Abstract
Spatial and non-spatial sensory information is hypothesized to be evaluated in parallel pathways. In this study, we tested the spatial and non-spatial sensitivity of auditory neurons in the ventrolateral prefrontal cortex (vPFC), a cortical area in the non-spatial pathway. Activity was tested while non-human primates reported changes in an auditory stimulus' spatial or non-spatial features. We found that vPFC neurons were reliably modulated during a non-spatial auditory task but were not modulated during a spatial auditory task. The degree of modulation during the non-spatial task correlated positively with the monkeys' behavioral performance. These results are consistent with the hypotheses that the vPFC is part of a circuit involved in non-spatial auditory processing and that the vPFC plays a functional role in non-spatial auditory cognition.
Keywords: non-spatial processing, spatial processing, vocalization
An important conceptual model in auditory neuroscience is that spatial (i.e., location) and non-spatial (i.e., sound type) information are processed in parallel processing streams (1–7). A “dorsal” pathway is preferentially involved in the processing of the location of a stimulus. A “ventral” pathway is preferentially involved in differentiating between sound types. The dorsal (spatial) pathway begins in the caudolateral belt of the auditory cortex and projects to the regions of the caudal dorsolateral prefrontal cortex. The ventral (non-spatial) auditory pathway begins in the anterolateral belt region of the auditory cortex, which projects to the ventrolateral prefrontal cortex (vPFC).
There is a great deal of electrophysiological data supporting this parallel-processing scheme in the auditory cortex. Neurons in the caudolateral belt are more sensitive to stimulus location than those in the anterolateral belt (8–10). Furthermore, neural activity in these dorsal areas is sufficient to account for an animal's psychophysical performance on a sound-localization task (11–13). In contrast, neurons in the anterolateral belt are more sensitive to the changes in stimulus type of an auditory stimulus than those in the caudolateral belt (8–10).
Although there is plenty of evidence from human-imaging studies for an extension of parallel functional streams into the parietal and prefrontal cortex (6), evidence from animal studies for selective processing in these structures is more limited. For example, single-unit studies have shown that vPFC and parietal neurons are modulated by both the location and the type of an auditory stimulus (14, 15). In contrast, other single-unit studies have found specialized representations for species-specific vocalizations (7, 16, 17). Thus, more evidence is needed to support the parallel-stream hypothesis in the PFC of nonhuman primates.
One possible explanation for the described above results is that the passive-listening tasks used in these vPFC and parietal studies (14, 15) do not appropriately engage the neurons in these cortical areas. Indeed, the well-known role of the PFC in executive function (18–21) and the parietal cortex in spatial cognition (22, 23) can only be demonstrated when subjects are actively engaged in a task. Thus, a true test of how the neurons in these areas code the location and type of an auditory stimulus requires that activity be tested while monkeys are in the process of selectively attending to stimulus location or stimulus type.
In the current study, we recorded from vPFC neurons while monkeys were engaged in a task in which they reported when the spatial or non-spatial features of an auditory stimulus changed. vPFC neurons were reliably modulated during a non-spatial auditory task but were not modulated during a spatial auditory task. We also found that the degree of modulation during the non-spatial task correlated positively with the monkeys' performance. These results are consistent with the hypotheses that the vPFC is part of a circuit that is involved in processing sound type (1, 4, 24) and that the vPFC has a functional role in non-spatial auditory cognition (25, 26).
Results
Rhesus monkeys participated in two tasks. In the first task, monkeys listened to a series of sounds and reported when the type of sound changed (i.e., from one vocalization to a different type of vocalization) but ignored changes in the location of a sound (e.g., from the right to the left). We called this the detect-type task. The task began with the presentation of an auditory stimulus, which was followed by a second stimulus. If the first and second stimuli were different types, the second stimulus was a cue for the monkeys to release a lever (a “release-S2” stimulus) to get a reward. If the first and second stimulus were the same type, the monkeys maintained their hold on a lever (a “hold-S2” stimulus), and a third stimulus (the “S3” stimulus) was presented. This third stimulus was always a different sound type than the first two stimuli and hence, a cue for the monkey to release a lever to get a reward. See Materials and Methods for more details.
In a second task, monkeys listened to a series of auditory stimuli and reported when the location of a sound changed but ignored changes in the type of sound. We called this the detect-location task (see Materials and Methods). The release-S2, hold-S2, and S3 stimuli are analogous to those described for the detect-type task.
To facilitate comparisons within a task and across tasks, a “vocalization-location target” (VLT) was chosen; this target stimulus was one of three different vocalizations at one of the three different locations. The VLT was systematically varied throughout the detect tasks so that it could be either the (hold- or release-) S2 or S3 stimulus.
Since the vPFC is part of the ventral (“what”) processing stream (1–6), we hypothesized that vPFC neurons would be preferentially modulated when monkeys were reporting changes in the type of sound (i.e., during the detect-type task). We recorded from 116 vPFC neurons while monkeys participated in interleaved blocks of the detect-type and the detect-location tasks. Of those 116 neurons, 78 were “auditory” (see Materials and Methods) and were analyzed further and discussed below.
Behavioral Data.
Analyses were conducted on the behavioral data that were generated from all of the recording sessions (i.e., recorded neurons) that are reported in this study.
During the detect-type task, the mean reaction time was 479.3 ms (standard deviation = 145 ms). During the detect-location task, the mean reaction time was 486.0 ms (standard deviation = 147 ms). These two mean values are not reliably different (t-test; P > 0.05). A neuron-by-neuron analysis indicated that the monkeys' reaction times did not reliably differ (paired t-test; P > 0.05) as a function of the two detect tasks.
We further quantified the monkeys' performance on the task using d′ (27). The average d′ of the trials in which the VLT was the release-S2 stimulus during the detect-type task was 0.40 (standard deviation = 0.26). The average d′ during the detect-location task was 0.42 (standard deviation = 0.25). Whereas there was substantial day-to-day variability in performance, both of these distributions of d′ values were reliably greater than chance (t-test; P < 0.05); this issue is addressed below. A neuron-by-neuron analysis indicated that the monkeys' d′ values did not reliably differ (paired t-test; P > 0.05) as a function of the two detect tasks.
Neurophysiological Data.
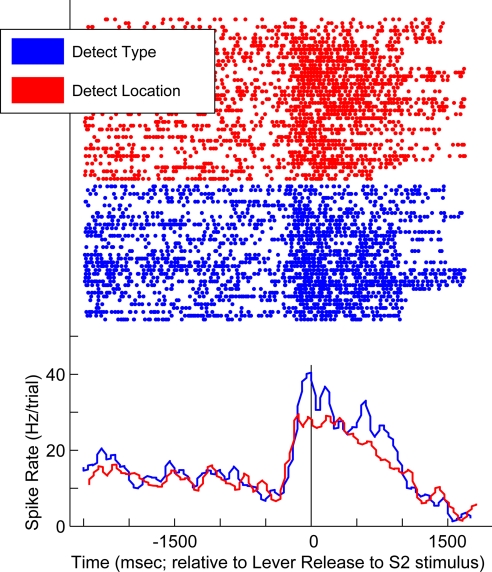
An example neuron is shown in Fig. 1. The data in blue were generated during trials of the detect-type task, whereas those in red were generated during the detect-location task. The peri-stimulus time histograms demonstrate that the neuron had a higher firing rate during trials of the detect-type task than during trials of the detect-location task. This divergence in firing rate began with onset of the VLT as the release-S2 stimulus (i.e., data to the left of the black vertical line) and continued during the time that the monkey was releasing the lever (i.e., data to the right of the black vertical line). The properties of this neuron are consistent with the hypothesis that the vPFC and the ventral (what) pathway preferentially process the non-spatial attributes of an auditory stimulus.
Fig. 1.
Response profile of a vPFC neuron recorded during the detect tasks. Data generated during the detect-type task are shown in blue; data generated during the detect-location task are shown in red. The rasters and peri-stimulus time histograms are aligned relative to the lever release initiated by the release-S2 stimulus; the vertical black line indicates this time point. The histograms were generated by binning spike times into 40-ms bins.
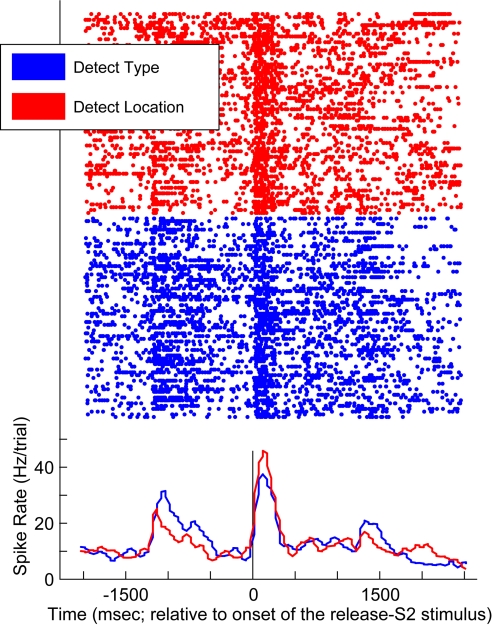
Not all of our vPFC neurons had such a response profile, as shown in Fig. 2. This neuron responded more vigorously during the VLT as the release-S2 stimulus during the detect-location task than during the detect-type task. The modulation of activity earlier in the task was due to the S1 stimulus. However, task-related differences are difficult to interpret since these S1 stimuli had potentially different spatial and non-spatial attributes.
Fig. 2.
Response profile of a vPFC neuron recorded during the detect tasks. Data generated during the detect-type task are shown in blue; data generated during the detect-location task are shown in red. The rasters and peri-stimulus time histograms are aligned relative to the onset of the release-S2 stimulus; the vertical black line indicates this time point. The histograms were generated by binning spike times into 40-ms bins.
These observations were quantified by calculating two modulation indices; see Materials and Methods for more details. Both indices were calculated on a neuron-by-neuron basis and on a task-by-task basis.
Stimulus Index.
The “stimulus index” quantified how neural activity was modulated by the VLT stimulus when it was a cue to release the lever, relative to when it was not a cue to release the lever (i.e., the release-S2 stimulus versus the hold-S2 stimulus). An index value of 1 implied that the neuron responded only to the release-S2 stimulus, whereas a value of −1 implied that the neuron responded only to the hold-S2 stimulus. A value of 0 meant that the neuron responded equally to both stimuli.
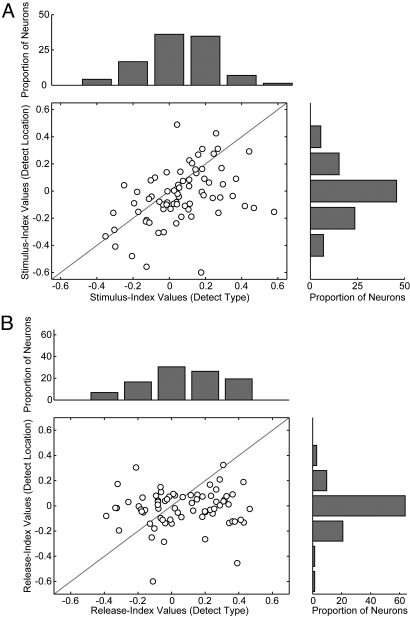
The distribution of stimulus-index values that were generated from neural activity recorded while monkeys participated in the detect-type task and the detect-location task are shown in Fig. 3A. The mean value of the stimulus index from the detect-type task was 0.06, a small value but one that was reliably greater than zero (t-test; P < 0.05). In contrast, the mean value of the stimulus index from the detect-location task was 0.03, a value that was not reliably different from zero (t-test; P > 0.05).
Fig. 3.
Modulation indices. (A) Stimulus index. The correlation between the stimulus-index values on a neuron-by-neuron basis. The stimulus-index values generated during the detect-type task are plotted on the x axis and the stimulus-index values generated during the detect-location task are plotted on the y axis. The solid gray line is the line of equal correlation (i.e., a line with a slope of 1). The marginal distributions are shown to the right of and on top of the scatterplot. (B) Release index. The correlation between the release-index values on a neuron-by-neuron basis. The release-index values generated during the detect-type task are plotted on the x axis and the release-index values generated during the detect-location task are plotted on the y axis. The solid gray line is the line of equal correlation (i.e., a line with a slope of 1). The marginal distributions are shown to the right of and on top of the scatterplot.
To test whether these index values differed on a neuron-by-neuron basis, we correlated the stimulus-index values that were generated during the detect-type task with those generated during the detect-location task (Fig. 3A). On average, vPFC neurons had reliably larger stimulus-index values during the detect-type task than during the detect-location task (Wilcoxon test; P < 0.05). That is, most of the data points on the scatterplot were below the line of equality.
Release Index.
The second index, the “release index” quantified how neural activity was modulated by the monkey's release of the lever during two different time points of the task (i.e., the release-S2 stimulus versus the S3 stimulus). An index value of 1 implied that the neuron responded only to the release-S2 stimulus, whereas a value of −1 implied that the neuron responded only to the S3 stimulus. A value of 0 meant that the neuron responded equally to both stimuli.
The distribution of release-index values that were generated during the two detect tasks are shown in Fig. 3B. The pattern for these values was analogous to that seen for the stimulus-index values: the mean value of the release index from the detect-type task (mean = 0.07) was reliably greater than zero (t-test; P < 0.05), whereas the mean value from the detect-location task (mean = −0.01) was not reliably different from zero (t-test; P > 0.05). Also, when correlated on a neuron-by-neuron basis, vPFC neurons had reliably larger release-index values during the detect-type task than during the detect-location task (Fig. 3B; Wilcoxon test; P < 0.05). That is, most of the data points on the scatterplot were below the line of equality.
Correlation between Behavior and Neural Activity.
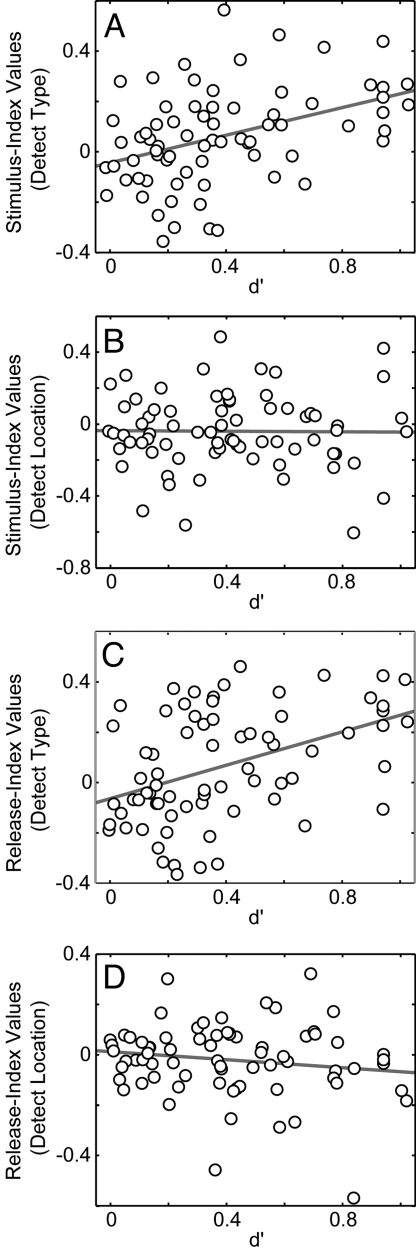
The relationship between the monkeys' behavioral performance and neural activity was tested by correlating, on a neuron-by-neuron basis, the aforementioned index values with the d′ values that were generated during the two detect tasks. The results of this analysis are shown in Fig. 4. When the monkeys were performing the detect-type task, a positive correlation between the d′ values and both the stimulus-index (r = 0.41; P < 0.05) and release-index values (r = 0.43; P < 0.05) (Fig. 4 A and C, respectively) was found. That is, as the monkeys' performance increased (d′ values increased), the index values increased. In contrast, during the detect-location task, we did not identify a correlation between the d′ values and stimulus-index (r = 0.11; P > 0.05) and release-index values (r = 0.13; P > 0.05) (Fig. 4 B and D, respectively).
Fig. 4.
Correlation between neural-modulation index values and behavioral performance. A–D shows the correlation between index values and d′ values. d′ values are shown on the x axis. Index values are shown on the y axis. A and B show data from the stimulus index. C and D show data from the release index. A and C show data from the detect-type task, whereas B and D show data from the detect-location task. In A–D, the solid gray line is a regression line fit to the data in the respective image.
Discussion
We tested vPFC activity while monkeys participated in the detect-type task and the detect-location task. We found that vPFC neurons were reliably modulated during the detect-type task but were not modulated during the detect-location task (Fig. 3). A positive correlation was also identified between the monkeys' behavioral performance and vPFC activity during the detect-type task (Fig. 4). This latter result indicates a direct functional link between vPFC activity and behavior during a non-spatial auditory task.
Comparison with Previous Studies: Relationship to Spatial and Non-Spatial Processing Streams.
Anatomical and electrophysiological studies in non-human primates first suggested that a pathway originating in the anterior belt of auditory cortex and ending in the vPFC was likely to be specialized for processing the non-spatial aspects of auditory stimuli (1, 4, 24). This hypothesis was later corroborated by a series of studies demonstrating that neurons in the anterior belt/parabelt regions were preferentially modulated by the non-spatial features of an auditory stimulus, whereas neurons in the caudal belt were modulated by the spatial features (8–10, 13).
Preferential non-spatial auditory processing in cortical areas more central than the superior temporal cortex has been harder to document in passively-listening monkeys (15). However, in the current study, when monkeys were asked to report changes in the spatial and non-spatial features of an auditory stimulus, we found that vPFC neurons were preferentially modulated by the non-spatial features of an auditory stimulus (see Fig. 3). This finding is consistent with the hypothesis that the vPFC and the pathway leading to the vPFC form a circuit for non-spatial processing (1–5, 17). These results are also consistent with human neuroimaging studies and recent behavioral work in the cat (6, 28).
More importantly, we found that vPFC activity was positively correlated with the monkeys' behavior during the detect-type task: vPFC modulation increased as d′ increased (Fig. 4 A and C). This result independently confirms and extends recent work from our group (25, 26) demonstrating that vPFC neurons are actively involved in non-spatial auditory cognition: vPFC activity reflects the decision-making processes during a phoneme-discrimination task. Together, these findings indicate that the vPFC plays a functional role in non-spatial auditory cognition, a role consistent with the large extant literature on PFC function (18–21, 29).
Caveats to Interpretations.
Since the index values were small and reliable modulation was only observed during the detect-type task (Fig. 3), it is conceivable that this pattern arose due to chance. However, we do not believe this to be valid for several reasons. First, the monkeys' performance (d′) and the associated reaction times were not reliably different during both detect tasks. This observation minimizes the possibility that differences between vPFC activity during the two detect tasks arose due to differences between task performance or task demands. Moreover, since the monkeys were trained simultaneously on both tasks, the modulation observed during the detect-type task cannot be associated with learning biases. Also, since the monkeys participated in interleaved blocks of the two detect tasks, differences in neural activity during these two tasks cannot be trivially attributed to changes in unit isolation, “baseline” firing rate, or other recording issues. Finally, the functional link that we identified between vPFC activity and behavior during the detect-type task (Fig. 4) further demonstrates that the reported neural modulation cannot be associated with uncontrolled variables.
Why then was the effect size so small? We favor two non-exclusive possibilities. First, all of the results were reported from a database that used a very minimal criterion for inclusion: responses to sounds. Consequently, the activity of some vPFC neurons was probably related to the task, whereas the activity of other neurons was not related to the task. Indeed, categorization studies from Miller's laboratory (30, 31) indicated that only approximately 20–25% of their PFC neurons were engaged in categorization. So, it is conceivable that only a small percentage of PFC neurons were engaged in a given task. Second, this was a highly demanding task since both spatial and non-spatial features could change on a given trial. We suspect that if the task was easier (e.g., during the detect-type task, the spatial location remained fixed but the non-spatial attributes would vary), we might have seen more neural modulation. Indeed, it is conceivable that although the monkeys were cued to attend to only one feature, they were tracking both features: PFC activity is modulated differently when monkeys are asked to attend to one visual feature versus multiple visual features simultaneously (32).
Conclusions
Our results in conjunction with previous studies (6, 7, 17, 24) establish firmly that the pathway leading from the primary auditory cortex via the anterior belt and parabelt to the vPFC forms a functional, hierarchical circuit involved in the coding and representation of an auditory stimulus' non-spatial features. More specifically, we hypothesize that whereas neurons in the auditory cortex preferentially represent the non-spatial, perceptual features of an auditory stimulus, neurons in the vPFC are actively involved in non-spatial auditory cognition (17, 25, 33). However, since the dorsal and ventral processing streams are highly interconnected (3, 34) and since both spatial and non-spatial information are found in both processing streams (14, 15, 32, 35), it is likely that spatial, non-spatial, and perhaps other types of information (e.g., information regarding “how” and “when” an event occurred) are integrated to form unified perceptual representations that guide goal-directed action and behavior (6, 32, 36, 37).
Materials and Methods
We recorded from vPFC neurons from two male rhesus monkeys (Macaca mulatta). Under isofluorane anesthesia, the monkeys were implanted with a scleral search coil, head-positioning cylinder, and a recording chamber. vPFC recordings were obtained from one monkey's left hemisphere and from the right hemisphere of the second monkey. All recordings were guided by pre- and post-operative magnetic resonance images of each monkey's brain; electrodes were placed in the rhesus brain and magnetic-resonance images verified their placement in the vPFC. The vPFC was identified by its anatomical location and its neurophysiological properties (15, 17, 38, 39). The vPFC is located anterior to the arcuate sulcus and area 8a and lies below the principal sulcus. vPFC neurons were further characterized by their strong responses to auditory stimuli. The Dartmouth Institutional Animal Care and Use Committee approved the experimental protocols.
Experimental Rig.
Recording sessions were conducted in a darkened room with sound-attenuating walls. The walls were covered with anechoic foam insulation (Sonomatt, Auralex). The monkeys were seated in a primate chair and placed in front of a stimulus array; since the room was darkened, the speakers producing the auditory stimuli were not visible to the monkeys. The monkeys were monitored during all sessions with an infrared camera.
The stimulus array consisted of three speakers (PLX32, Pyle) that formed a line centered on the monkey. The speakers were 1.2 m above the floor, which was at the approximate eye level of the monkeys. Relative to the monkey's position in the room, the three speakers were arranged such that the speaker-to-speaker separation was 20° in azimuth. A green and a red LED were mounted on the center speaker; one LED was illuminated before the start of each trial to cue the monkey about the trial's behavioral requirements (see below for more details).
Auditory Stimuli.
The stimuli were exemplars of three different rhesus vocalizations: a “coo,” a “grunt,” and a “warble”; spectrograms of these vocalizations are shown in Fig. S1. The duration of the coo was 459.6 ms, the duration of the grunt was 181.6 ms, and the duration of the warble was 462 ms. These vocalizations were recorded previously and digitized (40). The three stimuli differed by their spectrotemporal features.
Each auditory stimulus was presented at a sound level of 65-dB SPL. All of the stimuli were recorded to disk and sampled at 50 kHz. The stimuli were presented through a D/A converter (DA1, Tucker Davis Technologies), an anti-aliasing filter (FT6–92, Tucker Davis Technologies), an amplifier (SA1, Tucker Davis Technologies, and MPA-250, Radio Shack), and one of the speakers.
Behavioral Tasks.
Monkeys participated in two tasks: the detect-type task and the detect-location task. These tasks are analogous to those used by Recanzone et al. (11) and Maunsell and colleague (41, 42). In the detect-type task (Fig. S2A), monkeys listened to a series of auditory stimuli and reported when the sound type changed, independent of changes of stimulus location. In the detect-location task (Fig. S2B), monkeys listened to a series of auditory stimuli and reported when the spatial features of the auditory stimuli changed, independent of changes of stimulus type.
For both detect tasks, an auditory stimulus was first presented, the “S1” stimulus. Next, a second stimulus (the “S2” stimulus) was presented. The S2 stimulus might be identical to the S1 stimulus or have attributes different from the S1 stimulus. Depending on the relationship between the S1 and S2 stimuli and the task, the monkeys either (1) released the lever following onset of the second stimulus (the “release-S2” stimulus) to get a reward or maintained their grip on the lever (the “hold-S2” stimulus). When the second stimulus was a hold-S2 stimulus, a third stimulus (the “S3” stimulus) was presented; the S3 stimulus was always a cue for the monkeys to release the lever for a reward.
In trials of the detect-type task (Fig. S2A), monkeys released the lever when they detected changes in stimulus type of the (release) S2 or S3 stimuli. For example, if the S1 stimulus was a coo vocalization and the S2 stimulus was a grunt vocalization, the monkeys released the lever in response to the S2 stimulus to get a reward. If both the S1 and S2 stimuli were coo vocalizations, then the S3 stimulus could be a grunt to initiate a lever release by the monkey. In addition to changes in the stimulus type, the location of the stimulus could vary; although, changes in the location were irrelevant for successful task completion.
Trials of the detect-location task (Fig. S2B) had an analogous format. That is, monkeys released the lever when they detected changes in location of the (release) S2 or S3 stimuli. For example, if the S1 stimulus came from the left speaker and the S2 stimulus came from the center speaker, the monkeys released the lever in response to the S2 stimulus to get a reward. If both the S1 and S2 stimuli came from the left speaker, then the S3 stimulus could come from the center speaker to initiate a lever release by the monkey. In addition to changes in the location, the type of the stimulus could vary; although, changes in stimulus type were irrelevant for successful task completion.
The monkeys were cued that a trial was going to start by an LED that preceded every trial. This LED indicated that the monkey should grasp the lever to begin the trial. Also, the LED color cued the monkeys to the task type. A red LED signaled that the monkey was going to participate in the detect-type task. A green LED signaled that the monkey was going to participate in the detect-location task.
The inter-stimulus interval was 1,300–1,500 ms. Starting with onset of the stimulus, the monkeys had 800–900 ms to release the lever.
Recording Procedures.
Single-unit extracellular recordings were obtained with a tungsten microelectrode (1 MΩ at 1 kHz; Frederick Haer & Co.) seated inside a stainless-steel guide tube. The electrode signal was amplified (MDA-4I, Bak Electronics) and band-pass filtered (model 3362, Krohn-Hite) between 0.6–6.0 kHz. Single-unit activity was isolated using a two-window, time-voltage discriminator (Model DDIS-1, Bak Electronics). Neural events that passed through both windows were classified as originating from a single neuron. The time of occurrence of each action potential was stored for on- and off-line analyses.
Recording Strategy.
An electrode was lowered into the vPFC. To minimize sampling bias, any neuron that was isolated was tested. Extracellular action potentials from a single neuron were isolated using standard electrophysiological techniques (see above). Monkeys participated in interleaved blocks of the detect-type task and the detect-location task using the same VLT. Within each block, there were 18 trials. The monkeys continued to participate in blocks of trials until either unit isolation was lost or the monkey stopped participating in the tasks. We only report neurons in which greater than or equal to five successful trial blocks for each detect task were collected.
Data Analysis.
Behavioral Data.
For each correct trial, the monkey's reaction time was calculated. Reaction time was defined as the time between onset of the release-S2 stimulus and the lever release.
To further quantify the monkeys' behavior, we calculated d′ (27) from the monkeys' hits (correct release of lever) and false alarms (maintaining grip on lever) during trials of the detect tasks when the VLT was a release-S2 stimulus. d′ = Z(proportion of hits) − Z(proportion of false alarms), where Z is the inverse of the cumulative Gaussian distribution. A d′ of 0 implies that the monkey's hits and false alarms were equivalent. d′ increases when the proportion of hits increases and the proportion of false alarms decreases. d′ values were calculated as a function of the two detect tasks.
Neurophysiological Data.
A t-test tested whether a vPFC neuron had a reliably different firing rate during the period that began with onset of the VLT as the hold-S2 stimulus and ended with its offset relative to the same-duration period that occurred before test-stimulus onset. Neurons in which the null hypothesis (P < 0.05) was rejected were classified as “auditory” and were used in subsequent analyses; similar analyses using trials with the VLT as a release-S2 stimulus or the S3 stimulus yielded comparable results.
Two modulation indices were calculated: the “stimulus” index and the “release” index. The stimulus index quantified how neural activity was modulated by the VLT when it was the release-S2 stimulus versus when it was the hold-S2 stimulus. The release index quantified how neural activity was modulated by the VLT when it was the release-S2 stimulus versus when it was S3 stimulus.
The general formula for the modulation index was (A − B)/(A + B). A and B were the mean firing rates (number of action potentials divided by the duration of the task period; spikes/s) of a neuron during the presentation of a stimulus. When we computed the stimulus index, A was the firing rate of a vPFC neuron during a release-S2 stimulus and B was the firing rate during a hold-S2 stimulus. When we computed the release index, A was the firing rate of a vPFC neuron during a release-S2 stimulus and B was the firing rate during a S3 stimulus. For all cases, the firing rate was calculated over the duration of the stimulus.
Supplementary Material
Acknowledgments.
We thank Heather Hersh, James Saunders, Joji Tsunada, Jung Hoon Lee, and Kate Christison-Lagay for helpful comments on the preparation of this manuscript. This work was supported by grants from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (to Y.E.C.) and an National Research Service Award grant from the National Institute of Mental Health (to B.E.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907248106/DCSupplemental.
References
- 1.Romanski LM, et al. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hackett TA, Stepniewska I, Kaas JH. Prefrontal connections of the parabelt auditory cortex in macaque monkeys. Brain Res. 1999;817:45–58. doi: 10.1016/s0006-8993(98)01182-2. [DOI] [PubMed] [Google Scholar]
- 3.Kaas JH, Hackett TA. ‘What’ and ‘where’ processing in auditory cortex. Nat Neurosci. 1999;2:1045–1047. doi: 10.1038/15967. [DOI] [PubMed] [Google Scholar]
- 4.Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiol Neurootol. 1998;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- 5.Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1999;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romanski LM, Averbeck BB. The primate cortical auditory system and neural representation of conspecific vocalizations. Ann Rev Neurosci. 2009;32:315–346. doi: 10.1146/annurev.neuro.051508.135431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- 9.Rauschecker JP, Tian B. Processing of band-passed noise in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol. 2004;91:2578–2589. doi: 10.1152/jn.00834.2003. [DOI] [PubMed] [Google Scholar]
- 10.Tian B, Rauschecker JP. Processing of frequency-modulated sounds in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol. 2004;92:2993–3013. doi: 10.1152/jn.00472.2003. [DOI] [PubMed] [Google Scholar]
- 11.Recanzone GH, Guard DC, Phan ML, Su TK. Correlation between the activity of single auditory cortical neurons and sound-localization behavior in the macaque monkey. J Neurophysiol. 2000;83:2723–2739. doi: 10.1152/jn.2000.83.5.2723. [DOI] [PubMed] [Google Scholar]
- 12.Woods TM, Lopez SE, Long JH, Rahman JE, Recanzone GH. Effects of stimulus azimuth and intensity on the single-neuron activity in the auditory cortex of the alert macaque monkey. J Neurophysiol. 2006;96:3323–3337. doi: 10.1152/jn.00392.2006. [DOI] [PubMed] [Google Scholar]
- 13.Miller LM, Recanzone GH. Populations of auditory cortical neurons can accurately encode acoustic space across stimulus intensity. Proc Natl Acad Sci USA. 2009;106:5931–5935. doi: 10.1073/pnas.0901023106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gifford GW, III, Cohen YE. Spatial and non-spatial auditory processing in the lateral intraparietal area. Exp Brain Res. 2005;162:509–512. doi: 10.1007/s00221-005-2220-2. [DOI] [PubMed] [Google Scholar]
- 15.Cohen YE, Russ BE, Gifford GW, III, Kiringoda R, MacLean KA. Selectivity for the spatial and nonspatial attributes of auditory stimuli in the ventrolateral prefrontal cortex. J Neurosci. 2004;24:11307–11316. doi: 10.1523/JNEUROSCI.3935-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurophysiol. 2005;93:734–747. doi: 10.1152/jn.00675.2004. [DOI] [PubMed] [Google Scholar]
- 17.Russ BE, Ackelson AL, Baker AE, Cohen YE. Coding of auditory-stimulus identity in the auditory non-spatial processing stream. J Neurophysiol. 2008;99:87–95. doi: 10.1152/jn.01069.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller EK, Freedman DJ, Wallis JD. The prefrontal cortex: Categories, concepts, and cognition. Phil Trans R Soc Lond B Biol Sci. 2002;29:1123–1136. doi: 10.1098/rstb.2002.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- 20.Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller E, Cohen JD. An integrative theory of prefrontal cortex function. Ann Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 22.Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: A review. Vis Res. 2000;40:1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- 23.Kusunoki M, Gottlieb J, Goldberg ME. The lateral intraparietal area as a salience map: The representation of abrupt onset, stimulus motion, and task relevance. Vis Res. 2000;40:1459–1468. doi: 10.1016/s0042-6989(99)00212-6. [DOI] [PubMed] [Google Scholar]
- 24.Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russ BE, Orr LE, Cohen YE. Prefrontal neurons predict choices during an auditory same-different task. Curr Biol. 2008;18:1483–1488. doi: 10.1016/j.cub.2008.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Russ BE, Orr LE, Cohen YE. Prefrontal activity predicts monkeys' decisions during an auditory category task. Fron Integr Neurosci. 2009;3:16. doi: 10.3389/neuro.07.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: John Wiley and Sons Inc.; 1966. [Google Scholar]
- 28.Lomber SG, Malhotra S. Double dissociation of ‘what’ and ‘where’ processing in auditory cortex. Nat Neurosci. 2008;11:609–616. doi: 10.1038/nn.2108. [DOI] [PubMed] [Google Scholar]
- 29.Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405:347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- 30.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 31.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Visual categorization and the primate prefrontal cortex: Neurophysiology and behavior. J Neurophysiol. 2002;88:929–941. doi: 10.1152/jn.2002.88.2.929. [DOI] [PubMed] [Google Scholar]
- 32.Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Russ BE, Orr LE, Cohen YE. Prefrontal activity predicts monkeys' decisions during an auditory category task. Fron Integr Neurosci. 2009 doi: 10.3389/neuro.07.016.2009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzuto DS, Mamelak AN, Sutherling WW, Fineman I, Andersen RA. Spatial selectivity in human ventrolateral prefrontal cortex. Nat Neurosci. 2005;8:415–417. doi: 10.1038/nn1424. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths TD, Warren JD, Scott SK, Nelken I, King AJ. Cortical processing of complex sound: A way forward? Trends Neurosci. 2004;27:181–185. doi: 10.1016/j.tins.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Milner AD, Goodale MA. The Visual Brain in Action. Oxford: Oxford Univ. Pres; 1995. [Google Scholar]
- 38.Gifford GW, III, MacLean KA, Hauser MD, Cohen YE. The neurophysiology of functionally meaningful categories: Macaque ventrolateral prefrontal cortex plays a critical role in spontaneous categorization of species-specific vocalizations. J Cog Neurosci. 2005;17:1471–1482. doi: 10.1162/0898929054985464. [DOI] [PubMed] [Google Scholar]
- 39.Romanski LM, Goldman-Rakic PS. An auditory domain in primate prefrontal cortex. Nat Neurosci. 2002;5:15–16. doi: 10.1038/nn781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hauser MD. Functional referents and acoustic similarity: Field playback experiments with rhesus monkeys. Anim Behav. 1998;55:1647–1658. doi: 10.1006/anbe.1997.0712. [DOI] [PubMed] [Google Scholar]
- 41.Sereno AB, Maunsell JH. Shape selectivity in primate lateral intraparietal cortex. Nature. 1998;395:500–503. doi: 10.1038/26752. [DOI] [PubMed] [Google Scholar]
- 42.McAdams CJ, Maunsell JH. Attention to both space and feature modulates neuronal responses in macaque area V4. J Neurophysiol. 2000;83:1751–1755. doi: 10.1152/jn.2000.83.3.1751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.