Abstract
Dieting to control body weight involves cycles of deprivation from palatable food that can promote compulsive eating. The present study shows that rats withdrawn from intermittent access to palatable food exhibit overeating of palatable food upon renewed access and an affective withdrawal-like state characterized by corticotropin-releasing factor-1 (CRF1) receptor antagonist-reversible behaviors, including hypophagia, motivational deficits to obtain less palatable food, and anxiogenic-like behavior. Withdrawal was accompanied by increased CRF expression and CRF1 electrophysiological responsiveness in the central nucleus of the amygdala. We propose that recruitment of anti-reward extrahypothalamic CRF-CRF1 systems during withdrawal from palatable food, analogous to abstinence from abused drugs, may promote compulsive selection of palatable food, undereating of healthier alternatives, and a negative emotional state when intake of palatable food is prevented.
Keywords: eating disorders, obesity, palatability, palatable food dependence, withdrawal
Forms of obesity and eating disorders, similar to drug addiction, can be conceptualized as chronic relapsing conditions with alternating periods of abstinence (i.e., dieting to avoid “forbidden” palatable foods) and relapse (i.e., compulsive, often uncontrollable, eating of high-palatable foods) that continue despite negative consequences (1). Although the positive reinforcing properties of palatable foods are well known (2, 3), less attention has been given to their negative reinforcing properties (4–6), namely the increased probability of a behavioral response produced by removal of an aversive stimulus (e.g., intake of palatable food to relieve negative emotional states). Intermittent cycles of extended use of drugs of abuse can progressively lead to “affective dependence,” observed as a need for higher and/or more regular quantities of the drug to maintain a given emotional set point as well as a negative emotional state upon cessation of drug intake (7, 8). Such affective withdrawal may maintain use and motivate relapse via the negative reinforcing properties of continuing and resuming drug use, respectively (7, 8).
Extrahypothalamic corticotropin-releasing factor (CRF) brain stress systems are putatively involved in the transition from drug use to dependence, during which intake of abused drugs becomes increasingly motivated by these negative, rather than positive, reinforcement mechanisms. CRF plays a motivationally relevant role in withdrawal syndromes for every major drug of abuse, including alcohol, nicotine, cocaine, opiates, amphetamines, and tetrahydrocannabinol (7, 8). By analogy, repeated cycles of intermittent, extended access to highly palatable food were hypothesized to induce CRF system neuroadaptations similar to those seen in drug dependence models (4, 5, 9).
Results
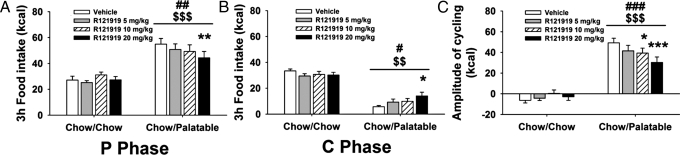
Intermittent, extended access to palatable food progressively leads to undereating of less preferred diets when palatable food is not available and to overeating of palatable food upon renewed access (10–12). To test the hypothesis that CRF1 systems mediate these feeding adaptations, male Wistar rats (n = 20) were provided a chow diet ad libitum (Chow/Chow) daily each week or were provided chow ad libitum for 5 days (C phase) followed by a highly palatable, sugary diet for 2 days (P phase) (Chow/Palatable) (see Fig. S1 for diet schedule and Fig. S2 for effects of diet schedule on food intake and body weight). After 7 weeks of diet cycling, rats received the non-peptide CRF1 receptor antagonist R121919 (0, 5, 10, and 20 mg/kg, s.c.) in a Latin-square design (13). Treatments were given 1 h before switches from palatable diet to chow or from chow to palatable diet. R121919 dose-dependently decreased palatable diet intake and increased chow food intake in Chow/Palatable rats (Diet Phase × Diet Schedule × Drug Dose: F3,54 = 7.25, P < 0.001), without altering intake of chow controls. R121919 decreased intake of the highly palatable diet upon renewed access to the palatable food (P phase) (Fig. 1A). In independent tests, the CRF1 receptor antagonist increased intake of the less palatable chow in Chow/Palatable rats withdrawn from the palatable diet (C phase) (Fig. 1B). Thus, by blunting both chow hypophagia and overeating of palatable food, R121919 attenuated the amplitude of intake cycling (the difference between intake during the first palatable P phase and first withdrawal to chow C phase: Diet Schedule × Drug Dose: F3,54 = 7.25, P < 0.001) (Fig. 1C). Supporting a progressive recruitment of CRF-CRF1 systems by the diet history, rather than by an acute diet effect, R121919 did not reduce palatable food intake after a single exposure to the diet or increase chow intake during a first withdrawal from palatable food (Fig. S3).
Fig. 1.
Effects of the CRF1 receptor antagonist R121919 (−1 h pretreatment, 0, 5, 10, and 20 mg/kg, s.c.) on cumulative 3-h food intake in (A) P phase (upon renewed access to the palatable food), (B) C phase (when rats had been withdrawn from the palatable diet to chow), and (C) amplitude of cycling (the difference between P phase and C phase intakes after a history of alternating Chow/Palatable diet access in male Wistar rats; n = 20) (see Materials and Methods or Fig. S1 for diet schedule). R121919 increased chow intake and decreased palatable diet intake in Chow/Palatable rats with no effect in Chow/Chow rats. Rats were tested after seven cycles of diet alternation. Doses were given in a within-subjects design before the first chow day or before the first palatable day of four consecutive cycles, respectively. Panels show M± SEM. $$, P < 0.01, $$$, P < 0.001, linear contrast dose effect; #, P < 0.05, ##, P < 0.01, ###, P < 0.001, main dose effect; *, P < 0.05, **, P < 0.01, ***, P < 0.001, different from vehicle.
Withdrawal from intermittent, extended access to palatable food also can increase anxiety-like behavior (11). To test the hypothesis that CRF1 receptors are involved in the negative emotional behavioral signs that follow withdrawal from palatable food, rats were administered R121919 (0, 20 mg/kg, s.c., 1-h pretreatment) and tested in a between-subjects design in the elevated plus-maze (14), 5–9 h after being switched from palatable diet to chow. Vehicle-treated Chow/Palatable rats exhibited less open arm time than chow-fed controls, reflecting an anxiogenic-like effect, during withdrawal from 7 weeks of diet cycling (Fig. 2A), an effect not yet seen after only two withdrawal cycles (Fig. S4). Pretreatment with R121919 (20 mg/kg, the dose that modulated both overeating of palatable food and undereating of chow) blocked the decrease in open arm exploration by Chow/Palatable rats at a dose that did not alter plus-maze behavior in chow controls (Diet Schedule × Dose: F1,43 = 7.25, P < 0.02; Fig. 2A Left). R121919 administration did not change general activity measured as closed arm entries. Therefore, R121919 blocked the increased anxiety-like behavior associated with withdrawal from intermittent, extended access to palatable food, without altering behavior of controls, suggesting recruitment of CRF1 systems.
Fig. 2.
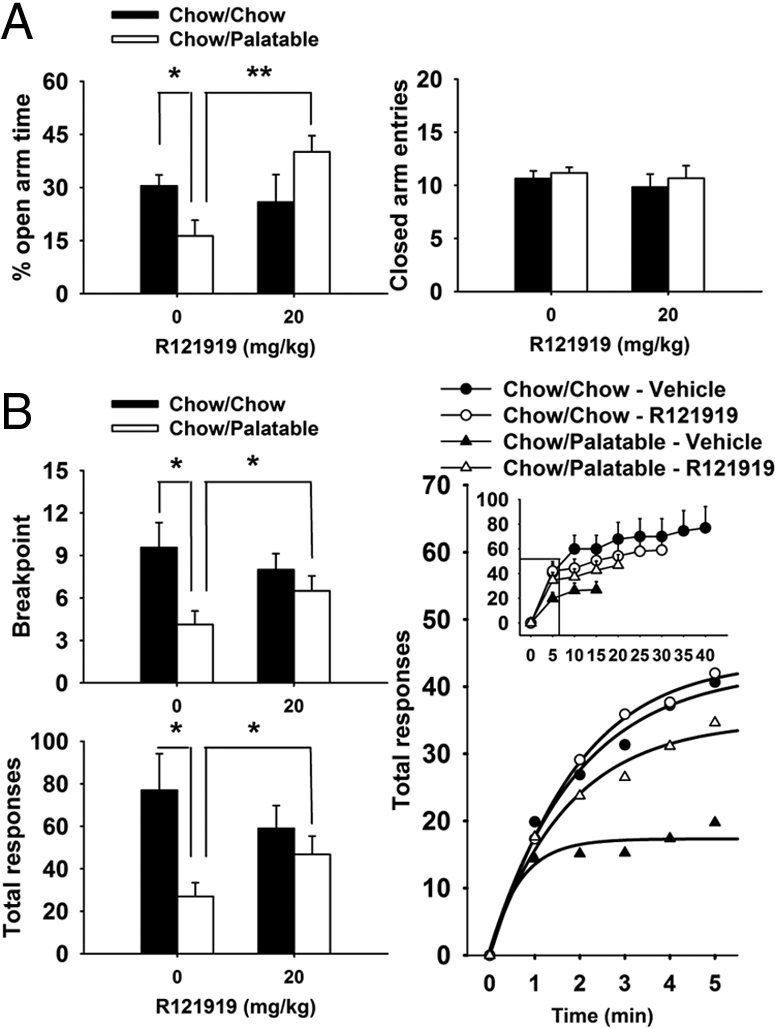
Effects of the CRF1 receptor antagonist R121919 (−1 h pretreatment, 0, 20 mg/kg, s.c.) on elevated plus-maze behavior (n = 47) and progressive-ratio responding for the less palatable food (n = 17) in male Wistar rats withdrawn from palatable food access. Rats were tested after 7 weeks of diet cycling. Panels show M ± SEM. (A) (Top) R121919 blocked the decrease in percentage of total arm time directed toward the open arms induced by withdrawing the highly palatable diet (less open arm time signifies more anxiogenic-like behavior). (Bottom) No effect was observed in the number of closed arm entries, an index of locomotor activity. (B) (Top Left) R121919 selectively blunted the deficits in breakpoint and (bottom left) total responses to obtain the less palatable chow in Chow/Palatable rats (lower scores signify hedonic-like deficit performance), without affecting performance of chow controls. (Right) Time-course of responding during the first 5 min. Inset shows the entire session (each symbol represents the mean number of cumulative reinforcers earned by subjects through that time point, irrespective of whether a subject had completed its session. The time course continued until each subject in the group stopped obtaining reinforcers). *, P < 0.05, **, P < 0.01, different from vehicle.
Withdrawal from intermittent, extended access to palatable food also can lead to motivational deficits to obtain less preferred diets, a potential index of hypohedonic-like behavior (10). Analogously, responding for less preferred gustatory reinforcers under progressive-ratio schedules of reinforcement has previously been used to index the motivational deficits seen during drug withdrawal (15). To determine the involvement of CRF1 receptors, we tested the effects of R121919 on the performance of diet-cycled rats to obtain their less preferred chow under a progressive-ratio schedule. Confirming previous findings (10), vehicle-treated Chow/Palatable rats exhibited reduced motivation to work to obtain the less palatable chow, reflected by a decreased breakpoint and decreased total responses emitted compared with Chow/Chow rats (10) (Fig. S5). R121919 pretreatment (20 mg/kg, the dose effective in increasing chow hypophagia, reducing palatable food hyperphagia and reducing anxiogenic-like behavior) selectively blunted the deficits in progressive-ratio performance in diet-cycled rats at a dose that was ineffective in chow controls (breakpoint: Diet Schedule × Drug: F1,15 = 8.17, P < 0.02; total responses: Diet Schedule × Drug: F1,15 = 9.14, P < 0.01; Fig. 2B, left). Against the alternative interpretation that R121919 facilitated performance in Chow/Palatable rats by decreasing postingestive satiation, R121919 blocked the deficits in responding as early as 5 min into the session (Diet Schedule × Drug: F1,15 = 2.55, P < 0.05) (Fig. 2B Right). Therefore, the CRF1 receptor antagonist blunted the motivational deficits in progressive-ratio responding for less preferred gustatory reinforcers that is seen in animals withdrawn from intermittent, extended access to highly palatable food.
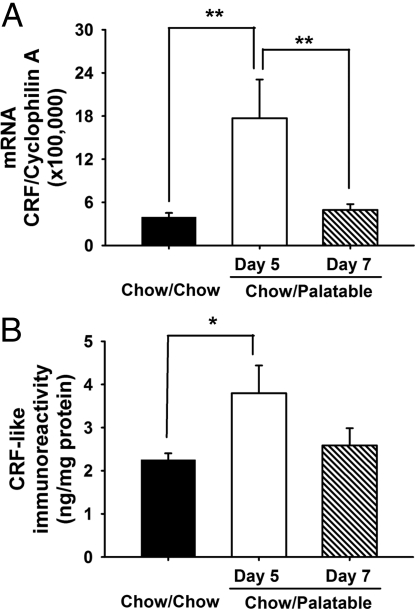
To test the hypothesis that withdrawal from palatable food might activate the stress-related extrahypothalamic CRF system, levels of CRF mRNA and peptide in the central nucleus of the amygdala were measured by quantitative real-time PCR and RIA, respectively. Rats were diet-cycled for 7 weeks or fed chow continuously. After anesthesia and decapitation, brain punches from the central nucleus of the amygdala were collected during withdrawal from and after renewing access to the palatable diet. Withdrawing palatable food in Chow/Palatable rats induced a five-fold increase in CRF mRNA expression in the central nucleus of the amygdala compared with Chow/Chow rats (Fig. 3A). Conversely, CRF mRNA returned to control-like levels with renewed access to palatable food (F2,19 = 6.97, P < 0.01). CRF mRNA expression in the central nucleus of the amygdala did not change when Chow/Palatable rats were cycled only once (Chow/Chow vs. Chow/Palatable: 5.5 ± 2.2 vs. 6.3 ± 1.7 n.s.), supporting a progressive recruitment of CRF-CRF1 systems by the diet history, rather than by an acute effect of the diet. In addition, CRF mRNA expression did not change in the nucleus accumbens, prefrontal cortex or insular cortex, supporting the regional specificity of findings (Fig. S6). Interestingly, no significant changes in CRF mRNA expression were observed in the paraventricular nucleus of the hypothalamus or in circulating corticosterone at the same withdrawal time point in Chow/Palatable rats (Figs. S6 and S7), suggesting the hypothesis that changes in amygdalar, rather than hypothalamic, CRF stress systems proximately subserved the behavioral adaptations. Moreover, CRF peptide immunoreactivity in the central nucleus of the amygdala of animals withdrawn from the palatable diet was 70% higher than in chow-fed animals, but returned to chow-fed control levels with access to the palatable diet (F2,24 = 4.01, P < 0.01) (Fig. 3B). Thus, withdrawing palatable food activated the stress-related CRF peptide system in the central nucleus of the amygdala, analogous to findings in models of drug and ethanol withdrawal (7, 8). Because renewed access to palatable food decreased extrahypothalamic CRF system activation in the central nucleus of the amygdala, wherein CRF activation is linked to anxiety (16), the present results also suggest that palatable food might acquire negative reinforcing properties by relieving negative affective consequences of abstinence (17).
Fig. 3.
Effects of palatable diet alternation on (A) CRF mRNA and (B) CRF peptide expression in the central nucleus of the amygdala. Rats (n = 45) were diet-cycled for 7 weeks, and central nucleus of the amygdala punches were collected. Both CRF mRNA and peptide expression in the central nucleus of the amygdala of Chow/Palatable rats increased when the palatable diet was withdrawn (day 5) and returned to chow-fed control levels with access to the palatable diet (day 7). Panels show M± SEM. *, P < 0.05, **, P < 0.01, different from Chow/Chow.
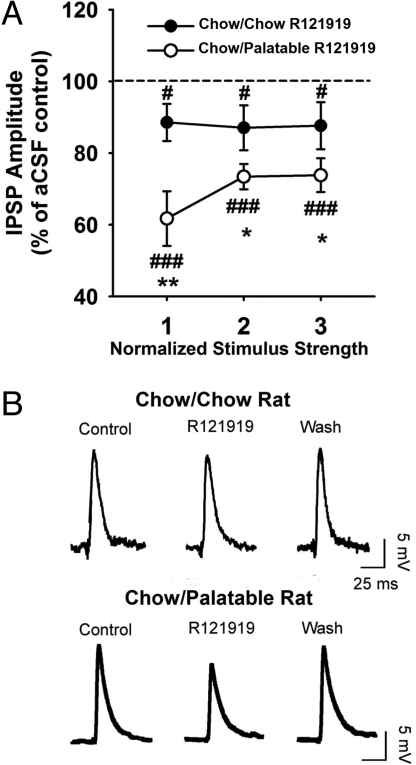
To test the hypothesis that rats withdrawn from palatable food might show increased sensitivity to CRF1 antagonist modulation of γ-aminobutyric acid (GABA) signaling in the central nucleus of the amygdala, which occurs during ethanol withdrawal (18), we examined the effect of R121919 on GABAergic transmission of central nucleus of the amygdala neurons in a slice preparation. Male Wistar rats (n = 14) were diet-cycled for 7 weeks and sacrificed after being switched to the less palatable chow. Basal GABAergic transmission in central nucleus of the amygdala synapses did not differ in relation to diet history (n = 23 cells) across all stimulus intensities used to evoke GABA-inhibitory postsynaptic potentials (IPSP). However, 20 min superfusion with R121919 (1 μM) induced a greater reduction in evoked GABAA-IPSPs in central nucleus of the amygdala neurons of Chow/Palatable rats (M± SEM: 30 ± 6%, n = 9 cells) than in those of chow-fed controls (M ± SEM: 12 ± 6%, P < 0.05, n = 11 cells) (Fig. 4). Following a 30 min washout period, IPSPs of both groups returned to similar, baseline-like levels. Therefore, consistent with overactivation of the amygdala CRF-CRF1 system and effects seen during ethanol withdrawal (18), diet-cycled rats showed an increased sensitivity to the inhibitory effects of a CRF1 receptor antagonist on central nucleus of the amygdala GABAergic transmission.
Fig. 4.
Effects of the CRF1 receptor antagonist R121919 on GABAA-IPSPs in the central nucleus of the amygdala after a history of alternating palatable diet access in male Wistar rats (n = 14) withdrawn from palatable food access. (A) R121919 significantly decreased the amplitude of IPSPs across three stimulus intensities in Chow/Palatable rats compared with Chow/Chow rats. The dotted line represents the mean IPSP amplitude of cells from the respective diet group in the pressure of a CSF only. (B) Representative IPSP traces taken from a Chow/Chow rat (top) and a Chow/Palatable rat (bottom). Panels show M ± SEM. *, P < 0.05, **, P < 0.01, differs from Chow/Chow; #, P < 0.05, ###, P < 0.001, different from baseline (100%).
Discussion
The collective results provide functional evidence that a history of intermittent, extended access to palatable food leads to progressive, motivationally relevant neuroadaptations in stress-related extrahypothalamic CRF-CRF1 systems. Specifically, the selective CRF1 receptor antagonist R121919 differentially and selectively affected feeding in diet-cycled rats, increasing regular chow intake and decreasing the intake of highly palatable food upon renewed access. The CRF1 receptor antagonist also selectively blocked the increased anxiety-like behavior and motivational deficits in responding for less preferred chow that were seen during withdrawal from the palatable diet. Withdrawing access to the palatable diet increased CRF gene and peptide expression in the central nucleus of the amygdala, effects that were eliminated with renewed access. Additionally, diet-cycled rats showed an increased sensitivity to the inhibitory effects of a CRF1 receptor antagonist on GABAergic transmission in the central nucleus of the amygdala, further suggesting overactivation of the amygdala CRF-CRF1 system. The overeating of palatable food upon renewed access may result from the increased CRF system activation of the just completed withdrawal period, seen as increased expression of CRF and electrophysiological sensitivity to CRF1 receptor blockade in the central nucleus of the amygdala. CRF1 antagonist pretreatment just before palatable food access is thereby interpreted to oppose the initially still present CRF-CRF1 system overactivation of withdrawal. The brief time course of palatable food overeating otherwise seen in untreated animals (10) may reflect the time course by which the expression, release, and effects of CRF peptide normalize once access to palatable food is regained, as seen in the present study. Thus, intermittently eating palatable diets may induce an allostatic shift in brain reward systems with recruitment of anti-reward CRF-CRF1 systems in the central nucleus of the amygdala.
These results have implications not only for compulsive eating, but also for motivation generally. The repeated activation of hedonic systems elicited opponent-like processes in the brain (i.e., recruitment of CRF1 circuitry) that were distinct from a simple loss of function in reward transmitter systems. Such between-system neuroadaptations (19) also occur during the transition to dependence on all major drugs of abuse (7, 8). The generalization to non-drug stimuli in the present study suggests that motivational processes can become perturbed in individuals who experience repeated contrasts in the intensity of hedonic stimuli over time (20). Adaptively, such processes may shift food-seeking and consummatory behavior toward energy-dense, high-reward foods, while devaluing efforts to obtain less energy-rich, low-reward foods (or non-foods), an adaptation evolutionarily useful when there are costs to foraging (e.g., predator exposure, limited time and energy resources). In today's environment, however, the same processes may drive intake of foods that promote obesity at the expense of less tasty, but perhaps more nutritious alternatives.
Thus, addiction-like changes in CRF1 systems may help drive (i) intake of energy-dense palatable foods, (ii) underconsumption of healthier alternatives, and (iii) the associated negative emotional state that occurs when access to palatable food is prevented (4, 5, 10–12, 17). Translated to the human condition, CRF system activation may promote relapse eating in obesity and related eating disorders as well as other negative motivational sequelae of cyclic abstinence from palatable food.
Materials and Methods
Subjects.
Male Wistar rats (n = 155, 180–230 g, 45 days old) were obtained from Charles River and single-housed upon arrival in wire-topped, plastic cages (19 × 10.5 × 8 inches) in a 12 h:12 h reverse light cycle (10:00 h lights off), humidity- (60%) and temperature-controlled (22 °C) vivarium. Rats had access to corn-based rodent chow [Harlan Teklad LM-485 Diet 7012: 65% (kcal) carbohydrate, 13% fat, 21% protein, metabolizable energy 341 cal/100 g] and water ad libitum for 1 week before the start of experiments. Experimental procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication number 85–23, revised 1996) and the “Principles of laboratory animal care” (http://www.nap.edu/readingroom/bookslabrats) and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs.
R121919 was synthesized as described in Chen et al. (21). R121919 is a high affinity (Ki = 3.5 nM) selective CRF1 antagonist with physiochemical properties superior to many other CRF1 antagonists (e.g., decreased logP and logD, increased water solubility) (13). For testing, R121919 was first solubilized in 1 M HCl (10% of final volume), then diluted to a final vehicle of 20% (wt/vol) 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich), back-titrated with NaOH to pH 4.5. The R121919 solution was administered s.c. (s.c.) in a volume of 2 mL/kg.
Ad Libitum Diet Alternation.
After acclimation, rats were divided into two groups matched for food intake, body weight, and feed efficiency from the previous 3–4 days. One group was provided a chow diet (“Chow”) ad libitum 7 days per week (Chow/Chow), and a second group was provided chow ad libitum for 5 days each week followed by 2 days of ad libitum access to the highly palatable, chocolate flavored, high-sucrose diet (“Palatable”; Chow/Palatable). The palatable diet is a nutritionally complete, chocolate-flavored, high-sucrose (50% kcal), AIN-76A-based diet that is comparable in macronutrient proportions and energy density to the chow diet [TestDiet; chocolate-flavored Formula 5TUL: 66.8% (kcal) carbohydrate, 12.7% fat, 20.5% protein, metabolizable energy 3.48 kcal/g; formulated as 45-mg precision food pellets to increase its preferredness (22, 23)]. For brevity, the first 5 days (chow only) and last 2 days (chow or palatable according to experimental group) of each week are referred to in all experiments as C and P phases. Diets were never concurrently available. Chow diet was either Harlan Teklad LM-485 Diet 7012 [65% (kcal) carbohydrate, 13% fat, 21% protein, metabolizable energy 341 cal/100 g] or 5TUM diet formulated as 4- to 5-g extruded pellets [65.5% (kcal) carbohydrate, 10.4% fat, 24.1% protein, metabolizable energy 330 cal/100 g; TestDiet]. Similar to previous studies, Harlan Teklad LM-485 chow was used in the feeding and elevated plus-maze experiments (11), while TestDiet 5TUM chow (10) was used in the progressive-ratio, CRF mRNA, CRF peptide content, corticosterone RIA and electrophysiological experiments.
As previously published (10), relative diet preferences, calculated as the percentage of daily intake (kcal) of the first diet in relation to the second diet, were the following: 5TUL Chocolate Diet (sugary Palatable diet) vs. Harlan LM-485 chow (M± SEM preference 90.7 ± 3.6%) and 5TUL Chocolate Diet (sugary Palatable diet) vs. 5TUM chow Diet (M ± SEM preference 91.2 ± 3.7%).
Elevated Plus-Maze.
The elevated plus-maze test was performed as described in Cottone et al. (24). Chow/Palatable rats were diet-cycled for at least 7 weeks and then were pretreated with either vehicle or 20 mg/kg R121919 (−1 h, s.c.) and tested 5–9 h after being switched from the palatable diet to chow (P→C phase). Chow/Chow control rats were tested concurrently in a between-subjects design (n = 47). Chow diet was available ad libitum until the time of testing. For further details, see the SI Text.
Progressive-Ratio Schedule of Reinforcement for Food.
The progressive-ratio schedule of reinforcement for food was performed as described in Cottone et al. (10). Animals received ad libitum A/I chow (5 g extruded pellets) in their home cages throughout the experiment unless otherwise specified. Food reinforcers were 45-mg chow-precision pellets, identical in composition to the extruded home cage chow diet. Sessions ended when subjects did not complete a ratio for 14 min, with the last completed ratio defined as the breakpoint. Chow/Palatable rats were diet-cycled for at least 7 weeks and then pretreated with R121919 (−1 h, s.c.) at the time of being switched from palatable diet to chow (P→C phase). Chow/Chow control rats were tested concurrently in a between-subjects design (n = 17). Doses of R121919 (0, 20 mg/kg body weight, s.c.) were given in a within-subjects, counterbalanced design across two diet cycles. For further details, see the SI Text.
Quantitative Real-Time PCR.
Rats (n = 20) were diet-cycled for 7 weeks, anesthetized, and decapitated during the two diet conditions (days 5 and 7 of each weekly cycle). Brains were quickly removed and sliced coronally in a brain matrix, and central nucleus of the amygdala, nucleus accumbens, insular cortex, and prefrontal cortex punches were collected on an ice-cold stage. Total RNA was prepared from each brain punch using a standard protocol for RNA extraction from animal tissues. Total RNA (1 μg) was then reverse transcribed in the presence of Oligo (dT)20 per the manufacturer's instructions. Quantitative RT-PCR reactions were carried out in a 20-μL volume using 0.5 μM primers and 4 mM MgCl2. Results were analyzed by second-derivative methods and expressed in arbitrary units, normalized to expression levels of the reference gene, CypA. All RT-PCR reactions for a given sequence were performed within the same run. For further details, see the SI Text.
Peptide Acid Extraction and CRF RIA.
Rats (n = 25) were diet-cycled for at least 7 weeks, anesthetized, and decapitated during the two diet conditions (days 5 and 7 of each weekly cycle). Brains were quickly removed and sliced coronally in a brain matrix, and central nucleus of the amygdala punches were collected on an ice-cold stage. Peptide acid extraction followed an already established procedure (25). Tissue CRF-like immunoreactivity was quantified with a sensitive and specific solid-phase RIA adapted from Zorrilla et al. (26). For further details, see the SI Text.
Corticosterone RIA.
Rats (n = 12) were diet-cycled for at least 7 weeks, and tail blood was sampled during the two diet conditions (days 5 and 7 of each weekly cycle). Plasma levels of corticosterone-like immunoreactivity were determined with a commercially available RIA kit, according to the manufacturer's instructions (MP Biomedicals, Inc.) (26). For further details, see the SI Text.
Electrophysiological Studies
Slice Preparation.
Central nucleus of the amygdala slices were prepared as previously described (27, 28) from rats (n = 7/group) that had been diet-cycled for at least 7 weeks, anesthetized, and decapitated 2–3 h after being withdrawn from palatable food. The brains were rapidly removed and placed into ice-cold artificial cerebrospinal fluid (aCSF) gassed with 95% O2 and 5% CO2. Slices were cut, incubated in an interface configuration for about 30 min, and completely submerged and continuously superfused with warm, gassed aCSF. Drugs were added to the aCSF from stock solutions to obtain known concentrations in the superfusate. At the 2–4 mL/min superfusion rates used, drug concentrations reach 90% of the reservoir concentration within 2 min.
Electrophysiology.
We recorded central nucleus of the amygdala neurons with sharp micropipettes using discontinuous voltage- or current-clamp mode. We held most neurons near their resting membrane potential. Data were acquired with a preamplifier and stored for later analysis using pClamp software. Pharmacologically isolated GABAA receptor-mediated inhibitory postsynaptic potentials (GABAA-IPSPs) were evoked by stimulating locally within the central nucleus of the amygdala through a bipolar stimulating electrode while superfusing the glutamate receptor blockers CNQX and APV and GABAB receptor blocker CGP 55845A. To determine the response parameters for each cell, we performed an input-output protocol. A range of currents was applied, starting at the threshold current required to elicit an IPSP up to the voltage required to elicit the maximum amplitude. We normalized three stimulus intensities of equal steps (threshold, half-maximal, and maximal) as 1–3×. Hyperpolarizing and depolarizing current steps (200-pA increments, 750-ms duration) also were applied to generate voltage-current (V-I) curves. We quantified the evoked IPSP amplitudes and V-I responses by using Clampfit software. All measures were taken before superfusion with the selective CRF1 receptor antagonist R121919 (1 μM), during its superfusion (20 min), and following washout (30 min). For further details, see the SI Text.
Statistics.
Group comparisons used Student's t-tests (two-group comparisons) or analysis of variance (ANOVA) (at least three-group comparisons), the latter interpreted by simple main effect analysis or Newman-Keuls comparisons after significant omnibus effects (P < 0.05). Data from the feeding experiment were analyzed by three-way mixed ANOVAs with Diet Schedule as the between-subjects factor and Dose and Diet Phase as within-subjects factors. Data from the elevated plus-maze experiment were analyzed by two-way ANOVAs with Diet Schedule and Dose as between-subjects factors. For the progressive-ratio schedule of reinforcement experiment, breakpoint and total responses were analyzed by two-way mixed ANOVAs with Diet Schedule as the between-subjects factor and Dose as the within-subjects factor. The time-course of responding during the first 5 min was analyzed by three-way mixed ANOVAs with Diet Schedule as the between-subjects factor and Dose and Time as within-subject factors. Data from the electrophysiological studies were analyzed with a between-subjects ANOVA or within-subjects ANOVA with repeated measures, as appropriate. Data from the corticosterone RIA where analyzed by two-way mixed ANOVA with Diet Schedule as the between-subject factor and Diet Phase as the within-subject factor. The statistical packages used were Instat 3.0, Prism 4.0 (GraphPad), Systat 11.0, and SPSS 11.5 (SPSS).
Supplementary Material
Acknowledgments.
We thank Mike Arends for editorial assistance, Mary Gichuhi for administrative assistance, and Bob Lintz, Jeanette Helfers, Stephanie Dela Cruz, and Molly Brennan for technical assistance. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK70118, DK26741, and P30DK56336; National Institute on Drug Abuse Grant DA023680; National Institute on Alcohol Abuse and Alcoholism Grants AA016731 and AA015566; National Institute of Neurological Disorders and Stroke Grant IT32NS061847-01A2; National Institute on Aging Grant AG028040; National Heart, Lung and Blood Institute Grant HL088083; the Ellison Medical Foundation; and the Pearson Center for Alcoholism and Addiction Research. A portion of this work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. This is manuscript number 19807 from The Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908789106/DCSupplemental.
References
- 1.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 2.Corwin RL. Bingeing rats: A model of intermittent excessive behavior? Appetite. 2006;46:11–15. doi: 10.1016/j.appet.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boggiano MM, et al. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: An animal model of lean vs obese binge-eating and obesity with and without binge-eating. Int J Obes. 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- 4.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2007;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–1029. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008;33:524–535. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- 7.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 9.Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: A role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent preferred food access reduces the reinforcing efficacy of chow in rats. Am J Physiol. 2008;295:R1066–1076. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2008;34:38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats With limited access to a sweet-fat diet. Obesity. 2008;16:1998–2002. doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- 13.Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- 14.Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Markou A, et al. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- 16.George O, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells AS, Read NW, Laugharne JD, Ahluwalia NS. Alterations in mood after changing to a low-fat diet. Br J Nutr. 1998;79:23–30. doi: 10.1079/bjn19980005. [DOI] [PubMed] [Google Scholar]
- 18.Cruz MT, et al. CRF1 receptor antagonists block the ethanol-induced release of GABA in the central amygdala in vitro and in vivo. Alcohol Clin Exp Res. 2008;32:6s1 P27A. [Google Scholar]
- 19.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 20.Flaherty CF, Grigson PS. From contrast to reinforcement: Role of response contingency in anticipatory contrast. J Exp Psychol. 1988;14:165–176. [PubMed] [Google Scholar]
- 21.Chen C, et al. Design of 2,5-dimethyl-3-(6-dimethyl-4-methylpyridin-3-yl)-7-dipropylaminopyrazolo[1, 5-a]pyrimidine (NBI 30775/R121919) and structure-activity relationships of a series of potent and orally active corticotropin-releasing factor receptor antagonists. J Med Chem. 2004;47:4787–4798. doi: 10.1021/jm040058e. [DOI] [PubMed] [Google Scholar]
- 22.Cooper SJ, Francis RL. Effects of acute or chronic administration of chlordiazepoxide on feeding parameters using two food textures in the rat. J Pharm Pharmacol. 1979;31:743–746. doi: 10.1111/j.2042-7158.1979.tb13649.x. [DOI] [PubMed] [Google Scholar]
- 23.Laboure H, Saux S, Nicolaidis S. Effects of food texture change on metabolic parameters: Short- and long-term feeding patterns and body weight. Am J Physiol. 2001;280:R780–R789. doi: 10.1152/ajpregu.2001.280.3.R780. [DOI] [PubMed] [Google Scholar]
- 24.Cottone P, Sabino V, Steardo L, Zorrilla EP. FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: Evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology. 2007;32:1069–1081. doi: 10.1038/sj.npp.1301229. [DOI] [PubMed] [Google Scholar]
- 25.Lahmame A, Grigoriadis DE, De Souza EB, Armario A. Brain corticotropin-releasing factor immunoreactivity and receptors in five inbred rat strains: Relationship to forced swimming behaviour. Brain Res. 1997;750:285–292. doi: 10.1016/s0006-8993(96)01368-6. [DOI] [PubMed] [Google Scholar]
- 26.Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology. 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- 27.Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.