Abstract
The capsaicin receptor TRPV1, one of the major transduction channels in the pain pathway, integrates information from extracellular milieu to control excitability of primary nociceptive neurons. Sensitization of TRPV1 heightens pain sensation to moderately noxious or even innocuous stimuli. We report here that oxidative stress markedly sensitizes TRPV1 in multiple species' orthologs. The sensitization can be recapitulated in excised inside-out membrane patches, reversed by strong reducing agents, and blocked by pretreatment with maleimide that alkylates cysteines. We identify multiple cysteines required for full modulation of TRPV1 by oxidative challenges. Robust oxidative modulation recovers the agonist sensitivity of receptors desensitized by prolonged exposure to capsaicin. Moreover, oxidative modulation operates synergistically with kinase or proton modulations. Thus, oxidative modulation is a robust mechanism tuning TRPV1 activity via covalent modification of evolutionarily conserved cysteines and may play a role in pain sensing processes during inflammation, infection, or tissue injury.
Keywords: covalent modification, pain
Animals living in aerobic environments face a constant challenge from oxidative stress. Chemical reactions of cellular constituents with oxidants alter protein functions, disrupt cellular communication signaling networks, or stimulate aberrant cell growth and differentiation through modification of genetic messages. Healthy cells have a wealth of reducing agents to combat environmental oxidative stress. The harm from oxidative chemicals is exacerbated by tissue injury, in that the damaged cell loses its physical barrier against reactive chemicals and fails to replenish cytoplasmic antioxidants.
Pain, the sensation for detecting tissue damage, also worsens during oxidative stress. Like other sensory modalities, pain displays short- and long-term plasticity. Application of oxidative chemicals evokes sharp pains by immediately activating pain-sensing neurons. In contrast, persistent or chronic oxidative challenge causes sustained pain intensified out of proportion to the severity of injury, or hyperalgesia, which increases our awareness of unrepaired tissue damage and assists us in actively avoiding incidental harms.
Sensory neurons use specialized transduction channels to convert environmental insults into electrical signals (1–5). Activation of both the capsaicin receptor TRPV1 and the mustard oil receptor TRPA1 is the principal excitatory drive for thermal or chemical nociceptors. Noxious heat, capsaicin, and acidic pH activate TRPV1; reactive chemical substances covalently modify and activate TRPA1 (6, 7). Excessive activation of TRPV1 and TRPA1 is an obligatory step in developing thermal hyperalgesia (8–11).
Chemical reactions between transduction channels and reactive chemicals have been recognized as effective means to alter their sensitivities to input stimuli. Covalent modification of TRPA1 by allyl-isothiocyanate or alkylating agents excites pain fibers, evoking acute pain and nocifensive behaviors. Hydrogen peroxide (H2O2) and α,β-unsaturated aldehydes acutely activate TRPA1 (12–15). The reactive compound allicin from garlic and nitric oxide activate TRPA1 and TRPV1 via covalent modification (16–22), providing examples for oxidative stress-related pain or hyperalgesia. H2O2 was discovered to mediate thermal hyperalgesia through TRPV1-dependent and -independent mechanisms; TRPV1 is required for long sustenance of thermal hypersensitivity after strong oxidative challenge (23).
Modulation of TRPV1 by oxidative chemicals in neurons is masked by prominent TRPA1 activation, owing to overlapping expression (3, 10) and complex cross desensitization between these two channels (24, 25). Membrane-permeable hydrogen peroxide potentiates heat activation of TRPV1, while membrane-impermeable oxidants are ineffective (26). The biochemical basis of H2O2 modulation, however, was undetermined. Oxidative environments also reduce capsaicin-evoked TRPV1 currents due to decreased effective agonist concentration caused by chemical reactions between capsaicin and oxidants (26, 27).
We found that TRPV1 activation by capsaicin in fact increases substantially following oxidative stress. The sensitization is long standing and involves covalent modification of conserved cysteines. Oxidation overrides receptor desensitization and operates synergistically with other excitatory modulations to supersensitize the receptor, helping to maintain thermal hyperalgesia related to oxidative damage. Covalent modification of cysteines is thus an important biochemical mechanism exploited by primary sensory neurons to increase their excitability, either by direct activation or by amplification of responses of nociceptive transduction channels.
Results
H2O2 Sensitizes TRPV1.
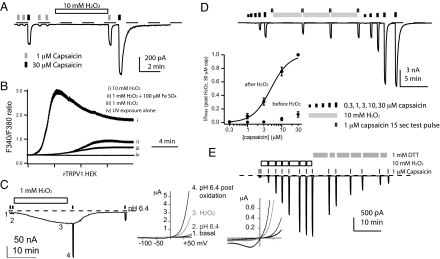
We used H2O2, a pharmacomimetic for general oxidative injuries, to study human or rat TRPV1 expressed in HEK293 cells. hTRPV1 current (1 μM capsaicin) was significantly potentiated in 5 min (5.34- ± 1.21-fold, n = 8) (Fig. 1A). H2O2 sensitization was not an untoward consequence of dialysis of cytoplasmic reducing agents during recording, since ratiometric Ca2+ imaging in intact HEK cells had the same outcome, a kinetically slow Ca2+ entry even at 10 mM H2O2. The maximal level of modulation in 15 min depends on H2O2 concentration; the small stimulation by 1 mM H2O2 can be augmented by additional 100 μM iron (II) sulfate (Fig. 1B, and compared with controls in Fig. S1). We also observed the potentiation of voltage-dependent, strongly outwardly-rectifying hTRPV1 currents (16.9- ± 4.6-fold, pH 6.4-induced, n = 6) (Fig. 1C) in Xenopus oocytes, which have large size and abundant antioxidant reserves to withstand prolonged recording with minimal changes of cellular contents. These results indicate that H2O2 slowly but strongly sensitizes TRPV1 in heterologous systems.
Fig. 1.
TRPV1 is slowly sensitized by hydrogen peroxide (H2O2). (A) hTRPV1 was potentiated by H2O2 in whole cell recording from HEK293 cells. (B) H2O2 dose-dependently stimulated rTRPV1 in ratiometric Ca2+ imaging (n = 193–408 cells). (C) H2O2 induced basal currents and sensitized acid-evoked hTRPV1 currents in Xenopus oocytes. Current-voltage plots at the labeled time points are displayed on the right, all showing strong outward rectification. (D) H2O2 sensitization of rTRPV1 displays a shift of capsaicin dose-response curves (n = 6 for each curve). Data were normalized to currents evoked by 30 μM capsaicin after sensitization to generate averaged curves shown in the inset. (E) H2O2 induced sensitization in an excised inside-out patch expressing rTRPV1. Dithiothreitol (DTT, 1 mM) slowly reversed the sensitization (n = 5).
Covalent Modification of Cysteines as the Mechanism of Oxidative Sensitization.
Slow modulation of TRPV1 can be due to intrinsically slow reaction kinetics between receptors and H2O2 or the requirement for activation of multiple pathways in different intracellular compartments. In excised inside-out patches, H2O2 modulated capsaicin-induced TRPV1 currents with similar robustness (32.9- ± 10.3-fold for 1 μM capsaicin, n = 8) and slow rate as in whole cells (Fig. 1 D and E), favoring the former hypothesis. The receptor exhibits a modest shift of EC50 and an increase of currents at 30 μM capsaicin (Fig. 1D, n = 6). H2O2 sensitization thus at least partly involves an increase of capsaicin efficacy. H2O2-potentiated TRPV1 stayed sensitized until application of strong reducing agents such as 2,3-dimercaptopropanol (BAL) or dithiothreitol (DTT) (Fig. 1E). Glutathione (GSH), a weaker reducing agent, did not effectively reverse H2O2 sensitization. The modulation in an excised membrane patch was as pronounced as that in a whole cell, followed similar kinetics, and could be slowly reversed by dithiol reducing agents, arguing for direct covalent modification of reactive cysteines as the underlying biochemical mechanism.
The slow rate of H2O2-catalyzed reaction required us to search for other cysteine-reactive compounds with faster reaction kinetics as chemical surrogates for mechanistic analysis. Since H2O2 reacts with cysteines to convert physically approximated cysteines into disulfides, we used dithio-bis-nitrobenzoic acid (DTNB) and phenylarsine oxide (PAO), two compounds that link thiols in physical proximity, to examine oxidation effects on TRPV1.
DTNB, a negatively charged membrane-impermeable chemical, is used to determine sidedness of reactive cysteines relative to plasma membrane. Besides forming mixed disulfides with proteins via a disulfide exchange mechanism, DTNB facilitates disulfide bond formation between vicinal cysteines. Although covalent bonds between DTNB and cysteines can be reversed by monothiol reducing agents like GSH, inter-cysteine disulfide bonds can only be reversed by stronger reducing agents (e.g., BAL or DTT) (28). DTNB potentiated basal, acid- or capsaicin-induced TRPV1 currents (3.26 ± 1.01 × 101-fold, n = 5, Figs. S2 and S3). Oxidation thus efficiently tunes TRPV1 responsiveness to noxious stimuli. We observed similar modulation in capsaicin-insensitive chicken TRPV1 (Fig. S4) (29). Sensitivity to oxidative chemicals appears to be a fundamental TRPV1 regulatory mechanism evolutionarily conserved in several vertebrate species.
In excised inside-out patches, DTNB enhanced basal currents and those induced by agonists. However, only rates but not maximal extents of stimulation depend on DTNB concentration (Fig. S5). DTNB stimulation is more likely due to a chemical reaction than a reversible receptor-ligand interaction. Reminiscent of the H2O2 effects, DTNB actions could only be reversed slowly by strong reducing agents, but not by GSH. These data favor formation of disulfide bonds between vicinal cysteines as the chemical basis of TRPV1 sensitization.
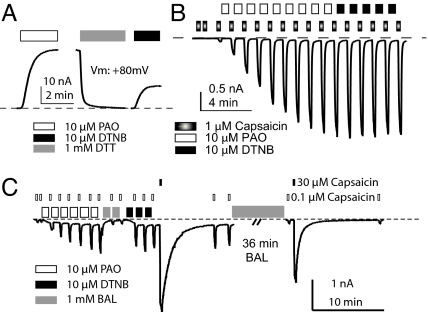
PAO forms cyclic dithiaarsenane adducts with thiols in X-C-X-X-C-X motifs to irreversibly inactivate many cellular signaling molecules (30–34). We thus exploited its membrane permeability and reactivity on vicinal cysteines (while sparing free monothiols) to dissect TRPV1 modulation. PAO sensitized capsaicin-evoked responses of recombinant receptors (7.58 ± 2.69 × 101-fold, n = 38) (Fig. S6A) and neuronal TRPV1 (19.0- ± 9.5-fold, n = 6) (Fig. S6B). It also stimulated basal or acid-evoked currents, notably to a greater degree than DTNB (Fig. 2A and Fig. S7A). PAO and DTNB share the same acceptor cysteines for their stimulatory effects on TRPV1, because neither could further potentiate the maximal effect of the other (Fig. 2B, n = 6, and Fig. S7B, n = 5). PAO occlusion of DTNB potentiation was abolished by BAL treatment, after which either PAO or DTNB could robustly potentiate TRPV1 (Fig. 2C, n = 4). By reducing inter-cysteine disulfide bonds back to free thiols to relieve PAO occlusion, BAL allows subsequent potentiation. Evidently, PAO and DTNB target a common set of cysteines in TRPV1 to evoke their modulatory effects.
Fig. 2.
PAO and DTNB modify the same substrates for oxidative sensitization of TRPV1. (A) PAO activated rTRPV1 basal currents (pH 7.4), which was reversed by DTT. DTNB later reactivated receptors in the same patch, albeit less effectively (IDTNB/IPAO = 0.30 ± 0.03, n = 8). (B) PAO-sensitized rTRPV1 is refractory to subsequent DTNB application. (C) PAO-potentiated hTRPV1 quickly recovered its DTNB sensitivity by brief BAL exposure. In contrast, reversal of DTNB sensitization required a long application of BAL (36 min). All experiments were conducted in inside-out patches.
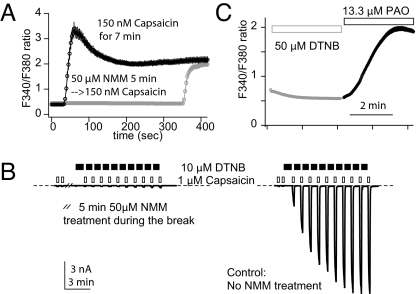
Cysteine forms covalent adducts with reactive electrophiles like mustard oil, unsaturated aldehydes, or maleimides to activate TRPA1. Nevertheless, N-methyl maleimide (NMM) treatment caused a small inhibition of TRPV1 (Fig. 3A). NMM did react with TRPV1 cysteines: it abolished further TRPV1 modulation by other oxidative chemicals (Fig. 3B, n = 5). Conversely, oxidant-potentiated TRPV1 becomes refractory to NMM (data not shown). Irrespective of the functional consequence of cysteine modification, the first cysteine-reactive chemical modified accessible thiols, protecting them from the attack by next compound. Although TRPV1 cysteines are amenable to modification by a host of reactive chemicals, only in some cases does this evoke channel modulation. Oxidative sensitization of TRPV1 apparently has stringent structural requirements.
Fig. 3.
Modulation-sensitive cysteines locate intracellularly. (A) In fura-2 Ca2+ imaging, N-methyl-maleimide (NMM) inhibited subsequent rTRPV1 responses to capsaicin (n = 3 experiments for each treatment). (B) In an inside-out patch, NMM-pretreatment abrogated DTNB-sensitization of rTRPV1 (n = 5). Recording from a patch not exposed to NMM is also shown for comparison (n = 4). (C) Extracellular DTNB neither activated rTRPV1 nor occluded subsequent receptor stimulation by PAO in Ca2+ imaging (n = 3 experiments).
We exploited differential membrane permeability of DTNB and PAO to locate reactive cysteines for sensitization. TRPV1 did not respond to extracellular DTNB in Ca2+ imaging. However, subsequent addition of PAO elicited a prominent response (Fig. 3C and Movies S1 and S2). Contrary to its effectiveness in inside-out patches, DTNB lacked activation or sensitization effects and failed to occlude the subsequent PAO action in imaging experiments. Modulation-sensitive cysteines must reside intracellularly, since DTNB cannot cross plasma membrane.
Conserved Cysteines Mediate Oxidative Sensitization of TRPV1.
To identify the cysteines involved in oxidative modulation, we examined oxidant sensitivity of chimeric channels between TRPV1 and TRPV2. A chimeric channel (ChV2-V1) containing transmembrane and C-terminal domains of TRPV1 successfully transferred potentiation to TRPV2 (Fig. S8). Seven out of eight transferred cysteines are distributed between the fifth transmembrane segment and the end of the C terminus. When this region was further replaced with TRPV2 sequence (Fig. S8B), the new chimera ChV2-V1-V2 lost the acquired oxidative sensitization. We initially expected to eliminate the sensitization by substituting all these seven cysteines in wild-type TRPV1. The septuple cysteine mutant rTRPV1–7C remained potentiated by oxidation, however (Fig. S8C). Although modification of cysteines within the TM5-CT region of TRPV1 contributes to channel sensitization, N-terminal cysteines apparently also participate in oxidative sensitization.
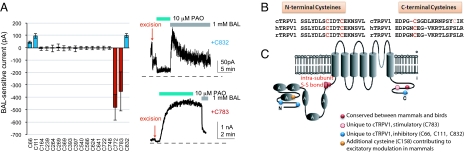
Due to dispersal of modulation-relevant cysteines throughout the sequence, we used cysteine reversion mutagenesis to delineate the full set of cysteine residues contributing to oxidative modulation. Our strategy was to remove all cysteine residues from TRPV1 and then evaluate the ability of various cysteine reversion mutants to undergo potentiation. Initial experiments with a cysteineless rat TRPV1 were confounded by a substantial reduction of functional expression of this channel. However, a cysteineless version of chicken TRPV1 (Cys-cTRPV1) still exhibited sufficient surface expression, and, as expected, was insensitive to oxidative modulation. Since oxidative modulations of mammalian and avian TRPV1 likely proceed via similar mechanisms (Fig. S8D), we mapped evolutionarily conserved sites of oxidant potentiation by reverting cysteines individually in Cys-cTRPV1, thereby identifying C772 and C783 as critical cysteines in cTRPV1 C terminus for sensitization (Fig. 4A). Because the potentiation is mediated by intramolecular disulfide bonds, sensitization of C772 or C783 single revertant mutants by PAO suggests that either residue forms inter-subunit disulfide bonds to stimulate TRPV1. Unexpectedly, our reversion analysis also reveals the inhibitory role of three cysteines unique to cTRPV1. Located close to the ends of N- or C terminus but absent in the mammalian TRPV1, Cys 66, 111, and 832 are oxidized by PAO to suppress basal cTRPV1 activities (Fig. 4A).
Fig. 4.
Multiple cysteines contribute to oxidative modulation of TRPV1. (A) Reversion analysis in cys-cTRPV1 unveils two opposite functional outcomes of cysteine oxidation, expressed as changes of current amplitudes after 1-min BAL treatment. For inhibition, BAL recovered the currents and received a positive score (e.g., +C832). For sensitization, BAL effect was channel suppression with a negative value assigned (e.g., +C783). (B) Sequence alignment of TRPV1 reveals three conserved cysteines (labeled red to contrast C783 shown in pink) among the four modulation-sensitive ones in cTRPV1. (C) Modulation-sensitive cysteines are shown in different colors to depict their functional conservation or divergence. Except for the pair labeled “intra-subunit S-S bond,” all cysteines form inter-subunit disulfide bonds to modulate TRPV1.
The absence of single revertants in N terminus to restore potentiation suggests that one or more pairs of cysteines in N terminus form intra-subunit disulfide bonds to potentiate cTRPV1. Subdivision of cysteines in N terminus into small clusters led to identification of modulation-sensitive Cys-cTRPV1 + 3C, the triple revertant with C369, C393, and C397. Among three combinations of double revertants, only the Cys-cTRPV1C393C397 showed PAO sensitization. Heterotetrameric channels formed by co-expression of Cys-cTRPV1C393 and Cys-cTRPV1C397 were insensitive to PAO, indicating that C393 and C397 form intra-subunit disulfide bonds to sensitize cTRPV1 (Fig. S9A). Presumably seven out of 18 cysteines participate in cTRPV1 modulation. Receptor potentiation dominates when stimulations and inhibitions coexist; inhibition becomes obvious only when sufficient stimulatory covalent modifications are ablated by mutations.
Three of four cysteines required for cTRPV1 oxidative sensitization are conserved in mammalian receptors (Fig. 4B). Surprisingly, PAO still potentiated mammalian TRPV1 triple mutants lacking the three conserved cysteines (C387, C391, and C767 in hTRPV1). Given that reactive chemical allicin activates rat TRPV1 by covalent modification of C157 (C158 in hTRPV1) (18) and that oxidative or alkylating electrophilic chemicals share the same reactant cysteines within TRPV1, we tested the potential reciprocal occlusion between allicin and PAO effects. We hence found C158 to be the missed acceptor cysteine in our reversion analysis (Fig. S9B). The quadruple mutant with further replacement of C158 became PAO and H2O2 resistant (Fig. S9C). Moreover, two hTRPV1 triple mutants (C158AC387SC767S and C158AC391SC767S) are also PAO insensitive (Fig. S9D), either expressed alone or co-expressed. It follows that C387 and C391 within the same subunit form a disulfide bond, while C158 or C767 forms inter-subunit disulfide bonds (Fig. 4C).
Oxidation Overrides Desensitization and Supersensitizes Receptors Prepotentiated by Other Pathways.
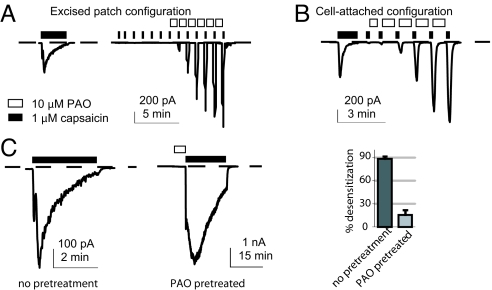
In an injured tissue, many forms of TRPV1 modulations frequently coexist. They may have additive or antagonistic effects on each other. How will oxidative sensitization affect TRPV1 for its signal integrator role? Ca2+-dependent receptor desensitization upon persistent exposure to agonists is an important mechanism to reduce TRPV1 activity. Desensitization blunts all modes of TRPV1 activation (35), partly explaining the therapeutic efficacy of capsaicin as a painkiller (36). We asked whether receptor desensitization would block subsequent potentiation by oxidation. We recorded from a cell-attached patch in the presence of extracellular Ca2+ to induce TRPV1 desensitization (Fig. 5A). The membrane patch was then excised into the inside-out configuration. Desensitized receptors remained refractory to agonist challenge after patch excision, but could later be strongly potentiated by the standard PAO stimulation protocol (Fig. 5A). The ability of oxidation to potentiate desensitized receptors was also observed in the cell-attached configuration (Fig. 5B). We also prestimulated rat TRPV1 by PAO in the cell-attached configuration and then challenged with capsaicin, to determine whether oxidized receptors become supersensitized to obliterate desensitization. Desensitization, although occurring in PAO-sensitized receptors, was less extensive than that from non-PAO treated cells within the same duration of capsaicin exposure (Fig. 5C). Oxidized receptors are more resistant to capsaicin-induced desensitization, while desensitized receptors can be primed by strong oxidative stresses to resume their capacity to sense noxious stimuli.
Fig. 5.
Oxidative sensitization overrides desensitization. (A and B) Capsaicin applied to a HEK293 cell recorded in the cell-attached mode introduces receptor desensitization. Desensitized receptors were potentiated by subsequent 10 μM PAO in either excised (n = 6) or cell-attached configuration (n = 9). (C) Desensitization is more marked in cells without PAO treatment. The bar graph shows averaged desensitization within 3-min capsaicin application from these two groups (P < 0.05, unpaired t-test, six patches from each group).
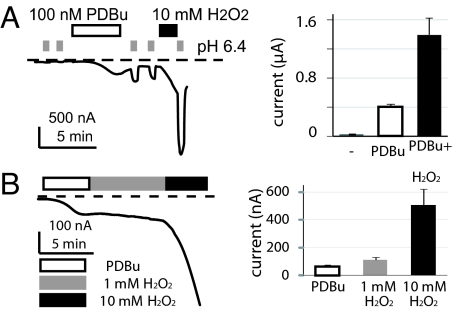
TRPV1 can be activated by several different modes of stimuli, including heat, capsaicin, and acid. By summing effects of small activating stimuli within each mode, TRPV1 acts as a signal integrator to effectively detect the coexistence of multiple harmful stimuli. Mild acidic pH (pH 6.4) or receptor phosphorylation alone is a weak activator for TRPV1 (35, 37). However, simultaneous presence of both stimuli elicits a response bigger than the sum of individual challenges. In our test for oxidized TRPV1 to amplify responses induced by other modulation pathways, we find a colossal synergism of oxidation, phosphorylation and tissue acidosis (Fig. 6A). However, sensitization of TRPV1 by H2O2 in phorbol-ester stimulated cells still has similar slow kinetics and concentration dependence (Fig. 6B). The oxidative sensitization of TRPV1 remains robust in phorbol ester-resistant TRPV1 mutants (Fig. S9E) (38). Hence, oxidation represents an independent pathway from phosphorylation, desensitization, and acidic extracellular pH, acting to increase the gain of this nociceptive transduction channel.
Fig. 6.
Oxidation auguments the potentiation effects of phorbol ester. Phorbol di-butyrate (PDBu, 100 nM) and H2O2 synergistically sensitizes (A) acid-activated (n = 5) or (B) basal hTRPV1 currents (n = 7) in oocytes [P < 0.05, paired t-test, for both (A) and (B)]. Averaged currents after each treatment are shown in the bar graphs.
Discussion
Molecules activating or sensitizing nociceptors are frequently involved in inflammation or tissue injuries. Oxidative stress is no exception (39). Reactive oxygen species (ROS) are known to cause persistent pain (40–44). Modifications of proteins by ROS are indiscriminative (45), compromising multiple cell types to exacerbate tissue inflammation and nociception. Although a significant part of hyperalgesia can arise from action of ROS on the spinal cord (44, 46), sensory transduction channels in peripheral nerves may also contribute to pain related to oxidation.
TRPV1 is the principal transduction channel serving as a polymodal detector. Many cellular signaling pathways modulate TRPV1 to alter the excitability of nociceptive neurons. Despite the importance of oxidative stress in pain sensitivity, its role in TRPV1 modulation was barely understood. We demonstrated that they evoke potent and long-lasting sensitization of TRPV1 via formation of inter-cysteine disulfide bonds within its cytoplasmic termini.
Sensitization of nociception from a single oxidative challenge displays multiple temporal components. TRPA1 is strongly activated by covalent cysteine modification, and suitable for detecting acute cellular injuries from reactive chemicals. In contrast, oxidative modulation of TRPV1 develops slower, primarily sensitizing instead of activating the receptor. However, the robustness of oxidative sensitization of TRPV1 is no less than that by other proalgesic substances. Direct modification of TRPV1 may thus contribute to elevated pain sensation under oxidative stress. The long-lasting sensitizing effect of TRPV1 oxidation may be partly from relative resistance of inter-cysteine disulfide bonds to glutathione, the most abundant cellular reducing agent. The large surface to volume ratio of nerve endings, where transduction occurs, presumably further increases the vulnerability of membrane proteins therein to oxidative damages. Products of oxidation may accumulate analogous to the accrual of oxidized proteins in aging (47–49) or other neurological disorders, like Alzheimer's disease, Parkinson's disease, and chronic neuropathic pain (44, 50–52). Unless neurons repair or remove oxidized TRPV1, covalently modified receptors may reside in sensory nerves for an unduly long time and contribute to the development of chronic pain.
Both TRPV1 and TRPA1 are important for thermal hyperalgesia. Covalent modification of cysteines is also a shared biochemical mechanism to tune activities of both channels. However, the structural requirements for channel activation, the magnitude and time course of the resulting effects differ. Oxidizing chemicals or sulfhydryl-reactive methanethiosulfonate (MTS) compounds can form disulfides to sensitize or activate TRPV1, while an irreversible thioether linkage with maleimide has essentially no stimulatory effect. In contrast, both types of cysteine modification activate TRPA1. The two noiceptive transduction channels can differentiate among reactive chemical species. The intrinsic sensitivity of TRPA1 to local Ca2+ entry results in positive feedback following initial exposure to oxidants, to evoke a large, self-potentiated acute activation rapidly (53, 54). This acute Ca2+-dependent potentiation is absent in TRPV1, accounting for the slower effects of oxidants. Oxidative modulation of TRPV1 involves modification of many different cysteines participating in intra- and inter-subunit disulfide bonds, which can contribute quantally to overall TRPV1 modulation according to their reaction kinetics and stability. TRPV1 may thus respond in a graded fashion to different levels of oxidative stress. Intrinsic differences in mechanisms of channel gating by oxidants make TRPA1 a rapid, highly sensitive detector of acute oxidative stress, and TRPV1 a sensor for a broad range of oxidant concentrations over a long time. It will be interesting to investigate the relative contribution of TRPA1 and TRPV1 in different phases of hyperalgesia induced by reactive chemicals, which will aid the development of therapeutic strategies to counter acute and chronic pain.
Materials and Methods
Cell Culture and Heterologous Expression.
HEK293 cells were cultured with MEM supplemented with 10% newborn calf serum and antibiotics. Cells were transfected with 300 ng wild-type or mutant receptors with or without 100 ng enhanced GFP plasmids using Lipofectamine reagents. Cells were plated on polyD-lysine coated coverslips and recorded 2–3 days following transfection. Stage VI oocytes [from Xenopus tropicalis anesthetized in 0.3% tricaine and prepared as described (55)] were microinjected with hTRPV1 or cTRPV1 cRNA (1 ng per oocyte) and kept in oocyte Ringer (OR-2) solution at room temperature. Recordings were done 5–10 days after microinjection.
Molecular Biology.
Chimeric receptors were generated by the overlapped extension PCR; single point mutants were constructed following QuikChange site-directed mutagenesis. cRNAs were synthesized using T7 mMessage Machine kit.
Ratiometric Ca2+ Imaging.
Cells expressing rat TRPV1 were loaded with fura-2 AM. (5 μM) for 2 h. Imaging experiments were carried out at room temperature (22 °C), with a sampling rate of one frame every 2 (for Fig. 3 A and C) or 5 s (for Fig. 1B), with 150 ms exposure time to either wavelength (340 and 380 nm) using a CCD camera driven by the Slidebook 4.2 software.
Electrophysiology.
Data are expressed as mean ± SEM. unless indicated otherwise. For oocyte recording, the extracellular solution contained (in mM) 5 HEPES or 5 PIPES, 96 NaCl, 1 MgCl2, and 1 BaCl2, titrated to pH 7.4 or pH 6.4 with NaOH. Data were acquired using the pClamp 10 software (Molecular Devices). For mammalian cell recording, the standard extracellular solution had (in mM) 10 HEPES, 145 NaCl or CsCl, 1 MgCl2, and 1 CaCl2, pH 7.4; the standard internal solution contained 10 HEPES, 145 Na gluconate (or 140 Na gluconate plus 6 NaCl for whole cell recordings), 1 Mg(gluconate)2, and 0.1 EGTA, pH 7.4. Access resistances of whole cell recording electrodes were 2–3 MΩ. Those of inside-out patch recordings were between 0.2 and 0.4 MΩ. Pulse and PulseFit software (HEKA) was used for data acquisition and analysis. Cells or patches were stimulated with voltage ramps from -120 to + 80 mV. Time lapsed changes in current amplitudes at the holding potential (−60 mV) were displayed.
Additional Information.
For additional information on materials and methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank our colleagues for reading and commenting on this manuscript. This work is supported by Cornell University Startup Funds and a Scientist Development Grant from American Heart Association to H.-h. Chuang.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902675106/DCSupplemental.
References
- 1.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 3.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibers through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 4.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 5.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 6.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 8.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 9.Davis JB, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 10.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Trevisani M, et al. 4-hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawada Y, Hosokawa H, Matsumura K, Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci. 2008;27:1131–1142. doi: 10.1111/j.1460-9568.2008.06093.x. [DOI] [PubMed] [Google Scholar]
- 15.Cruz-Orengo L, et al. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol Pain. 2008;4:30. doi: 10.1186/1744-8069-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macpherson LJ, et al. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Bautista DM, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar H, et al. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci. 2008;11:255–261. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cebi M, Koert U. Reactivity recognition by TRPA1 channels. Chembiochem. 2007;8:979–980. doi: 10.1002/cbic.200700113. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi N, et al. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels. 2008;2:287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida T, et al. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 22.Bessac BF, et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keeble JE, et al. Hydrogen peroxide is a novel mediator of inflammatory hyperalgesia, acting via transient receptor potential vanilloid 1-dependent and independent mechanisms. Pain. 2009;141:135–142. doi: 10.1016/j.pain.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Filomeni G, Rotilio G, Ciriolo MR. Molecular transduction mechanisms of the redox network underlying the antiproliferative effects of allyl compounds from garlic. J Nutr. 2008;138:2053–2057. doi: 10.1093/jn/138.11.2053. [DOI] [PubMed] [Google Scholar]
- 25.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135:271–279. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Susankova K, Tousova K, Vyklicky L, Teisinger J, Vlachova V. Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol Pharmacol. 2006;70:383–394. doi: 10.1124/mol.106.023069. [DOI] [PubMed] [Google Scholar]
- 27.Jin Y, et al. Thimerosal decreases TRPV1 activity by oxidation of extracellular sulfhydryl residues. Neurosci Lett. 2004;369:250–255. doi: 10.1016/j.neulet.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 28.Gitler C, Mogyoros M, Kalef E. Labeling of protein vicinal dithiols: Role of protein-S2 to protein-(SH)2 conversion in metabolic regulation and oxidative stress. Methods Enzymol. 1994;233:403–415. doi: 10.1016/s0076-6879(94)33047-6. [DOI] [PubMed] [Google Scholar]
- 29.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 30.Shekels LL, Smith AJ, Van Etten RL, Bernlohr DA. Identification of the adipocyte acid phosphatase as a PAO-sensitive tyrosyl phosphatase. Protein Sci. 1992;1:710–721. doi: 10.1002/pro.5560010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiedemann C, Schafer T, Burger MM. Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J. 1996;15:2094–2101. [PMC free article] [PubMed] [Google Scholar]
- 32.Gerhard R, John H, Aktories K, Just I. Thiol-modifying phenylarsine oxide inhibits guanine nucleotide binding of rho but not of rac GTPases. Mol Pharmacol. 2003;63:1349–1355. doi: 10.1124/mol.63.6.1349. [DOI] [PubMed] [Google Scholar]
- 33.Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 34.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tominaga M, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 36.Szallasi A, Blumberg PM. Vanilloid receptors: New insights enhance potential as a therapeutic target. Pain. 1996;68:195–208. doi: 10.1016/s0304-3959(96)03202-2. [DOI] [PubMed] [Google Scholar]
- 37.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 38.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 39.Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite, and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006;34:965–970. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- 40.Chung JM. The role of reactive oxygen species (ROS) in persistent pain. Mol Interv. 2004;4:248–250. doi: 10.1124/mi.4.5.3. [DOI] [PubMed] [Google Scholar]
- 41.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hacimuftuoglu A, et al. Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav Brain Res. 2006;173:211–216. doi: 10.1016/j.bbr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 43.Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: A key role for superoxide anion. Eur J Pharmacol. 2006;548:167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Kim HK, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab Rev. 1998;30:225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- 46.Siniscalco D, et al. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res. 2007;55:158–166. doi: 10.1016/j.phrs.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 48.Herndon LA, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 49.Annunziato L, et al. Modulation of ion channels by reactive oxygen and nitrogen species: A pathophysiological role in brain aging? Neurobiol Aging. 2002;23:819–834. doi: 10.1016/s0197-4580(02)00069-6. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- 51.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 1996;47:S161–S170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- 53.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 54.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 55.Goldin AL. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 1992;207:266–279. doi: 10.1016/0076-6879(92)07017-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.