Abstract
In the recently halted HIV type 1 (HIV-1) vaccine STEP trial, individuals that were seropositive for adenovirus serotype 5 (Ad5) showed increased rates of HIV-1 infection on vaccination with an Ad5 vaccine. We propose that this was due to activation and expansion of Ad5-specific mucosal-homing memory CD4 T cells. To test this hypothesis, Ad5 and Ad11 antibody titers were measured in 20 healthy volunteers. Dendritic cells (DCs) from these individuals were pulsed with replication defective Ad5 or Ad11 and co-cultured with autologous lymphocytes. Cytokine profiles, proliferative capacity, mucosal migration potential, and susceptibility to HIV infection of the adenovirus-stimulated memory CD4 T cells were measured. Stimulation of T cells from healthy Ad5-seropositive but Ad11-seronegative individuals with Ad5, or serologically distinct Ad11 vectors induced preferential expansion of adenovirus memory CD4 T cells expressing α4β7 integrins and CCR9, indicating a mucosal-homing phenotype. CD4 T-cell proliferation and IFN-γ production in response to Ad stimulation correlated with Ad5 antibody titers. However, Ad5 serostatus did not correlate with total cytokine production upon challenge with Ad5 or Ad11. Expanded Ad5 and Ad11 memory CD4 T cells showed an increase in CCR5 expression and higher susceptibility to infection by R5 tropic HIV-1. This suggests that adenoviral-based vaccination against HIV-1 in individuals with preexisting immunity against Ad5 results in preferential expansion of HIV-susceptible activated CD4 T cells that home to mucosal tissues, increases the number of virus targets, and leads to a higher susceptibility to HIV acquisition.
Keywords: STEP trial, vaccine, alpha 4 beta 7
Replication-defective recombinant Ad5 vectors have been considered as potential vaccine delivery vehicles for HIV-1 (1). There were great expectations of the recent HIV-1 vaccine phase IIb clinical trial known as the STEP carried out in individuals at high risk of HIV infection. Because preexisting vector immunity was expected to blunt the effectiveness of vaccination, volunteers were divided into two groups based on their Ad5 antibody titers (less and above 200). The vaccine consisted of three first-generation replication defective Ad5 vectors expressing HIV-1 Gag, Pol, and Nef. In September 2007, the study was halted as the vaccine was declared ineffective. More worryingly however, individuals who received the vaccine were more susceptible to HIV-1 infection compared to the placebo arm, this was most significant in vaccinees with high antibody titers to the vector (2), [Robertson, http://www.hvtn.org/fgm/1107slides/Robertsonfinal.pdf, reviewed in (3, 4)].
There is an urgent need to understand the mechanisms underlying the increased HIV infection rate in Ad5-seropositive vaccinees to ascertain whether adenoviral or any viral-vector-based approach may ever be suitable for HIV vaccination. Natural Ad5 infection occurs via the nasopharynx or gut and replicates in epithelial cells of mucosal tissues, inducing mucosal immunity. Thus we hypothesized that vaccination of individuals immune to Ad5 with adenovirus vectors would activate and expand T cells expressing a mucosal homing phenotype, and these cells would migrate to the gut mucosa, increasing the number of permissive HIV-1 target cells and the risk of infection.
Results
Adenovirus-Specific Cytokine Responses Do Not Correlate with Ad5 Antibody Titers.
To investigate the relationship between Ad5 serostatus and Ad-specific cellular immune responses, Ad5 and Ad11 antibody titers were measured in 20 healthy volunteers (Fig. 2A). IFN-γ ELISPOT was performed on 15 of these donors (Fig. S1A). In 73.3% (mean response of 103.7 ± 30.8 SFCs/106 PBMCs) and 66.7% (mean response of 98.1 ± 46.7) of individuals, there were responses against Ad5 and Ad11, respectively. For comparison, responses against inactivated influenza were also measured. All individuals but one showed influenza-specific IFN-γ ELISPOT responses (mean response of 820.2 ± 261.7). To determine whether Ad5 serostatus is a surrogate for Ad5- or Ad11-induced IFN-γ production by T lymphocytes, Ad5 and Ad11 IFN-γ responses were plotted against Ad5 antibody titers (Fig. S1B). We observed no significant correlations between Ad5 or Ad11 IFN-γ ELISPOT responses and Ad5 antibody titers. Furthermore, there was no correlation between responses generated against Ad5 and Ad11 (Fig. S1C). To identify which cell populations were responsive to Ad5 and Ad11, we undertook multiparameter flow cytometric analysis for the detection of intracellular IFN-γ, IL-2, and TNF-α. Dendritic cells (DCs) from all 20 donors were pulsed with Ad5, Ad11, tetanus toxoid, heat-inactivated influenza, or Staphylococcus enterotoxin B (SEB), and cytokines were measured in lymphocytes. The majority of the IFN-γ response against Ad5 and Ad11 was mediated by CD8 T cells (means of 0.149 ± 0.04 and 0.216 ± 0.06%, respectively) in comparison to CD4 T cells (means of 0.043 ± 0.001%, P = 0.043 and 0.058 ± 0.01, P = 0.078, respectively) (Fig. S1D). Similarly, CD8 T cells produced significantly more TNF-α in response to Ad5 or Ad11 (means of 0.211 ± 0.04, P = 0.002 and 0.257 ± 0.05, P = 0.016, respectively) (Fig. S1D). In contrast, we detected no or very little IL-2 production in response to Ad5 and Ad11 by either CD4 or CD8 cells (means of 0.028 ± 0.005, 0.044 ± 0.025 and 0.029 ± 0.008, 0.033 ± 0.008, respectively) (Fig. S1D). Furthermore, we observed no significant correlation between Ad5 serostatus and IL-2 or TNF-α production in response to Ad5 or Ad11, while a moderate but statistically significant correlation was seen between CD4 T-cell IFN-γ production and Ad5 antibody titers (r = 0.547, P = 0.012, Spearman test) (Fig. S2).
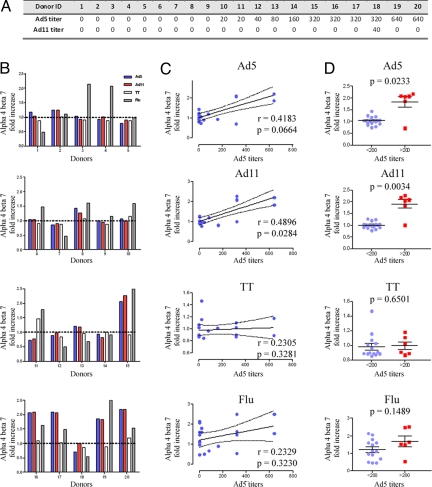
Fig. 2.
The expression of α4β7 in response to Ad5 and Ad11 correlates with Ad5 titers. (A) Ad5 and Ad11 antibody titers of 20 donors were measured as indicated in Materials and Methods. (B) T lymphocytes from donors were co-cultured with unpulsed DC, or Ad5, Ad11, tetanus toxoid, or influenza-pulsed DCs. The specific increase in α4β7 expression by CD4 T cells in response to Ad5 (blue bars), Ad11 (red bars), tetanus toxoid (white bars), or influenza (shaded bars) were divided by the background expression of α4β7 of T cells that were cultured with unpulsed DCs. (C) The fold increases in α4β7 expression by T cells in response to Ad5, Ad11, tetanus toxoid or influenza were plotted against the respective Ad5 antibody titers. R and P values were obtained using the nonparametric Spearman test. (D) The fold increases in α4β7 expression in response to Ad5, Ad11, tetanus toxoid, or influenza were stratified by Ad5 titers (<200 and >200). Plots are shown with lines representing means ± SD whereas P values were obtained using the Mann Whitney U test.
The functional quality of the responding Ad5 and Ad11-specific CD4 T cells is depicted in Fig. 1E. No significant differences were found between Ad5- and Ad11-responding CD4 T cells in terms of cytokine profiles with cells predominantly producing TNF-α alone, or in combination with IFN-γ or IL-2. Cytokine-producing CD8 T cells exhibited almost identical profiles in response to Ad5 or Ad11. The majority of responding cells produced IFN-γ, TNF-α, or both, indicating an effector phenotype (Fig. S1E).
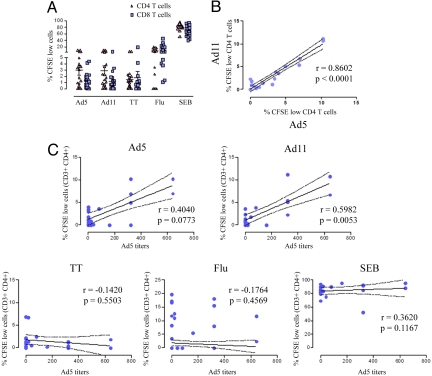
Fig. 1.
Ad-induced T-cell expansion correlates with Ad5 serostatus. (A) CFSE-labeled lymphocytes from 20 individuals were co-cultured for 5 days with Ad5, Ad11, tetanus toxoid, influenza, or SEB-stimulated DCs. The mean percentages ± SD of proliferating (CFSE low) CD4 (triangles) and CD8 (squares) T cells are shown. (B) The percentages of proliferating CD4 T cells in response to Ad5 stimulation (x axis) were plotted against those in response to Ad11-pulsed DCs (y axis). P and R values were obtained using the spearman correlation test. (C) The percentages of expanded Ad5, Ad11, tetanus toxoid, influenza, and SEB-specific CD4 T cells (y axis) were plotted against the individuals' Ad5 antibody titers (x axis). Continued and dotted lines represent the best fit line and 95% confidence intervals respectively, whilst R and P values were obtained using the Spearman test.
Adenovirus-Induced T-Cell Proliferation Correlates with Ad5 Antibody Titers.
We next addressed the ability of Ad vectors to induce T-cell expansion ex vivo. DCs were pulsed with Ad5, Ad11, tetanus toxoid, influenza, or SEB then co-cultured with autologous CFSE-stained T cells. Unlike cytokine production, which was mainly mediated by CD8 T cells, the majority of proliferating T cells in response to Ad5 or Ad11 stimulation were CD4 T cells (means of 2.942 ± 0.748, and 2.901 ± 0.754%, respectively) in comparison to just 1.360 ± 0.275 and 1.213 ± 0.367 of proliferating (CFSE low) CD8 T cells (Fig. 1A and Fig. S3). No major differences were seen between the percentages of expanded CD4 and CD8 T lymphocytes in response to tetanus toxoid and influenza (Fig. 1A). We observed a strong correlation between Ad5 and Ad11-mediated CD4 T-cell proliferation (Spearman r = 0.8602, P < 0.0001) (Fig. 1B), likely due to conserved CD4 epitopes in viruses from serogroups C and B. There was a trend toward a positive correlation between Ad5- or Ad11-induced CD4 T lymphocyte proliferation and Ad5 serostatus (r = 0.404, P = 0.077 and r = 0.598, P = 0.005 respectively) (Fig. 1C) that was statistically significant when Ad5 titers >200 were analyzed (Fig. S4). As expected, CD4 T-cell expansion in response to tetanus toxoid, influenza, or SEB was unrelated to the individuals' Ad5 titers (Fig. 1C).
α4β7 Expression by Expanded Adenovirus-Specific Memory CD4 T Cells.
Lymphocyte migration to gastrointestinal tissues is dependent on the expression of a heterodimer comprising of α4 and β7 integrins that bind to MAdCAM-1 on endothelial cells in the gastrointestinal tract (5–9). We therefore measured expression of these molecules on expanded CFSE low CD4 T cells co-cultured for 5 days with Ad5, Ad11, second-generation Ad5 vector, influenza, tetanus toxoid, or SEB-pulsed DCs. Representative profiles are shown in Fig. S5, whereas Fig. S6 shows the cumulative results from a total of 14 out of 26 samples (four samples from buffy coats for which we did not have neutralizing antibody data) that exhibited a proliferative response against adenovirus stimulation. The majority of the expanded Ad5-specific CD4 T cells co-expressed α4 and β7 (means of 71.5 ± 3.6%) (Fig. S6), whereas undivided cells (CFSE high) CD4 T cells exhibited minimal expression levels (Fig. S5). Moreover, stimulation with Ad11 or second-generation Ad5 (n = 4), considered to produce less adenovirus-encoded protein than first-generation vectors, resulted in a similar expansion of α4+ β7+ CD4+ T cells (means of 72.1 ± 3.3 and 78.8 ± 12.2%, respectively). Results with the second-generation vector suggest that low levels of newly synthesized adenovirus proteins were not augmenting stimulation. Conversely, CD4 T lymphocytes that proliferated in response to un-pulsed or SEB-stimulated DCs showed a higher expression of α4 but not β7 (Fig. S5). Since SEB stimulation results in proliferation of a heterogeneous population of naive and memory T cells, while responses against Ad5 or Ad11 would presumably be mediated by memory CD4 T cells, we repeated the SEB stimulations using purified memory CD4 T cells (n = 2). In both samples tested, memory CD4 T cells that proliferated against SEB resulted in higher expression of α4 but not β7 (Fig. S7), indicating that the lower levels of β7 expression were not skewed by naive T-cell expansion.
Memory CD4 T lymphocytes that proliferated in response to influenza exhibited a mean percentage of cell expressing α4β7 of 58.72 ± 4.50. Although this was lower than Ad5 or Ad11-induced α4β7 expression, it was not statistically significant (Fig. S6). However, proliferating T cells against tetanus toxoid exhibited significantly lower expression levels of α4β7 (mean percentage of 41.7 ± 5.9) (Fig. S6). Taken together, this suggests that antigens originally encountered through mucosal surfaces, adenovirus, and influenza, expanded memory CD4 T cells with a mucosal homing phenotype, while tetanus toxoid, which most individuals initially encounter as a systemic intramuscular vaccine, expanded memory CD4 T cells that are predominantly negative for mucosal homing markers.
Adenovirus-Induced α4β7 Increases Correlate with Ad5 Titers.
We next investigated the relationship between increased α4β7 expression by Ad-specific memory CD4 T cells and preexisting Ad5 neutralizing antibodies. For this purpose, we determined the fold increases in α4β7 expression by total antigen-stimulated CD4 T cells in relation to background expression levels of unstimulated CD4 T lymphocytes as the latter varied considerably among the donors tested (Fig. 2B). We observed a positive correlation between the fold increases in total CD4 T lymphocytes expressing α4β7 in response to Ad5 or Ad11 and Ad5 antibody titers (P = 0.066 and P = 0.028, respectively) (Fig. 2C). Conversely, no correlations with neutralizing antibody to Ad5 were observed when tetanus toxoid or influenza was used as a stimulus (Fig. 2C). Furthermore, when data were stratified by Ad5 titers as per the STEP study (<200 or >200), individuals with Ad5 titers >200 showed significantly higher percentages of CD4 T cells expressing α4β7 in comparison to donors with Ad5 titers <200 in response to Ad5 and Ad11 stimulation (P = 0.023, and P = 0.003, respectively) (Fig. 2D). This finding was specific to adenoviral challenge as no differences were seen between the two groups when tetanus toxoid or influenza was used as a stimulus (Fig. 2D).
To further address the possibility that the observed increases in α4β7 expression was an experimental artifact, we plotted α4β7 fold increases against the percentages of proliferating cells in response to the different antigens utilized in this study. As can be seen in Fig. S8, there was a positive and significant correlation between Ad5-, Ad11-, and influenza-induced proliferation and α4β7 expression, while tetanus toxoid-mediated expansion was unrelated to α4β7 increases, indicating that expression of these molecules was restricted to Ad5, Ad11, and flu-specific memory CD4 T cells rather than a non-specific property of proliferating cells.
CCR9 and CCR5 Upregulation in Response to Ad5 and Ad11 Challenge.
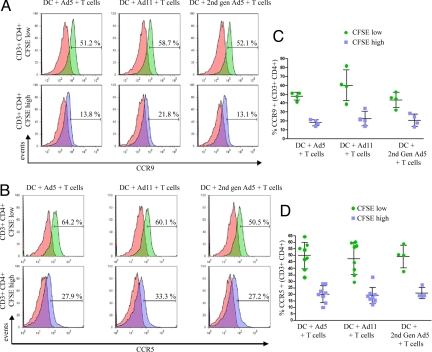
Migration to mucosal tissues is also facilitated by the chemokine receptor CCR9 in response to its ligand CCL25 which is constitutively expressed in the small intestine (10, 11). We examined the levels of CCR9 expression by activated adenoviral-specific memory CD4 T cells in 4 Ad5 responders by flow cytometry. There were significantly increased levels of surface CCR9 in cells expanded by stimulation with first- and second-generation Ad5 vectors and Ad11 vectors in comparison to non-proliferating T cells (Fig. 3 A and C). CCR5 expression is upregulated by T cells with effector/memory phenotype and Th1 cells (12, 13) and is expressed on gut mucosal CD4 T cells (14). As CCR5 serves as a co-receptor for HIV-1 entry, we investigated CCR5 expression on expanded adenovirus-specific memory T cells. CCR5 was upregulated on divided CFSE-low CD4 T cells when cultured with first- or second-generation Ad5, or Ad11-pulsed autologous DCs in comparison to CFSE-high undivided T lymphocytes (Fig. 3 B and D). However as expected, unlike α4β7 receptor, which was expressed by a greater number of adenovirus-specific T cells, increased CCR5 expression by CD4 T cells was seen in response to tetanus toxoid, influenza virus, and SEB (Fig. S9).
Fig. 3.
CCR5 and CCR9 expression by expanded Ad-specific CD4 T cells. CFSE-labeled T cells were co-cultured with Ad5, Ad11, or second-generation Ad5-pulsed DCs. CCR9 (A) and CCR5 (B) expression by divided CFSElow (green histograms) or undivided CFSEhigh CD3+ CD4+ T lymphocytes (blue histograms) was measured by flow cytometry. Red histograms represent the non-specific staining using the appropriate isotype controls. Plots are representative of four samples for CCR9 and eight samples for CCR5. (C and D) The cumulative data for CCR9 and CCR5, respectively, are shown with lines representing means ± SD.
Re-Stimulated Adenovirus-Specific CD4 T Cells Are More Permissive to HIV-1 Infection.
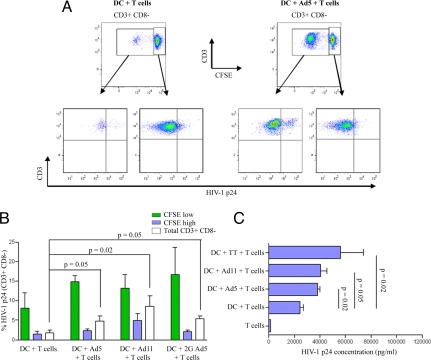
Since expanded adenovirus-specific memory CD4 T cells express higher levels of CCR5, the co-receptor required for infection by R5 strains of HIV-1 (15, 16), we tested whether these cells were susceptible to HIV-1 infection. DCs from buffy coats (n = 4) that showed a proliferative response against Ad5 were pulsed with first- or second-generation Ad5 or Ad11 and co-cultured with autologous lymphocytes for 3 days; they were then infected with the R5 virus, HIV-1BAL, for an additional 4 days. Infection was monitored by intracellular staining for p24 and by ELISA for p24 in the supernatant. As can be seen in Fig. 4 A and B, intracellular staining for HIV-1 p24 was significantly higher in the proliferating CFSE-low CD4 T cells compared to undivided CFSE-high cells. ELISA showed significantly higher levels of p24 in adenovirus stimulated cultures (Fig. 4C). Furthermore, we observed similar levels of HIV-1 p24 in culture supernatants from Ad5- or Ad11-stimulated lymphocytes.
Fig. 4.
HIV-1BAL preferentially infects expanded Ad-specific CD4 T cells. CFSE-stained lymphocytes were co-cultured with autologous DCs that were unpulsed or pulsed with Ad5, Ad11, or second-generartion Ad5 for 3 days. Cells were then cultured in the presence of infectious HIV-1BAL for an additional 4 days. HIV-1 infection of T helper cells was then measured by intracellular labeling of CD3+ CD8− cells for p24 gag. The gating strategy to identify HIV-1 infected Ad-specific CD4 T cells is shown in (A) where quadrants were based on p24 stained uninfected T cells subjected to the same conditions. (B) The mean percentages of HV-1 p24 positive divided CFSElow (green bars), undivided CFSEhigh (blue bars), or total CD3+ CD8− cells (white bars) are shown (n = 4). Error bars, SEM; P values were obtained using the Mann Whitney U test. (C) Lymphocytes from 4 Ad5-responders were either cultured alone or co-cultured with unstimulated or Ad5, Ad11, or tetanus toxoid-stimulated autologous DCs for 3 days. Cells were then infected with HIV-1BAL for an additional 7 days. HIV-1 p24 levels were then measured in cell culture supernatants by ELISA as indicated in Materials and Methods. The means are given; error bars, SEM. P values were obtained using the Mann Whitney U test.
Discussion
Recent reports showed no correlation between Ad5 serostatus and Ad5 stimulated cytokine production suggesting that activation of adenovirus-specific memory cells could not explain the increased susceptibility to HIV infection in the STEP trial (17–19). We confirmed these observations as measured by total IFN-γ ELISPOTS and by flow cytometry and found no correlation between Ad5 neutralizing antibody and IL-2 and TNF-α production. However, by flow cytometry, we observed a moderate but significant correlation between IFN-γ secretion by CD4 T cells and Ad5 antibody. This discrepancy may reflect the use of antigen-pulsed DC versus PBMC stimulations.
In contrast to cytokine responses, CD4 T-cell proliferation stimulated by Ad5 or Ad11 correlated with Ad5 antibody titers, particularly in individuals with Ad5 neutralizing antibody titers above 200. In an early study, Chirmule et al. reported an association between CD4 T-cell proliferation and Ad5- neutralizing antibodies in 74 donors (20). We observed a highly significant association between CD4 T-cell expansion in response to Ad5 and Ad11, most likely reflecting conserved CD4 T-cell epitopes across the two sero subgroups (21–23). Given that there are 51 known human strains of adenovirus, one might expect most individuals to have encountered at least one strain and thus CD4 memory T cells from Ad5-seronegative individuals may be expected to proliferate in response to conserved epitopes on Ad5. Although we do not have data to explain this observation it could be due to differences in immunodominance hierarchy such that conserved epitopes may not be the most immunodominant in all strains of adenovirus. This would also be influenced by HLA composition. Also of relevance to this question are observations of clear differences between Ad5-seronegative subjects (who may be seropositive for other Ad subtypes) and donors with preexisting Ad5 immunity with regards to NF-κB and cell death pathways and IRF1/IRF1-binding sites as revealed by gene microarrays. In addition, the finding of higher levels of activated CD4 T cells in Ad5-seropositive than in Ad5-seronegative individuals who received the saline placebo in the STEP trial (24, Zak et al., www.hivvaccineenterprise.org/conference/archive/2008/Presentations/Wednesday/Oral%20Abstract%2009/1330%20DE%20Zak.pdf),* indicates possible genetic characteristics that are specific for Ad5-seropositive individuals.
We show that memory CD4 T cells expanded by adenovirus stimulation exhibited elevated expression of the gut-homing molecules α4β7 and CCR9 with expression of α4β7 being significantly higher in individuals with Ad5 antibody titers greater than 200. There is no commercially available antibody that recognizes this dimer in humans, and we therefore used separate antibodies against the two subunits. However, the β7 integrin is only known to dimerize with α4 or αE subunits, both of which have been associated with T-cell trafficking or retention in mucosal sites (8, 25), and thus our approach should identify mucosal homing cells.
In the current investigation, we also show that Ad5 or Ad11 re-stimulated memory CD4 T cells are more susceptible to HIV-1 infection than unstimulated cells. HIV-1 preferentially replicates in activated T cells (26), and therefore, CD4 T cells activated in vitro to any antigen could be infected by HIV. However, our key observation with respect to the STEP trial is that Ad5-activated memory cells would home to mucosal tissue, the site of HIV transmission. In a recent study by Fauci's group (27), it has been shown that the α4β7 receptor binds to HIV-1 gp120 and may serve as a co-receptor for HIV-1 uptake. Thus, it is plausible that the increased expression of α4β7 by re-challenged Ad5 memory CD4 T cells may have contributed toward their increased susceptibility to HIV-1 infection.
Other hypotheses have been put forward to explain the STEP trial outcome, including a report from Perreau et al. demonstrating in in vitro studies that Ad5 immune complexes activate DCs that in turn would promote Ad5-specific CD4 T-cell activation and a higher susceptibility to HIV-1 infection (28). However, vaccine recipients who were Ad5-seronegative at baseline would have developed anti Ad5 neutralizing antibodies after the first immunization. Since the majority of volunteers in the STEP study received all three immunizations, all vaccinees would have become Ad5-seropositive regardless of their baseline serostatus and shown increased rates of HIV infection.
In the Merck vaccine trial individuals with neutralizing Ad5 antibody titers of less than 18 showed no increase in infection rates compared with the placebo group (2, Robertson, http://www.hvtn.org/fgm/1107slides/Robertsonfinal.pdf). Given that in the vaccine group these individuals would have been expected to become strongly Ad5-positive after three intramuscular vaccinations, why did they not show increase risk of infection? Naturally acquired adenovirus replicates in epithelial cells in mucosal tissue and would induce CD4 T cells that home to the site of infection. On re-exposure to adenovirus, expansion and activation of a mucosal homing CD4 memory population would occur, increasing in the number of potential target cells at the site of infection and increasing the incidence of infection. The vaccine was given by intramuscular injection and in individuals with very low or no immunity to adenovirus, this would lead to the generation of activated CD4 cells that are susceptible to HIV-1 infection but which do not home to mucosal tissue and thus should not increase the risk of infection. Thus a testable prediction of this hypothesis is that in vitro stimulation with adenovirus vectors of PBMCs from vaccinated individuals who had no preexisting neutralizing antibodies for Ad5 would cause expansion of CD4 T cells expressing low levels of mucosal homing markers. Interestingly in vaccines, Ad5-specific memory CD4 T cells were found to exhibit lower levels of α4β7 in individuals seronegative for Ad5 at baseline compared to those that were Ad5-seropositive before vaccination (18). Immunological analysis of the STEP trial showed that the percentage of activated adenovirus-specific CD4 T cells in the blood was lower in the group with preexisting immunity to Ad5 (19; Robertson, CROI 2008, http://www.retroconference.org/2008/data/files/retro2008_frameset.html) and may reflect recruitment of Ad5-specific mucosal CD4 T cells to mucosal tissues, as suggested by this study. Antigen-induced CD4 T-cell activation is transient and fades as the antigen disappears; however, there was increased susceptibility to infection in the Ad-seropositive group for several months. This may reflect known persistence of adenovirus, which is reported to last for several years, thus causing more prolonged stimulation (29). Additionally, Tatsis et al. demonstrated that replication-incompetent Ad5 vectors, such as the one used in the STEP trial, persist over a year in vaccinated mice (30).
Finally, our data also suggests that because there are conserved CD4 epitopes in different adenovirus serotypes, the Ad vaccination problem may not be overcome by vectors derived from rarer serotypes. As proposed above, analysis of the mucosal homing phenotype of Ad5 vaccinated, Ad5-seronegative and -seropositive individuals may provide further relevant data. Our findings suggest a cautious approach in the development of adenovirus virus vector vaccines for HIV.
Materials and Methods
A detailed description of materials and methods is given in the SI Materials and Methods but is briefly as follows:
Vectors and Virus Neutralizing Antibody.
First-generation E1- and E3-deleted Ad5, E1-deleted Ad11, and less “leaky” second generation E1-, E3-, polymerase-, and pTP-deleted Ad5 vectors all carrying the gene for green fluorescent protein (GFP) were propagated in packaging cell lines and purified by CsCl gradient centrifugation. Titers of neutralizing antibody to Ad5 and Ad11 were determined in 20 healthy volunteers by adding vectors [1,000 virus particles (vp)/cell] treated with doubling dilutions of serum for 30 min to A549 cells and assessing infection after 48 h.
Proliferation of Ad-Specific Memory T Cells.
Monocytes were isolated from the blood of healthy volunteers with CD14 immunomagnetic beads and used to generate DCs by culture with GM-CSF and IL-4. After 7 days, DCs were pulsed with 2,500 vp/cell of first-generation Ad5 or second-generation Ad5 or Ad11 vectors and co-cultured with carboxyfluorescein succinimidyl ester (CFSE)-labeled autologous lymphocytes for 5 days. At the end of culture, cells were labeled with fluorescently labeled antibodies to CD3, CD4, CD8, CD11c, CD195 (CCR5), CD49d (α4), β7, and CCR9 and analyzed by flow cytometry. Responses to other antigens including tetanus toxoid, influenza and the super antigen SEB were also assessed. Susceptibility to HIV infection was determined by culturing CFSE-labeled lymphocytes cells with Ad vector-pulsed DCs for 3 days, infecting with HIV-1 Bal, and culturing for a further 4–7 days. Cells were then labeled for cell surface antigens, fixed, stained for HIV p24, and analyzed by flow cytometry. Supernatant was analyzed for p24 by ELISA.
Cytokine Analysis.
DCs were pulsed for 3 h with Ad vectors or other antigens described above, matured with LPS for 3 h, and co-cultured with autologous lymphocytes for 24 h. Brefeldin A was added for the last 16 h and then cells were fixed, labeled for IFN-γ, IL-2, or TNF-α, and analyzed by flow cytometry using the gating strategy indicated in Fig. S10. Antigen-pulsed, DC-stimulated cells were also analyzed for IFN-γ secretion by ELISPOT.
Supplementary Material
Acknowledgments.
We thank Drs. Adriano Boasso, Nick Mathews, and Philip Bergin for their intellectual input and Dr. Mario Roederer for providing Simulation Program with Integrated Circuit Emphasis (SPICE) and Pestle software. This work was conducted as part of the Collaboration for AIDS Vaccine Discovery with support from the Bill and Melinda Gates Foundation and The Stephens Trust, Chelsea and Westminster Hospital.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907898106/DCSupplemental.
Zak DE, AIDS Vaccine 2008, October 18, 2008, Cape Town, South Africa.
References
- 1.Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomized, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu S. Human versus HIV: Round 2 defeat in AIDS vaccine development. Expert Rev Vaccines. 2008;7:151–153. doi: 10.1586/14760584.7.2.151. [DOI] [PubMed] [Google Scholar]
- 4.Sekaly RP. The failed HIV Merck vaccine study: A step back or a launching point for future vaccine development? J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlin C, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 6.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 7.Hesterberg PE, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin α4β7. Gastroenterology. 1996;111:1373–1380. doi: 10.1053/gast.1996.v111.pm8898653. [DOI] [PubMed] [Google Scholar]
- 8.Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev. 2007;215:226–242. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 9.Wagner N, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 10.Kunkel EJ, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zabel BA, et al. Human G protein-coupled receptor GPR-9–6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–1256. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037–2045. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 14.Agace WW, et al. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur J Immunol. 2000;30:819–826. doi: 10.1002/1521-4141(200003)30:3<819::AID-IMMU819>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 15.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 16.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: Fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 17.Koup RA, et al. Replication-defective adenovirus vectors with multiple deletions do not induce measurable vector-specific T cells in human trials. J Virol. 2009;83:6318–6322. doi: 10.1128/JVI.00384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutnick NA, et al. Baseline Ad5 serostatus does not predict Ad5 HIV vaccine-induced expansion of adenovirus-specific CD4(+) T cells. Nat Med. 2009;15:876–878. doi: 10.1038/nm.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien KL, et al. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. NatMed. 2009;15:873–875. doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chirmule N, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 21.DiPaolo N, et al. Evaluation of adenovirus vectors containing serotype 35 fibers for vaccination. Mol Ther. 2006;13:756–765. doi: 10.1016/j.ymthe.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- 23.Onion D, et al. The CD4+ T-cell response to adenovirus is focused against conserved residues within the hexon protein. J Gen Virol. 2007;88:2417–2425. doi: 10.1099/vir.0.82867-0. [DOI] [PubMed] [Google Scholar]
- 24.McElrath MJ, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: A case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw SK, Brenner MB. The beta 7 integrins in mucosal homing and retention. Semin Immunol. 1995;7:335–342. doi: 10.1016/1044-5323(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arthos J, et al. HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 28.Perreau M, Pantaleo G, Kremer EJ. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J Exp Med. 2008;205:2717–2725. doi: 10.1084/jem.20081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garnett CT, Erdman D, Xu W, Gooding LR. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002;76:10608–10616. doi: 10.1128/JVI.76.21.10608-10616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatsis N, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: Implications for their use as vaccines. Blood. 2007;110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.