Abstract
The vertebrate central nervous system is organized in modules that independently execute sophisticated tasks. Such modules are flexibly controlled and operate with a considerable degree of autonomy. One example is locomotion generated by spinal central pattern generator networks (CPGs) that shape the detailed motor output. The level of activity is controlled from brainstem locomotor command centers, which in turn, are under the control of the basal ganglia. By using a biophysically detailed, full-scale computational model of the lamprey CPG (10,000 neurons) and its brainstem/forebrain control, we demonstrate general control principles that can adapt the network to different demands. Forward or backward locomotion and steering can be flexibly controlled by local synaptic effects limited to only the very rostral part of the network. Variability in response properties within each neuronal population is an essential feature and assures a constant phase delay along the cord for different locomotor speeds.
Keywords: basal ganglia, brainstem, computational model, lamprey, spinal CPG
Vertebrate behavior depends on different sets of neuronal networks specialized to coordinate different motor tasks like locomotion, breathing, and the expression of emotions (1–5). The activity of these networks is governed by brainstem command centers that are, in turn, under the control of the basal ganglia (1, 2). Although the critical role of these networks is well established, their intrinsic mode of operation has, for the most part, remained enigmatic. The lamprey nervous system provides one of the few examples in which detailed knowledge is available, not only of the different types of the participating neurons, but also their membrane properties and synaptic connectivity, particularly with regard to the spinal network (6, 7). Even with this knowledge at hand, it is very difficult, if not impossible, to establish, without the help of modeling, whether the experimental data available are actually sufficient to account for the dynamic operation of the network—and enable exploration of the particular role of different cellular and synaptic components to make predictions of their functional role. To achieve this, we report here a biophysically realistic model of the locomotor network and its control from the brain. The spinal network is composed of excitatory and inhibitory model neurons that, in considerable detail, behave as their biological counterparts. It proved to be important to introduce within each pool of model neurons the known biological variability in neuronal size and membrane properties. Thanks to recent developments in computer technology, we have been able to make a full-scale model of the network with 100 model neurons per segment, each of the Hodgkin–Huxley type—altogether 10,000 neurons connected via 760,000 synapses in the 100 segments. We represent the brainstem command system, as well as the forebrain control from the basal ganglia with an additional 3,500 neurons. Simulations limited to subsamples of neurons have previously been presented, not only from the lamprey (8–10), but also for the amphibian tadpole and the feline nervous system (11–13) and different invertebrate central pattern generators (CPGs) (14).
The biological network is flexible and operates over a wide range of frequencies, producing undulatory locomotor movements that travel along the body with a constant phase lag between each segment (≈1% of the cycle duration), resulting in a total lag from head to tail of approximately a full undulatory wave (see Fig. 2A). The speed and direction of the locomotor wave is, however, modifiable and can be reversed, because it occurs in backwards swimming when the animal needs to retract backwards (15). The question of how the phase lag is generated has previously been addressed both experimentally and by using computational models (8, 9, 16–20). Previous biophysical models have had difficulties in reproducing the apparent frequency independence of the phase lag, which is a characteristic of normal swimming at different speeds. We show here how this can be achieved, using known asymmetric anteroposterior synaptic projections in interneuronal populations that have a biologically realistic degree of variability in neuronal size and membrane properties. The direction of the locomotor wave (i.e., forward or backward) along the entire spinal cord can be controlled in the model by signals directed exclusively to the very rostral part of the spinal cord (one-tenth of its length; see Fig. 2C Inset), which corresponds to the rich brainstem innervation of the rostral cord (21). Under these conditions, the spinal CPG network will be able to adjust the phase lag appropriately along the entire spinal cord due to inherent network properties. We show that the variability in cellular properties (Fig. 1A) within a given neuronal population is of critical importance for this local control of the coordination.
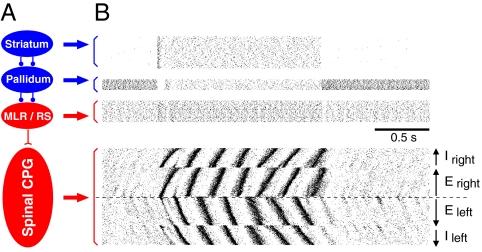
Fig. 2.
Control of the direction of swimming. (A) Pattern of neuron activity for the forward and backward swimming in specified segments on one side of the spinal cord. (B) Activity in four simulated populations, the left and right E and I interneurons. Dots correspond to the spikes in single neurons. Neurons are ordered in a rostrocaudal direction according to the arrows. (C and D) Controlling the intersegmental phase lag by the rostral command. Somatic current injection is applied to interneurons in the first 10 segments, with a linear decay (C Inset). The magnitude of the intersegmental phase lag varies continuously with the current injection for both the hemispinal cord (open squares) and intact spinal cord networks (filled circles; C). Only the left E population of the intact cord network is shown (D) in five different cases with different stimulation levels from +0.05 nA to −0.1 nA. In each sequence, the most rostral segment is represented in the uppermost position and the neurons in segment 100 in the lowermost position. Note that the positive phase lag is the largest with +0.05 nA applied to the 10 most rostral segments and the most negative with −0.1 nA.
Fig. 1.
Full-scale simulation of the spinal locomotor network of lamprey. (A) Mean firing rate (thick line) as a function of the somatic current injection. The shaded area shows maximum variation of the spike frequency in the simulated population. (B and C) Organization of the synaptic connections in transverse (B) and longitudinal (C) views of the spinal cord. Caudally directed projections dominate. E neurons project ipsilaterally, i.e., their axons do not cross the midline, and I neurons project contralaterally.
Results
Here, we use a full-scale model of the whole lamprey spinal CPG simulated on an IBM BlueGene/L supercomputer to investigate mechanisms underlying a flexible and biologically feasible control of the coordination along the spinal cord as an emergent property of the distributed spinal neural network. Each excitatory (E) and inhibitory (I) neuron is modeled in a biophysically detailed manner (22) (see supporting information (SI) Movie S1, Movie S2, and Movie S3). Populations of 6,000 E and 4,000 I cells are simulated by using an 86-compartmental neuronal model (22, 23) and a total of 760,000 synapses. The synapses and model neurons have properties that very closely resemble those of their biological counterparts, causing them to respond in the same way to synaptic input. The different types of ion channels established experimentally are also modeled with voltage-dependent Na+, subtypes of Ca2+, K+ channels and Na+- and Ca2+-gated K+ channels (22). Cells are placed randomly within a space corresponding to the spinal cord (see Methods).
There is a considerable degree of variability in neuronal size and membrane properties within each pool of interneurons in the spinal cord, and the corresponding variability was introduced in each pool of model neurons. Fig. 1A shows how the response to current injection varies between different neurons within one pool. The excitatory and inhibitory synaptic connections are set, based on experimental data (see Fig. 1 B and C and Network Model in Methods). The model network could be activated either by (i) simulating the commonly used experimental procedure by applying glutamate agonists in the bath, or (ii) by letting a population of reticulospinal model neurons (n = 200) activate the network as would occur under physiological conditions. The activity of each of the 10,000 neurons in the model spinal cord are displayed as color-coded spheres with blue indicating inhibition, red spheres cells indicating firing action potentials, and green indicating a subthreshold depolarization (see Movie S1 and Movie S2).
Simple Control Principles Determine Direction of Locomotor Wave (Forward–Backward).
The simulated network (Fig. 1 B and C) produces alternating locomotor-like output in E and I interneuron populations on both the left and right sides of the spinal cord (Fig. 2B) in response to a tonic excitation supplied to all spinal neurons via excitatory (AMPA) synapses. As in experiments and previous models (10, 24–27), the frequency of the rhythm increases with the intensity of the tonic drive, i.e., increased reticulospinal drive or agonist drive. The activity of E and I interneurons is represented in Fig. 2B for both the left and right sides at different distances from the rostral to the most caudal level (indicated by arrows to the right) in E and I interneurons, respectively. Note that with the asymmetric axonal distribution used here (28), there is a constant phase lag along the entire simulated spinal cord, and the total phase lag from head to tail is close to one cycle as during normal locomotion.
Experimentally, the overall phase lag along the spinal cord can be increased, reduced, or even reversed if the excitability of a few segments at the head or tail end of the spinal cord is modified (19). In Fig. 2 C and D, we show that the intersegmental phase lag along the entire spinal model network can be modified by applying additional positive or negative synaptic current to only the 10 most rostral segments (Fig. 2C, drawing; see also Movie S3 and Movie S4). Fig. 2D illustrates that a local rostral command can modify the rostrocaudal delay in a broad range from a positive to a negative phase lag value along the entire spinal cord. The control activity (0 nA) with tonic excitatory drive to the whole spinal cord shows the uniform phase lag of neuronal activity from head segments to the tail. When +0.05 nA is added to the few rostral segments, there is a uniform increase of the phase lag, whereas, if instead, a range of negative currents from −0.025 to −0.1 nA is applied, there is a progressive reduction of the phase lag, followed by a reversal to a posterior–anterior direction—again along the entire spinal cord. The graph in Fig. 2C shows that the segmental phase lag can be controlled in a graded way between +2% and −2%, similar to the range obtained experimentally (19). It would thus be possible to switch between forward and backward locomotion (19) just by command signals acting on a few rostral segments and not to the entire or caudal part of the spinal cord, as sometimes has been assumed.
To explore the possible role of reciprocal inhibition for the longitudinal coordination, we modeled not only the whole spinal cord but also the hemispinal cord network (23, 29), which consists of only segments of the left or right side where there is no inhibition from the contralateral side, but in which a unilateral phase lag is still known to occur experimentally. Fig. 2C (open symbols) shows that also in the hemicord the phase lag can be controlled from the rostral segments, but in a narrower range and with much greater sensitivity to changes in the degree of activation. The dynamic range is thus much broader in the intact cord than in the hemicord, suggesting that the reciprocal inhibition between hemisegments is important for a reliable and efficient control of the phase lag of the swimming pattern. Furthermore, in simulations of the intact spinal cord, a change of the rostral command was applied during stable forward swimming activity, which resulted in a rapid change in the rostrocaudal coordination within a few cycles. In a simulated hemicord, the pattern can also be reversed, but the process is in this case much slower, extending over many cycles, and it is also less stable. This is also illustrated in Fig. S1. The overall quality of the pattern, such as the constancy of the intersegmental phase lag along the body, is also lower in the hemicord. This corroborates the conclusion that the reciprocal inhibition between the two sides of the spinal cord is vital, not only for assuring a left–right alternation but also for achieving a reliable intersegmental coordination.
Higher-Level Control of the Locomotor CPG–Brainstem Locomotor Command Regions and the Basal Ganglia.
There is a two-level control hierarchy (1, 2, 7), whereby the brain controls the spinal locomotor network (Fig. 3A). The direct control is exerted by evolutionary conserved locomotor command regions (MLR) that via reticulospinal neurons (RS) directly regulate the level of activity in the spinal CPG. The level of activity in these command regions is, in turn, under the control of the basal ganglia (striatum and pallidum), through a selective and gradual release from tonic inhibition (30, 31). Stimulation of the lamprey striatum can elicit locomotion, and the locomotor command regions are known to be under tonic inhibition at rest (30). Here, we model the entire control system that would normally be involved in activating the locomotor behavior, extending from striatum to the end of the spinal cord.
Fig. 3.
Initiation of locomotor activity from the basal ganglia–brainstem. (A) Scheme of the basal ganglia–brainstem control of locomotor behavior. The neurons in the basal ganglia output stage, the pallidum, are during resting conditions tonically active, thus keeping motor centra in the brainstem (MLR, mesencephalic locomotor region and reticulospinal neurons, RS) under tonic inhibition. It is only after a strong excitatory input to the basal ganglia input stage, the striatum, that the striatal projections neurons can inhibit the tonic activity in the pallidal neurons, thus releasing the brainstem command centra from tonic inhibition. (B) This control of the spinal CPG is simulated. The resting CPG network receives a low synaptic drive from the brainstem motor centra (reticulospinal neurons, RS) because these are inhibited by pallidal cells. When the activity is suddenly released in the reticulospinal neurons because of disinhibition through the basal ganglia, the locomotor activity in the spinal network appears. Both an appropriate locomotor frequency and coordination is, as a consequence, immediately achieved.
In the simulations of Fig. 3B we have merged the brainstem locomotor command region with the reticulospinal level (MLR/RS) to one level of command neurons. Each such neuron activates both inhibitory and excitatory neurons along the entire spinal cord with a projection probability of ≈10%. Fig. 3B shows the neuronal activity at the different hierarchical levels as raster plots. It shows that when a population (n = 200) of reticulospinal model neurons increase their level of activity (see Fig. 3 legend) the locomotor network turns on almost instantaneously (Fig. 3B Lower) with appropriate intersegmental coordination. The activity of the E and I neurons on both sides is activated in an orderly fashion from the most rostral segment to the most caudal segments (illustrated as in Fig. 2B). Clearly, the spinal network can be controlled in a very efficient way with practically no delay by using a simulated reticulospinal drive.
The enhanced activity in the reticulospinal model neurons (MLR/RS) in Fig. 3B results, in turn, from a removal of inhibitory activity from the modeled output stage of the basal ganglia (pallidum). Biologically, it is well documented that these inhibitory neurons are spontaneously active under resting conditions and that they target the locomotor command regions from lamprey to mammals and that an experimental blockade of the tonic inhibition releases locomotor activity (30, 31). We have included the basal ganglia control by modeling the pallidal output neurons (n = 300), spontaneously active at rest, and the striatal (n = 3000) neurons being silent at rest. Fig. 3B shows that when the striatal model neurons are activated, the pallidal neurons are turned off, and thereby the MLR/RS activity is enhanced and the spinal locomotor network activated.
We have also shown that an additional modulation of the rostral part of only the spinal cord as in Fig. 2 C and D can be mediated by a subset of rostrally projecting reticulospinal neurons (see also Movie S3 and Movie S4). They are, in turn, activated through a disinhibition from the basal ganglia. They can modify the intersegmental coordination—to enhance or reduce the phase lag along the entire spinal cord.
The activity of the spinal CPGs can thus be turned on and off, their level of activity varied, and the size and direction of the phase lag controlled from the level of the basal ganglia. Although we do not, as yet, have as detailed information on the cellular and synaptic properties of these neurons, as for the model neurons at the spinal level, these simulations provide a proof of principle. The basal ganglia structure and function are conserved in considerable detail between lamprey and mammals (1, 32, 33), even at the level of cellular properties, transmitters, and afferent inputs.
Steering of Locomotion.
A turning response (left or right) during locomotion will occur when there is an enhanced activity of the reticulospinal neurons on one side, leading to a more pronounced motor activity on that side (see ref. 7). In Fig. 4 we have modeled this process as initiated from the striatum by including two separate subpopulations of striatal and pallidal neurons that control the level of activity of a portion of the reticulospinal neurons on the left and right side, respectively. When symmetric locomotor activity (MN) has been introduced through striatal activation (blue trace; same model design as in Fig. 3; see also legend), the subpopulation controlling the right side (green) is activated for a short period leading to an enhanced activity of the reticulospinal on the right side and a more prominent bursting on that side. This would lead to a turning response. We thus show that an enhanced reticulospinal activity on one side would lead to increased bursts on this side, which corresponds to the experimental findings. With regard to the striatal subpopulation that is assumed to generate the turning response, this is a prediction of the model that needs to be tested experimentally.
Fig. 4.
Left–right steering of locomotion initiated from the basal ganglia. Locomotor activity was initiated by an enhanced activity in striatal neurons (blue trace) that, in turn, inhibits the tonic activity in pallidum (blue trace). The disinhibition of MLR/RS leads to an enhanced activity that turns on the spinal locomotor activity. Alternating activity is indicated by motoneuronal activity (MN) on the left (red) and right side (green). Activity of the neuronal pools is shown as relative changes of the firing rate in each population. Left and right MN activity is represented as the summed activity over the 10 most rostral segments. The drive signal to the striatal neurons is symmetric and bilateral and leads via the pallidum to a symmetric activation of the left and right MLR/RS populations. The model design for forward locomotion (blue trace) is identical to that in Fig. 3, but in addition, we have introduced two separate unilateral population of striatal neurons one for the left (red) and one for the right side (green), that will inhibit the corresponding unilateral subpopulations in the pallidum, which project specifically to the right or left side MLR/RS cells. The increased activity of the striatal right population (green) leads to the expected changes in the pallidal subpopulation and an elevation of the right MLR/RS population and finally an enhanced asymmetric MN alternations with the right side dominating. This would cause a turning to the right in the living animal. When the steering command is terminated, the network returns to the forward swimming mode.
Cellular Basis of Segmental Phase Coupling—Importance of Variability of Cellular Properties.
The ability of the spinal cord to generate an approximately constant phase lag (percent of cycle duration) along the spinal cord during locomotion at different frequencies is a nonobvious feature that needs to be considered. During actual locomotion, interneurons often fire only one spike per cycle (29), which has been difficult to account for in previous biophysically detailed models. This also applies to the simulated conditions, when neurons often fired no more than one spike per activity cycle, and the overall pattern was shaped by population bursts due to the spike synchronization and cell recruitment in the interneuronal subpopulations (23). To incorporate the variability in neuronal properties found experimentally (29, 34), normally distributed values for single-cell properties were used (see Fig. 1A, inhomogeneous population). This yielded a triangle-like bursting output pattern with a characteristic gradual rise and decay phase in the simulated motoneurons (Fig. 5A, solid line), similar to those obtained experimentally (35–37). This feature is observed in a broad frequency range. The model motoneurons integrate the output from the presynaptic E and I populations extending over several segments (Fig. 1C). For comparison, when more homogenous parameter values (Fig. 5A, dotted red line) were used, a different membrane potential trajectory with sharper onset and a shape somewhat similar to single excitatory postsynaptic potentials (EPSP) occurred. The biophysically detailed models (e.g., refs. 8 and 9) used previously had interneurons that fired more intensely and displayed square pulse, or relaxation oscillator-like, locomotor bursts (see also ref. 38).
Fig. 5.
Intersegmental coordination of neural activity. (A) Intensity of neural activity in a hemisegment, measured as subthreshold depolarization in motorneurons, for homogeneous network and a network with distributed parameters. (B) In the inhomogeneous network, the time delay d between the beginning of the depolarization and the crossing of the threshold for synaptic interaction between the segmental populations scales linearly with the cycle duration T. (C) Intersegmental phase lag as function of oscillation frequency. The relation for the homogeneous network is close to linear, y = kx (thick dashed line). The parameter variability in the inhomogeneous network changes this dependence to a near-constant relation, y = const (thick solid line). Shaded area shows the range of adaptation of the intersegmental delay.
The triangular wave shape suggests a possible way to adaptively control the intersegmental phase lag from segment to segment. If it is assumed that each segment must have reached a certain threshold (Fig. 5B) with regard to the activity level before it can effectively recruit the next segment, the result will be that a triangular-shaped membrane potential trajectory will reach the threshold after a delay, which is proportional to the cycle duration (compare green and black traces in Fig. 5B). If so, the intersegmental phase lag will result from a relative time delay, and can therefore be preserved at an approximately constant proportion of the cycle as the locomotor frequency changes (see also Fig. 5 legend). In contrast, an EPSP-shaped (red trace in Fig. 5A) or square pulse-like burst will cross the interaction threshold almost immediately upon being induced. This will instead lead to an approximately constant or less varying time delay between the activation of subsequent segments. The triangular wave shape can therefore contribute importantly to the generation of a constant intersegmental phase lag that remains the same at different burst frequencies.
Fig. 5C compares the intersegmental lag produced at different burst frequencies with an inhomogenous interneuron population (triangular-shaped membrane potential trajectory) with that of a homogenous population. The former remains constant over an extended burst frequency range to decrease somewhat at lower frequencies, whereas the latter increases progressively with burst frequency. Consistent with the theoretical predictions, the intersegmental coordination in the inhomogeneous network simulations was near-perfect between 3 and 15 Hz. This frequency range corresponds to the fast rhythm observed in the experiments on fictive locomotion (7, 29).
Discussion
The intention of this study has been to explore through biophysically detailed large-scale modeling, whether the detailed experimental findings obtained through a large number of experimental studies regarding connectivity and cellular and synaptic properties can actually account for the operation of the spinal CPG with its characteristic intersegmental constant phase lag. In addition, we would like to test whether this lag in the model, as in biology, can be flexibly set to different positive and negative values to allow for backward and forward locomotion, respectively. Moreover, we have considered the contribution of the basal ganglia and brainstem control in the control of the CPG, thereby representing essential features of the entire control system for goal-directed locomotion, including steering.
The simulated spinal network, consisting of populations of excitatory and inhibitory neurons (100 per each of 100 segments), can clearly generate a replica of the biological motor pattern with a constant phase lag that can be set to different values with a simple mechanism acting on the most rostral segments. Each of the 10,000 multicompartmental neurons have biophysically realistic properties and closely resemble their biological counterparts. One critical factor for the overall network function is the importance of the experimentally observed variability in neuronal size and membrane properties within each population (6, 38, 39). The asymmetric anteroposterior distribution of the axonal projections of the interneurons (see Fig. 1) contributes importantly to the constant phase lag along the spinal cord, whereas the variability is required to generate the phase lag over a range of frequencies, and it thus confers important properties to the network. The adaptive control of the phase lag is achieved through a progressive recruitment and derecruitment (23) of neurons during each cycle due to the large range of membrane properties in each segmental pool of interneurons (see Fig. 5). In addition, our study highlights the importance of reciprocal inhibition in providing for a wide adaptive range and stability of the phase lag to facilitate the control of the locomotor pattern (Fig. 2C). This suggests a previously uncharacterized role for the reciprocal inhibition in addition to its role in generating alternating activity between antagonist muscle groups. In accordance with experiments highlighting local control of the phase lag (20), the present simulations also rely primarily on local interaction.
The fact that the coordination along the entire spinal network can be modified by interacting with just a few segments near the head end of the spinal cord has obvious advantages from a command point of view. The neuronal explanation in the model lies in the rostrocaudal axonal branches of both E and I interneurons that extend over several segments (see Fig. 1C). When additional excitability is provided to the most rostral segments, they will enhance their basic burst rate somewhat, and the other segments along the cord will follow, albeit with a longer phase lag due to their lower degree of excitation (see Fig. 2 C and D). Conversely, if the rostral segments are instead depressed to be active at a somewhat lower frequency, this will also lead to repercussions on the more caudal segments with higher excitability. They will all be slowed to some degree and align themselves so that a constant negative phase lag will build up along the entire spinal cord, with the tail end leading. In the living animal, it has previously been shown (19) that by increasing or reducing the excitability in a limited number of segments within the spinal cord, the phase lag can be modified or even reversed. Similar conclusions have been reached in experiments with mechanical entrainment (40). The model establishes that a very simple neural mechanism, consistent with the biological findings, can account for the ability to rapidly determine whether the leading segment will be at the head or tail end by just acting on a few rostral segments. We thus predict that to generate backwards locomotion, a set of short reticulospinal neurons acting on the rostral spinal cord are activated that either are inhibitory in themselves or act through inhibitory interneurons. Although we have not as yet identified biologically the presumed reticulospinal neurons controlling the excitability in the rostral spinal cord, it is noteworthy that a large proportion of the reticulospinal fibers project only to the most rostral segments (21). Another group project throughout the spinal cord, presumably to activate the entire spinal network. In previous simulations, we have shown that backward coordination could be achieved by additional excitation applied to the caudal part of the spinal cord network, and similarly, in pieces of spinal cord up to 24 segments, the coupling can be reversed by extra excitation to the caudal segments of the spinal cord (19). However, in pieces of 50 segments Sigvardt and Williams (41) reported that it was difficult to reverse the coupling over the entire spinal cord. This was also the case in the present simulations, suggesting that a rostral command as discussed here would be better suited to exert an efficient control.
The spinal CPG is normally turned on by brainstem reticulospinal cells that provide excitation along the spinal cord to the different spinal neurons and the motoneurons. Similarly here, the large population of reticulospinal model neurons activate (through glutamate receptors) a proportion of the model interneurons in each segment along the entire network and thereby turn on the model CPG. The same effect can also be achieved by simulating the effect of applying glutamate on the spinal cord, which is analogous to the reticulospinal activation. Each of the left and right locomotor command regions in mesencephalon (MLR) exert a bilateral symmetric excitatory effect on the reticulospinal neurons (26, 42), which in turn, drives the CPG; therefore, each side can induce symmetric locomotion. This provides the rationale for lumping MLR and the reticulospinal level into one (see Fig. 4). The two different locomotor command regions both receive tonic input from the inhibitory output neurons of the pallidum—an evolutionarily conserved organization found from lamprey to mammals (43). By including a population of pallidal neurons in the simulations, it has been possible to elicit CPG activity through a release from pallidal inhibition of a reticulospinal population involved in the control of locomotion. This, in turn, is produced by activation of the striatal cell population. Under physiological conditions, striatum has a decision-making role (1, 2, 15) and is summing inputs from pallium (cortex in mammals) and thalamus.
Turning results from an enhanced locomotor activity on one side due to a transient asymmetric reticulospinal drive (44). This can be induced directly by somatosensory input (N. Trigeminus) already at the lower brainstem level. Normally, however, steering would result from orienting movements elicited at the level of the optic tectum through projections to the brainstem. Tectum is activated by direct retinotopic input and tonic inhibitory input originating from the pallidum. In the lamprey as well as mammals, there is a prominent pallidal innervation from this area—and the eye and orienting movements are released when the pallidal inhibition is removed (45).
In conclusion, central and local control mechanisms for establishing and modifying the coordination within the spinal CPG have been established. The rostral part of the CPG network can efficiently determine the direction of the movement, whereas local intrinsic properties allow for an adaptive coordination of the intersegmental phase lag.
Methods
CPG Neuronal Populations.
Populations of 6,000 E and 4,000 I cells were simulated by using an 86-compartment model with the different types of ion channels as established experimentally (22). The cell model is available from Model DB (accession code 93319): http://senselab.med.yale.edu/modeldb/. Cells were placed randomly within a space corresponding to the spinal cord (2 mm wide, 0.25 mm thick, and 100 mm long). The populations of model neurons can be activated by simulated reticulospinal AMPA/NMDA synapses (200 reticulospinal model neuron; see below) or by simulating the experimental procedure of bath application of glutamate agonists modeled by a permanent opening of a fraction (30%) of AMPA channels (23). Poisson-distributed noise with a mean intensity of 200 Hz was applied uniformly to all cells through AMPA synapses with 1-nS conductance.
Network Model.
The synaptic connectivity within the spinal CPG is shown in Fig. 1 B and C. Models for the excitatory glutamatergic (AMPA and NMDA) and inhibitory glycinergic (Glyc) synapses were based on values used previously (23), as are the synaptic conductances gsynAMPA = 0.5 nS, gsynNMDA = 0.25 nS, and gsynGlyc = 0.5 nS. Synaptic sites were spread evenly over the cell surface, with 38 inputs per cell for each type of synaptic projection. In total, this resulted in 760,000 synapses between the CPG neurons. Synapses were distributed uniformly along the axons. Axons extended over 4 segments rostrally and 8 segments caudally for the E cells, and 5 segments rostrally and 15 segments caudally for I cells. The synaptic delay for each connection was calculated based on the distance between the connected interneurons (mean conduction velocity 0.7 m/s for E cells and 1 m/s for I cells). A normal distribution of the parameter values was assigned individually with regard to axonal conduction velocity, compartment area, decay time constant, and influx rate of the ionic pools. The Gaussian function was truncated at 1/4 and 4 times the mean value (standard deviation 0.5). In some experiments, the output of the network was integrated in the form of up to 10 motoneurons per hemisegment (see Fig. 4). This pool of motoneurons received the same excitatory and inhibitory input as the E population received in a segment and was used to visualize the segmental output. The motoneurons were designed not to generate spikes.
Brainstem Activation.
To activate the CPG network synaptically from the brainstem, a population of reticulospinal model neurons (n = 200) was used. They project to the spinal neurons with a projection probability of ≈10% with synapses distributed evenly over dendritic and soma compartments.
Basal Ganglia Control.
The brainstem spinal cord was explored by adding a simplified pallidum (n = 300 neurons) and striatum (n = 3000 neurons) population with properties similar to the reticulospinal population. The pallidal neurons were tonically active, whereas the striatal neurons (which should correspond to the medium spiny neurons) were quiet during rest. The model neurons in the basal ganglia and brainstem were similar, because detailed information on the cellular and synaptic properties is not yet available. These simulations provide a platform, however, for investigating supraspinal control of the spinal CPG.
Simulation Environment.
Calculations are performed using a specialized library for parallel simulations of biophysically detailed large-scale neural networks, SPLIT (46), on a 2048 CPU IBM Blue Gene/L supercomputer.
Supplementary Material
Acknowledgments.
We thank R. Hill and P. Wallén for valuable comments on the manuscript. This work was supported by the Swedish Science Research Council, Center for Parallel Computing, Royal Institute of Technology, European Commission and the Wallenberg Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906722106/DCSupplemental.
References
- 1.Grillner S, Hellgren J, Ménard A, Saitoh K, Wikström MA. Mechanisms for selection of basic motor programs–roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Hikosaka O. GABAergic output of the basal ganglia. Prog Brain Res. 2007;160:209–226. doi: 10.1016/S0079-6123(06)60012-5. [DOI] [PubMed] [Google Scholar]
- 3.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 4.Feldman J, Del Negro CA. Looking for inspiration: New perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts A, Li WC, Soffe SR, Wolf E. Origin of excitatory drive to a spinal locomotor network. Brain Res Rev. 2008;57:22–28. doi: 10.1016/j.brainresrev.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan JT. Contributions of identifiable neurons and neuron classes to lamprey vertebrate neurobiology. Prog Neurobiol. 2001;63:441–466. doi: 10.1016/s0301-0082(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 7.Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 8.Hellgren Kotaleski J, Grillner S, Lansner A. Neural mechanisms potentially contributing to the intersegmental phase lag in lamprey. I. Segmental oscillations dependent on reciprocal inhibition. Biol Cybern. 1999;81:317–330. doi: 10.1007/s004220050565. [DOI] [PubMed] [Google Scholar]
- 9.Hellgren Kotaleski J, Grillner S, Lansner A. Neural mechanisms potentially contributing to the intersegmental phase lag in lamprey. II. Hemisegmental oscillations produced by mutually coupled excitatory neurons. Biol Cybern. 1999;81:299–315. doi: 10.1007/s004220050564. [DOI] [PubMed] [Google Scholar]
- 10.Hellgren J, Grillner S, Lansner A. Computer simulation of the segmental neural network generating locomotion in lamprey by using populations of network interneurons. Biol Cybern. 1992;68:1–13. doi: 10.1007/BF00203132. [DOI] [PubMed] [Google Scholar]
- 11.Tunstall MJ, Roberts A, Soffe SR. Modelling inter-segmental coordination of neuronal oscillators: synaptic mechanisms for uni-directional coupling during swimming in Xenopus tadpoles. J Comput Neurosci. 2002;13:143–158. doi: 10.1023/a:1020114324350. [DOI] [PubMed] [Google Scholar]
- 12.McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daun S, Rubin JE, Rybak IA. Control of oscillation periods and phase durations in half-center central pattern generators: a comparative mechanistic analysis. J Comput Neurosci. 2009;27:3–36. doi: 10.1007/s10827-008-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanchenko MV, Nowotny T, Selverston AI, Rabinovich MI. Pacemaker and network mechanisms of rhythm generation: Cooperation and competition. J Theor Biol. 2008;253:452–461. doi: 10.1016/j.jtbi.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Islam SS, Zelenin PV, Orlovsky GN, Grillner S, Deliagina TG. Pattern of motor coordination underlying backward swimming in the lamprey. J Neurophysiol. 2006;96:451–460. doi: 10.1152/jn.01277.2005. [DOI] [PubMed] [Google Scholar]
- 16.Matsushima T, Grillner S. Intersegmental co-ordination of undulatory movements—A “trailing oscillator” hypothesis. NeuroReport. 1990;1:97–100. doi: 10.1097/00001756-199010000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Williams TL, Sigvardt KA, Kopell N, Ermentrout GB, Remler PM. Forcing of coupled nonlinear oscillators: Studies of intersegmental coordination in the lamprey locomotor central pattern generator. J Neurophysiol. 1990;64:862–871. doi: 10.1152/jn.1990.64.3.862. [DOI] [PubMed] [Google Scholar]
- 18.Cohen AH, et al. Modelling of intersegmental coordination in the lamprey central pattern generator for locomotion. Trends Neurosci. 1992;15:434–438. doi: 10.1016/0166-2236(92)90006-t. [DOI] [PubMed] [Google Scholar]
- 19.Matsushima T, Grillner S. Neural mechanisms of intersegmental coordination in lamprey: Local excitability changes modify the phase coupling along the spinal cord. J Neurophysiol. 1992;67:373–388. doi: 10.1152/jn.1992.67.2.373. [DOI] [PubMed] [Google Scholar]
- 20.Sigvardt KA, Miller WL. Analysis and modeling of the locomotor central pattern generator as a network of coupled oscillators. Ann NY Acad Sci. 1998;860:250–265. doi: 10.1111/j.1749-6632.1998.tb09054.x. [DOI] [PubMed] [Google Scholar]
- 21.Bussières N. Univ of Montreal, Montreal: Faculté des Études Supérieures; 1994. Les Systèmes Descendants chez la Lamproie. Etude Anatomique et Fonctionnelle. PhD thesis. [Google Scholar]
- 22.Huss M, et al. Roles of ionic currents in lamprey CPG neurons: A modeling study. J Neurophysiol. 2007;97:2696–2711. doi: 10.1152/jn.00528.2006. [DOI] [PubMed] [Google Scholar]
- 23.Kozlov AK, Lansner A, Grillner S, Hellgren Kotaleski J. A hemicord locomotor network of excitatory interneurons: A simulation study. Biol Cybern. 2007;96:229–243. doi: 10.1007/s00422-006-0132-2. [DOI] [PubMed] [Google Scholar]
- 24.Brodin L, Grillner S, Rovainen CM. N-Methyl-D-aspartate (NMDA), kainate and quisqualate receptors and the generation of fictive locomotion in the lamprey spinal cord. Brain Res. 1985;325:302–306. doi: 10.1016/0006-8993(85)90328-2. [DOI] [PubMed] [Google Scholar]
- 25.Tråvén HG, et al. Computer simulations of NMDA and non-NMDA receptor-mediated synaptic drive: Sensory and supraspinal modulation of neurons and small networks. J Neurophysiol. 1993;70:695–709. doi: 10.1152/jn.1993.70.2.695. [DOI] [PubMed] [Google Scholar]
- 26.Brocard F, Dubuc R. Differential contribution of reticulospinal cells to the control of locomotion induced by the mesencephalic locomotor region. J Neurophysiol. 2003;90:1714–1727. doi: 10.1152/jn.00202.2003. [DOI] [PubMed] [Google Scholar]
- 27.Kozlov A, Hellgren Kotaleski J, Aurell E, Grillner S, Lansner A. Modeling of substance P and 5-HT induced synaptic plasticity in the lamprey spinal CPG: Consequences for network pattern generation. J Comput Neurosci. 2001;11:183–200. doi: 10.1023/a:1012806018730. [DOI] [PubMed] [Google Scholar]
- 28.Kopell N, Ermentrout GB. Coupled oscillators and the design of central pattern generators. Math Biosci. 1988;90:87–109. [Google Scholar]
- 29.Cangiano L, Grillner S. Mechanisms of rhythm generation in a spinal locomotor network deprived of cross connections: the lamprey hemicord. J Neurosci. 2005;25:923–935. doi: 10.1523/JNEUROSCI.2301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ménard A, Grillner S. Diencephalic locomotor region in the lamprey—Afferents and efferent control. J Neurophysiol. 2008;100:1343–1353. doi: 10.1152/jn.01128.2007. [DOI] [PubMed] [Google Scholar]
- 31.Takakusaki K. Forebrain control of locomotor behaviors. Brain Res Rev. 2008;57:192–198. doi: 10.1016/j.brainresrev.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Pombal MA, El Manira A, Grillner S. Organization of the lamprey striatum - transmitters and projections. Brain Res. 1997;766:249–254. doi: 10.1016/s0006-8993(97)00701-4. [DOI] [PubMed] [Google Scholar]
- 33.Thompson RH, Ménard A, Pombal M, Grillner S. Forebrain dopamine depletion impairs motor behavior in lamprey. Eur J Neurosci. 2008;27:1452–1460. doi: 10.1111/j.1460-9568.2008.06125.x. [DOI] [PubMed] [Google Scholar]
- 34.Buchanan JT. Electrophysiological properties of identified classes of lamprey spinal neurons. J Neurophysiol. 1993;70:2313–2325. doi: 10.1152/jn.1993.70.6.2313. [DOI] [PubMed] [Google Scholar]
- 35.Shupliakov O, Wallén P, Grillner S. Two types of motoneurons supplying dorsal fin muscles in lamprey and their activity during fictive locomotion. J Comp Neurol. 1992;321:112–123. doi: 10.1002/cne.903210110. [DOI] [PubMed] [Google Scholar]
- 36.Wallén P, Shupliakov O, Hill RH. Origin of phasic synaptic inhibition in myotomal motoneurons during fictive locomotion in the lamprey. Exp Brain Res. 1993;96:194–202. doi: 10.1007/BF00227099. [DOI] [PubMed] [Google Scholar]
- 37.Buchanan JT, Kasicki S. Segmental distribution of common synaptic inputs to spinal motoneurons during fictive swimming in the lamprey. J Neurophysiol. 1999;82:1156–1163. doi: 10.1152/jn.1999.82.3.1156. [DOI] [PubMed] [Google Scholar]
- 38.Djurfeldt M, Ekeberg Ö, Lansner A Large-scale modeling—A tool for conquering the complexity of the brain. Front Neuroinformatics. 2008;2:1. doi: 10.3389/neuro.11.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker D, Bevan S. Modulation of cellular and synaptic variability in the lamprey spinal cord. J Neurophysiol. 2007;97:44–56. doi: 10.1152/jn.00717.2006. [DOI] [PubMed] [Google Scholar]
- 40.Tytell ED, Cohen AH. Rostral versus caudal differences in mechanical entrainment of the lamprey central pattern generator or locomotion. J Neurophysiol. 2008;99:2408–2419. doi: 10.1152/jn.01085.2007. [DOI] [PubMed] [Google Scholar]
- 41.Sigvardt KA, Williams TL. Effects of local oscillator frequency on intersegmental coordination in the lamprey locomotor CPG: Theory and experiment. J Neurophysiol. 1996;76:4094–4103. doi: 10.1152/jn.1996.76.6.4094. [DOI] [PubMed] [Google Scholar]
- 42.Dubuc R, et al. Initiation of locomotion in lampreys. Brain Res Rev. 2008;57:172–182. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Robertson B, Auclair F, Ménard A, Grillner S, Dubuc R. GABA distribution in lamprey is phylogenetically conserved. J Comp Neurol. 2007;503:47–63. doi: 10.1002/cne.21348. [DOI] [PubMed] [Google Scholar]
- 44.Grillner S, et al. Modeling a vertebrate motor system: Pattern generation, steering and control of body orientation. Prog Brain Res. 2007;165:221–234. doi: 10.1016/S0079-6123(06)65014-0. [DOI] [PubMed] [Google Scholar]
- 45.Saitoh K, Ménard A, Grillner S. Tectal control of locomotion, steering, and eye movements in lamprey. J Neurophysiol. 2007;97:3093–3108. doi: 10.1152/jn.00639.2006. [DOI] [PubMed] [Google Scholar]
- 46.Hammarlund P, Ekeberg Ö. Large neural network simulations on multiple hardware platforms. J Comput Neurosci. 1998;5:443–459. doi: 10.1023/a:1008893429695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.