Abstract
Restricting transgene expression to maturing erythroid cells can reduce the risk for activating oncogenes in hematopoietic stem cells (HSCs) and their progeny, yet take advantage of their robust protein synthesis machinery for high-level protein production. This study sought to evaluate the feasibility and efficacy of reprogramming erythroid cells for production of a lysosomal enzyme, α-L-iduronidase (IDUA). An erythroid-specific hybrid promoter provided inducible IDUA expression and release during in vitro erythroid differentiation in murine erythroleukemia cells, resulting in phenotypical cross-correction in an enzyme-deficient lymphoblastoid cell line derived from patients with mucopolysaccharidosis type I (MPS I). Stable and higher than normal plasma IDUA levels were achieved in vivo in primary and secondary MPS I chimeras for at least 9 months after transplantation of HSCs transduced with the erythroid-specific IDUA-containing lentiviral vector (LV). Moreover, long-term metabolic correction was demonstrated by normalized urinary glycosaminoglycan accumulation in all treated MPS I mice. Complete normalization of tissue pathology was observed in heart, liver, and spleen. Notably, neurological function and brain pathology were significantly improved in MPS I mice by erythroid-derived, higher than normal peripheral IDUA protein. These data demonstrate that late-stage erythroid cells, transduced with a tissue-specific LV, can deliver a lysosomal enzyme continuously at supraphysiological levels to the bloodstream and can correct the disease phenotype in both viscera and CNS of MPS I mice. This approach provides a paradigm for the utilization of RBC precursors as a depot for efficient and potentially safer systemic delivery of nonsecreted proteins by ex vivo HSC gene transfer.
Keywords: hematopoietic stem cells, neurological function, gene therapy, lysosomal storage diseases, RBC precursors
Mucopolysaccharidosis type I (MPS I) or Hurler syndrome, a common lysosomal storage disease (LSD), is caused by defective α-L-iduronidase (IDUA) (EC3.2.1.76) and consequent systemic accumulation of the unprocessed glycosaminoglycans (GAGs) (1). Clinical features in patients with MPS I include cardiac, hepatic, and soft tissue defects as well as CNS abnormalities in severely affected patients who would die by the age of 10 years if untreated. Allogeneic hematopoietic stem cell (HSC) transplantation (BMT) from healthy donors provides therapeutical benefits, including prolonging life and improving some of the visceral manifestations, by metabolic cross-correction from intercellular enzyme transfer (2). Furthermore, BMT early in life (<2 years) leads to significant improvement in CNS outcomes, even though minimal or no response has been obtained for the reversal of preexisting CNS abnormalities (2–5). Despite these benefits, allogeneic transplantation is limited by a procedure-related mortality rate between 20–30%, late complications such as graft-versus-host disease, and the need to find an HLA-matched donor. A pharmaceutical IDUA product is now available and is being used to ameliorate visceral manifestations of MPS I in some patients. However, it is limited by likely poor penetration to the CNS, the need for frequent i.v. infusions for a lifetime, and tremendous costs. A therapeutical approach with lower mortality and morbidity, and with the capacity to correct CNS deterioration, is needed.
Ex vivo HSC gene transfer followed by autologous transplantation is an attractive alternative for LSD treatment that could provide lifelong therapeutical effects without the morbidity and mortality of allogeneic transplantation. Previously, we showed the feasibility of transducing human primitive hematopoietic progenitors from MPS I patients (6). However, in general, the frequencies of transduced and successfully engrafted HSCs have been low in gene therapy clinical trials. Strong in vivo selection pressure for genetically corrected cells appears to be necessary to obtain clinically relevant long-term functional corrections, as demonstrated in gene therapy trials for children with inherited immunodeficiencies (7–10). Unfortunately, inadvertent activation of cellular proto oncogenes by provirus insertion resulted in secondary leukemogenesis in two otherwise successful clinical trials (11–13). In addition, selective advantage is not available for most other diseases.
Restricting transgene expression to late erythroblasts and RBCs may reduce the risk for activating oncogenes in HSCs and their offspring in all lineages (14, 15). Moreover, healthy individuals can produce 2.4 × 1011 RBCs/d with a daily output of 7.2 g hemoglobin. Redirecting a portion of the formidable protein synthesis machinery in maturing erythroid cells toward the expression of a transgene may provide an efficient approach for long-term protein delivery to the circulation. The high efficiency of protein synthesis may compensate for the generally low HSC gene transfer frequency. Recent studies by Chang et al. (16) demonstrated the feasibility of long-term secretion of therapeutical levels of human factor IX in plasma from HSC-derived erythroid cells using the human β-globin promoter/enhancer. Our previous work has identified an ankyrin-1–based erythroid-specific hybrid promoter/enhancer (IHK) that could introduce high erythroid-specific expression in vivo in primary and secondary murine BMT recipients (17).
In the present study, we hypothesize that maturing erythroid cells derived from genetically modified HSCs can provide long-term systemic production of IDUA and lead to phenotypic cross-correction in an animal model of MPS I. Using lentiviral vectors (LVs), we demonstrated that the IHK promoter/enhancer provided inducible lysosomal IDUA expression and release during in vitro erythroid differentiation in murine erythroleukemia (MEL) cells, resulting in cross-correction in lymphoblastoid cells derived from an MPS I patient (LCLmps). We further showed in vivo that stable and higher than normal IDUA activity levels could be achieved in the plasma of primary or secondary MPS I chimeras for at least 9 months after transplantation of MPS I HSCs transduced with an IDUA-containing erythroid-specific LV. Long-term systemic metabolic correction and complete normalization of visceral pathology were attained in MPS I mice 5 months after treatment. Moreover, significant improvement of neurological function and brain pathology were achieved in MPS I mice by the erythroid-derived higher than normal peripheral IDUA protein. This approach would provide a paradigm for the utilization of RBC precursors as a depot for efficient and systemic delivery of proteins that are not conventionally secreted.
Results
Inducible IDUA Expression and Enzyme Release from IHK Promoter During in Vitro Erythroid Differentiation in MEL Cells.
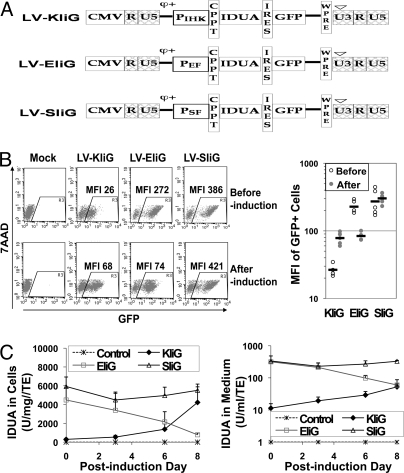
To determine if cells from the erythroid lineage could produce and release lysosomal IDUA during erythroid differentiation, an erythroid MEL cell line was used to compare IDUA expression and enzyme release from 3 LV constructs containing the same expression cassette with 3 different promoters, i.e., erythroid-specific IHK promoter, ubiquitous cellular promoter of human elongation factor (EF)-1α, and LTR promoter of spleen focus-forming virus (SF) (Fig. 1A). Progressive erythroid differentiation during hexamethylene bisacetamide (HMBA) induction of MEL cells was confirmed by morphologic evaluation and by histochemical staining with benzidine showing an increasing number of hemoglobin-expressing cells (Fig. S1). The mean fluorescent intensity (MFI) of GFP in stably transduced MEL-KIiG increased from a mean of 27 to 78 by day 8 of inductive culture, whereas the MFI in MEL-EIiG decreased from 228 to 84 and no significant change of MFI was observed in MEL-SIiG during erythroid induction (Fig. 1B). IDUA expression from IHK promoter was relatively low (5% of SF and 8% of EF) in uninduced MEL-KIiG but increased 15-fold following induction, reaching an intracellular level similar to that obtained with the strong LTR promoter of SF (Fig. 1C). After erythroid induction, IDUA expression from the EF promoter decreased to 17% of the uninduced levels, whereas the levels from SF promoter remained unchanged. A similar pattern was found in IDUA activity in the media from transduced MEL cells during induction. The endogenous IDUA levels of untransduced MEL control cells were very low (1.1 ± 0.7 U/mg) and decreased to negligible levels during erythroid induction; no IDUA activity was ever found in culture medium. These results demonstrate that maturing erythroid cells can increasingly overexpress IDUA during differentiation to levels comparable to those of strong SF promoter and that a portion of the IDUA can be released from these cells.
Fig. 1.
Transgene expression and release during erythroid induction in MEL cells. (A) Illustration of LVs. PIHK, erythroid-specific hybrid promoter/enhancer containing intron 8 erythroid-specific enhancer of human ALAS2 (I), HS40 core element from human α-globin locus control region (H), and human ankyrin-1 promoter (K); PEF, human EF-1α promoter; PSF, LTR of SF; IRES, internal ribosome entry site. (B) Representative FACS plots (Left) and quantitative analysis (Right) of GFP expression in transduced MEL cells before and after inductive culture. The solid bar represents the mean of MFI derived from 2–3 experiments. (C) Intracellular IDUA activities (Left) and extracellular IDUA release (Right). Culture media were harvested 24 h after inoculation of cells at 105 cells per 100 μL. All enzyme levels were normalized by transduction efficiency determined by FACS analysis for GFP+% (mean of 48% for KIiG, 75% for EIiG, and 67% for SIiG). Data were derived from 2–3 experiments in duplicate wells and shown as mean ± SEM.
Erythroid-Released Enzyme Cross-Corrected Lysosomal Defect in Cells Derived from a Patient with MPS I.
IDUA is synthesized in the endoplasmic reticulum as a 653-aa precursor that undergoes posttranslational glycosylation and extensive proteolytic processing to produce at least 10 polypeptides during passage through the endosome–lysosome compartments (18). The enzyme is normally targeted to the lysosome via the cation-independent mannose 6-phosphate (M6P) receptor (MPR) (19). To test if this endogenous uptake pathway remains effective for IDUA protein released by erythroid cells, lymphoblastoid cells derived from an MPS I patient were exposed to medium preconditioned by induced MEL-KIiG (Fig. S2). The intracellular IDUA levels increased from undetectable to 0.8 U/mg (Fig. S2A). This uptake process was inhibitable by the presence of M6P competitor. To determine the functional integrity of IDUA generated by erythroid cells, in situ immunostaining was performed using a fluorescent dye that could be endocytosed into lysosomes (Fig. S2B). In contrast to normal LCL cells, untreated LCLmps cells contained more lysosomes (i.e., stronger fluorescent intensity), and these compartments might be smaller in size, as suggested by more uniform staining. The majority of LCLmps cells exposed to erythroid-released IDUA exhibited a normalized lysosomal pattern, and this was not seen in the presence of M6P. These data demonstrate that IDUA released from maturing erythroid cells can use the MPR lysosomal enzyme trafficking system and can also restore a normal pattern of lysosomal distribution and morphology in cells derived from MPS I patients.
Long-Term Supraphysiological Levels of IDUA Were Achieved in Plasma of MPS I Mice Transplanted with LV-KIiG–Transduced Enzyme-Deficient HSCs.
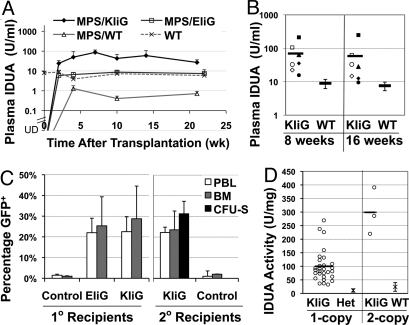
Next, we evaluated in vivo systemic IDUA production by erythroid-specific LVs in MPS I mice (Fig. 2). Lin− bone marrow cells from MPS I mice were isolated by lineage depletion with 92–97% purity, followed by transduction twice with LV-KIiG or LV-EIiG for a total multiplicity of infection (MOI) of 20 or 18, respectively. Starting 2 weeks after BMT, plasma IDUA activity levels increased from undetectable levels to 27 ± 9 U/mL and persisted at supraphysiological levels (4-fold higher than WT) until the end of the 5-month observation period (Fig. 2A). Only 0.7 ± 0.2 U/mL plasma IDUA was present in MPS I mice that received WT marrow, and 7 ± 4 U/mL was found in those receiving LV-EIiG–transduced Lin− cells. These results show that a lysosomal enzyme can be produced and released into the circulation in vivo by erythroid cells using tissue-specific LV, even though erythroid cells are not normally regarded as cells that secrete plasma proteins.
Fig. 2.
Long-term expression of IDUA in LV-KIiG–transduced MPS I chimeras. (A) Plasma IDUA levels over 5 months after BMT in primary MPS I recipients. MPS I mice were transplanted at 8–9 weeks of age with WT bone marrow (MPS/WT), LV-KIiG–transduced MPS Lin− cells (MPS/KIiG), or LV-EIiG–transduced MPS Lin− cells (MPS/EIiG). An undetectable level of IDUA was found in MPS I mice. Data were derived from 5–7 mice per group. (B) Plasma IDUA levels in secondary MPS I chimeras harboring LV-KIiG or WT marrow. Each symbol represents a secondary MPS I BMT recipient, and the solid line represents the mean. (C) Transgene frequencies determined by real-time qPCR in PBLs and bone marrow from primary (1°) and secondary (2°) BMT recipients 4–5 months after transplantation. A CFU-S assay was conducted with bone marrow from 5 primary donors, each into 6–7 secondary mice. (D) IDUA levels in GFP+ CFU-S colonies in correlation with vector copy number. Mean of IDUA levels from CFU-S colonies derived from heterozygous (Het) or WT HSCs is also shown.
To determine whether gene transfer had occurred in primitive HSCs and could sustain long-term erythroid IDUA “secretion,” we conducted secondary transplantation in MPS I mice using bone marrow from primary recipients of LV-KIiG–transduced cells 5 months after primary BMT (Fig. 2B). Stable erythroid IDUA expression derived from primary transduced HSCs was attained in all secondary recipients sampled 8 and 16 weeks after transplantation. Long-term plasma IDUA levels achieved in the secondary MPS I recipients were about 8-fold higher than WT levels.
To determine transgene frequency in primary and secondary BMT recipients, we evaluated GFP transgene frequency by real-time quantitative PCR in peripheral blood leukocytes (PBLs) and total bone marrow 4–5 months after transplantation (Fig. 2C). Similar levels of transgene frequency were obtained with both EIiG and KIiG vectors in primary recipients, averaging 22 ± 7% and 23 ± 8% in PBLs and 24 ± 12% and 28 ± 16% in bone marrow, respectively. Stable gene transfer in HSCs was ascertained in secondary recipients for KIiG with 22 ± 3% GFP+ in PBLs and 24 ± 9% in bone marrow.
Five months after primary transplantation, spleen colony-forming unit (CFU-S) assays were carried out to determine transgene frequency and functional IDUA expression in the clonal progeny of LV-KIiG–transduced pluripotent HSCs/progenitor cells after secondary transplants (Fig. 2 C and D). Of 112 CFU-S colonies analyzed, 35 CFU-S were positive for the provirus determined by real-time qPCR (31%) and all expressed elevated IDUA as determined by enzyme assay. Of these, 32 colonies contained a single copy and 3 colonies contained 2 copies of provirus. The IDUA activity levels in MPS I CFU-S colonies harboring a single-copy KIiG insertion were 100 ± 59 U/mg, which were 9-fold higher than those derived from heterozygous mice (11 ± 6 U/mg). The mean IDUA level in LV-KIiG–transduced 2-copy CFU-S colonies was 299 ± 86 U/mg. These observations were consistent with robust levels of IDUA detected in plasma of secondary MPS I recipients transplanted with primary LV-KIiG–transduced HSCs.
To determine whether erythroid-specific IDUA expression may affect normal erythropoiesis, a complete blood count was performed in primary or secondary MPS I chimeras 5–6 months after transplantation. Erythrocyte parameters were indistinguishable between MPS I chimeras receiving KIiG-transduced HSCs and those receiving WT bone marrow (Table S1). These results suggest that no significant perturbation of erythropoiesis occurred from IDUA transgene expression in these animals.
Erythroid-Specific Expression of IDUA Predominantly in Late Stages of Erythroblasts.
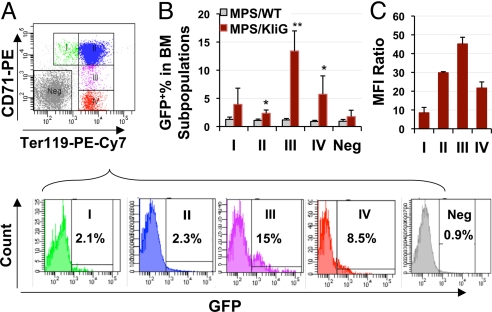
To investigate whether IDUA expression from LV-KIiG is erythroid-specific and to define its expression pattern during erythroid differentiation in vivo, GFP expression (as bicistronic gene downstream from IDUA) was evaluated in fresh bone marrow cells stained with the erythroid-specific cell surface markers Ter119 (glycophorin A-associated protein) and CD71 (transferrin receptor) (Fig. 3A). The progressive maturation in erythroid precursor subpopulations, labeled as I–IV on the histograms, has been examined previously by us (20) and others (21, 22). Population I corresponded mainly to proerythroblasts and early basophilic erythroblasts. Population II contained a mixture of basophilic, polychromatophilic, and orthochromatic erythroblasts. Population III contained reticulocytes and a fraction of mature RBCs, whereas population IV was mostly RBCs. GFP-expressing cells became detectable (i.e., significantly higher than background levels) starting from subpopulation II and further increased with greater percentage representation in later stages of erythroid differentiation (Fig. 3B). Only background levels of expression were observed in nonerythroid populations (Ter119−CD71− fraction). The GFP expression levels, as determined by MFI, increased in population II, peaked in population III, and decreased again in population IV (Fig. 3C). These results demonstrate predominant transgene expression in late stages of erythroid differentiation and confirm the erythroid restriction imparted by the IHK promoter as previously found with related β-globin–encoding vectors (17).
Fig. 3.
Transgene expression pattern in erythroid precursors of primary MPS I BMT recipients. (A) (Top) Representative flow cytogram of bone marrow cells immunostained for Ter119 and CD71, showing gating for various stages of erythroid cells (subpopulations I–IV). (Bottom) Representative histograms for GFP expression in gated subpopulations of treated MPS I mice. Neg, Ter119−CD71− fraction. Background GFP levels in MPS/WT controls are 0.9–1.3% in all subpopulations. (B) Frequency of detectable GFP+ cells in various erythroid progenitors. *, P ≤ 0.05; **, P ≤ 0.01. (C) Relative expression is shown as fold increase of mean MFI in GFP+ cells over GFP− cells in the same subpopulation (n = 5 for MPS/KIiG and n = 3 for MPS/WT).
To evaluate transgene expression in the clonal progeny of HSCs, we examined the cellular composition of individual CFU-S colonies and the GFP expression pattern in transduced colonies (Fig. S3). Twenty CFU-S colonies were immunostained with erythroid markers CD71 and Ter119, and all of them contained 48–91% erythroblasts/reticulocytes (CD71+) (Fig. S3 A and B), suggesting that 12-day CFU-S colonies were either erythroid or multilineage colonies. Moreover, GFP expression was restricted in the middle/late stages of erythroblasts and reticulocytes (Ter119+) (Fig. S3C).
Long-Term Systemic Metabolic Correction in MPS I Mice.
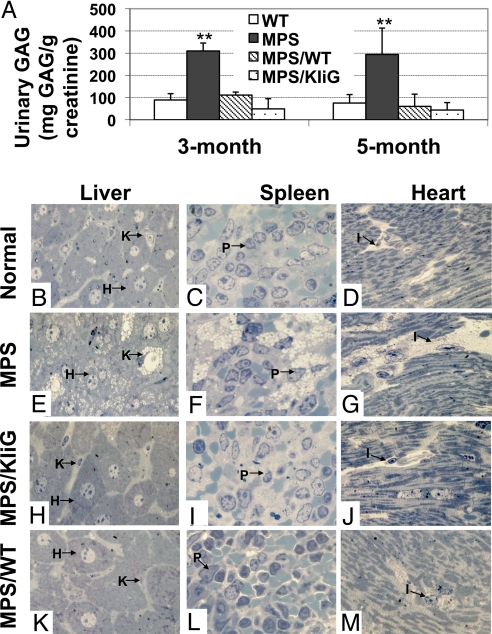
To evaluate the therapeutical effect of gene transfer, the GAG levels, a parameter for systemic metabolic accumulation, were determined in urine of treated MPS I groups in comparison with age-matched untreated MPS I and normal mice (Fig. 4A). At 3 and 5 months after transplantation, the mean GAG levels for all transplanted groups were not different from those in normal controls. Untreated MPS I mice had significantly higher urinary GAG accumulation than all other groups (P < 0.01). These data indicate that significant systemic metabolic correction of storage disease can be achieved in MPS I chimeras receiving MPS I HSCs transduced with an erythroid-specific LV or receiving WT HSCs.
Fig. 4.
Long-term systemic correction in treated MPS I mice. (A) Urinary GAG levels were determined by a direct 1,9-dimethylmethylene blue dye-binding assay 3 or 5 months after transplantation (n = 4–6 for all groups). **, P < 0.002 between MPS I mice and all other groups. (B–M) Representative views of histopathology of liver, spleen, and heart from Epon-embedded tissue sections (0.5–1 μm thick) with Toluidine blue staining. H, hepatocytes; I, interstitial cells; K, Kupffer cell; P, perisinusoidal cells. For MPS/KIiG and MPS/WT groups, sections of peripheral organs from the animal that exhibited the lowest plasma IDUA among the group are shown.
To evaluate the potential therapeutical effects of erythroid-derived IDUA on multiorgan deficits in MPS I mice, histological examinations of liver, spleen, and heart were performed on 2 mice from the KIiG group (one with the highest plasma IDUA and the other with the lowest) and 2 MPS I mice transplanted with WT marrow, and the findings were then compared with those from untreated MPS I and WT animals (Fig. 4 B–M). Cytoplasmic vacuoles, which represent distended lysosomes from which the GAG contents have been leached by fixation, are the pathognomonic feature of MPS. In age-matched untreated MPS I mice, lysosomal inclusions were most marked in Kupffer cells that were distended by massive vacuoles as well as in hepatocytes (Fig. 4E). Extensive pathological vacuoles were also observed in scattered perisinusoidal cells of the spleen (Fig. 4F). The interstitial space between myocardial cells was distended by aggregates of vacuolated interstitial cells in the heart of untreated MPS I mice (Fig. 4G). In contrast, tissues of all tested peripheral organs from MPS I mice transplanted with LV-KIiG-transduced MPS HSCs or those transplanted with WT HSCs were indistinguishable in regions examined from those of age-matched WT controls. These results suggest that erythroid-generated recombinant IDUA enzyme can correct lysosomal storage pathology completely in the liver, spleen, and heart.
Significant Improvement in Neurological Function and Brain Pathology in MPS I Mice with Higher Than Normal IDUA Activities in Peripheral Blood.
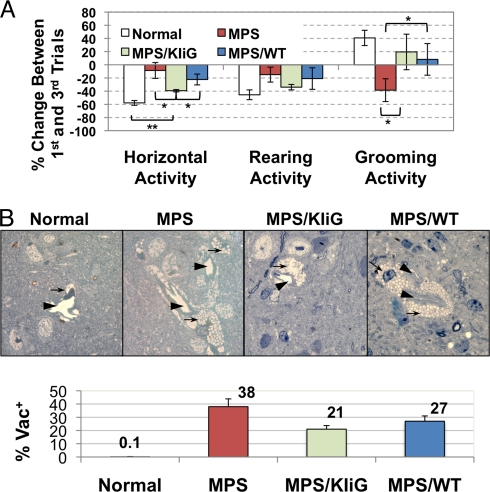
To determine if the supraphysiological levels of IDUA in the circulation could lead to functional neurological improvement in MPS I mice, a repeated open-field test was conducted (Fig. 5A). This test has been shown previously to characterize nonaversive and nonassociative memory deficits in MPS I mice without gender differences (23). Mice were exposed to the same open field for 3 repeated trials, with 30-min intertrial intervals. The normal mice showed a 58% reduction in horizontal locomotor activity, whereas the MPS I mice only showed a 9% reduction in activity (P < 0.001). Importantly, mice from the MPS/KIiG group exhibited 39% reduction in locomotor activity, a significant improvement toward normal behavior; whereas the MPS/WT group showed no significant improvement. In addition, the normal mice spent 41% more time grooming in the final trial than in the initial trial; however, the untreated mice spent 39% less time in the final trial (P < 0.001). Both treated groups showed significantly normalized grooming behavior, with 20% more time grooming for MPS/KIiG mice and 4% more for the MPS/WT group. Treated mice also had a greater reduction in rearing on the last trial compared with untreated controls. These observations suggested a significant improvement of the memory deficit in MPS I mice by erythroid-derived IDUA in peripheral blood.
Fig. 5.
Supraphysiological plasma IDUA levels lead to partial CNS correction in MPS I mice. (A) Repeated open-field test. Age-matched untreated MPS I (n = 8), KIiG-treated MPS I (n = 5), MPS I transplanted with WT bone marrow (n = 6), and normal littermates (n = 8) were evaluated 5 months after transplantation. Data are presented as mean ± SEM. (B) Histopathology of cerebral cortex in Epon-embedded tissue sections (0.5 μm) with Toluidine blue staining. (Top) Representative brain microvessels (arrowhead) and perivascular cells (arrow) are shown. (Bottom) Percentages of brain microvessels that are associated with vacuolated perivascular cells in the brain (%Vac+). The mean of scoring data from 6 slides (of 2 animals) is shown for each group. P < 0.01 by the Student's t test among all groups.
We then compared the histological appearance of forebrain tissues (Fig. 5B). Cells that were distended with pathological vacuoles were still visible in cerebral cortex of MPS/KIiG and MPS/WT chimeras; however, there appeared to be a reduction in the number of vacuolated cells. To evaluate the change more objectively, more than 500 microvessels were assessed for their association with vacuolated perivascular cells from 9 sections randomly selected from 3 slides of each animal. Significantly fewer brain capillaries were found to be associated with vacuolated perivascular cells from slides of both MPS/KIiG and MPS/WT groups than those from slides of MPS I controls (P < 0.01). Interestingly, the MPS/KIiG mice exhibited significantly less pathological accumulation than the MPS/WT group (P < 0.01). Taken together, these results demonstrate that behavioral deficits and CNS pathology can be improved but not cured by long-term supraphysiological IDUA in peripheral blood derived from erythroid cells.
Discussion
We have successfully demonstrated in depth a unique gene therapy approach that leads to extremely efficient, long-term, systemic delivery of a nonsecreted lysosomal enzyme at supraphysiological levels in the circulation. We showed that a lysosomal enzyme could be produced at high levels and “secreted” by erythroid cells during in vitro and in vivo definitive erythroid differentiation. Remarkably, with a relatively low vector copy number (0.2–0.3 copy per cell), IDUA levels 4- or 8-fold higher than WT were achieved in the blood circulation of primary or secondary MPS I chimeras during the 9 months of observation. These levels are at least 40-fold higher than those observed in MPS I mice fully engrafted with normal donor cells. Considering that 5% of normal plasma IDUA levels is therapeutical, based on allogeneic BMT experience in MPS I patients (5), one can speculate that only 0.3% transduced HSCs would be needed by this erythroid-specific gene therapy approach to achieve a similar therapeutical plasma level following autologous transplantation. Thus, this highly efficient erythroid-specific gene expression approach would make it substantially feasible to achieve clinical benefits even with the generally low levels of HSC gene transfer frequency (<1%) commonly obtained in most HSC gene therapy clinical trials.
Unlike intracellular IDUA polypeptides, the released form of IDUA from normal or enzyme-overexpressing cells appears not to be proteolytically processed and exhibits a unique molecular weight that is not found in cell lysate (18, 24). We showed in vitro that IDUA-overexpressing MEL/KIiG cells could release IDUA in an inducible pattern but to a lesser extent than in intracellular enzyme production. Moreover, the released form of IDUA is fully functional with normal lysosomal enzyme trafficking and is suitable for uptake by other cells via receptor-mediated endocytosis, resulting in cross-correction of phenotypic defects in cells from MPS I patients. Importantly, we also demonstrated in vivo that the IDUA produced by erythroid cells could lead to long-term systemic metabolic correction as well as complete normalization of tissue pathology in all tested peripheral organs of treated MPS I mice.
Although proviral integration into HSCs by randomly integrating viral vectors has the potential to provide a lifelong therapeutical effect, it also carries the risk for insertional oncogenesis from the strong viral enhancers that can ubiquitously activate transgene expression (25, 26). Vector genotoxicity has dampened the clinical success of ex vivo stem cell gene therapy for children with severe X-linked combined immunodeficiency (7, 8) and X-linked chronic granulomatous disease (10). Subsequent studies have demonstrated that the ability of LTR promoters/enhancers to transactivate genes over large distances in both directions, largely attributed to the increased risk for transforming potential of vectors (27). Thus, the use of vectors with intact LTRs now has limited clinical utility. As an alternative, promoters from cellular housekeeping genes may provide ubiquitous multilineage transgene expression and reduce the frequency of transactivating oncogenes. The EF-1α promoter is one of the strongest such promoters in HSCs tested in vitro and in vivo (28). Yet, by restricting transgene expression to a single lineage, the erythroid-specific hybrid promoter evaluated here generated 4-fold higher plasma IDUA levels than those derived from EF-1α promoter. Moreover, this tissue-specific vector may provide additional safety benefits compared with ubiquitous promoters. To begin with, the possibility of transactivating neighboring genes is limited to a much smaller number of integration sites in transgene-containing progeny of transduced HSCs, reducing the risk for insertional oncogenesis. In addition, highly efficient IDUA expression and release by IHK promoter would reduce the demand for the high vector copy numbers that are often associated with increased risks for genotoxicity. Finally, we showed that IHK promoter-derived transgene expression was predominantly restricted to late stages of erythroid differentiation. Thus, the time frame for active transcription from the IHK promoter during precursor maturation is relatively brief (3–4 days). This is followed by expulsion of the nucleus as the cells become reticulocytes, which, arguably, is one of the most radical safety features imaginable.
Several factors may have contributed to the high efficiency of the erythroid cell-derived systemic lysosomal IDUA production reported in this study. To begin with, RBCs are the most abundant blood cells and are constantly replenished at a rate of more than 2 × 106 cells/sec under normal hematopoiesis (29). The enormous cell mass and rapid turnover are likely to boost IDUA production at any given time and contribute to the high plasma enzyme levels. In addition, Sadelain and coworkers (16) have demonstrated the feasibility of introducing long-term secretion of a secreted clotting factor, human factor IX, using a β-globin promoter and its locus control region. In this study, we chose to use a hybrid promoter/enhancer containing the core sequence from human ankryin-1 gene promoter (30), a strong enhancer HS40 variant upstream from human embryonic ζ-globin gene (31), and the intron 8 enhancer of erythroid ALAS gene (32). This promoter has been shown in vivo to drive high erythroid-specific GFP expression and to retain viral titers because of its relatively small size in comparison to other erythroid promoters (17).
Although reprogramming erythroid cells for highly efficient continuous lysosomal enzyme production in circulation with phenotypic corrections is in itself an important finding, the improvement in brain pathology and behavioral deficit in MPS I mice after long-term peripheral IDUA delivery is one of the most compelling observations of this study. It has been generally believed that the blood–brain barrier (BBB) in the mature brain is largely impermeable to lysosomal enzymes, and that the CNS benefits observed in some patients with LSD receiving allogeneic BMT treatment early in life (<2 years old) may depend on diapedesis of donor HSC-derived macrophage/monocytes into the brain (33). Recently, a study performed on mice with another LSD, metachromatic leukodystrophy, showed that gene-marked HSCs overexpressing relatively high levels of the aryl sulfatase A enzyme (ARSA) were far more efficient at reversing the preexisting CNS deficits (demyelination) than BMT using normal HSCs (34). Higher than normal ARSA levels were achieved in serum by transplantation of transduced HSCs (using an LTR promoter). The gene-modified, donor-derived, ARSA-overexpressing microglia cells were proposed to be the exclusive source of ARSA in the CNS. However, we showed here that CNS benefit could be obtained when the sole source of IDUA was in the peripheral circulation. One possible reason could be that migrating WBCs in the CNS are “supercharged” with IDUA by endocytosis from constant high enzyme levels in serum before crossing the BBB. It has been suggested that CNS pathology in several MPS conditions (including MPS I) contains an inflammatory component, which encourages more diapedesis than that occurring under healthy conditions (35). On the other hand, several studies in some LSD models have shown evidence of partial clearance of CNS storage after multiple infusions of large doses of synthetic corrective enzyme in adult mice (36). Low levels of brain entry were implicated to account for the effects, even though disappearance of these proteins from serum was reported to occur in minutes. More recently, it has been suggested that slowing clearance of the recombinant enzyme from the circulation could further improve CNS pathology in MPS VII mice (37, 38). The LV-mediated erythroid-specific gene therapy approach developed here could provide continuously higher than normal IDUA in the circulation with potential lifelong CNS therapeutical benefits, although the precise mechanisms for CNS entry are to be determined.
In summary, our results demonstrate that late-stage erythroid cells transduced with a tissue-specific LV not only can produce and release a lysosomal enzyme successfully and continuously at supraphysiological levels in circulation but can achieve phenotypic correction in peripheral organs and the CNS of MPS I mice. This approach may break the conundrum of achieving high efficacy with high copy numbers, thereby increasing the risk for oncogenesis. Our study has important practical implications for treatment of many LSDs involving neurological defects, although the efficacy of this approach in large animal models remains to be assessed. These studies could also open a door for the utilization of RBC precursors as a depot for efficient, seemingly safer, systemic delivery of nonsecreted proteins by ex vivo HSC gene transfer.
Materials and Methods
LV Construction.
Three bicistronic self-inactivating LVs were constructed by insertion into a third-generation LV backbone pLV-TW (39) with EF-1α promoter (GenBank database, accession no. AF403737, 1–1192), LTR promoter/enhancer from SF (40), or an erythroid-specific hybrid IHK promoter (17). The expression cassette IDUA-ires-GFP, containing human IDUA cDNA (41) and GFP, was inserted into the HpaI site. The transfer LVs were generated using the 4-plasmid system and concentrated as previously described (42).
Further Details.
Additional information on experimental methods may be found in SI Methods.
Supplementary Material
Acknowledgments.
We are grateful for the technical assistance of Shuna Chen, Erin Ballard, and the Comprehensive Mouse Core. We thank Dr. Tal Kafri (Chapel Hill, NC) for LV packaging plasmids and Dr. Christopher Baum for providing the SF promoter/enhancer. This work was supported by the National Institutes of Health Grants AI061703, NS064330, HL70135–01, and U54 HL06–008 and by University Research Council at the University of Cincinnati.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908528106/DCSupplemental.
References
- 1.Hopwood JJ, Morris CP. The mucopolysaccharidoses. Diagnosis, molecular genetics and treatment. Mol Biol Med. 1990;7:381–404. [PubMed] [Google Scholar]
- 2.Souillet G, et al. Outcome of 27 patients with Hurler's syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- 3.Staba SL, et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 4.Grewal S, et al. Continued neurocognitive development and prevention of cardiopulmonary complications after successful BMT for I-cell disease: A long-term follow-up report. Bone Marrow Transplant. 2003;32:957–960. doi: 10.1038/sj.bmt.1704249. [DOI] [PubMed] [Google Scholar]
- 5.Peters C, et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- 6.Pan D, Stroncek DF, Whitley CB. Improved gene transfer and normalized enzyme levels in primitive hematopoietic progenitors from patients with mucopolysaccharidosis type I using a bioreactor. J Gene Med. 2004;6:1293–1303. doi: 10.1002/jgm.621. [DOI] [PubMed] [Google Scholar]
- 7.Hacein-Bey-Abina S, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 8.Aiuti A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 9.Gaspar HB, et al. Successful reconstitution of immunity in ADA-SCID by stem cell gene therapy following cessation of PEG-ADA and use of mild preconditioning. Mol Ther. 2006;14:505–513. doi: 10.1016/j.ymthe.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Ott MG, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 11.Hacein-Bey-Abina S, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 12.Howe SJ, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacein-Bey-Abina S, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer. 2003;3:477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- 15.von Kalle C, Baum C, Williams DA. Lenti in red: Progress in gene therapy for human hemoglobinopathies. J Clin Invest. 2004;114:889–891. doi: 10.1172/JCI23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang AH, Stephan MT, Sadelain M. Stem cell-derived erythroid cells mediate long-term systemic protein delivery. Nat Biotechnol. 2006;24:1017–1021. doi: 10.1038/nbt1227. [DOI] [PubMed] [Google Scholar]
- 17.Moreau-Gaudry F, et al. High-level erythroid-specific gene expression in primary human and murine hematopoietic cells with self-inactivating lentiviral vectors. Blood. 2001;98:2664–2672. doi: 10.1182/blood.v98.9.2664. [DOI] [PubMed] [Google Scholar]
- 18.Scott HS, et al. Human alpha-L-iduronidase: cDNA isolation and expression. Proc Natl Acad Sci USA. 1991;88:9695–9699. doi: 10.1073/pnas.88.21.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao KW, Faull KF, Kakkis ED, Neufeld EF. Carbohydrate structures of recombinant human alpha-L-iduronidase secreted by Chinese hamster ovary cells. J Biol Chem. 1997;272:22758–22765. doi: 10.1074/jbc.272.36.22758. [DOI] [PubMed] [Google Scholar]
- 20.Kalfa TA, et al. Rac1 and Rac2 GTPases are necessary for early erythropoietic expansion in the bone marrow but not in the spleen. Haematologica. 2009 doi: 10.3324/haematol.2009.006239. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath KE, Bushnell TP, Palis J. Multispectral imaging of hematopoietic cells: Where flow meets morphology. J Immunol Methods. 2008;336:91–97. doi: 10.1016/j.jim.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Socolovsky M, et al. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 23.Pan D, Sciascia A, 2nd, Vorhees CV, Williams MT. Progression of multiple behavioral deficits with various ages of onset in a murine model of Hurler syndrome. Brain Res. 2008;1188:241–253. doi: 10.1016/j.brainres.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks DA, et al. Glycosidase active site mutations in human alpha-L-iduronidase. Glycobiology. 2001;11:741–750. doi: 10.1093/glycob/11.9.741. [DOI] [PubMed] [Google Scholar]
- 25.Baum C, et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003;101:2099–2114. doi: 10.1182/blood-2002-07-2314. [DOI] [PubMed] [Google Scholar]
- 26.Bushman FD. Retroviral integration and human gene therapy. J Clin Invest. 2007;117:2083–2086. doi: 10.1172/JCI32949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metais JY, Dunbar CE. The MDS1-EVI1 gene complex as a retrovirus integration site: Impact on behavior of hematopoietic cells and implications for gene therapy. Mol Ther. 2008;16:439–449. doi: 10.1038/sj.mt.6300372. [DOI] [PubMed] [Google Scholar]
- 28.Chang AH, Sadelain M. The genetic engineering of hematopoietic stem cells: The rise of lentiviral vectors, the conundrum of the LTR, and the promise of lineage-restricted vectors. Mol Ther. 2007;15:445–456. doi: 10.1038/sj.mt.6300060. [DOI] [PubMed] [Google Scholar]
- 29.Chasis JA, Mohandas N. Erythroblastic islands: Niches for erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabatino DE, et al. A minimal ankyrin promoter linked to a human gamma-globin gene demonstrates erythroid specific copy number dependent expression with minimal position or enhancer dependence in transgenic mice. J Biol Chem. 2000;275:28549–28554. doi: 10.1074/jbc.M004043200. [DOI] [PubMed] [Google Scholar]
- 31.Huang BL, et al. Derepression of human embryonic zeta-globin promoter by a locus-control region sequence. Proc Natl Acad Sci USA. 1998;95:14669–14674. doi: 10.1073/pnas.95.25.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surinya KH, Cox TC, May BK. Identification and characterization of a conserved erythroid-specific enhancer located in intron 8 of the human 5-aminolevulinate synthase 2 gene. J Biol Chem. 1998;273:16798–16809. doi: 10.1074/jbc.273.27.16798. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy DW, Abkowitz JL. Mature monocytic cells enter tissues and engraft. Proc Natl Acad Sci USA. 1998;95:14944–14949. doi: 10.1073/pnas.95.25.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biffi A, et al. Gene therapy of metachromatic leukodystrophy reverses neurological damage and deficits in mice. J Clin Invest. 2006;116:3070–3082. doi: 10.1172/JCI28873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohmi K, et al. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci USA. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogler C, et al. Overcoming the blood–brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2005;102:14777–14782. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sly WS, et al. Enzyme therapy in mannose receptor-null mucopolysaccharidosis VII mice defines roles for the mannose 6-phosphate and mannose receptors. Proc Natl Acad Sci USA. 2006;103:15172–15177. doi: 10.1073/pnas.0607053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grubb JH, et al. Chemically modified beta-glucuronidase crosses blood–brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2008;105:2616–2621. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worsham DN, Schuesler T, von Kalle C, Pan D. In vivo gene transfer into adult stem cells in unconditioned mice by in situ delivery of a lentiviral vector. Mol Ther. 2006;14:514–524. doi: 10.1016/j.ymthe.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baum C, et al. cis-Active elements of Friend spleen focus-forming virus: From disease induction to disease prevention. Acta Haematol. 1998;99:156–164. doi: 10.1159/000040830. [DOI] [PubMed] [Google Scholar]
- 41.Pan D, Aronovich E, McIvor RS, Whitley CB. Retroviral vector design studies toward hematopoietic stem cell gene therapy for mucopolysaccharidosis type I. Gene Ther. 2000;7:1875–1883. doi: 10.1038/sj.gt.3301298. [DOI] [PubMed] [Google Scholar]
- 42.Pan D, et al. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol Ther. 2002;6:19–29. doi: 10.1006/mthe.2002.0630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.