Abstract
Store-operated Ca2+ entry (SOCE) is activated by redistribution of STIM1 into puncta in discrete ER-plasma membrane junctional regions where it interacts with and activates store-operated channels (SOCs). The factors involved in precise targeting of the channels and their retention at these specific microdomains are not yet defined. Here we report that caveolin-1 (Cav1) is a critical plasma membrane scaffold that retains TRPC1 within the regions where STIM1 puncta are localized following store depletion. This enables the interaction of TRPC1 with STIM1 that is required for the activation of TRPC1-SOCE. Silencing Cav1 in human submandibular gland (HSG) cells decreased plasma membrane retention of TRPC1, TRPC1-STIM1 clustering, and consequently reduced TRPC1-SOCE, without altering STIM1 puncta. Importantly, activation of TRPC1-SOCE was associated with an increase in TRPC1-STIM1 and a decrease in TRPC1-Cav1 clustering. Consistent with this, overexpression of Cav1 decreased TRPC1-STIM1 clustering and SOCE, both of which were recovered when STIM1 was expressed at higher levels relative to Cav1. Silencing STIM1 or expression of ΔERM-STIM1 or STIM1(684EE685) mutant prevented dissociation of TRPC1-Cav1 and activation of TRPC1-SOCE. However expression of TRPC1-(639KK640) with STIM1(684EE685) restored function and the dissociation of TRPC1 from Cav1 in response to store depletion. Further, conditions that promoted TRPC1-STIM1 clustering and TRPC1-SOCE elicited corresponding changes in SOCE-dependent NFkB activation and cell proliferation. Together these data demonstrate that Cav1 is a critical plasma membrane scaffold for inactive TRPC1. We suggest that activation of TRPC1-SOC by STIM1 mediates release of the channel from Cav1.
Store-operated calcium entry (SOCE) is activated by depletion of endoplasmic reticulum (ER) Ca2+ stores and regulates a variety of critical cellular functions (1). Ca2+ depletion in the ER lumen is detected by the Ca2+-binding protein STIM1, which oligomerizes into puncta and relocates to discrete ER-plasma membrane (ER-PM) junctional regions (2, 3) where it associates with and activates store-operated channels including Orai1 and TRPC1, which are components of CRAC and SOC channels, respectively (4–13). Therefore, the location of these channels in the plasma membrane is likely to be critical for their interaction with peripheral STIM1 and activation. However, mechanisms involved in the precise targeting and retention of the channels at the domains where STIM1 puncta are located are not well-understood.
Distinct regions of STIM1 determine aggregation and targeting of the protein to ER-PM junctional domains as well as its clustering with and gating of Orai1 and TRPC1 at these sites. The SAM and coiled-coiled domains are involved in STIM1 aggregation while the polybasic C-terminal region of STIM1 is suggested to target STIM1 to ER-PM junctional regions, which is the likely site for SOCE in native cells (3, 9–11, 14). Thus, it can be predicted that SOCs are either localized in this region or in close proximity to it so that they can be readily recruited following store depletion. Clustering of STIM1 in ER-PM junctional regions results in relatively high local concentrations of STIM1 at these sites, which appears to be sufficient to drive the diffusion of Orai1 into this region where it binds to STIM1, resulting in CRAC channel activation (11, 12).
STIM1 also associates with and regulates store-operated TRPC channels, including TRPC1-SOC (6–8, 13, 15–19). Several studies show that association between TRPC1 and STIM1 increases following store depletion (6, 16, 19, 20), although a recent study was unable to demonstrate this (21). While the ezrin/radixin/moesin (ERM) domain of STIM1 appears to bind to TRPC1, the C-terminal 684KK685 residues are involved in gating the channel via electrostatic interaction with TRPC1(639DD640) (8, 13). We have reported earlier that lipid raft domains (LRD) provide a platform for regulation of TRPC1-SOCE (22). Further, we demonstrated that peripheral STIM1 puncta are anchored in LRD which facilitates TRPC1-STIM1 association required for activation of TRPC1-SOCE (6). This has now been suggested in several other studies (10, 18, 23). The role of LRD in the regulation of TRPC1-SOC suggested by these recent findings are consistent with previous reports which showed that plasma membrane localization of TRPC1 depends on its association with the cholesterol-binding protein caveolin-1 (Cav1), which promotes retention of TRPC1 within the LRD (6, 18, 22–25). Together, these findings suggest a critical role for Cav1 and LRD in the association of TRPC1 with STIM1 within ER-PM junctional regions that is required for SOCE (10, 26–28). However, the precise molecular interactions involving Cav1, STIM1, and TRPC1 that are triggered by store depletion and are critical for TRPC1-SOCE have not yet been defined.
Here we have examined the contribution of Cav1 and STIM1 in the regulation of TRPC1-SOCE that occurs within the ER-PM junctional region in response ER-Ca2+ store depletion. We report that Cav1 is a critical plasma membrane scaffold that retains TRPC1-SOC within the ER-PM region where STIM1 puncta are localized following store depletion. Retention of TRPC1 in this region facilitates the interaction of STIM1 with the channel that is required for activation of SOCE. Activation of TRPC1 by STIM1 also releases the channel from Cav1.
Results
Caveolin-1 Is Required for Plasma Membrane Expression of TRPC1 and Clustering with STIM1.
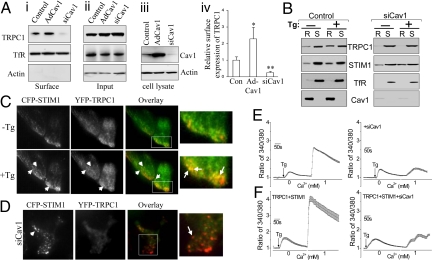
Compared to the surface expression of TRPC1 in control HSG cells overexpression of Cav1 increased (>2-fold), while silencing Cav1 expression decreased (>60%) surface expression of TRPC1 in HSG cells (Fig. 1A i–iii show representative blots and iv shows quantitation). Further, partitioning of TRPC1 into LRD, but not that of STIM1, in response to store depletion was decreased in cells treated with siCav1 (Fig. 1B). We characterized the Cav1 binding site in further detail and show that Cav1 interacts with an N-terminal region of TRPC1 (Fig. S1). Directed mutation of this domain ablated binding of TRPC1 to Cav1, decreased but did not eliminate plasma membrane localization of the channel; and reduced SOCE. Thus, scaffolding of TRPC1-SOC by Cav1 is important for its activation by store depletion.
Fig. 1.
Cav1 is required for plasma membrane expression of TRPC1 and clustering with STIM1. (A i–iii) Biotinylation of TRPC1 in control, Cav1-expressing (Ad-Cav1) and Cav1-silenced (siCav1) HSG cells. (Aiv) Relative surface expression of TRPC1, normalized to expression of transferin receptor (TfR). * and ** represent significant differences relative to control (Con), P < 0.05, and 0.01, respectively. (B) Tg-mediated recruitment of TRPC1 and STIM1 into lipid raft domains in control or siCav1 cells (R, insoluble fraction representing LRD; S, soluble fractions representing non-rafts). (C) TIRFM imaging of CFP-STIM1 (red) and YFP-TRPC1 (green) in resting (-Tg) and stimulated (+Tg) cells. Co-localization, yellow, is seen in overlay images (arrows indicate TRPC1-STIM1 clusters, enlarged areas shown; also see Movie S1). (D) Localization of CFP-STIM1 and YFP-TRPC1 in siCav1-treated cells after Tg stimulation (arrows indicate STIM1 puncta, red, which are not seen before stimulation). (E and F). Fura 2 fluorescence measurements in control and TRPC1+STIM1 expressing HSG cells (siCav1 treatment is indicated in traces on right). Each trace represents the average obtained from at least 50 cells.
Activation of TRPC1 in response to store depletion is dependent on the association of the channel with SOCE regulatory protein, STIM1 (8, 6, 13, 16). Here we show that thapsigargin (Tg) stimulation of HSG cells induced clustering of YFP-TRPC1 and CFP-STIM1 (YFP-TRPC1-green; CFP-STIM1-red) (Fig. 1C; see Movie S1). Notably, TRPC1-STIM1 clustering was dramatically reduced in cells treated with siCav1 (Fig. 1D) which was primarily due to decrease of YFP-TRPC1 in the plasma membrane region (compare TRPC1 localization in Fig. 1 C and D). Note that STIM1 puncta was not affected by siCav1. Consistent with the effect on TRPC1-STIM1 clustering, knockdown of Cav1 in HSG cells reduced TRPC1-mediated SOCE (Fig. 1E). SOCE was also reduced to the same level in cells expressing TRPC1+STIM1 (Fig. 1F). Together these data suggest that Cav1 determines plasma membrane expression of TRPC1 in LRD in unstimulated cells, which is critical for clustering of the channel with STIM1 following Ca2+ store depletion. It was recently suggested (18) that STIM1 regulates localization of TRPC1 in LRD. However, since STIM1 associates with LRD only after stimulation, it is unlikely to be involved in TRPC1 localization within LRD in unstimulated cells.
Functional Consequence of Cav1-TRPC1 Association.
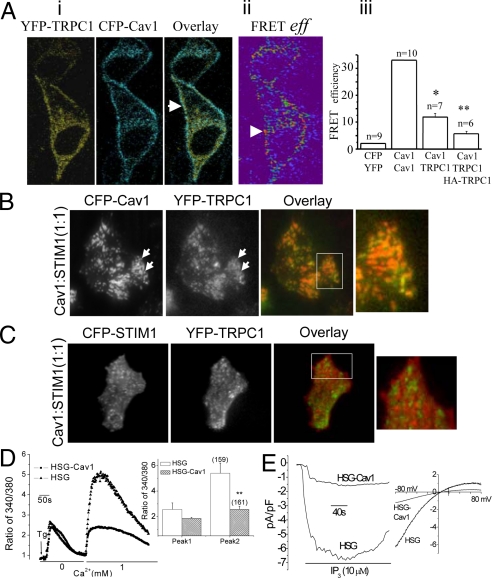
The relative contributions of STIM1 and Cav1 in TRPC1-SOCE were assessed by examining localization and interactions between these proteins within the ER-PM domains where SOCE is activated. Fluorescence resonance energy transfer (FRET) signal was detected in the periphery of resting cells expressing YFP-TRPC1 and CFP-Cav1 (Fig. 2A i and ii, quantitation is shown in iii). TIRFM was used to determine the localization of TRPC1, STIM1, and Cav1 in the ER-PM junctional region, imaging was done using YFP-TRPC1/CFP-Cav1 or YFP-TRPC1/CFP-STIM1 (TRPC1 shown in green, CFP-Cav1 and -STIM1 shown in red). In cells expressing YFP-TRPC1 and CFP-Cav1 together with HA-STIM1, TRPC1, and Cav1 were co-localized in clusters in the ER-PM junctional region of cells before stimulation and this did not change in response to Tg stimulation (Fig. 2B). When Cav1 was expressed together with YFP-TRPC1 and CFP-STIM1, although STIM1 puncta were formed after Tg stimulation (see enlarged images), clustering of TRPC-STIM1 normally observed in stimulated cells was greatly reduced (compare Figs. 1C and 2C; see Movie S2). Expression of Cav1, which reduced TRPC1-STIM1 clustering, attenuated endogenous SOCE and ISOC in HSG cells (Fig. 2 D and E). These findings suggest that although overexpression of Cav1 increases plasma membrane expression of TRPC1, the function of the channel is dependent on its interaction with STIM1.
Fig. 2.
Functional consequence of TRPC1-Cav1 association. (A) FRET measurements in HSG cells expressing YFP-TRPC1 and CFP-Cav1. (i) Localization of the proteins (shown by arrows). (ii) Detection of FRET. (iii) Quantitation of data (FRETeff ± SEM); a significant difference (*, P < 0.05) was noted between Cav1-TRPC1 vs. YFP-CFP or Cav1-TRPC1-HATRPC1; and (**, P < 0.05) between Cav1-TRPC1 vs. Cav1-TRPC1-HATRPC1. (B) Localization of CFP-Cav1 (red) and YFP-TRPC1 (green) in Tg-stimulated HSG cells expressing HA-STIM1 (1:1 cDNA ratio of STIM1 relative to CFP-Cav1). Images before stimulation are not shown since there was no change with stimulation. (C) Localization of CFP-STIM1 and YFP-TRPC1 in Tg-stimulated cells expressing HA-Cav1 (1:1 cDNA ratio of STIM1 and Cav1). (D) Tg-stimulated calcium changes in HSG cells transfected with Cav1, bar graph shows quantitation. (E) ISOC measurements in Cav1-expressing HSG cells with IP3 in the pipette solution (similar data were obtained with Tg).
Ca2+ Store Depletion Induces STIM1-Dependent Dissociation of TRPC1 from Cav1.
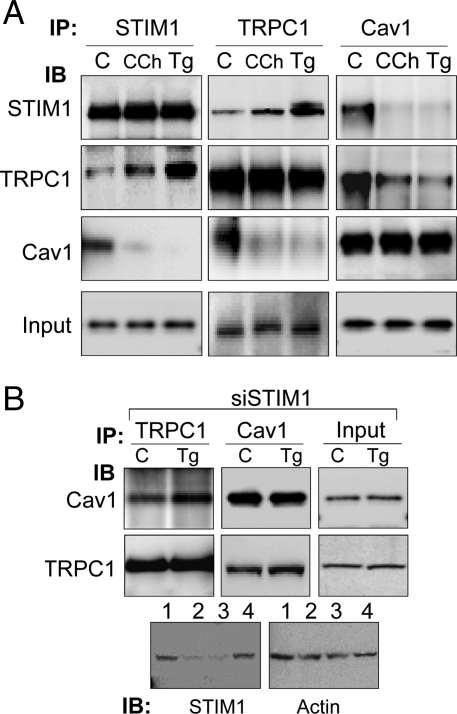
The data presented above demonstrate that Cav1 scaffolds TRPC1 in ER-PM domains but does not activate it. We hypothesized that inactive TRPC1 is retained by Cav1 in the plasma membrane, and that following store depletion, STIM1 relocates to these domains resulting in channel activation. In cells overexpressing Cav1, although more inactive TRPC1 can be expected to accumulate in the plasma membrane by scaffolding to Cav1, the amount of STIM1 is low relative to Cav1 and insufficient to fully activate TRPC1-SOCE. To confirm this, we examined the effect of stimulating the cells on the interactions between endogenous TRPC1, STIM1, and Cav1. A key finding in this study was that Ca2+ store depletion increased the association of endogenous TRPC1 and STIM1, but decreased that of TRPC1 and Cav1 (Fig. 3A). It is important to note that knockdown of STIM1 prevented stimulation-induced dissociation of TRPC1 from Cav1 (Fig. 3B, lower blots show knockdown of STIM1 in cells treated with siSTIM1). Together these data demonstrate that STIM1 is required for activation of TRPC1-SOCE and dissociation of the channel from Cav1. Further, our data indicate that while STIM1 might contribute to retention of TRPC1 within LRD in stimulated cells; that is, when the channel is no longer scaffolded by Cav1, it is insufficient for recruitment of TRPC1 in the absence of Cav1 (see data in Fig. 1).
Fig. 3.
Store depletion induces STIM1-dependent dissociation of TRPC1 from Cav1. (A) Co-immunoprecipitation of endogenous STIM1, TRPC1, and Cav1 from control (C), or Tg or CCh-stimulated HSG cells. (B) Co-immunoprecipitation of TRPC1 and Cav1 in STIM1-silenced HSG cells (siSTIM1) under unstimulated (C) or stimulated (Tg) conditions. Bottom panel shows STIM1 levels in control (lane 1), siSTIM1-treated (lanes 2 and 3, respectively), and non-targeting siRNA (lane 4), and actin as control.
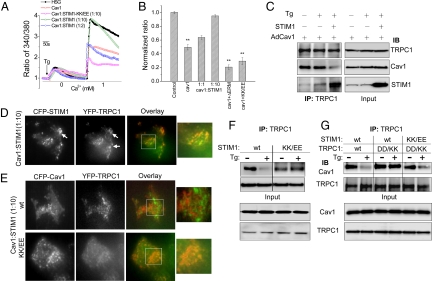
Expression of STIM1 at higher levels relative to Cav1 (cDNA ratio of Cav1 and STIM1 was 1:10, 5- to 10- fold higher STIM1 than used for the experiments shown in Fig. 2C (see Fig. 4C, blots on the right show input) resulted in the recovery of SOCE (Fig. 4 A and B). However, when STIM1 and Cav1 were expressed at 1:1 or 1:2 ratio (as used for experiments shown in Fig. 2) there was no recovery of SOCE (Fig. 4 A and B). SOCE was also not recovered by expression of ΔERM-STIM1 (aa 251–535 were deleted), which neither interacts with TRPC1 nor forms peripheral STIM1 puncta in response to Ca2+ store depletion (8). Protein associations under these conditions were examined by immunoprecipitation. Expression of Cav1 alone suppressed the dissociation of Cav1 from TRPC1 that is triggered by store depletion (Fig. 4C; compare with Fig. 2). However, when STIM1 was co-expressed at higher levels relative to Cav1 (Fig. 4C, see input blots shown on the right) there was a marked increase in TRPC1-STIM1 which was correlated with a substantial decrease in TRPC1-Cav1 association. Under these conditions, Tg stimulation induced formation of YFP-TRPC1/CFP-STIM1 clusters (Fig. 4D). Conversely, when HA-STIM1 was expressed at a higher ratio (10:1) to Cav1, there was minimal clustering between CFP-Cav1 and YFP-TRPC1 (Fig. 4E, upper panels). These findings suggest that when STIM1 relocates into ER-PM junctional regions in response to Ca2+ store depletion it associates with TRPC1 and mediates release of TRPC1 from its plasma membrane scaffold, Cav1.
Fig. 4.
Overexpression of functional STIM1 induces recovery of TRPC1-SOCE. (A and B) Fura 2 measurements in HSG cells expressing Cav1, Cav1+wt-STIM1 (1:2 cDNA ratio), Cav1+wt-STIM1 (1:10 cDNA ratio), Cav1+ΔERM-STIM1, and STIM1-KK/EE (each at 1:10 cDNA ratio of Cav1 relative to STIM1). ** indicates statistically significant (P < 0.05) difference from control values. (C) Co-immunoprecipitation of TRPC1 and Cav1 from cells expressing Cav1 alone or with STIM1 in resting and Tg-stimulations. (D) Localization of CFP-STIM1+YFP-TRPC1 in HA-Cav1-expressing cells. (E) Localization of CFP-Cav1+YFP-TRPC1 in wt-STIM1 or STIM1-KK/EE expressing cells (in D and E STIM1:Cav1 was used at 10:1). (F) Immunoprecipitation of TRPC1 from cells expressing either wt-STIM1 or STIM1-KK/EE. Input protein levels are shown in the lower blots. (G) Immunoprecipitation of TRPC1 from cells expressing wt-STIM1 with wt-TRPC1; wt-STIM1 with TRPC1-DD/KK; or STIM1-KK/EE with TRPC1-DD/KK. Input protein levels are shown in the lower blots. Anti-TRPC1 was used for IP in Fig. 4 F and G.
The positively charged 684KK685 in the C terminus of STIM1 gates TRPC1-SOC via electrostatic interaction with TRPC1(639DD640), while the ERM domain of STIM1 is suggested to bind to TRPC1 but not gate the channel (13). We examined the effect of STIM1(684EE685) mutant (STIM1-KK/EE) on TRPC1-Cav1 association as well as activation of TRPC1-SOCE. STIM1-KK/EE (expressed at 10:1 ratio with Cav1) did not disrupt Cav1-TRPC1 clustering as did wt-STIM1 (Fig. 4E, bottom panel) nor did it induce recovery of SOCE (Fig. 4 A and B). Importantly, in cells expressing STIM1-KK/EE, Cav1 remained associated with the TRPC1 even after stimulation with Tg, as opposed to cells expressing wt-STIM1 (Fig. 4F). However, expression of the mutant TRPC1(639KK640) (TRPC1-DD/KK) together with STIM1-KK/EE restored SOCE (Fig. S2) and induced Cav1 dissociation from TRPC1 following Tg stimulation (Fig. 4G). These interesting data demonstrate that the same aa residues of STIM1 that are involved in the gating of TRPC1 are also critical for mediating release of TRPC1 from Cav1 and activation of TRPC1-SOCE.
NF-κB Activation and Cell Proliferation Is Inhibited by Cav1 Expression, but Rescued by STIM1.
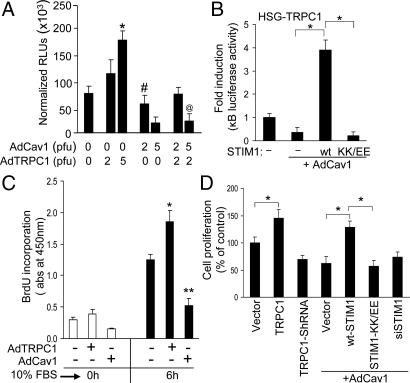
Functional consequences of Cav1 and STIM1 on SOCE were further examined by measuring NF-κB-driven luciferase reporter activity, NF-κB (p65 subunit) nuclear translocation, and cell proliferation, both of which are regulated by SOCE (29, 30). Store depletion promoted nuclear translocation of p65 subunit of NF-κB, which was enhanced by TRPC1 expression, but was inhibited by La3+ (Fig. S3E). Similarly, TRPC1 expression significantly increased the NF-κB -driven luciferase reporter activity (>3–4-fold), which was decreased by TRPC1 silencing (Fig. 5A and Fig. S3A). Further, graded-expression of Cav1 decreased reporter activity in control cells and those expressing TRPC1 (Fig. 5 A and B, expression levels of the protein is shown in Fig. S3C) are consistent with decreased SOCE under these conditions. Importantly, expression of wt-STIM1 but not STIM1-KK/EE, significantly activated NF-κB in cells expressing TRPC1 alone or TRPC1+Cav1 (Fig. 5B; see Fig. S3B for basal activity), reflecting the SOCE measured under these conditions (STIM1 expression is shown in Fig. S3D). Cell proliferation is yet another parameter to evaluate the physiological impact of SOCE. BrdU labeling of cells (indicative of G1-S phase transition in cell cycle) was increased by expression of TRPC1 and suppressed by Cav1 expression (Fig. 5C). Cell proliferation was also increased by TRPC1-expression but inhibited by TRPC1-knockdown or expression of Cav1 (Fig. 5D). Further, expression of wt-STIM1 but not STIM1-KK/EE significantly enhanced cell proliferation that was inhibited by Cav1. Thus, SOCE-dependent cellular functions accurately reflect the level of TRPC1-SOCE.
Fig. 5.
Relative effects of STIM1 and Cav1 on TRPC1-SOCE dependent NF-κB activation and cell proliferation. (A) NF-κB activity in HSG cells expressing TRPC1, Cav1, or TRPC1+Cav1. Normalized relative luciferase units (RLUs) are plotted as mean ± SD. *, P < 0.01 indicates value significantly different from control HSG cells without any exogenous expression. # and @ denote values significantly different (P < 0.01) from TRPC1-overexpressing cells and also significantly different (P < 0.05) from control cells. (B) NF-κB activity in cells expressing wt-STIM1 or STIM1-KK/EE and Cav1. Under these expression conditions, the basal NF-κB activities before serum stimulation are presented in Fig. S3B. Data are normalized to luciferase activity in control HSG cells. *P < 0.01 indicates difference of values compared to cells overexpressing Cav1 alone. (C) BrdU incorporation in control, TRPC1 and Cav1 over-expressing HSG cells. * and ** indicate values significantly different from control HSG cells (P < 0.05). (D) Cell proliferation (MTT assays) performed on asynchronously growing HSG cells transfected with the desired plasmids and AdCav1 (2 pfu). Relative cell proliferation is shown. *, P < 0.05 indicates values significantly different from corresponding vector controls.
Discussion
The data presented above demonstrate that interaction of TRPC1 with Cav1 targets and retains the channel within the ER-PM regions where STIM1 puncta are formed in response to Ca2+ store depletion. Our previous studies and several other reports (5, 6, 9, 10, 22, 26–28) provide strong evidence that the microdomains involved in regulation of TRPC1-SOCE are plasma membrane LRD. Here we show that TRPC1 clusters with Cav1 in LRD before stimulation of the cells while after Ca2+-store depletion, the channel clusters with STIM1. Importantly, these findings reveal that this molecular rearrangement involving TRPC1 (i.e., that store depletion induces association of TRPC1-STIM1 and dissociation of TRPC1-Cav1) is involved in the activation of TRPC1-SOCE by STIM1. A major finding of this study is that dissociation of TRPC1 from Cav1 is an essential step in the activation of TRPC1-SOCE. Further, our data show that this dissociation is dependent on the localization of STIM1 in the ER-PM junctional regions following Ca2+-store depletion. Together, these findings demonstrate that SOCE activation is dependent on interaction of STIM1 with TRPC1 and not on TRPC1-Cav1 interaction per se. Most significantly, we have identified that C-terminal 684KK685 residues of STIM1 that are involved in gating TRPC1 (13) also mediate release of the channel from Cav1. Since STIM1-KK/EE has an intact ERM domain, these data also suggest that binding of ERM domain to TRPC1 is insufficient to dissociate the TRPC1-Cav1 complex. How exactly the interaction of STIM1 with TRPC1 results in Cav1 dissociation from the channel is not yet clearly understood. The STIM1 binding domains on TRPC1 are in the C-terminal region of the channel while the scaffolding action of Cav1 is mediated via an N-terminal domain (discussed above). It is interesting to note that a second putative Cav1 binding domain that we have previously described (22, 24) overlaps with the C-terminal TRPC1 domain involved in electrostatic interaction with STIM1 (26). Based on our findings we hypothesize that interaction of STIM1 with TRPC1 induces a conformational change in the channel that results in its dissociation from Cav1 and STIM1-mediated gating of Ca2+ influx. However, further studies will be required to understand the structural details of this regulation.
Our findings suggest that differential effects of STIM1 and Cav1 on TRPC1 determine the magnitude of SOCE which correspondingly impacts downstream signaling events and cellular functions. We have shown that activation of NF-κB as well as cell proliferation reflect changes in TRPC1-SOCE induced by Cav1 or STIM1. We also suggest that the relative levels of TRPC1-Cav1 or TRPC1-STIM1 complexes in LRD represent inactive and active channels respectively. Our data do not rule out an additional contribution of STIM1 in the retention of TRPC1 within LRD in stimulated cells since TRPC1 association with Cav1 is decreased under these conditions. However, further studies will be required to determine whether STIM1 or some other protein(s) is involved in retaining active TRPC1-SOCs within LRD.
In conclusion, the data presented herein show that clustering of TRPC1 with STIM1 and subsequent activation of TRPC1-SOCE are dependent on precise targeting of TRPC1-SOCs to the ER-PM domains where STIM1 puncta are located following store depletion. This is achieved by binding of the channel to Cav1 and its retention at these sites. Scaffolding of TRPC1 by Cav1 localizes TRPC1 within LRD which serve as a platform for STIM1 aggregation at the cell periphery as well as for STIM1 association with TRPC1-SOC. The activation of TRPC1 by STIM1 also mediates dissociation of the channel from Cav1. We suggest that targeting of the channels and STIM1 to the same microdomains governs both specificity and rate of interaction between these proteins that are essential for activation of SOCE.
Methods
Details of all materials and methods including cell culture, plasmids and transfection, calcium imaging, TIRFM, electrophysiology, as well as biochemical techniques such as immunoprecipitation, surface biotinylation, cell growth, and NF-κB assays are described in the SI Methods.
Supplementary Material
Acknowledgments.
We thank Dr. Tobias Meyer (Stanford University, CA) for the CFP-STIM1, YFP-STIM1, and D76A-STIM1 plasmids; Drs. Shmuel Muallem (University of Texas Southwestern Medical Center, TX) for the ΔERM-STIM1, STIM1- KK/EE, and TRPC1-DD/KK; Jonathan Soboloff (Temple University School of Medicine, PA) for the HA-STIM1; and the confocal facility at the University of North Dakota for assistance. We also thank Katherine Johnson for her excellent technical support. This work was supported by National Science Foundation Grant 0548733; National Institutes of Health Grants DE017102 and 5P20RR017699 (to B.B.S.); National Institute of Dental and Craniofacial Research, Division of Intramural Research (I.S.A.), and University of North Dakota and North Dakota-Experimental Program to Stimulate Competitive Research Student fellowships (to B.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905002106/DCSupplemental.
References
- 1.Putney JW, Jr, Broad LM, Braun F-J, Lievremont J-P, Bird GSJ. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 2.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong HL, et al. Relocalization of STIM1 for activation of store-operated Ca2+ entry is determined by the depletion of subplasma membrane endoplasmic reticulum Ca2+ store. J Biol Chem. 2007;282:12176–12185. doi: 10.1074/jbc.M609435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pani B, et al. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) J Biol Chem. 2008;283:17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 9.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaumer L. The TRPC class of ion channels: A critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 11.Park CY, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan JP, et al. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng W, et al. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium. 2007;42:173–182. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong HL, et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao Y, et al. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci USA. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alicia S, Angelica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: Moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008;44:479–491. doi: 10.1016/j.ceca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Jardin I, Lopez JJ, Salido GM, Rosado JA. Orai1 mediates the interaction between STIM1 and hTRPC1 and regulates the mode of activation of hTRPC1-forming Ca2+ channels. J Biol Chem. 2008;283:25296–25304. doi: 10.1074/jbc.M802904200. [DOI] [PubMed] [Google Scholar]
- 20.Ng LC, et al. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol. 2009 doi: 10.1113/jphysiol.2009.172254. doi: 10.1113/jphysiol.2009.172254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehaven W, et al. TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009 doi: 10.1113/jphysiol.2009.170431. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockwich TP, et al. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 23.Liao Y, et al. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci USA. 2009;106:3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278:27208–27215. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murata T, et al. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem. 2007;282:16631–16643. doi: 10.1074/jbc.M607948200. [DOI] [PubMed] [Google Scholar]
- 26.Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009 doi: 10.1016/j.ceca.2009.02.009. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambudkar IS, et al. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium. 2006;40:495–504. doi: 10.1016/j.ceca.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Ambudkar IS, Ong HL, Singh BB. Molecular and functional determinants of Ca2+ signaling microdomains. In: Sitaramayya A, editor. Signal Transduction: Pathways, Mechanisms and Diseases. Heidelberg: Springer; 2009. in press. [Google Scholar]
- 29.Pani B, et al. Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+-ATPase 2 gene silencing promotes cell survival: A potential role for TRPC1 in Darier's disease. Mol Biol Cell. 2006;17:4446–4458. doi: 10.1091/mbc.E06-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.