalized normal cell line derived from the peripheral zone of a benign prostate, was maintained in PrEGM medium (Lonza). Human embryonic kidney 293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) containing 10% FBS. LY294002 was purchased from Cayman, and TNF-α was purchased from Invitrogen.
The Institutional Review Board approved the tissue procurement protocol in this study and appropriate informed consent was obtained from all patients. Benign prostatic hyperplasia specimens were obtained from transurethral prostatic resection and primary cancer specimens (Gleason score 6–9) were obtained from prostatectomies performed in our institute. All of the metastatic samples provided by R. L. Vessella (University of Washington, Seattle, WA) were obtained from patients with bony metastases with a Gleason score = 9. The DAB2AIP mRNA levels from each sample were determined by qRT-PCR analysis. see SI Text for details.
RNA Interference for DAB2IP.
Three pairs of siRNA oligonucleotides for human DAB2IP (5′-GGAGCGCAACAGUUACCUGTT-3′ [siRIP1-A]; 5′-GGUGAAGGACUUCCUGACATT-3′ [siRIP1-B]; 5′- GGACUUGUUUUUUGUCACATT-3′ [siRIP1-C]) and control siRNA (5′-CTGGACTTCCAGAAGAACA-3′) were synthesized by Invitrogen. siRNA (20 μM) was transfected into cells by using LipofectAMINE 2000 (Invitrogen) according to the manufacturer's protocol.
Analyses of Effect of DAB2IP on Cell Cycle or Apoptosis.
Cell cycle analysis and Cyclins/DNA multiparameter flow cytometry were performed as described previously (27, 40).
For apoptosis, cells after treatment with LY (40 μM) for 24 h or TNF-α (100 ng/mL plus CHX 10 μg/mL) for 6 h were harvested and fixed in ice-cold 80% ethanol at −20 °C for at least 24 h. Annexin V/FITC staining was performed with kit from Bender Medsystems. TUNEL assay was performed with In Situ Cell Death Detection kit, Fluorescein from Roche.
PI3K Activity Assay.
A nonradioactive competitive ELISA-based assay (Echleon Biosciences) was used to assess the PI3K activity. Relative PI3K activity was calculated using untreated cells (= 1).
Animal Model.
All experimental procedures have been approved by the Institutional Animal Care and Use Committee. For the s.c. model, 5 × 105 cells were injected on the left and right flanks of animal. Tumor volume (mm3) was measured by caliper and calculated by using the ellipsoid formula (π/6 × length × width × depth). Tissue specimens were subjected to immunohistochemistry. See SI Text for details.
Statistical Analysis.
All error bars in graphical data represent mean ± SEM. Student's two-tailed t test was used for the determination of statistical relevance between groups with P < 0.05 considered as significant.
Supplementary Material
Acknowledgments.
We thank Drs. Lai Wang and Sudan He for their technical advice. This work was supported in part by United States Army Grant W81XWH-04-1-0222 (to J.-T.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908458106/DCSupplemental.
References
- 1.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 2.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, et al. Regulation of the p85/p110 phosphatidylinositol 3-kinase: Stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueki K, Algenstaedt P, Mauvais-Jarvis F, Kahn CR. Positive and negative regulation of phosphoinositide 3-kinase-dependent signaling pathways by three different gene products of the p85 α regulatory subunit. Mol Cell Biol. 2000;20:8035–8046. doi: 10.1128/mcb.20.21.8035-8046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rordorf-Nikolic T, Van Horn DJ, Chen D, White MF, Backer JM. Regulation of phosphatidylinositol 3-kinase by tyrosyl phosphoproteins. Full activation requires occupancy of both SH2 domains in the 85-kDa regulatory subunit. J Biol Chem. 1995;270:3662–3666. doi: 10.1074/jbc.270.8.3662. [DOI] [PubMed] [Google Scholar]
- 6.Alessi DR, et al. Characterization of 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 7.Philp AJ, et al. The phosphatidylinositol 3′-kinase p85α gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–7429. [PubMed] [Google Scholar]
- 8.Moscatello DK, Holgado-Madruga M, Emlet DR, Montgomery RB, Wong AJ. Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J Biol Chem. 1998;273:200–206. doi: 10.1074/jbc.273.1.200. [DOI] [PubMed] [Google Scholar]
- 9.Moore SM, et al. The presence of a constitutively activate phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70S6k dependent pathway. Cancer Res. 1998;58:5239–5247. [PubMed] [Google Scholar]
- 10.Okudela K, et al. K-ras gene mutation enhances motility of immortalized airway cells and lung adenocarcinoma cells via Akt activation: Possible contribution to non-invasive expansion of lung adenocarcinoma. Am J Pathol. 2004;164:91–100. doi: 10.1016/S0002-9440(10)63100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe G, et al. Analysis of the P13K signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- 12.Chang F, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 13.Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 15.Ichijo H, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, et al. The mechanism of growth-inhibitory effect of DOC-2/DAB2 in prostate cancer. Characterization of a novel GTPase-activating protein associated with N-terminal domain of DOC-2/DAB2. J Biol Chem. 2002;277:12622–12631. doi: 10.1074/jbc.M110568200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, et al. AIP1 mediates TNF alpha-induced ASK1 activation by facilitating dissociation of ASK1 from its inhibitor 14–3-3. J Clin Invest. 2003;111:1933–1943. doi: 10.1172/JCI17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Toyooka S, Gazdar AF, Hsieh JT. Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in prostate cancer cell lines. J Biol Chem. 2003;278:3121–3130. doi: 10.1074/jbc.M208230200. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Tu S, Hsieh JT. Down regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J Biol Chem. 2005;280:22437–22444. doi: 10.1074/jbc.M501379200. [DOI] [PubMed] [Google Scholar]
- 20.Dote H, et al. Aberrant promoter methylation in human DAB2 interactive protein (hDAB2IP) gene in breast cancer. Clin Cancer Res. 2004;10:2082–2089. doi: 10.1158/1078-0432.ccr-03-0236. [DOI] [PubMed] [Google Scholar]
- 21.Dote H, et al. Aberrant promoter methylation in human DAB2 interactive protein (hDAB2IP) gene in gastrointestinal tumour. Br J Cancer. 2005;92:1117–1125. doi: 10.1038/sj.bjc.6602458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yano M, et al. Aberrant promoter methylation of human DAB2 interactive protein (hDAB2IP) gene in lung cancers. Int J Cancer. 2005;113:59–66. doi: 10.1002/ijc.20531. [DOI] [PubMed] [Google Scholar]
- 23.Qiu GH, et al. Differential expression of hDAB2IPA and hDAB2IPB in normal tissues and promoter methylation of hDAB2IPA in hepatocellular carcinoma. J Hepatol. 2007;46:655–663. doi: 10.1016/j.jhep.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Duggan D, et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. J Natl Cancer Inst. 2007;99:1836–1844. doi: 10.1093/jnci/djm250. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, et al. AIP1/DAB2IP, a novel member of the Ras-GAP family, transduces TRAF2-induced ASK1-JNK activation. J Biol Chem. 2004;279:44955–44965. doi: 10.1074/jbc.M407617200. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, et al. RIP1-mediated AIP1 phosphorylation at a 14-3-3-binding site is critical for TNF-induced ASK1-JNK/p38 activation. J Biol Chem. 2007;282:14788–14796. doi: 10.1074/jbc.M701148200. [DOI] [PubMed] [Google Scholar]
- 27.Darzynkiewicz Z, Gong J, Juan G, Ardelt B, Traganos F. Cytometry of cyclin proteins. Cytometry. 1996;25:1–13. doi: 10.1002/(SICI)1097-0320(19960901)25:1<1::AID-CYTO1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Gong J, Ardelt B, Traganos F, Darzynkiewicz Z. Unscheduled expression of cyclin B1 and cyclin E in several leukemic and solid tumor cell lines. Cancer Res. 1994;54:4285–4288. [PubMed] [Google Scholar]
- 29.Kulik G, et al. Tumor necrosis factor alpha induces BID cleavage and bypasses antiapoptotic signals in prostate cancer LNCaP cells. Cancer Res. 2001;61:2713–2719. [PubMed] [Google Scholar]
- 30.Zhang H, et al. AIP1 functions as an endogenous inhibitor of VEGFR2-mediated signaling and inflammatory angiogenesis in mice. J Clin Invest. 2008;118:3904–3916. doi: 10.1172/JCI36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3′-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res. 1999;59:2891–2897. [PubMed] [Google Scholar]
- 32.Kim HL, et al. Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer Res. 2001;61:2833–2837. [PubMed] [Google Scholar]
- 33.Levresse V, Butterfield L, Zentrich E, Heasley LE. Akt negatively regulates the c-Jun N-terminal kinase pathway in PC12 cells. J Neurosci Res. 2000;62:799–808. doi: 10.1002/1097-4547(20001215)62:6<799::AID-JNR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Datta SR, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 35.Cardone MH, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 36.Tonissen KF, Di Trapani G. Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Mol Nutr Food Res. 2009;53:87–103. doi: 10.1002/mnfr.200700492. [DOI] [PubMed] [Google Scholar]
- 37.Gotoh Y, Cooper JA. Reactive oxygen species and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- 38.Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol. 2002;191:1091–1099. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- 39.Saitoh M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protocols. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.