Abstract
Cytoplasmic inclusions containing α-synuclein (α-Syn) fibrils, referred to as Lewy bodies (LBs), are the signature neuropathological hallmarks of Parkinson's disease (PD). Although α-Syn fibrils can be generated from recombinant α-Syn protein in vitro, the production of fibrillar α-Syn inclusions similar to authentic LBs in cultured cells has not been achieved. We show here that intracellular α-Syn aggregation can be triggered by the introduction of exogenously produced recombinant α-Syn fibrils into cultured cells engineered to overexpress α-Syn. Unlike unassembled α-Syn, these α-Syn fibrils “seeded” recruitment of endogenous soluble α-Syn protein and their conversion into insoluble, hyperphosphorylated, and ubiquitinated pathological species. Thus, this cell model recapitulates key features of LBs in human PD brains. Also, these findings support the concept that intracellular α-Syn aggregation is normally limited by the number of active nucleation sites present in the cytoplasm and that small quantities of α-Syn fibrils can alter this balance by acting as seeds for aggregation.
Keywords: Parkinson's disease, pathology, protein misfolding
Alpha-synuclein (α-Syn) is a highly soluble natively unfolded protein expressed throughout the CNS. Although the close association of α-Syn with lipid membranes and enrichment at synaptic terminals suggest a role in synaptic maintenance and neurotransmitter release (1, 2), the precise physiological functions of α-Syn remain uncertain. Also, animals lacking the α-Syn gene (SNCA) show no obvious defects (3). In contrast, intracellular accumulations comprised of highly organized α-Syn amyloid fibrils define a family of neurological disorders (the synucleinopathies) that includes Parkinson's disease (PD), dementia with Lewy bodies (LBs), multiple systems atrophy, and neurodegeneration with brain iron accumulation type 1 (4).
Purified α-Syn readily assembles into amyloid-like fibrils similar to those in LBs under defined conditions in vitro and has been studied extensively (5, 6). Fibrillization occurs through a two-step polymerization process, whereby soluble monomer is converted into conformationally distinct oligomeric intermediates, which then serve as nuclei for subsequent elongation (7). Curiously, although α-Syn aggregation and pathology are prominent in humans and in animal models of synucleinopathies (8–10), overexpression of α-Syn in neuronal and nonneuronal cells, as well as primary neurons derived from α-Syn transgenic mice, does not lead to significant α-Syn inclusion formation (11). Indeed, this absence of cell models that recapitulate the morphological and biochemical features of LBs is a serious impediment to elucidating the pathological events or disease pathways leading to α-Syn aggregation in vivo.
Given its naturally unfolded state, we hypothesized that even highly elevated levels of α-Syn overexpression in cultured cells may not generate sufficient amounts of oligomeric or protofibrillar nuclei required to seed fibril elongation. We tested this hypothesis by asking whether or not cellular α-Syn could be recruited and converted into insoluble forms with features typical of intracellular LB-like inclusions after the introduction of α-Syn nucleating structures into the cytoplasm. Our findings here demonstrate that introduction of exogenously assembled α-Syn fibrils catalyzes intracellular α-Syn aggregation in various cells engineered to overexpress α-Syn. Although monomeric and oligomeric α-Syn showed little effect, α-Syn fibrils rapidly recruited endogenous soluble α-Syn protein, converting this into detergent-insoluble inclusions. Also, pathological hyperphosphorylated and ubiquitinated α-Syn species were featured abundantly in fibril-seeded inclusions; thus, recapitulating key features of human LBs in a cell culture model.
Results
Intracellular α-Syn Fibrils, but Not Soluble α-Syn Species, Seed the Formation of LB-Like Inclusions.
To effectively serve as seeds for aggregation, exogenously added α-Syn fibrils must first localize to cellular compartments accessible to soluble endogenous α-Syn and persist for a sufficient period to allow recruitment of endogenous α-Syn and promote growth of inclusions. Recent studies have revealed that fibrils comprised of polyglutamine repeats, as well as of tau protein accumulating in various neurodegenerative disorders, can be internalized by cells on addition to culture medium (12, 13), although the mechanism by which fibrils reach the cytoplasm is not clearly understood.
We investigated whether monomeric and fibrillar forms of fluorescently labeled α-Syn (α-Syn594) could be efficiently introduced into the cytoplasm of QBI-293 cells to generate a cell culture model system of LB formation. In contrast to prior studies (12–14), we were unable to demonstrate meaningful internalization of either monomeric or fibrillar α-Syn594 by mere addition of protein preparations to the culture medium. However, intracellular α-Syn594 was readily detectable in cells by fluorescence- and differential interference contrast (DIC)-microscopy 24 h after transduction using cationic-liposomes optimized for intracellular protein delivery (Fig. 1 A–F; Fig. S1 f–k) (15). Transduced α-Syn594 monomers were transiently distributed throughout the cytoplasm as small punctate structures, whereas internalized α-Syn594 preformed-fibrils (PFFs) were present as large irregular foci. Although nearly all monomeric α-Syn594 was degraded by 48 h, α-Syn594 PFFs persisted for days within cells. When unlabeled WT α-Syn PFFs (Fig. S1a) were transduced into QBI-293 cells stably expressing WT α-Syn (QBI-WT-Syn cells), we observed large cytoplasmic inclusions within 48 h by using multiple antibodies against α-Syn that differed from the smaller irregular foci observed shortly after PFF transduction (Figs. 1 G–L and 2). Confocal microscopy confirmed that these inclusions resided within the boundaries of the plasma membrane (Fig. 1 J–O). Similar results were also obtained in cells expressing a disease-associated α-Syn mutants (QBI-A53T-Syn) (16).
Fig. 1.
Intracellular fibrils seed α-Syn aggregation. (A–F) Monomeric fluorescently labeled α-Syn (α-Syn594) or α-Syn594 PFFs were delivered into QBI-WT-Syn cells by using cationic-liposome reagent (Bioporter). Cells were passaged after 4 h and visualized under DIC and fluorescence-microscopy after fixation at 24 h. Both transduced α-Syn594 monomer (A–C, arrowheads) and fibrils (D–F, arrows) could readily be detected within cell boundaries (dashed lines) indicating efficient intracellular delivery. (G–L) QBI cells stably expressing A53T-Syn were transduced with WT-α-Syn PFFs, fixed at 48 h, and immunostained with either a pan-α-Syn antibody (SNL4; G) or a monoclonal antibody specifically recognizing misfolded α-Syn (Syn506; J). Colabeling with Alexa Fluor 488 conjugated PHΑ-L (PHA) was used to reveal the plasma membrane. Confocal microscopy shows large α-Syn-positive intracellular inclusions in fibril-seeded cells (G–I and J–L). (M–O) A 3D view of an inclusion-bearing cell reconstructed from serial confocal images. Removal of the PHA signal (green) reveals the juxtanuclear position of a single prominent α-Syn aggregate and confirmed its intracellular location. (P) Quantification of α-Syn inclusions in QBI-WT-Syn cells seeded with either WT-Syn monomer or PFFs (data from three separate transductions from two independent experiments; n > 500 cells per condition). [Scale bars, 10 μm (F); 6 μm (G); 15 μm (j).]
Fig. 2.
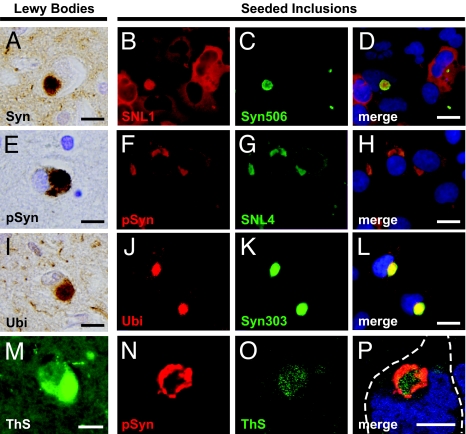
Seeded inclusions resemble human LBs. LBs in the cingulate cortex of a PD patient with dementia showing strong immunoreactivity for α-Syn (A), phosphorylated α-Syn (pSyn) (E), ubiquitin (Ubi) (I), and positive staining with the amyloid-specific dye ThS (M). Intracellular inclusions formed by seeding with recombinant WT-α-Syn fibrils also stained positively with a monoclonal antibody specific to misfolded α-Syn (Syn 506; B–D). Inclusions were also strongly phosphorylated (F–H) and ubiquitinated (J–L), and detectable by using additional α-Syn antibodies SNL4 (G) and Syn303 (K). Confocal images demonstrating intense ThS labeling in the core region of inclusions, in contrast to phosphorylation, which was strongest in the periphery (N–P). Cell boundary is indicated in white. [Scale bars, 10 μm (A, E, I, and M); 10 μm (D, H, and L); 5 μm (P).]
Inclusions were detectable as early as 24 h after PFF treatment and typically round in shape, ranging from 2 to 5 μm in diameter (Figs. 1 and 2). More than 40% of PFF-transduced QBI-WT-Syn cells harbored these LB-like inclusions (Fig. 1P). Notably, only one inclusion was observed per cell, and, similar to LBs in PD, inclusions abutted or indented the nucleus in most cells. Interestingly, no inclusions were detected in cells transduced with nonfibrillar α-Syn comprised of either monomers or oligomers generated by incubation with dopamine (Figs. S1e and S2 d–l) despite previous reports that certain species of oligomeric α-Syn may initiate intracellular α-Syn aggregation (17). Similarly, transduction with an unrelated soluble protein (β-galactosidase) did not result in inclusion formation. Thus, exogenously generated α-Syn fibrils specifically induce intracellular inclusion formation.
Fibril-Seeded Intracellular α-Syn Inclusions Display Properties of LBs in Human Disease.
Immunostaining of PFF-transduced QBI-WT-Syn cells with pan-α-Syn antibodies (e.g., SNL1 and SNL4) revealed intensely stained inclusions surrounded by diffuse cytoplasmic α-Syn, indicating that α-Syn was a major constituent of these aggregates (Fig. 2 B and G). Inclusions were also specifically labeled with antibodies recognizing misfolded forms of α-Syn (Syn506 and Syn303; Fig. 2 C and K), suggesting that α-Syn present within inclusions assumes pathological conformations similar to α-Syn in LBs of PD, but distinct from normal cellular α-Syn.
To further confirm that transduced α-Syn PFFs are intracellular and to determine whether the resulting seeded-inclusions recapitulate posttranslational modifications found in human LBs, we next asked whether α-Syn within inclusions inside transduced cells underwent hyperphosphorylation (18) and ubiquitination (4). Dual immunofluorescence with anti-α-SynpSer129 revealed that nearly all inclusions showed strong immunoreactivity for α-Syn phosphorylated at Ser-129, thereby resembling authentic LBs in PD (Fig. 2 E–H; Fig. S3). Similar phosphorylated α-Syn inclusions also formed after transduction with α-Syn PFFs in other cell lines overexpressing α-Syn, including HeLa and SH-SY5Y neuroblastoma cells (Fig. S4), indicating that this phenomenon extends to multiple cell types. The majority of the α-Syn inclusions were also ubiquitinated, as evidenced by ubiquitin-immunostaining (Fig. 2 I–L). Because recombinant α-Syn PFFs are neither phosphorylated or ubiquitinated before transduction, these modifications occur de novo after the transduction process and further confirm the intracellular location of α-Syn inclusions, as well as their verisimilitude to α-Syn fibrils found in PD brains. Staining with the amyloid-specific dye thioflavin S (ThS) revealed that most inclusions contained significant β-pleated sheet content like that found in authentic LBs (Fig. 2 M–P). Together, these observations indicate that the intracellular α-Syn inclusions in our cell culture system closely resemble LBs in PD with respect to their key defining features.
Exogenous Fibrils Seed Intracellular Inclusion Formation via Recruitment and Conversion of Soluble Cytoplasmic α-Syn.
Given the substantial size of the intracellular inclusions within fibril-transduced cells and the observation that anti-α-SynpSer129 primarily labeled the inclusion periphery, we surmised that endogenously expressed α-Syn is recruited to the sites of internalized α-Syn PFFs. To test this hypothesis, and to determine the relative contributions of exogenous and cellular α-Syn to the formation of intracellular inclusions, QBI-α-Syn-A53T cells were transduced with fibrils assembled from recombinant Myc-tagged α-Syn (Fig. S1d). Double-immunofluorescence with anti-Myc (labeling the internalized α-Syn PFFs) and anti-α-SynpSer129 revealed that Myc-positive PFF seeds are located at the center of inclusions (Fig. 3 A–C). Also, Myc-labeled cores were surrounded by a region labeled with α-SynpSer129 that was devoid of Myc staining, indicating that this phosphorylated α-Syn, which represents a significant proportion of inclusions, was comprised largely, if not exclusively, of endogenous α-Syn. Indeed, the lack of colocalization between Myc and α-SynpSer129 suggested that the α-Syn PFF seeds were not significantly phosphorylated after internalization.
Fig. 3.
Soluble endogenous Syn is recruited into inclusions by fibrils. (A–C) QBI-WT-Syn cells were seeded with fibrils generated by using recombinant α-Syn containing a C-terminal Myc-tag. Double staining for Myc and anti-α-SynpSer129 (pSyn) revealed that fibril seeds form the core of inclusions whereas pSyn predominates in the periphery regions. (D) Immunoblot of detergent-soluble (TriX) and detergent-insoluble (SDS) fractions of cell lysates from control unseeded QBI-WT-Syn cells. (E and F) Lysates from cells transduced with Syn-Myc fibrils contained both WT (black arrowhead) and Syn-Myc (white arrowhead) in the insoluble fraction, indicating that α-Syn originating from the cell comprise the majority of α-Syn within inclusions. A smear representing high molecular weight α-Syn species (**) could also be detected in the SDS fraction. Remaining Syn-Myc seeds within the SDS-fraction of transduced lysates were immunoprecipitated with anti-Myc (9E10). (G) Antibodies against α-Syn (SNL-4) indicate efficient pull-down of Syn-Myc seeds. (H) Probing with anti-pSyn indicates that phosphorylation occurs overwhelmingly in endogenous α-Syn but not exogenous fibrils. (I and J) Inclusions detected in cells stably expressing Myc-tagged α-Syn (QBI-Syn-Myc) transduced with WT α-Syn PFFs. Positive staining for Myc (I) and Syn303 (J) indicates that inclusions contain α-Syn of cellular origin. [Scale bars, 2.5 μm (A); 5 μm (I).] *, IgG light chain; FT, flow-through fraction.
The α-Syn within LBs isolated from human PD brains has been demonstrated to be insoluble (10, 19). Endogenous α-Syn from untransduced QBI-WT-Syn cells was highly soluble and entirely recovered after 1% Triton X-100 extraction (Fig. 3D). However, transduction with α-Syn-Myc PFFs led to the appearance of a significant amount of Triton-insoluble WT α-Syn as well as higher Mr species, which required SDS for solubilization (Fig. 3E). A weaker band consistent with α-Syn-Myc was also recovered in the SDS fractions of α-Syn-Myc PFF-transduced cells, the identity of which was confirmed on reprobing blots with anti-Myc antibody (Fig. 3 E and F). The relative intensities of the endogenous α-Syn band, which are several fold-higher than α-Syn-Myc in the SDS-soluble fraction from transduced cells (Fig. 3E), further support that the intracellular aggregates are comprised largely of recruited endogenous α-Syn. The insolubility of endogenous α-Syn recruited by α-Syn-Myc PFFs was also apparent by immunofluorescence after Triton X-100 extraction of transduced QBI-WT-Syn cells, which effectively removed all diffuse cytoplasmic α-Syn while leaving inclusions intact as shown by immunostaining with anti-α-SynpSer129 or Syn506 (Fig. S5 a–f).
Inclusion Formation Is Mediated by the Core Amyloid-Forming Region of α-Syn.
Concordant with our colocalization data, we were unable to detect significant phosphorylation of Myc-tagged α-Syn PFFs after immunoprecipitation with anti-Myc and probing with α-SynpSer129 antibody, further confirming that exogenously introduced α-Syn fibrils are not phosphorylated (Fig. 3 G and H). Consistent with the majority of α-Syn found within detergent-insoluble inclusions representing newly recruited protein of cellular origin, cells stably expressing α-Syn-Myc also formed Myc-positive inclusions after transduction with WT α-Syn fibrils (Fig. 3 I–K).
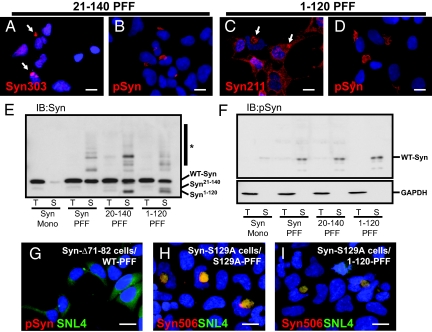
To better understand the molecular interaction between recruited endogenous α-Syn and the PFFs, we transduced QBI-A53T-Syn cells with fibrils lacking either the N-terminal (α-Syn21–140) or C-terminal (α-Syn1–120) region of α-Syn. These PFFs are indistinguishable from WT α-Syn PFFs on EM evaluation ((Figs. S1 a–c and S5g). Transduction with N or C terminus truncated α-Syn PFFs resulted in robust formation of inclusions that were detected by antibodies that recognizing only endogenous full-length α-Syn. For example, Syn303 recognizes an N-terminal epitope not present in α-Syn21–140 PFFs (Fig. 4 A and B), whereas α-Syn1–120 PFFs lack the epitope detected by anti-α-SynpSer129 (Fig. 4 C and D). The abundance of endogenous α-Syn in the Triton-insoluble fractions from these cells confirms that recruitment has a central role in α-Syn inclusion formation (Fig. 4E).
Fig. 4.
Inclusion formation does not require the α-Syn N- or C-terminal regions in seeds. (A–D) QBI-A53T-Syn cells were transduced with α-Syn PFFs lacking either the N-terminal (α-Syn21–140) or C-terminal domain (α-Syn1–120). Transduced cells were immunostained using antibodies recognizing either the extreme N-terminal (Syn303; A) or C-terminal (Syn211; C); thus, detecting only endogenous α-Syn. Both truncated forms of fibrils recruited cellular α-Syn as indicated by inclusion formation. Inclusions were phosphorylated, indicating that their formation does not depend on seed phosphorylation or interaction with membranes. (E and F) Lysates from cells transduced with either WT-α-Syn monomer (Mono), full-length (Syn PFF), or truncated α-Syn fibrils were probed with antibodies against α-Syn (SNL4) and α-SynpSer129. Immunoblot with SNL-4 (E) shows full-length endogenous α-Syn within Triton-insoluble fractions of cells transduced with WT, α-Syn21–140, and α-Syn1–120 PFFs, indicating that cellular α-Syn is converted by fibril seeds. Transduction also resulted in the appearance of high molecular weight α-Syn species (*) consistent with ubiquitination. Fibril transduction also led to a dramatic increase in the amount of phosphorylated α-Syn found almost exclusively within SDS-soluble fractions (F Upper) consistent with its location within insoluble inclusions. GAPDH loading controls are also shown (F Lower). (G) QBI-Δ71-82-Syn cells transduced with WT fibrils did not form inclusions, indicating that the core fibril assembly region of α-Syn is critical to recruitment and incorporation. (H and I) QBI-cells stably expressing α-Syn mutated at Ser-129 (S129A) were transduced with PFFs prepared from α-Syn-S129A (H) or α-Syn1–120 (I), which lack this phosphorylation site. Double immunostaining with Syn506 and SNL4 indicate the formation of misfolded α-Syn inclusions. [Scale bars, 5 μm (A–D); 5 μm (G–I).]
Interestingly, phosphorylated α-Syn was found only in the Triton-insoluble fraction, which parallels studies of pathological α-Syn in PD brains (Fig. 4F). The ability of truncated α-Syn PFFs to seed further aggregate formation implies that the central portion of α-Syn is sufficient for the recruitment and subsequent incorporation of α-Syn into inclusions. Residing within this region is a hydrophobic sequence (residues 71–82) that forms the core of α-Syn fibrils (20). To test whether this segment is required for the association of endogenous α-Syn, we transduced WT α-Syn PFFs into cells stably expressing α-Syn lacking this region. As predicted based on our previous α-Syn fibrillization studies (20), we were unable to detect inclusions in transduced QBI-α-SynΔ71–82 cells (Fig. 4G), thereby confirming that binding of endogenous α-Syn to PFFs requires the region of α-Syn critical for fibrillization in vitro.
The observation that phosphorylated α-Syn was present only in the Triton-insoluble fraction suggested that this modification occurs after recruitment to the growing inclusion. To confirm that phosphorylation of α-Syn at Ser-129 is not necessary for recruitment, we transduced either α-Syn1–120 or α-SynS129A PFFs into cells stably expressing phosphorylation-incompetent α-SynS129A. Inclusions resembling those formed with WT α-Syn were detected, further revealing that phosphorylation is not required for inclusion seeding nor the subsequent recruitment of endogenous α-Syn (Fig. 4 H and I).
Ultrastructure of Fibril-Seeded α-Syn Inclusions.
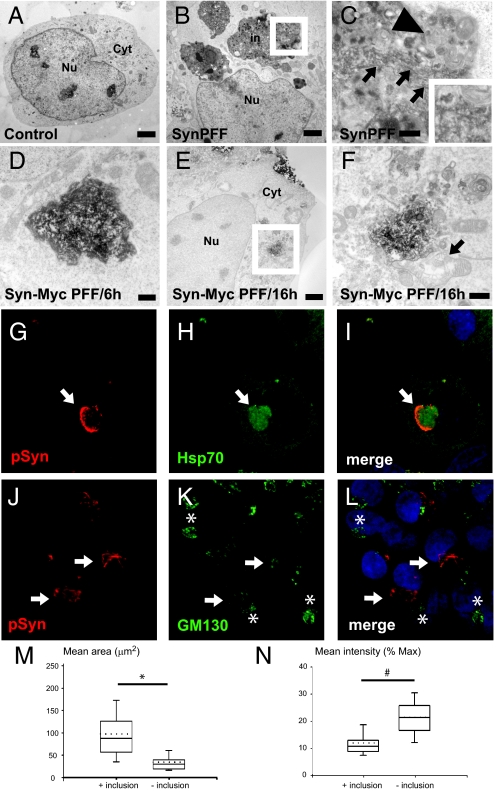
Cells containing α-Syn inclusions were next examined by EM. In monomer-transduced QBI-Syn-A53T cells, major organelles appeared intact and the cytoplasm was clear of any large accumulations (Fig. 5A). In contrast, electron-dense cytoplasmic inclusions were prominent after PFF-transduction (Fig. 5B). Inclusions were perinuclear, although no disruption of the nuclear membrane was observed. Examination at higher magnifications revealed the presence of distinct fibrillar structures ≈10–15 nm in diameter located at the center of inclusions (Fig. 5C). Inclusions also contained many vesicles, of which some were multilamellar and in contact with the fibrillar core. We used immuno-EM to further monitor the localization of transduced α-Syn-Myc PFFs in QBI-Syn-A53T cells and to analyze the recruitment and incorporation of cellular α-Syn to fibrils. At 6 h after transduction, anti-Myc staining revealed accumulations of exogenous fibrils inside the cytoplasm within close proximity of the nucleus (Fig. 5D), where nearly all inclusions are subsequently located. Uranyl acetate counterstaining also revealed that transduced fibrils at this stage were not associated with vesicles typical of mature inclusions. By 16 h, PFF-transduced cells contained Myc-positive structures that were surrounded by various membrane bound organelles comparable with the arrangement observed in EM samples from cells obtained at later times (Fig. 5 E and F). These findings further verify that exogenously introduced PFFs reach the cytoplasmic space where they serve as a nidus for the formation of complex intracellular inclusions. Also, cytoplasmic vesicles appear to associate with fibrils during the inclusion formation process similar to what occurs in human LBs (21). Because our immunofluorescence data indicate that insoluble α-Syn within inclusions extends considerably beyond the PFF core (Fig. 3 A–C), these vesicles may contain hyperphosphorylated α-Syn and, thus, form an integral part of inclusions.
Fig. 5.
Fibril-seeded α-Syn inclusions contain vesicular bodies. (A–C) EM images of cells stably expressing A53T-α-Syn 24 h after transduction with either WT-α-Syn monomer (A) or PFFs (B and C). Electron dense inclusions (in) were found only in the cytoplasm of PFF-seeded cells. Nucleus (Nu) and cytoplasm (Cyt) are also indicated. (C) High-power magnification of the region delineated in B revealing a fibrillar core (black arrows and Inset) consistent with fibrils serving as a nidus for recruiting endogeous α-Syn. Multilamellar bodies are also present in the surrounding cytoplasm (arrowhead). (D and E) Immuno-EM using anti-Myc (9E10) in QBI-Syn-A53T cells transduced with Syn-Myc PFFs. Exogenous (Myc-tagged) fibrils are localized to the perinuclear region 6 h after treatment (D). At 16 h, vesicular organelles, likely containing α-Syn, are recruited to PFF seeds (D and E). [Scale bars, 2 μm (A, B, and E); 400 nm (C); 0.5 μm (D and F).] Double-immunostaining against pSyn and Hsp70 (G–I) reveal molecular chaperones colocalized to inclusions 48 h after transduction with Syn-Myc PFFs. Whereas pSyn is found at the periphery, Hsp70 is detected throughout inclusions, suggesting it is also recruited to PFF seeds. (J–L) Antibodies against α-SynpSer129 (J) and GM130 (K) were used to label inclusions and the Golgi matrix, respectively. Cells lacking aggregates display compact Golgi morphology (asterisks), whereas inclusion-bearing cells show fragmented GM130 staining (arrows). (M and N) Golgi dispersal was assessed by measuring the area stained by GM130 (M) and average pixel intensity (N) in Myc-Syn PFF-transduced cells with or without phosphorylated α-Syn inclusions. The majority of inclusion-bearing cells displayed fragmentation as reflected by an increase in area positive for GM130 with a concomitant decrease in staining intensity. *, P < 0.001; #, P < 0.005 t test (results obtained from three separate transduction experiments, n = 200).
We examined the consequences of LB-like α-Syn inclusions on cellular architecture and organization. Although we were unable to detect colocalization of α-SynpSer129-positive inclusions with various cytoskeletal markers, inclusions were positive for Hsp70 and Hsp90 (Fig. 5 G–I; Fig. S6), members of the heat-shock protein family widely reported to accumulate in LBs and misfolded protein inclusions in multiple neurodegenerative disorders. In contrast to phosphorylated α-Syn, which was distributed primarily at the periphery of inclusions but excluded from the core, Hsp70 was present throughout inclusions, suggesting that chaperones recognize both α-Syn PFF seeds and subsequently recruited endogenous α-Syn. Last, we examined whether the presence of LB-like inclusions in QBI-Syn-A53T cells disrupts trafficking pathways, including ER-Golgi transport and secretory function as recently shown in yeast models of α-Syn overexpression (21, 22). Immunostaining for α-SynpSer129 and the Golgi matrix protein GM130 in α-Syn PFF-transduced cells revealed that the majority of α-Syn inclusions were located near the cis-Golgi (Fig. 5 J–L). Significantly, GM130 staining in inclusion-bearing cells exhibited a dispersed pattern compared with the dense stacked morphology in cells without inclusions. Quantitative analyses of the mean Golgi area and intensity also revealed significant differences between cells with and without α-Syn inclusions (Fig. 5 M and N). Thus, as in yeast models of synucleinopathies, seeded α-Syn inclusions alter normal cellular processes.
Discussion
Our findings here clearly demonstrate that intracellular inclusion formation in α-Syn overexpressing cells can be initiated by the presence of fibrillar α-Syn seeds. Once inside cells, fibrillar seeds actively recruit and convert soluble endogenous α-Syn into a misfolded state, leading to the formation and growth of detergent-insoluble structures closely resembling LBs in the brains of patients with PD and other synucleinopathies. Importantly, the α-Syn inclusions in our cell culture model also undergo several modifications characteristic of human LBs, including hyperphosphorylation, ubiquitination, and the accumulation of cytoplasmic vesicles around the periphery of the inclusions. The striking morphological and biochemical similarities between LBs and the intracellular accumulations in this model suggest that fibrillar seeds may have a fundamental role in the initial formation of LBs and other disease-associated filamentous inclusions. Also, the accumulation of assembly-competent α-Syn nucleation seeds may be an important rate-limiting factor for LB formation. Although the precise series of events leading to inclusion formation remain unclear, our data indicate α-Syn recruitment depends on the presence of an amyloidogenic sequence. Together with the observation that the majority of α-Syn within inclusions is endogenous, these findings suggest that endogenous α-Syn recruitment to fibrillar α-Syn seeds underlies the formation of these inclusions in our cell culture system, and we speculate that similar processes lead to the formation and growth of LBs in PD and related synucleinopathies.
The absolute number of nucleation sites introduced into individual cells in our model has not been determined, although our biochemical data suggest that the amount of protein transduced represents a minor fraction of the endogenous α-Syn pool. Thus, small quantities of misfolded and fibrillar α-Syn may be sufficient to seed aggregation in the context of long-lived postmitotic cells such as neurons. However, little is known regarding how fibrillar nuclei initially arise in neurons and glia in vivo. Misfolded α-Syn could arise in a cell-autonomous manner via increased synthesis as seen in individuals with α-Syn gene amplification (23) or by mutations that accelerate α-Syn misfolding itself (e.g., the familial A53T mutation) (16). Generation of rapidly aggregating C-terminally truncated α-Syn species, as reported in PD brains (24), may also contribute to this process. Likewise, impairment of α-Syn degradation pathways or insults that alter the degradation or function of α-Syn could result in the accumulation of a critical mass of seeds. Indeed, our results indicate that, even in rapidly dividing cells, α-Syn fibrils remain longer in the intracellular space compared with soluble species, which may further promote its ability to recruit and convert endogenous α-Syn.
Another possibility is that α-Syn seeds enter from neighboring cells or the extracellular space as suggested by recent studies demonstrating that both neuronal and nonneuronal cells participate in the release and uptake of soluble α-Syn species (14). Supporting this notion, data from autopsied PD brains suggest that LBs appear in a progressive temporospatial pattern between closely connected regions of the nervous system (25). Also, recent studies indicate that embryonic dopaminergic neurons grafted into PD patients develop α-Syn inclusions, suggesting that pathology is conferred by proximity to pathological tissue (26). However, it remains unclear whether released α-Syn or some other agent is responsible for the development of α-Syn deposits in the transplanted cells. Significantly, we were unable to detect inclusion formation in α-Syn overexpressing cells, even when cocultured in direct contact with cells already containing prominent inclusions (Fig. S7). Our data, together with the observation that α-Syn pathology within grafts is seen after extended periods, suggest that transmission of misfolded seeds is a rare event.
The capacity for exogenously introduced amyloids to seed intracellular aggregation has also been recently reported for two neurodegenerative disease-related proteins. In contrast to our results with α-Syn, fibrils comprised of either tau (13) or polyglutamine-expanded proteins (12) appear to be actively taken into cells, including neurons, without reagent-mediated transduction. Significantly, when injected into the brains of transgenic mice expressing WT tau, which do not otherwise develop tau lesions, tissue homogenates containing misfolded mutant tau induce conformational changes and tau neuropathology, even in areas beyond the injection site (27). It is not clear why α-Syn fibrils were not efficiently introduced into cells in the absence of transduction reagent, although it is likely that not all fibrils are internalized in a similar manner. For example, it has been suggested that tau aggregates undergo endocytosis (13), whereas polyglutamine fibrils are internalized via an endosome-independent process (12). It is also possible that different cell types employ different mechanisms of fibril internalization.
We have expanded significantly on these recent studies, in particular focusing on molecular processes that occur once α-Syn fibrils gain entry into cells. Our data support the view that the sequence of events leading up to LB formation can be recapitulated in cultured cells. Within all cell types examined in this study, the vast majority of inclusions occupied a juxtanuclear position, although they failed to colocalize with any classically defined compartment. However, inclusions consistently colocalized with ubiquitin and multiple chaperones, which strongly suggests that they elicit the misfolded protein response and protein degradation pathways. Also, the α-Syn inclusions disrupt Golgi integrity, indicating that insoluble α-Syn inclusions are not benign. Further characterization of these intracellular changes should uncover how α-Syn inclusions influence key cellular processes. Intriguingly, although some previous studies suggest a possible link between α-Syn phosphorylation and cytotoxicity, our data show that inclusion formation does not require this modification. Nonetheless, the extent of α-Syn hyperphosphorylation seen in our model and human LBs suggests that it may be an important postaggregation event.
Our findings, coupled with other recent results (12–14, 27), strongly suggest that several different amyloid fibrils can act as potent catalysts for the conversion of soluble proteins into amyloid fibrils, and highlight the importance of developing agents that prevent the formation of nucleating cores (or block further aggregate growth from existing seed structures) as a therapeutic strategy for the treatment of patients with neurodegenerative protein misfolding diseases. To this end, our model provides significant insights into the events that regulate the formation of intracellular α-Syn inclusions and represents an invaluable tool for further elucidating the pathological mechanisms underlying this major family of diseases.
Materials and Methods
Recombinant α-Syn Proteins and Protein Labeling.
Recombinant WT, Myc-tagged, and mutant human α-Syn proteins used in this study are detailed in Table S1 and purified as described in ref. 20 and SI Materials and Methods.
Fibril Assembly.
Fibrils were prepared in reactions (200 μL per tube) containing 360 μM (≈5 mg/mL) α-Syn monomer in assembly buffer (50 mM Tris/100 mM NaCl, pH 7.0). Reactions were incubated at 37 °C with constant agitation (1,000 rpm) in an orbital mixer. Fluorescent fibrils were assembled by including 1.8 μM α-Syn594 in WT α-Syn assembly reactions. Reactions were stopped after 5 days, aliquoted, and stored at −80 °C until use. The presence of amyloid fibrils was confirmed by using thioflavin fluorimetry and EM.
Cell Culture and Fibril Transduction in Mammalian Cells.
Cells stably expressing α-Syn used in this study are listed in Table S2. Generation of stable QBI-HEK-293 (QBiogene) and SH-SY5Y cell lines in SI Materials and Methods. QBI cells were plated in 35-mm tissue culture plates and allowed to reach 80–90% confluence for transduction experiments. Cells were transferred to serum-free media 1 h before transduction. For each plate, 10 μL of cationic-liposomal protein transduction reagent (Bioporter; Sigma) was added to a 1.5-mL Microfuge tube and evaporated as per the manufacturer's guidelines. The resulting reagent dry-film was then directly resuspended with 80 μL of PBS containing α-Syn fibrils (100 μg/mL) fragmented by brief sonication with a hand-held probe. Protein:reagent complexes were allowed to form at room temperature for 10 min, after which the mixture was further diluted in OptiMEM (Invitrogen) and added to cells. Cells were then further incubated for 4 h, washed twice with Versene and 0.5% trypsin/EDTA to remove extracellular α-Syn fibrils, and transferred onto 6-well tissue culture plates or poly(d-lysine)-coated glass coverslips. Transduced cells were maintained in media containing 0.5% FBS unless otherwise indicated.
Immunocytochemistry and ThS Staining.
Fluorescence immunocytochemistry was performed by using primary antibodies listed in Table S3. Procedures for immuno and ThS staining are described in SI Materials and Methods.
Immunoblot Analysis and Immunoprecipitation.
Procedures for protein extractions and immunoblot analyses are detailed in SI Materials and Methods.
EM.
Procedures for sample preparation and acquisition of EM images are described in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank J. Guo and S. Tanik for discussions regarding this manuscript, and I. P. Mills for technical assistance. This work was supported by National Institutes of Health Grants AG09215 (to V.M.-Y.L) and NS053488 (to J.Q.T.), the Picower Foundation, and the Benaroya Foundation. V.M.-Y.L. is the John H. Ware, III, Professor of Alzheimer's Disease Research. J.Q.T. is the William Maul Measey-Truman G. Schnabel, Jr., Professor of Geriatric Medicine and Gerontology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908005106/DCSupplemental.
References
- 1.Maroteaux L, Campanelli JT, Scheller RH. Synuclein - A neuron-specific protein localized to the nucleus and presynaptic nerve-terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:120–129. [PubMed] [Google Scholar]
- 3.Abeliovich A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 5.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 6.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 7.Wood SJ, et al. alpha-synuclein fibrillogenesis is nucleation-dependent - Implications for the pathogenesis of Parkinson's disease. J Biol Chem. 1999;274:19509–19512. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]
- 8.Masliah E, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: Implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 9.Lee MK, et al. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 → Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci USA. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giasson BI, et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 11.Kahle PJ, Neumann M, Ozmen L, Haass C. Physiology and pathophysiology of alpha-synuclein. Cell culture and transgenic animal models based on a Parkinson's disease-associated protein. Ann NY Acad Sci. 2000;920:33–41. 33–41. doi: 10.1111/j.1749-6632.2000.tb06902.x. [DOI] [PubMed] [Google Scholar]
- 12.Ren PH, et al. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–U232. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost B, Ollesch J, Wille H, Diamond MI. Conformational diversity of wild-type tau fibrils specified by templated conformation change. J Biol Chem. 2009;284:3546–3551. doi: 10.1074/jbc.M805627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelphati O, et al. Intracellular delivery of proteins with a new lipid-mediated delivery system. J Biol Chem. 2001;276:35103–35110. doi: 10.1074/jbc.M104920200. [DOI] [PubMed] [Google Scholar]
- 16.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 17.Danzer KM, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 19.Baba M, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 20.Giasson BI, Murray IVJ, Trojanowski JQ, Lee VMY. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 21.Soper JH, et al. alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19:1093–1103. doi: 10.1091/mbc.E07-08-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper AA, et al. alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singleton AB, et al. alpha-synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 24.Li W, et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proc Natl Acad Sci USA. 2005;102:2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson's disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 26.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 27.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.