Abstract
I reject the Darwinian assumption that larvae and their adults evolved from a single common ancestor. Rather I posit that, in animals that metamorphose, the basic types of larvae originated as adults of different lineages, i.e., larvae were transferred when, through hybridization, their genomes were acquired by distantly related animals. “Caterpillars,” the name for eruciforms with thoracic and abdominal legs, are larvae of lepidopterans, hymenopterans, and mecopterans (scorpionflies). Grubs and maggots, including the larvae of beetles, bees, and flies, evolved from caterpillars by loss of legs. Caterpillar larval organs are dismantled and reconstructed in the pupal phase. Such indirect developmental patterns (metamorphoses) did not originate solely by accumulation of random mutations followed by natural selection; rather they are fully consistent with my concept of evolution by hybridogenesis. Members of the phylum Onychophora (velvet worms) are proposed as the evolutionary source of caterpillars and their grub or maggot descendants. I present a molecular biological research proposal to test my thesis. By my hypothesis 2 recognizable sets of genes are detectable in the genomes of all insects with caterpillar grub- or maggot-like larvae: (i) onychophoran genes that code for proteins determining larval morphology/physiology and (ii) sequentially expressed insect genes that code for adult proteins. The genomes of insects and other animals that, by contrast, entirely lack larvae comprise recognizable sets of genes from single animal common ancestors.
Keywords: hybridization, insect evolution, interphyletic crosses, larval transfer, metamorphosis
Darwin (1), Haeckel (2), and most zoologists assume that any larva and its adult evolved from a single common ancestor. My larval transfer hypothesis, by contrast, claims that the basic forms of all larvae were transferred as adults of other taxa. Larvae originated when adult genomes were acquired by other different animals probably primarily by sexual hybridization (3, 4). The dual genomes of the merged lineages are expressed in a temporal sequence. An egg fertilized by sperm from an animal of another species can hatch as a larva that resembles one parent who later metamorphoses and matures into an adult morphologically indistinguishable from the second parent. Larvae were later additions to established animal phylogenies and merged lineages continue to evolve. My idea is that metamorphosis represents an evolutionary legacy: A change in genetic expression during development from one taxon to another, and it is testable.
The prevalent common ancestor assumption of monophyly and my larval transfer hypothesis are contrasted here in relation to the evolutionary origins of caterpillar larvae. Although caterpillars as acquired larvae were mentioned earlier (3–5), derivatives of caterpillars were not considered. This exposition that includes new evidence from micropterigid moths and a Cambrian lobopod presents the concept for testability with molecular biological methods. This specific example of my larval transfer thesis is: The onychophoran origin of caterpillars is now amenable to verification or to disproof by genome analysis.
Haeckel's “biogenetic law” (2) developed from the common ancestor assumption; it postulates that larvae represent ancestral adults and ontogeny is a short, rapid recapitulation of phylogeny. Garstang (6) proposed that modern larvae represent ancestral larvae rather than adults. He posited that adults in one taxon that resemble larvae in another are “persistent larvae,” i.e., forms that were originally larvae but now mature without metamorphosis. I regard his persistent larvae as relatives of adult animals that were sources of larvae in distant lineages.
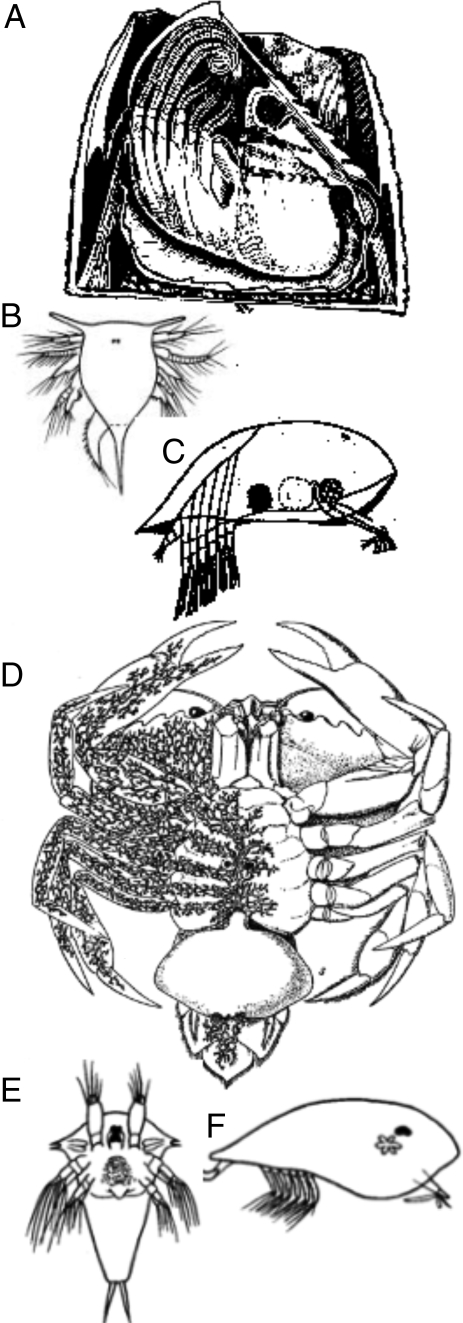
“Even the illustrious Cuvier did not perceive that a barnacle was, as it certainly is, a crustacean; but a glance at the larva shows this to be the case in an unmistakable manner” wrote Darwin (ref. 1, p. 420), and barnacles provide a clear example of the differences between Darwin's attitude to larvae and mine. Barnacles (cirripedes) are crustaceans because of their adult characteristics, but I claim that because all larvae evolved as later additions to life histories, emphasis on the larval morphologies often lead to misclassification. Cirripede larvae (Fig. 1) comprise nauplii with frontal horns and cypris larvae with bivalved carapaces (Fig. 1 B and C), but such larvae also occur in rhizocephalans (literally “root heads”), which infect crabs or hermit crabs (Fig. 1 D and F). An adult rhizocephalan consists of a bulbous projection on the ventral side of the crab abdomen and tubules that ramify throughout the soft tissues (Fig. 1D). It lacks a chitinous cuticle and limbs, and it does not molt. Although called “parasitic barnacles,” rhizocephalans lack any characteristics of adult barnacles, crustaceans, or arthropods. Both the nauplius and cypris larvae of rhizocephalans (Fig. 1 E and F) closely resemble the corresponding larval growth stages of barnacles. Rhizocephalans are not arthropods yet their development is explicable if an ancestor acquired larvae by hybridization with a barnacle. Cambrian nauplii, in my view, were adults of noncrustacean arthropods (3, 8), and the Cambrian arthropod Canadaspis (10) resembled a huge cypris larva.
Fig. 1.
A barnacle, a rhizocephalan, and their larvae. (A) The barnacle Balanus tintinnabulum in longitudinal section. (B) Nauplius and (C) cypris larvae of Balanus sp. (D) The rhizocephalan Sacculina carcini infesting the crab Carcinus maenas. Right side of crab shown transparent to illustrate ramifications of parasite. (E) Nauplius and (F) cypris larvae of Sacculina carcini. A after Darwin (7); B, C, E, and F after Williamson (8), with permission of Brill (publishers); D after Boas (9).
The 2 contrasting concepts to explain rhizocephalans can be experimentally distinguished. If rhizocephalans are parasitic barnacles, i.e., adults that lost all barnacle morphology by reduction yet their larvae retain virtually all features of larval barnacles, their genomes should be typical of cirripedes. Alternatively, if, as I suggest, rhizocephalans are not arthropods but acquired arthropod larvae by hybrid transfer, at least 3 genomes should be detected. Those that code for nauplius and cypris larvae should be similar to those in cirripedes, while the third “adult” genome should differ distinctively from that of cirripedes.
Caterpillars.
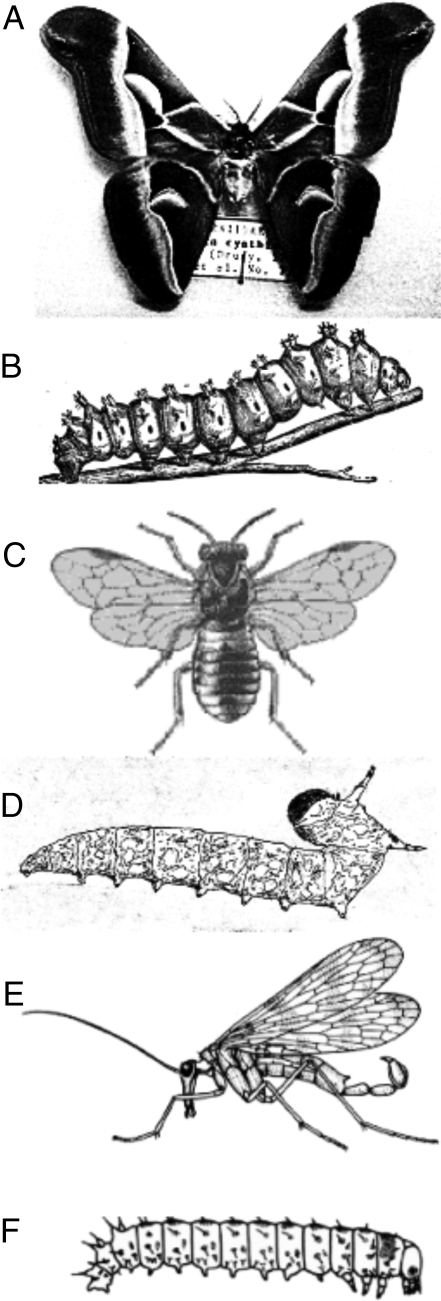
Caterpillar larvae are found in many insect orders. They usually have a pair of 3-jointed legs on each of the 3 thoracic segments and paired unjointed prolegs on some or all of the 10 abdominal segments. The prolegs are extended by hydrostatic pressure. Such larvae occur in some species of the insect orders Lepidoptera (butterflies and moths), Hymenoptera (ants, bees, wasps, and sawflies), and Mecoptera (scorpion flies and hanging flies). Caterpillars present many variations. Larvae of Trichoptera (caddisflies), Coleoptera (beetles), and some Hymenoptera lack abdominal prolegs. Nearly all butterflies and moths (Lepidoptera) have caterpillar larvae (Fig. 2 A and B), but larvae of the superfamily Nepticuloidea are apodous. Typical lepidopteran caterpillars have prolegs on abdominal somites 3–6 and 10, each with a flattened tip armed with a series of crochets. In the Geometridae, however, prolegs occur on somites 6 and 10 only, and in the Micropterigidae, the 3 pairs of thoracic appendages and 8 pairs of abdominal appendages of Micropterix (Fig. 3A) are similar prolegs, each ending in a single claw. The abdominal appendages are vestigial in other micropterigids, such as Epimartyria (12).
Fig. 2.
Insects with caterpillar larvae. (A and B) Lepidoptera: (A) Adult of the moth Samia cynthia. (B) Larva of Samia cecropia. (C and D) Hymenoptera: adult (C) and larva (D) of the sawfly Diprion similis. (E and F) Mecoptera: adult (E) and larva (F) of the scorpion fly Panorpa sp. (A from http://en.wikipedia.org/wiki/Samia_cynthia. B, C, and D adapted from photographs by Steven Katovich, USDA Forest Service. E and F from John R. Meyer, Department of Entomology, North Caroline State University, with permission.)
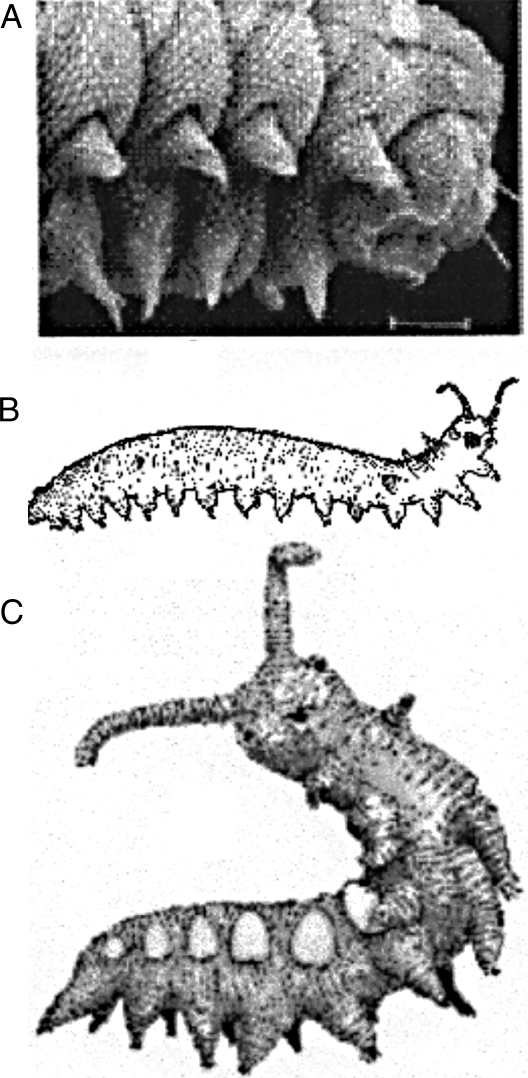
Fig. 3.
A caterpillar, an onychophoran, and a lobopod. (A) Caterpillar of moth Micropterix sp (Lepidoptera): anterior end, ventrolateral view. (B) Tasmanian onychophoran, Ooperipatellus sp. (C) Cambrian lobopod, Microdictyon sp. A from SEM by Donald R. Davis, National Museum of Natural History, Smithsonian Institution, Washington, DC, with permission; B from Tasmanian Multipedes website, http://www.qvmag.tas.gov.au/zoology/multipedes/mulintro.html, with permission; C from Brasier (11), with permission.
Hymenopterans of suborder Apocrita, which includes wasps, bees, and ants, have larvae that lack legs, prolegs, and ocelli. Larvae of suborder Symphyta, which includes sawflies, horntails, and woodwasps, have large compound eyes, 3 pairs of thoracic legs, and 6–10 pairs of abdominal prolegs without terminal crochets (Fig. 2 C and D). Compound eyes also occur in the 3 types of mecopteran larvae. Caterpillar-like larvae, with abdominal prolegs, occur in the mecopteran families Panorpidae (Fig. 2 E and F) and Bittidae. Larvae of the Panorpodidae lack abdominal prolegs and are scarabaeiform, i.e., they resemble the larvae of scarab beetles. The aquatic larvae of the Nannochoristidae look like the wireworm larvae of click beetles (Elateridae). The Meropeidae and Eomeropeidae probably have no larvae (13).
From these specific examples, it can be seen that a range of larvae exists from those with appendages on all thoracic and abdominal segments, through examples in which varying numbers of appendages have been lost, to maggots, without appendages. Loss of appendages, apparently independently, evolved several times, such that maggots are typical larvae of both social hymenopterans (bees and ants) and cyclorrhaphan dipterans (some flies). This scenario suggests that caterpillars first appeared with their full complement of appendages; they did not gradually evolve from legless ancestors. This favors larval genome acquisition (larval transfer) over the assumption of Darwinian gradual descent with modification.
Metamorphosis.
Insects mature in one of 3 ways. Ametabolous species develop gradually, without metamorphosis. Hemimetabolous species hatch as aquatic nymphs, which metamorphose directly into winged adults. Holometabolous species pass through a pupal phase of limited mobility between the last larval stage and the winged adult, and all insects with caterpillar larvae or derivatives fall in this category. In the pupa, the inner tissues and organs of the larva disintegrate to form a structureless “soup.” In most holometabolous insects, the cuticle, legs, wings, and nerves of the imago develop from imaginal discs formed in the last larval stage. The adult gut, digestive gland, and other internal organs grow from the pupal soup of dedifferentiated cells, i.e., cells that have returned to the stem cell stage. The imaginal discs of Drosophila and other cyclorrhaphan dipterans differ from those of most holometabolous insects in general shape and arrangement, and nearly all of the adult head and thorax is formed from them. Cyclorrhaphan dipterans uniquely have histoblasts, formed during embryonic life, which remain undifferentiated until the pupal phase, when they develop into most of the adult abdomen (14).
Metamorphosis in holometabolous insects is an example of “start again metamorphosis.” This term was originally applied to marine bryozoans (moss animalcules), in which all larval tissues and organs revert to stem cells, and the juvenile (miniature adult) grows from these stem cells (3). In holometabolous insects, imaginal discs and histoblasts play no part in larval development, and larval tissues undergo histolysis and cytolysis to produce “pupal soup.” No larval components or organs contribute directly to adult components or organs. A life history that involves dismantling a complex larva then starting again to differentiate an adult seems bizarre on the “descent with modification” assumption. I question the assumption that holometabolous insects evolved solely by lineal descent. Rather my hypothesis of larval transfer, i.e., that 1 or more hybridizations transferred caterpillar larvae to insects (3, 4), is more consistent with evidence of radical metamorphosis. Caterpillars and their apodous descendants differed too dramatically from adult insects into which they develop for gradual metamorphosis to have evolved by accumulation of mutations. Rather the pupal phase permitted metamorphosis by cellular dedifferentiation and redifferentiation.
Onychophorans and Lobopods.
Onychophorans or velvet worms are terrestrial “worms with legs,” and they show a combination of features of arthropods and annelids. The thin cuticle consists of α-chitin and various proteins, and they molt like arthropods. The appendages, however, are unjointed and are extended by hydrostatic pressure, as in parapodia of annelid. The excretory system and the musculature of onychophorans also resemble those of annelids. All species have 1 pair of antennae and a pair of oral papillae. Different species have from 13 to 43 pairs of stub feet. Specimens gain a pair of feet at the first molt in some species, and in others the males, with fewer feet, are smaller than females (15). Ooperipatellus (Fig. 3B) is a Tasmanian genus with 14 pairs of feet in both sexes. Onychophorans occur today in Central and South America, West and South Africa, East Asia, and Australasia, but specimens from Eocene amber suggest that they previously had a wider distribution (16).
Lobopods, e.g., Microdictyon (Fig. 3C), resembled onychophorans. Whittle et al. (17) give a table of known fossil lobopods from Lower Cambrian to Eocene, and they point out that “Helenodora and specimens found from the Cretaceous onwards have been interpreted as onychophorans.” Helenodora was described from the Upper Carboniferous beds of Mazon Creek, Illinois (18). Mazon Creek has yielded a mixture of marine, freshwater, and terrestrial fossils (19), implying that it may have been an estuary, where lobopods could have made the transition from aquatic to terrestrial.
The segmental dorsolateral sclerites of Microdictyon (Fig. 3C) may be homolgous to similarly placed spines of other fossil lobopods, such as Xenusion and Hallucinogenia, and the spines or clusters of spines of modern caterpillars (Fig. 2). No corresponding structures exist in extant onychophorans.
Discussion
Terrestrial onychophorans most likely evolved from aquatic lobopods by increased desiccation resistance. A succinct statement of my testable hypothesis is that “An onychophoran was the evolutionary ancestor of caterpillars.” Modern velvet worms in this view, are surviving relatives of the onychophoran that hybridized with an insect, which, as a result, acquired caterpillar larvae, perhaps in the late Carboniferous period, as suggested by Labandeira and Phillips (20) and Shear and Kukalova-Peck (19). This hybridization event occurred before lepidopterans became established as a taxon, and larvae resembling onychophorans occur in Micropterix (Fig. 2A), probably the most archaic of extant lepidopterans (21).
Neither Haeckel (2) nor Garstang (6) discussed insect larvae, but, under Haeckel's recapitulation theory, holometabolous insects would have evolved from adult caterpillars, and under Garstang's variant of it, holometabolous insects would have evolved from animals with caterpillar larvae. The sudden appearance of fully formed caterpillars is inexplicable under either theory. Onychophorans cannot be accepted as an example of Garstang's persistent larvae, because they evolved from marine lobopods that predated insects by hundreds of millions of years.
Many corollaries of my hypothesis are testable. If insects acquired larvae by hybrid transfer, the total base pairs of DNA of exopterygote insects that lack larvae will be smaller than those of endopterygote (holometabolous) species that have both larvae and pupae. Genome sequences are known for the fruitfly, Drosophila melanogaster, the honeybee, Apis mellifera, the malarial mosquito, Anopheles gambiae, the red flour beetle, Tribolium castaneum, and the silkworm, Bombyx mori: holometabolous species, with marked metamorphoses. I predict that an earwigfly (Mercoptera Meropeidae), an earwig (Dermaptera), a cockroach (Dictyoptera), or a locust (Orthoptera) will have not necessarily fewer chromosomes but will have fewer base pairs of protein-coding chromosomal DNA than have these holometabolans. Also the genome of an onychophoran that resembles extant species will be found in insects with caterpillar or maggot-like larvae. Onychophoran genomes will be smaller than those of holometabolous insects. Urochordates, comprising tunicates and larvaceans, present a comparable case. Larvaceans are tadpoles throughout life. Garstang (6) regarded larvaceans as persistent tunicate larvae, and, if so, their genomes would resemble those of tunicates. But if larvaceans provided the evolutionary source of marine tadpole larvae, their genomes would be smaller and included in those of adult tunicates. The genome of the larvacean Oikopleura dioica is about one-third that of the tunicate Ciona intestinalis, consistent with my thesis (22, 23).
I theorize that the first insect to acquire caterpillar larvae did so by hybridizing with an onychophoran, possibly in the Upper Carboniferous period. A laboratory hybridization between extant members of these groups would be of great interest. I urge biologists with access to live onychophorans to carry out such experiments. As an initial trial, it should be possible to attach an onychophoran spermatophore to the genital pore of a female cockroach and see if fertilized eggs are laid.
The origin of caterpillars, discussed here, is but one example of larval transfer. Dipluran (campodeiform) larvae provide another example from holometabolous insects, and I claim that the basic forms of all known larvae were later additions to animal life histories and were transferred from animals in other taxa (3). Balfour, in 1881, (24) deduced that virtually all larvae are “secondary.” He claimed they were “introduced” into the life histories of animals. He was unclear of the source of his secondary larvae, but he and I independently concluded that larvae had been introduced or transferred into ontogenies. That larvae have been “intercalated” into the life histories of mollusks and other lophotrochozoans was suggested by Page (25). She, too, independently concluded that larvae may have been introduced, transferred, or intercalated into the life histories of animals.
My larval transfer hypothesis was originally proposed as an explanation of anomalies in the development of marine invertebrates (26, 27), and only later expanded to cover all larvae (3, 4, 28, 29). The Biological Bulletin (30) devoted an entire issue to a “Virtual Symposium: Biology of Marine Invertebrate Larvae” this year. Although several symposium papers, including that of Page (25), discuss issues pertinent to larval transfer, apparently my hypothesis is not familiar to her. Many biologists appear unwilling to consider that animals with metamorphosing larva descended from hybrids, who evolved in separate lineages before interspecific, interfamilial, or even interphyletic hybridization. If larval transfer occurred the evolution of animals with larvae should be depicted by reticula rather than by dichotomous branching. Nature does not consider the convenience of taxonomists (3). Several contributors to the Virtual Symposium accept Lophotrochozoa as a taxon, but my theory posits both lophophores and trochophore larvae were later additions to life histories, so “Lophotrochozoa” in relation to animal evolution is an inappropriate taxon. I urge genomicists to test my larval transfer hypothesis by analysis of genomes (i) of animals with larvae, (ii) of related animals without larvae, and (iii) of relatives of the proposed adult source of the larval form.
The hypothesis of the onychophoran origin of caterpillar larvae and their apodous descendants provides a clear testable example of my larval transfer concept. Component transfer, by my hypothesis, also resulted from sexual hybridization and was an essential feature of the Cambrian explosion (11, 31). Both larval transfer and component transfer exemplify evolution by merger of genomes. So too does the origin of eukaryotic cells by symbiogenesis represent an example of acquisition of foreign genomes in the microbial world and its larger descendants (5, 32). These saltatory evolutionary processes differ from “Darwinian gradual descent with modification followed by natural selection,” but they are additions to and not replacements of Darwin's great insight into the history of life and the generation of its diversity.
Acknowledgments.
I wish to thank Celeste A. Asikainen, Barthold Bouricius, Martin Brasier, Andrew Fleming, Farley Fleming, Lynn Margulis, James MacAllister, Frank P. Ryan, Robert Sternberg, and Sonya Vickers for constructive contribution and Donald R. Davis, Bob Mesibov, and John R. Meyer for illustrations.
Footnotes
The author declares no conflict of interest.
References
- 1.Darwin C. On the Origin of Species by Means of Natural Selection or the Preservation of Favoured Races in the Struggle for Life. London, UK: John Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- 2.Haeckel E. Generelle Morphologie der Organismen: Algemeine Grundzügge der organischen Formen-Wissenschaft, mechanisch begründet durch die von Charles Darwin reformirte Descencendenz-Theorie. Berlin, Germany: Georg Reimer; 1866. [Google Scholar]
- 3.Williamson DI. The Origins of Larvae. Dordrecht, The Netherlands: Kluwer; 2003. [Google Scholar]
- 4.Williamson DI, Vickers SE. The origins of larvae: Mismatches between the forms of adult animals and their larvae may reflect fused genomes, expressed in sequence in complex life histories. Amer Sci. 2007;95:509–517. [Google Scholar]
- 5.Margulis L, Sagan D. Acquiring Genomes: A Theory of the Origins of Species. New York: Basic Books; 2002. [Google Scholar]
- 6.Garstang W. The theory of recapitulation: A critical re-statement of the Biogenic Law. Zool J Linnean Soc. 1922;35:81–101. [Google Scholar]
- 7.Darwin C. The Balanidae and Verrucidae. London, UK: Ray Society; 1854. A Monograph on the Sub-Class Cirripedia. [Google Scholar]
- 8.Williamson DI. The origins of crustacean larvae. In: Forest J, von Vaupel Klein JC, editors. Treatise on Zoology, The Crustacea. Vol 2. Leiden, The Netherlands: Brill; 2006. pp. 461–482. [Google Scholar]
- 9.Boas JEV. Lehrbuch der Zoologie für Studierende. Jena, Germany: Gustav Fischer; 1920. [Google Scholar]
- 10.Briggs D. The morphology, mode of life, and affinities of Canadaspis perfecta (Crustacea: Phyllocarida), Middle Cambrian, Burgess Shale, British Columbia. Phil Trans R Soc Lond B. 1978;281:439–487. [Google Scholar]
- 11.Brasier M. Darwin's Lost World: The Hidden History of Animal Life. Oxford: Oxford Univ Press; 2009. [Google Scholar]
- 12.Davis DR. Lepidoptera. In: Stehr FW, editor. Immature Insects. Vol 1. Dubuque, IA: Kendall/Hunt; 1987. pp. 288–596. [Google Scholar]
- 13.Byers GW, Thornhill R. Biology of the Mecoptera. Annu Rev Entomol. 1983;28:203–228. [Google Scholar]
- 14.Nijhout HF. Insect Hormones. Princeton: Princeton Univ Press; 1998. [Google Scholar]
- 15.Margulis L, Chapman MJ. Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth. Burlington, MA: Academic; 2009. [Google Scholar]
- 16.Poinar G. Fossil velvet worms in Baltic and Dominican amber: Onychophoran evolution and biogeography. Science. 1996;273:1370–1371. [Google Scholar]
- 17.Whittle RJ, Gabbott SE, Aldridge RJ, Theron J. An Ordovician lobopodian from the Soom Shale Lagerstätte. South Africa Palaeontology. 2009;52:561–567. [Google Scholar]
- 18.Thompson I, Jones DS. A possible onychophoran from the Middle Pennsylvanian Mazon Creek Beds of Northern Illinois. J Paleontol. 1980;54:588–596. [Google Scholar]
- 19.Shear WA, Kukalova -Peck J. The ecology of Paleozoic terrestrial arthropods: The fossil evidence. Can J Zool. 1990;68:1807–1834. [Google Scholar]
- 20.Labandeira CC, Phillips TL. A Carboniferous insect gall: Insight into early ecologic history of the Holometabola. Proc Natl Acad Sci USA. 1996;93:8470–8474. doi: 10.1073/pnas.93.16.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scoble MJ. The Lepidoptera: Form, Function and Diversity. Oxford: Oxford Univ Press; 1992. [Google Scholar]
- 22.Seo H-C, et al. Miniature genome in the marine chordate Oikopleura dioica. Science. 2001;294:2506–2516. doi: 10.1126/science.294.5551.2506. [DOI] [PubMed] [Google Scholar]
- 23.Dehal P, et al. The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- 24.Balfour FM. A Treatise on Comparative Embryology. 2 vols. London: Macmillan; 1880–81. [Google Scholar]
- 25.Page LR. Molluscan larvae: Pelagic juveniles or slowly metamorphosing larvae. Biol Bull. 2009;216:216–225. doi: 10.1086/BBLv216n3p216. [DOI] [PubMed] [Google Scholar]
- 26.Williamson DI. Incongruous larvae and the origin of some invertebrate life-histories. Progr Oceanogr. 1988;19:87–116. [Google Scholar]
- 27.Williamson DI. Larvae and Evolution: Toward a New Zoology. London: Chapman and Hall; 1992. [Google Scholar]
- 28.Williamson DI. Larval transfer in evolution. In: Syvanen M, Kado C, editors. Horizontal Gene Transfer. New York and London: Chapman and Hall; 1998. pp. 436–453. [Google Scholar]
- 29.Williamson DI. Larval transfer and the origins of larvae. Zool J Linnean Soc. 2001;131:111–122. [Google Scholar]
- 30.Emlet RB, et al. Biological bulletin virtual symposium: Biology of marine invertebrate larvae. Biol Bull. 2009;216:201–385. doi: 10.1086/BBLv216n3p201. [DOI] [PubMed] [Google Scholar]
- 31.Williamson DI. Hybridization in the evolution of animal form and life-cycle. Zool J Linnean Soc. 2006;148:585–602. [Google Scholar]
- 32.Margulis L. Symbiosis in Cell Evolution: Microbial Communities in Archean and Proterozoic Eons. San Diego: W H Freeman; 1993. [Google Scholar]