Abstract
Intracellular amyloid-β peptide (Aβ) has been implicated in neuronal death associated with Alzheimer's disease. Although Aβ is predominantly secreted into the extracellular space, mechanisms of Aβ transport at the level of the neuronal cell membrane remain to be fully elucidated. We demonstrate that receptor for advanced glycation end products (RAGE) contributes to transport of Aβ from the cell surface to the intracellular space. Mouse cortical neurons exposed to extracellular human Aβ subsequently showed detectable peptide intracellularly in the cytosol and mitochondria by confocal microscope and immunogold electron microscopy. Pretreatment of cultured neurons from wild-type mice with neutralizing antibody to RAGE, and neurons from RAGE knockout mice displayed decreased uptake of Aβ and protection from Aβ-mediated mitochondrial dysfunction. Aβ activated p38 MAPK, but not SAPK/JNK, and then stimulated intracellular uptake of Aβ-RAGE complex. Similar intraneuronal co-localization of Aβ and RAGE was observed in the hippocampus of transgenic mice overexpressing mutant amyloid precursor protein. These findings indicate that RAGE contributes to mechanisms involved in the translocation of Aβ from the extracellular to the intracellular space, thereby enhancing Aβ cytotoxicity.
Keywords: β-amyloid, Alzheimer's disease, mitochondrial dysfunction, p38 MAPK
Alzheimer's disease (AD) is a progressive neurodegenerative process characterized by senile plaques, neurofibrillary tangles, and neuronal loss (1, 2). Deposition of amyloid-β peptide (Aβ), a 39–43-amino acid peptide derived from the transmembrane amyloid precursor protein (APP), is found in extracellular senile plaque cores and is associated with neurodegeneration in later stages of AD. In contrast, recent studies suggest that accumulation of intraneuronal Aβ may be an early event in the pathogenesis of AD (3–16). Addition of Aβ to human neuronal-like cells caused significant mitochondrial damage (17). Furthermore, our recent study revealed that binding of Aβ to Aβ-binding alcohol dehydrogenase (ABAD) or cyclophilin D (10, 11) intracellularly triggered events leading to neuronal apoptosis through a mitochondrial pathway (12, 13, 18, 19). However, mechanisms through which Aβ produced at the plasma membrane and released into the extracellular space reaches the intracellular milieu remain to be elucidated.
Receptor for advanced glycation end products (RAGE) is a multiligand receptor of the Ig superfamily of cell surface molecules (20–22). RAGE acts as a counter-receptor for several quite distinct classes of ligands, such as AGEs, S100/calgranulins, HMG1 (high mobility group 1 or amphoterin), and the family of crossed β-sheet fibrils/macromolecular assemblies, which activate receptor-mediated signal transduction pathways. These ligand-receptor interactions are believed to exert pathogenic effects through sustained cellular perturbation in a range of chronic disorders, including the secondary complications of diabetes, inflammation, and neurodegenerative processes (23, 24). RAGE, a cell surface binding site for Aβ (25), is expressed at higher levels in an Aβ-rich environment (26, 27). Targeted neuronal overexpression of a wild-type RAGE transgene in AD-type mice also expressing mutant human APP (mAPP) amplified Aβ-mediated neuronal dysfunction. The latter was shown by early abnormalities in spatial learning/memory and exaggerated neuropathologic changes not seen in single transgenics (such as transgenics expressing mAPP alone at the same ages). These data support the hypothesis that RAGE might function as a cofactor for Aβ-induced neuronal perturbation in AD (28). Interaction of Aβ with RAGE expressed on brain endothelial cells initiates cellular signaling leading to the trafficking of monocytes across the blood-brain barrier (BBB) (29). Furthermore, RAGE has been shown to mediate Aβ transport across the BBB and to contribute to pathologic accumulation of the amyloid peptide in brain (30). Herein, we demonstrate that RAGE contributes to translocation of Aβ across the cell membrane from the extracellular to the intracellular space in cortical neurons. We also present evidence that Aβ-initiated RAGE signaling, especially stimulation of p38 mitogen-activated protein kinase (MAPK), has the capacity to drive a transport system delivering Aβ as a complex with RAGE to the intraneuronal space.
Results
Extracellular Aβ Translocates into Mitochondria in Cortical Neurons.
We have recently demonstrated that Aβ, endogeneously generated from a mutant APP transgene, interacts with ABAD within mitochondria and leads to apoptosis-like cell death in vivo and in vitro using a murine system (12, 13). Addition of exogenous Aβ, both 1–40 (Aβ1–40) and 1–42 (Aβ1–42), to culture media caused mitochondrial dysfunction and apoptotic-like cell death in cortical neurons prepared from wild-type and transgenic (Tg) ABAD mice (Fig. S1). However, evidence of Aβ-induced neuronal perturbation was significantly enhanced in the ABAD-expressing cells, indicating that an enzyme in the mitochondrial matrix (ABAD) appears to exert toxic effects in response to the exogenous Aβ. These data suggested the possibility that Aβ added to the extracellular milieu gained access to the intracellular space and, subsequently, interacted with its intracellular target. These findings led us to probe mechanisms through which Aβ gains access to intracellular compartments.
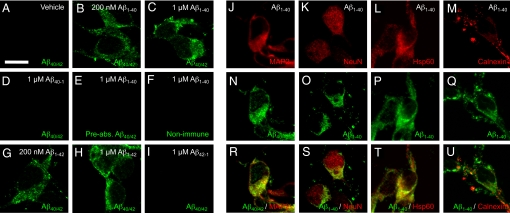
To evaluate cellular uptake of Aβ, we first measured levels of intracellular Aβ in neurons treated with the synthetic human Aβ peptides by ELISA using an antibody specific for the human form of Aβ to differentiate it from endogenous mouse Aβ. To remove Aβ bound to the cell surface, cells were treated with trypsin for 5 min before harvest for measurement of the intracellular human Aβ. As shown in Figs. S2 and S3, intracellular human Aβ content was at background levels in vehicle-treated neurons, whereas levels of intracellular human Aβ were significantly increased in mouse cortical neurons incubated with human Aβ1–40 and Aβ1–42. The accumulation of both Aβ1–40 and Aβ1–42 peptides occurred in a time- (Figs. S2 A and B and S3 A and C) and dose-dependent (Figs. S2C and S3 A and C) manner. Biochemical subcellular fractionation further revealed that the majority of the intracellular Aβ was detected in the mitochondria-enriched fractions (Fig. S3F) as compared with plasma membrane (Fig. S3E) and cytosolic fractions (Fig. S3G). As a complementary approach, we performed confocal microscopy using double immunofluorescence with antibodies to Aβ and intracellular markers, such as MAP-2 (neuronal marker), NeuN (neuronal marker), Hsp60 (mitochondrial marker), and calnexin (endoplasmic reticulum marker). After exposure (60 min) to human Aβ1–40 and Aβ1–42, but not Aβ40–1 (Fig. 1H) and Aβ42−1 (Fig. 1I), neurons displayed immunoreactivity to anti-human Aβ antibody (clone 4G8) in a cytosolic-like distribution, in addition to a cell surface-like staining pattern (Fig. 1 B, C, G, and H; double staining images with Hoechst 33342, Fig. S4 J and K). In contrast, immunoreactivity to anti-human Aβ antibody (clone 4G8) preabsorbed with Aβ1–40 (Fig. 1E) or to non-immune serum (Fig. 1F), was background level in the cells exposed to human Aβ1–40. Cells without Aβ treatment also showed no specific staining patterns (Fig. 1A). Intracellular Aβ1–40 was observed in cells stained positively for two neuronal markers, MAP2 (Fig. 1R) and NeuN (Fig. 1S). Further analysis using the mitochondrial marker Hsp60 demonstrated extensive colocalization with Aβ epitopes (Fig. 1T), although to a lesser extent with the endoplasmic reticulum marker calnexin (Fig. 1U). To confirm localization of Aβ to the intracellular space, we performed immunogold electron microscopy on cultured neurons. Immunogold particles labeled Aβ and were present in the intracellular space, such as the cytosolic compartment and mitochondria, after exposure of neurons to Aβ1–42. In contrast, the number of immunogold particles was significantly diminished in RAGE-deficient (RAGE−/−) neurons (Fig. 2C), as compared with wild-type (WT) neurons (Fig. 2 A and B). Gold particles were virtually absent when cells were treated with vehicle alone (without treatment of Aβ, Fig. S5 A and B) or Aβ1–42 antibody was replaced by non-immune IgG (Fig. S5 C and D). Quantification of the total number of gold particles per field, based on analysis of multiple images, confirmed a significant decrease Aβ-immunogold particles in RAGE−/− neurons as compared with WT neurons (Fig. 2D). These data suggest that exogenous Aβ gains access to intracellular compartments, such as mitochondria, and that absence of RAGE reduces Aβ transport to the intracellular compartment. Neurons exposed to 1 μM Aβ1–42 (Fig. S6, part 1) and lower concentration (200 nM) of Aβ1–40 (Fig. S6, part 2) showed a similar intracellular distribution of the peptide.
Fig. 1.
Confocal images of Aβ, MAP2, NeuN, Hsp60, and calnexin in cortical neurons after exposure to Aβ-related peptides. Cells were exposed to the indicated concentration [or 1μM (J−U)] of human Aβ1−40, Aβ1−42, reversed Aβ (Aβ40−1 and Aβ42−1), or vehicle for 60 min, fixed in 3% PFA, and stained by [anti-human Aβ (clone 4G8) (A–D, G−I, N, and R), anti-Aβ1–40 (O–P and S–U), preabsorbed anti-Aβ (clone 4G8) (E) or non-immune IgG (F)]/Alexa Fluor 488 anti-IgG (green), anti-MAP2/Alexa Fluor 568 anti-IgG (red) (J and R) and [anti-NeuN (K and S), anti-Hsp 60 (L and T), or anti-calnexin (M and U)]/Alexa Fluor 546 anti-IgG (red). Scale bar, 10 μm. Hoechst 33342 staining and phase contrast images of the same field of cells in panels of A, C, H, D, or Fig. S4 A–D are represented in Fig. S4 E–H and M–P, respectively.
Fig. 2.
Immunoelectron microscopy of Aβ in cortical neurons after exposure to Aβ. Cells were prepared from wild-type (WT) (A and B) and RAGE−/− mice (C), exposed to human 1 μM Aβ1–42 for 60 min, fixed in 4% PFA and 0.1% glutaraldehyde, and the ultra-thin sections were stained with rabbit anti-Aβ1–42/donkey anti-rabbit IgG conjugated to colloidal gold (18 nm particle). Arrows denote mitochondria. (Scale bar, 200 nm.) Two negative controls, in which cells were treated with vehicle or stained with non-immune IgG (NI-IgG), are represented in Fig. S5. (D) Quantification of Aβ immunogold particles in WT and RAGE−/− neurons after exposure to Aβ. Numbers of gold particles were counted per field of each microscopic image including two negative controls and expressed as mean ± SEM; ***, P < 0.001, versus WT; Unpaired t-test.
Blockade of RAGE Diminishes Aβ Uptake and Aβ-Induced Mitochondrial Dysfunction.
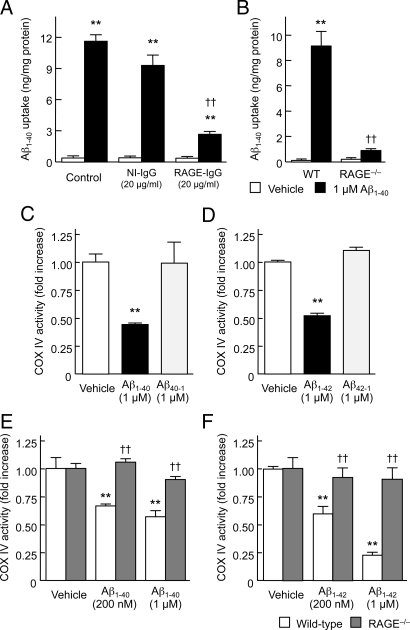
To determine the potential role of RAGE in neuronal Aβ transport, the effect of a blocking antibody to the receptor on Aβ uptake and neurotoxicity was examined in mouse cortical neuron cultures. Pretreatment of neuronal cultures with anti-RAGE IgG (N-16) for 2 h attenuated uptake of human Aβ1–40 (Fig. 3A) and Aβ1–40-induced mitochondrial dysfunction, at the level of MTT reduction (Fig. S7A). In contrast, non-immune IgG had no effect on either uptake of Aβ or MTT reduction. To further examine RAGE-dependent neuronal Aβ transport, neurons prepared from RAGE−/− mice were used. Neurons lacking RAGE showed a marked decrease in uptake of Aβ1–40 (Fig. 3B) and complete preservation of MTT reduction in the presence of Aβ1–40 (Fig. S7B). To examine the effect of RAGE on Aβ-induced mitochondrial dysfunction, we measured mitochondrial respiratory key enzyme cytochrome c oxidase (COX IV) activity in RAGE-deficient neurons as compared with COX IV activity in WT neurons. After exposure (24 h) to human Aβ1–40 (Fig. 3 C and E) and Aβ1–42 (Fig. 3 D and F), but not their reversed sequence peptides, neurons displayed a significant dose-dependent reduction in COX IV activity. Notably, RAGE deficiency completely reversed the Aβ1–40- and Aβ1–42-induced reduction in COX IV activity (Fig. 3 E and F), which is in agreement with the results of MTT reduction activity. These data indicate that RAGE contributes to transport of Aβ from the cell membrane to the intracellular space, and subsequent induction of mitochondrial dysfunction.
Fig. 3.
Blocking RAGE or genetic deletion of the receptor suppresses Aβ uptake and minimizes Aβ-induced mitochondrial dysfunction in cortical neurons. Intracellular levels of human Aβ1–40 (A and B) and COX IV activity (C–F) were assayed 60 min (A and B) and 24 h (C–F) after exposure to the indicated Aβ peptides. (A) Effect of a neutralizing antibody to RAGE. Cells were pre-treated with 20 μg/mL of anti-RAGE (N-16) IgG or NI-IgG for 2 h, and then exposed to 1 μM human Aβ1–40. (B, E, and F) Effect of genetic deletion of RAGE. Cells prepared from WT or RAGE−/− mice were exposed to the indicated concentrations of human Aβ1–40 (B and E) or Aβ1–42 (F). (C and D) Aβ-related peptides with the reverse sequence have no effect on mitochondrial function in cortical neurons. Cells prepared from wild-type mice were exposed to 1 μM human Aβ1–40 or Aβ40–1 (C), and 1 μM human Aβ1–42 or Aβ42–1 (D). Data represent mean ± SEM; **, P < 0.01, versus vehicle- and reversed Aβ-treated cells (A–D), or Aβ-treated RAGE−/− neurons (E and F); ††, P < 0.01, versus control (A and B) or WT (E and F).
Aβ/RAGE-Mediated Signaling Contributes to Aβ Transport and Internalization.
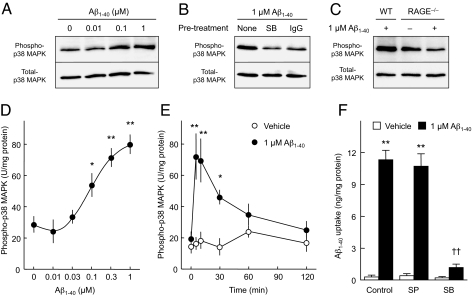
In many contexts, RAGE appears to function as a signal transduction receptor, activating multiple downstream intracellular pathways (22, 31). Thus, we sought to determine if RAGE-mediated cellular activation of such intracellular mechanisms might impact on neuronal Aβ transport. We started by examining the effect of Aβ treatment on phosphorylation of SAPK/JNK and p38 MAPK. Exposure of neurons to Aβ1–40 for 10 min did not affect levels of total or phosphorylated forms of SAPK/JNK (Fig. S8A). In contrast, neurons exposed to Aβ1–40 displayed a dose-dependent increase in phosphorylated p38 MAPK as compared to vehicle-treated controls (Fig. 4 A and D), although Aβ1–40 did not affect total protein levels of p38 MAPK (Fig. 4A). Aβ1–42 also stimulated p38 MAPK phosphorylation in a similar dose-dependent manner (Fig. S8B). Activation of p38 MAPK was observed immediately after Aβ1–40 treatment and for up to 30 min (Fig. 4E). Pretreatment of neuronal cultures with the p38 MAPK inhibitor SB203580 blocked Aβ1–40-stimulated p38 MAPK phosphorylation (Fig. 4B). Consistent with these data, neurons pretreated with SB203580, but not a SAPK/JNK inhibitor (SP600125), showed strong inhibition of Aβ1–40 uptake (Fig. 4F) and MTT reduction in response to Aβ1–40 (Fig. S8C). A role for RAGE in Aβ-mediated activation of p38 MAPK was indicated by inhibition of p38 phosphorylation in cortical neurons from wild-type mice exposed to Aβ in the presence of anti-RAGE IgG (N-16) (Fig. 4B) and in RAGE-deficient cortical neurons derived from RAGE−/− mice (Fig. 4C).
Fig. 4.
Aβ-stimulated p38 MAPK activation is required for Aβ uptake in cortical neurons. (A–C) Immunoblot analyses of phospho-p38 MAPK in cortical neurons treated with Aβ1–40. Cells were exposed to the indicated concentrations of human Aβ1–40 for 10 min, lysed, and subjected to SDS/PAGE. Typical immunoblot images detected by antibodies against phospho-p38 MAPK (A–C, upper) and total-p38 MAPK (A–C, lower) are shown from 3–6 independent experiments. (A) Dose-dependency. (B) The p38 MAPK inhibitor SB 203580 (1 μM; SB) and anti-RAGE (N-16) IgG (20 μg/mL; IgG) were added 30 min and 2 h before exposure to Aβ1–40, respectively. (C) Phospho-p38 MAPK levels in neurons from RAGE−/− mice after exposure to Aβ1–40. WT, wild-type. (D and E) Phospho-p38 MAPK levels were determined by ELISA. (D) Dose-dependency. Cells were exposed to the indicated concentration of human Aβ1–40 for 10 min. (E) Time course. Cortical neurons were exposed to 1 μM of human Aβ1–40 for the indicated time. (G) Effects of JNK and p38 MAPK inhibitors on intracellular levels of human Aβ1–40 in cultured neurons exposed to Aβ. Cells were pretreated with the JNK inhibitor SP 600125 (SP, 20 μM), or SB 203580 (SB, 1 μM), for 30 min, and then exposed to 1 μM human Aβ1–40 for 60 min. Intracellular Aβ1–40 concentrations were determined by ELISA. Data represent mean ± SEM; *, P < 0.05, **, P < 0.01, versus none (0 μM Aβ1–40) (D and F) and 0-time (E); ††, P < 0.01, versus control (no inhibitor).
Membrane RAGE Acts as an Aβ Carrier and Co-Internalizes with Aβ.
To determine molecular mechanisms underlying neuronal Aβ transport, we biotinylated neuronal cell surface proteins, incubated the labeled cells with Aβ1–40, and then analyzed internalized biotinylated proteins. First, we assessed the distribution of biotin in labeled cells before Aβ treatment. Cells fixed immediately after biotinylation and permeabilized with detergent displayed a cell surface and focal [the latter were probably surface accumulations of biotin since they were removed by sodium 2-mercaptoethanesulfonate (MesNa) treatment; see below] distribution of the biotin (Fig. S9D) and, as expected, the absence of Aβ (Fig. S9 A and G). Next, we examined the intracellular distribution of biotin and Aβ in the cells after Aβ treatment. After biotinylation, cells were incubated with vehicle or Aβ1–40 for 60 min, treated with MesNa (the latter to remove biotin remaining on the cell surface), fixed and permeabilized with detergent. Cells exposed to Aβ1–40 displayed an overlapping intracellular distribution of Aβ (Fig. S9 C and I) and biotinylated-proteins (Fig. S9 F and I), while control cells treated with vehicle alone showed no specific signal (Fig. S9 B, E, and H), suggesting that Aβ is able to interact with cell surface proteins.
To analyze internalized proteins in cells exposed to Aβ, we performed Western blotting. After biotinylation of surface proteins, cells were incubated with vehicle or Aβ1–40 for 60 min, treated with MesNa, and then whole cell lysates were collected and subjected to immunoprecipitation. Cell lysates contained same amount of total protein, in each case and from both groups, and were reacted with streptavidin followed by SDS/PAGE. Silver staining of gels revealed a broad array of protein bands, especially in cells exposed to Aβ1–40, compared with controls (Fig. S10A). Interestingly, immunoblotting with anti-RAGE IgG demonstrated >8-fold more RAGE antigen had been immunoprecipitated from cells exposed to Aβ1–40, compared with non-treated control (Fig. S10B). To determine whether RAGE and Aβ were in the cytosol, we performed immunoprecipitation with anti-Aβ IgG-conjugated beads using the cytosolic fraction from neurons exposed to Aβ. Such cytosolic fractions were obtained by ultracentrifugation (13, 32) and showed virtually undetectable levels of the membrane marker Na+/K+-ATPase, compared with presence of the latter in whole cell lysates or membrane-enriched fractions (Fig. S10C). Immunoprecipitation analysis was also applied to cytosolic fractions using anti-Aβ IgG-conjugated beads or non-IgG-conjugated beads as a control for nonspecific binding. SDS/PAGE of these immunopreciptates was followed by immunoblotting with anti-RAGE IgG. While there was only a weak signal with immune precipitates prepared in the presence of non-IgG beads, the immune precipitates prepared with anti-Aβ IgG beads demonstrated a strong immunoreactive RAGE band (Fig. S10D). Based on image analysis, there was >4-fold more RAGE antigen detected in the immune precipitates with anti-Aβ IgG beads compared with non-IgG beads. These data are consistent with the hypothesis that Aβ stimulates internalization of RAGE, and that during this process, RAGE and Aβ interact closely.
To further assess possible colocalization of RAGE and Aβ, and the spatial topography of these two molecules after internalization of Aβ, we performed dual fluorescence confocal microscopy. Incubation of Aβ1–40 with neurons for 60 min demonstrated extensive colocalization of epitopes visualized with anti-RAGE and anti-Aβ antibodies (Fig. S11).
Aβ Colocalizes with RAGE in Hippocampus of Aged Tg-mAPP Mice.
To extrapolate these findings to the in vivo setting, we turned to a mouse model of AD-like pathology, transgenic mice overexpressing the human APP isoforms (APP695 and APP751/770) with the familial Alzheimer's dementia mutation (Tg mAPP) and Aβ. Immunohistochemical studies were performed to colocalize intracellular Aβ and RAGE in brains from 9- to 10-month-old mice after permeabilizing the cell membrane with detergent. Compared with wild-type controls (Fig. S12 A and C), low power immunofluorescence images of brain sections from aged Tg mAPP mice displayed increased staining for Aβ (Fig. S12B) and RAGE (Fig. S12D) antigens in the hippocampus, especially in the pyramidal cell layer. Plaques in Tg mAPP mice displayed strong staining for Aβ (Fig. S12B). High power confocal immunofluorescence images of the hippocampal CA3 region in Tg mAPP mice further demonstrated that Aβ and RAGE co-localized in an apparently intracellular distribution in pyramidal cells (Fig. S12 F, H, and J).
Discussion
Our studies address a paradigm in which Aβ binding to cell surface RAGE translocates the ligand into the cytosolic compartment. Our in vitro studies show that: (i) exogenous Aβ translocates from the cell surface to the cytosol, with at least some of the peptide eventually localizing in mitochondria; (ii) such translocation is dependent on RAGE, as it is prevented by blocking antibodies to the receptor and does not occur to an appreciable extent in neurons devoid of RAGE (from RAGE−/− mice); (iii) RAGE-mediated cellular activation at the level of p38 MAPK has a central role in internalization of the receptor-ligand complex; and, (iv) the presence of Aβ within the cytosol and mitochondria is associated with functional consequences, including mitochondrial dysfunction. Immunoprecipitation of cytosolic fractions after Aβ treatment showed that RAGE itself interacts closely with Aβ, consistent with the concept that the receptor may be the actual Aβ transporter/carrier. As a counterpart to these observations in cell culture, immunohistochemical studies showed colocalization of Aβ and RAGE in an apparently intracellular distribution in hippocampal pyramidal cells in the brains of AD-type transgenic mice expressing mAPP/Aβ.
Increasing evidence points to a role for intraneuronal Aβ in the pathogenesis of early neural dysfunction and AD pathology. Several observations have indicated that APP localizes not only to the plasma membrane, but also to the trans-Golgi network, endoplasmic reticulum, and endosomal, lysosomal, and mitochondrial membranes (5, 7, 33). Thus, two possible pathways could underlie the accumulation of intraneuronal Aβ: (i) Aβ secreted into extracellular space is subsequently taken up by neurons (and/or other cells); and, (ii) Aβ produced intracellularly remains within the neuron. Our results provide insight into the former pathway, which involves neuronal internalization of both Aβ1–40 and Aβ1–42. Initially, based on in vitro studies, it was thought that Aβ1–42 was more neurotoxic than Aβ1–40, in part because of the propensity of Aβ1–42 to form large aggregates and fibrils. However, more recently, it has been appreciated that oligomeric and prefibrillar Aβ1–40 and Aβ1–42 have similar cytotoxic effects (34) and such soluble forms of Aβ are believed to play a critical role in the pathogenesis of AD. Recent work has demonstrated that oligomeric Aβ1–42, at a concentration of 200 nM, is capable of blocking long-term potentiation at cortical synapses in the hippocampus and entorhinal cortex (10, 28, 35, 36). Taken together, our findings suggest that via RAGE, neuronal transmembrane transport of Aβ1–40 and Aβ1–42 carries soluble assemblies of amyloid peptide into the cell.
The present study revealed that intraneuronal accumulation of Aβ could be sustained during exposure to the peptide, especially in mitochondria, as previously reported (10, 12, 37–40). Considerable studies over the past decade have emerged indicating that some intracellular enzymes, insulin-degrading enzyme, endothelin-converting enzyme (ECE)-1b and ECE-2, as well as membrane enzymes, such as neprilysin, ECE-1a, ECE-1c, ECE-1d, matrix metalloproteinase (MMP)-2, MMP-3, and MMP-9, can cleave Aβ at either a single or multiple sites and cleavage products of Aβ resulting from such catabolism are less likely to aggregate and are less neurotoxic than Aβ itself (41). Moreover, a mitochondrial peptidase, PreP peptidasome, has been recently shown to be capable of degrading Aβ (42). As these various amyloid-degrading enzymes have distinct subcellular localization, Aβ metabolism may influence the subcellular accumulation of Aβ and its neurotoxicity. The mechanism through which intraneuronal Aβ is metabolized will require further study to elucidate.
Recent studies demonstrate that several plasma membrane receptors, such as N-methyl-D-aspartate receptors (14), α7 nicotinic acetylcholine receptors (15), and low-density lipoprotein receptor-related proteins (LRP) (16), have the capacity to bind to Aβ and, potentially, promote intracellular accumulation of Aβ. Previous studies have shown that RAGE binds monomeric, oligomeric, and even fibrillar forms of Aβ at the neuronal cell surface (22, 27, 43). Moreover, RAGE promotes Aβ-induced neuronal dysfunction in a mouse model of AD-type pathology (28). Subsequent to Aβ binding to RAGE on the cell surface, we have found that the amyloid peptide is internalized in a RAGE-dependent manner; blocking RAGE or deletion of the receptor attenuates Aβ internalization and Aβ-induced mitochondrial dysfunction in cortical neurons. These findings strongly suggest a role for RAGE as a cell surface-binding site and a potential transporter for Aβ which facilitates intracellular transfer of the peptide.
RAGE-ligand interaction has been shown to activate multiple intracellular signaling pathways including the MAPKs (ERK1/2, p38 MAPK and SAPK/JNK), rho-GTPases, phosphoinositol-3-kinase, and the JAK/STAT pathway in various cells (23, 43). In addition, the RAGE-ligand interaction has been shown to directly cause generation of reactive oxygen species via NADPH oxidases (44). As a consequence of Aβ−RAGE interaction, activation of p38 MAPK, SAPK/JNK, and NF-κB was observed in sporadic AD cybrids (45). In addition, Arancio et al. (28) reported increased phosphorylation of CREB, ERK1/2, p38 MAPK, and CaMKII in hippocampal extracts from Tg mice overexpressing RAGE and mAPP. RAGE-dependent activation of p38 signal transduction also plays an important role in Aβ-mediated synaptic failure (35, 36). However, direct links between RAGE-mediated signaling pathways and Aβ neurotoxicity remain to be fully elucidated. The present study indicates that the Aβ-RAGE interaction rapidly activates p38 MAPK, but not SAPK/JNK, and further demonstrates a link between activation of p38, intracellular Aβ accumulation, and Aβ-induced cytotoxicity in cortical neurons.
In the BBB endothelial cells, RAGE and LRP1 have shown to be critical for regulation of Aβ homeostasis in the central nervous system (46). RAGE binds soluble Aβ at the apical side of human BBB, and promotes transport of soluble Aβ from blood to brain via endocytosis and transcytosis. These events promote Aβ accumulation in brain parenchyma (29, 47). Our biotinylation study revealed that Aβ stimulated internalization of neuronal plasma membrane proteins, including RAGE, and that RAGE−Aβ complex was present intracellularly. These finding suggest that the interaction of Aβ with RAGE activates an endocytosis-like pathway that causes rapid internalization of Aβ−RAGE complex. Consistent with these in vitro results, recent studies in brains of AD patients (48) and another mouse AD model (49) displayed striking accumulation of Aβ in hippocampal pyramidal cells.
In conclusion, our study demonstrates that Aβ induces a RAGE-dependent pathway that involves activation of p38 MAPK, resulting in internalization of Aβ and leading to mitochondrial dysfunction in cultured cortical neurons. We propose that Aβ internalization may be associated with RAGE-mediated endocytosis and that RAGE itself may act as a carrier in transmembrane Aβ transport. The mechanism through which Aβ gains access to the cytosol and enters mitochondria will require further study to elucidate. Cytosolic Aβ may enter mitochondria through the TOM pathway as recently reported (39) leading to mitochondrial stress. The results of our studies contribute to a growing body of evidence demonstrating that RAGE can act as a receptor magnifying intraneuronal Aβ cytotoxicity. Blockade of RAGE may have a beneficial effect by limiting intracellular accumulation of amyloid in AD brain and serves a potential therapeutic target for AD.
Materials and Methods
For full description of this study's materials and methods, see SI Materials and Methods.
Animals.
RAGE knockout (RAGE−/−) mice have been described previously (35, 50).
Cell Culture.
Cortical neurons were prepared from embryos at 17 days of gestation of C57BL/6J mice, transgenic mice overexpressing the human full-length ABAD (Tg-ABAD mice) and homozygous RAGE−/− mutant mice.
Biochemical Determination of Neuronal Perturbation.
Neuronal perturbation after Aβ treatment was determined by generation of reactive oxygen species (ROS), mitochondrial membrane potential, caspase activity, DNA fragmentation, MTT reduction, and cytochrome c oxidase (COX IV) activity assays.
Determination of Membrane Aβ Transport.
Transport of Aβ into cytosol through the plasma membrane was measured by ELISA and detected by confocal immunofluorescence and immunoelectron microscopies using anti-Aβ IgG.
Measurement of Phospho-MAPKs.
Aβ-stimulated phosphorylation of SAPK/JNK and p38 MAPK was detected by Western blot analysis or measured by ELISA.
Analysis of Internalization of Membrane Surface Proteins.
Internalization of membrane surface proteins after Aβ treatment was detected by Western blot analysis using biotinylation and immunoprecipitation.
Immunohistochemistry.
Immunohistochemistry was executed in hippocampal sections from Tg mAPP mice (9- to 10-month-old) and age- and strain-matched wild-type mice using anti-Aβ IgG and anti-RAGE IgG.
Statistics.
Statistical analysis of the experimental data were carried out using GraphPad Prism 4 for Macintosh (GraphPad Software). The significance of differences was determined by a one-way ANOVA, followed by the Dunnett's or Tukey's multiple comparison test for multigroup comparisons. Unpaired t-test was used for two-group comparisons. The criterion for statistical significance was P < 0.05.
Supplementary Material
Acknowledgments.
This study was supported in part by a grant for the 21st Century Centers of Excellence Program (1640102) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a Grant-in-Aid for Scientific Research (18590050) from the Japan Society for the Promotion of Science, and a grant from Takeda Science Foundation. This work was also supported by grant from the U.S. Public Health Service (PO1AG17490).
Footnotes
Conflict of interest statement: D. Stern is a consultant for TransTech Pharma.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905686106/DCSupplemental.
References
- 1.Yankner BA. Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 2.LaFerla FM, Oddo S. Alzheimer's disease: Aβ, tau, and synaptic dysfunction. Trends Mol Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Gouras GK, et al. Intraneuronal Aβ42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi RH, et al. Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:869–879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 6.Reddy PH, Beal MF. Amyloid β, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin MT, Beal MF. Alzheimer's APP mangles mitochondria. Nat Med. 2006;12:1241–1243. doi: 10.1038/nm1106-1241. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Stern D, Yan SD. In: Neurobiology of Alzheimer's Disease. 3rd Ed. Dawbarn D, Allen SJ, editors. New York: Oxford Univ. Press; 2007. pp. 227–244. [Google Scholar]
- 9.Caspersen C, et al. Mitochondrial Aβ: A potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 10.Du H, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lustbader JW, et al. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 13.Takuma K, et al. ABAD enhances Aβ-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- 14.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 15.Nagele RG, D'Andrea MR, Anderson WJ, Wang HY. Intracellular accumulation of β-amyloid1–42 in neurons is facilitated by the α7 nicotinic acetylcholine receptor in Alzheimer's disease. Neuroscience. 2002;110:199–211. doi: 10.1016/s0306-4522(01)00460-2. [DOI] [PubMed] [Google Scholar]
- 16.Zerbinatti CV, et al. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Aβ42 accumulation in amyloid model mice. J Biol Chem. 2006;281:36180–36186. doi: 10.1074/jbc.M604436200. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer's disease cybrids enhances Aβ toxicity. J Neurochem. 2004;89:1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 18.Ren Y, et al. Endophilin I expression is increased in the brains of Alzheimer disease patients. J Biol Chem. 2008;283:5685–5691. doi: 10.1074/jbc.M707932200. [DOI] [PubMed] [Google Scholar]
- 19.Yao J, et al. Interaction of amyloid binding alcohol dehydrogenase/Aβ mediates up-regulation of peroxiredoxin II in the brains of Alzheimer's disease patients and a transgenic Alzheimer's disease mouse model. Mol Cell Neurosci. 2007;35:377–382. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Neeper M, et al. Cloning and Expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 21.Schmidt AM, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- 22.Chen X, et al. RAGE: A potential target for Aβ-mediated cellular perturbation in Alzheimer's disease. Curr Mol Med. 2007;7:735–742. doi: 10.2174/156652407783220741. [DOI] [PubMed] [Google Scholar]
- 23.Bucciarelli LG, et al. RAGE is a multiligand receptor of the immunoglobulin superfamily: Implications for homeostasis and chronic disease. Cell Mol Life Sci. 2002;59:1117–1128. doi: 10.1007/s00018-002-8491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bierhaus A, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 25.Yan SD, et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 26.Yan SD, et al. Nonenzymatically glycated tau in Alzheimer's disease induces neuronal oxidant stress resulting in cytokine gene expression and release of Aβ. Nat Med. 1995;1:693–699. doi: 10.1038/nm0795-693. [DOI] [PubMed] [Google Scholar]
- 27.Lue LF, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer's disease: Identification of a cellular activation mechanism. Exp Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- 28.Arancio O, et al. RAGE potentiates Aβ-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giri R, et al. β-Amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol. 2000;279:C1772–C1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- 30.Deane R, et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurt CM, Feng FY, Kobilka B. Cell-type specific targeting of the α2c-adrenoceptor: Evidence for the organization of receptor microdomains during neuronal differentiation of PC12 cells. J Biol Chem. 2000;275:35424–35431. doi: 10.1074/jbc.M006241200. [DOI] [PubMed] [Google Scholar]
- 33.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cecchi C, et al. Increased susceptibility to amyloid toxicity in familial Alzheimer's fibroblasts. Neurobiol Aging. 2007;28:863–876. doi: 10.1016/j.neurobiolaging.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Origlia N, et al. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-β-mediated cortical synaptic dysfunction. J Neurosci. 2008;28:3521–3530. doi: 10.1523/JNEUROSCI.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Origlia N, et al. Aβ-dependent Inhibition of LTP in different intracortical circuits of the visual cortex: The role of RAGE. J Alzheimers Dis. 2009;17:59–68. doi: 10.3233/JAD-2009-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manczak M, et al. Mitochondria are a direct site of Aβ accumulation in Alzheimer's disease neurons: Implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 38.Yao J, et al. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0903563106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansson Petersen CA, et al. The amyloid β-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci USA. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crouch PJ, et al. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-β1–42. J Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miners JS, et al. Aβ-degrading enzymes in Alzheimer's disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falkevall A, et al. Degradation of the amyloid β-protein by the novel mitochondrial peptidasome, PreP. J Biol Chem. 2006;281:29096–29104. doi: 10.1074/jbc.M602532200. [DOI] [PubMed] [Google Scholar]
- 43.Ding Q, Keller JN. Evaluation of rage isoforms, ligands, and signaling in the brain. Biochim Biophys Acta. 2005;1746:18–27. doi: 10.1016/j.bbamcr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Wautier MP, et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 45.Onyango IG, Tuttle JB, Bennett JP., Jr Altered intracellular signaling and reduced viability of Alzheimer's disease neuronal cybrids is reproduced by β-amyloid peptide acting through receptor for advanced glycation end products (RAGE) Mol Cell Neurosci. 2005;29:333–343. doi: 10.1016/j.mcn.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Deane R, et al. Clearance of amyloid-β peptide across the blood-brain barrier: Implication for therapies in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackic JB, et al. Human blood-brain barrier receptors for Alzheimer's amyloid-β 1–40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102:734–743. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Andrea MR, Derian CK, Santulli RJ, Andrade-Gordon P. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology. 2001;38:120–134. doi: 10.1046/j.1365-2559.2001.01082.x. [DOI] [PubMed] [Google Scholar]
- 49.Oakley H, et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: Potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakaguchi T, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–972. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.