Abstract
Oxidatively truncated phospholipids are present in atherosclerotic lesions, apoptotic cells, and oxidized low density lipoproteins. Some of these lipids rapidly enter cells to induce apoptosis by the intrinsic pathway, but how such lipids initiate this process is unknown. We show the truncated phospholipid hexadecyl azelaoyl glycerophosphocholine (Az-LPAF), derived from the fragmentation of abundant sn-2 linoleoyl residues, depolarized mitochondria of intact cells. Az-LPAF also depolarized isolated mitochondria and allowed NADH loss, but did not directly interfere with complex I function. Cyclosporin A blockade of the mitochondrial permeability transition pore partially prevented the loss of electrochemical potential. Depolarization of isolated mitochondria by the truncated phospholipid was readily reversed by the addition of albumin that sequestered this lipid. Ectopic expression of the anti-apoptotic protein Bcl-XL in HL-60 cells reduced apoptosis by the truncated phospholipid by protecting their mitochondria. Mitochondria isolated from these cells were also protected from Az-LPAF-induced depolarization. Conversely mitochondria isolated from Bid−/− animals that lack this pro-apoptotic Bcl-2 family member were resistant to Az-LPAF depolarization. Addition of recombinant full-length Bid, which has phospholipid transfer activity, restored this sensitivity. Thus, phospholipid oxidation products physically interact with mitochondria to continually depolarize this organelle without permanent harm, and Bcl-2 family members modulate this interaction with full-length Bid acting as a co-factor for pro-apoptotic, oxidatively truncated phospholipids.
Vascular cells are exposed to oxidizing radicals during normal metabolism, but especially so during physiologic and pathologic inflammatory processes. The double bonds of polyunsaturated fatty acyl residues are particularly prone to attack by radicals because the C-H bond situated between two double bonds is relatively weak, allowing a more facile abstraction of hydrogen to produce a radical (1). Because polyunsaturated fatty acyl residues are abundant and are generally esterified in the sn-2 position of the glycerol backbone, common products of oxidative attack on cells and circulating lipoproteins are phospholipids that have been peroxidized at their sn-2 position. These peroxy radicals abstract hydrogen to form hydroperoxy phospholipids, may be reduced to the corresponding alcohol, rearrange (2, 3), or fragment to generate a host of oxidatively truncated phospholipids (4–7).
The shortened sn-2 residue of truncated phospholipids, which may also contain a newly introduced polar oxygen function, does not intercalate into the membrane well and is energetically favored to protrude into the aqueous phase, a conformation that disorders phospholipid packing into a bilayer (8–10). Oxidatively truncated phospholipids are more water soluble than their phospholipid precursors and readily associate with plasma albumin (11), plasma membranes (12), and even traffic into cells to lysosomes (12) or mitochondria (13) depending on the structure of the truncated phospholipid.
Phospholipid oxidation products can be cytotoxic (14, 15), and at least some of these are toxic because they initiate the apoptotic process of regulated cell death (13). The manner by which oxidatively truncated phospholipids alter cell viability has been ascribed to solubilization of the plasma membrane (14), adduction of mitochondrial proteins (17), temporary physical distortion of the plasma membrane (18), or activation of acid sphingomyelinase activity that alters plasma membrane microdomains by generating ceramide (15, 19). We found that a common oxidatively truncated phospholipid, containing a 9-carbon azelaoyl fragment derived from fragmentation of sn-2 linoleoyl residues, induces apoptosis by the intrinsic caspase cascade with loss of mitochondrial function and not, apparently, from damage of the plasma membrane (13).
Members of the Bcl-2 family modulate mitochondria-dependent apoptosis either by promoting apoptosis (Bid, Bad, and Bax) or obstructing this event (Bcl-2 and Bcl-XL). Aggregation of Bax on the mitochondrial outer membrane forms ion conducting pores and Bcl-XL associates with mitochondrial outer membranes to suppress this Bax activity (20). In contrast, Bid promotes apoptosis after cleavage to truncated Bid, a regulatory event catalyzed by activated caspase 8 (21). Bid, alone among Bcl-2 family members, displays homology to plant lipid transfer proteins and both truncated and full-length Bid will incorporate fluorescent phospholipids, and not the cognate fluorescent fatty acid, into mitochondrial membranes (22).
We determined whether mitochondrial integrity or function were directly affected by oxidatively truncated phospholipids, and then whether Bcl-2 family members alter these effects as they do in other, established apoptotic signaling pathways. We find that truncated phospholipids accumulated from the extracellular environment depolarize intracellular mitochondria, that these bilayer challenged phospholipids reversibly interact with mitochondria to continually reduce their transmembrane potential, and that Bcl-2 family members modulate this interaction.
EXPERIMENTAL PROCEDURES
Materials
JC-1, annexin V, propidium iodide, and the mitoprobe transition pore assay kit were from Invitrogen. Recombinant murine Bid was from ProteinX Lab (San Diego, CA). Tetramethylrhodamine methyl ester (TMRM),2 CCCP (carbanoyl cyanide m-chlorophenyl hydrazone), NADH, coenzyme Q10, rotenone, phosphate-buffered saline (PBS), cyclosporin A, bovine serum albumin (BSA), and other reagents were from Sigma. The Cleveland Clinic medium core prepared RPMI 1640 medium. Fetal bovine serum was from Fisher Scientific (Pittsburgh, PA). [acetyl-3H]PAF was supplied by PerkinElmer Life Sciences. Az-LPAF and butyroyl-PAF (C4PAF) were from Cayman Chemical (Ann Arbor, MI). Lyso-PAF, carbamoyl-PAF, PAF, and BEL were from BioMol Research Laboratories (Plymouth Meeting, PA). Palmitoyl glutaroyl phosphatidylcholine, palmitoyl oxovaleroyl phosphatidylcholine, and lysophosphatidic acid were from Avanti Polar Lipids, Inc. (Alabaster, AL).
Cell Culture
HL-60 cells were cultured in RPMI 1640 supplemented with streptomycin (100 units/ml), penicillin (100 μg/ml), and 10% fetal bovine serum. The cells were maintained in log phase growth at 37 °C in a humidified atmosphere of 5% CO2.
Mitochondria Preparation
Livers from adult Sprague-Dawley rats, or C57 BL6 wild type or Bid−/− mice (generously provided by Dr. Xiao-Ming Yin, University of Pittsburg) in a protocol approved by the Cleveland Clinic IACUC were excised and minced on ice in EB medium (200 mm d-mannitol, 70 mm sucrose, 20 mm Hepes, pH 7.4, 0.5 g/liter of defatted BSA, and 1 mm EGTA). Homogenates were centrifuged at 1,000 × g for 5 min twice before these supernatants were centrifuged at 9,500 × g for 10 min. The resulting pellets were resuspended in the same volume of EB medium without BSA and centrifuged at 9,500 × g for 10 min. The final pellet was resuspended in EB medium to 35 mg of protein/ml. HL-60 cells (3 × 108) stably transfected with Bcl-XL or its empty vector were washed twice in PBS and once in EB medium before the cells were suspended in 10 ml of EB medium containing BSA (2 mg/ml), and then mechanically homogenized and mitochondria isolated as above. Cellular organelles were prepared from liver homogenates pre-cleared by centrifugation (1,000 × g) and then fractionated by centrifugation through a sucrose density gradient. Sucrose concentrations of the recovered fractions were determined by refractometry.
Phospholipid Hydrolysis
Density purified subcellular fractions were recovered and assayed for PAF hydrolysis with or without a 30-min preincubation with the iPLA2 inhibitor BEL at 10 μm to achieve complete inhibition. Hydrolysis of [acetyl-3H]PAF was quantitated by separation and recovery of [3H]acetate over C8-silica cartridges as described (23). Hydrolysis of Az-LPAF used freezing and thawing to lyse mitochondria (40 mg). This was diluted into Hanks' balanced salt solution containing 400 nm Az-LPAF and incubated in triplicate at 37 °C for the stated times before the reaction was stopped with methanol and the lipid recovered by extraction (24). [2H]PAF was used as an internal standard. Quantitation of samples or defined amounts of Az-LPAF used liquid chromatography/electrospray ionization/tandem mass spectrometry with a Quattro Ultima triple quadrapole mass spectrometer (Micromass, Wythenshawe, UK) with collision-induced dissociation by argon gas. Analyses were performed using electrospray ionization in the positive-ion mode with multiple reaction monitoring. The multiple reaction monitoring transitions used to detect the choline phospholipids were the mass to charge ratio (m/z) for the molecular ion [M + H]+ and the m/z 184 phosphocholine daughter ion.
Complex I Activity Measurement
Mitochondria were frozen and thawed before these lysed mitochondria (1 mg) were added to 1 ml of a reaction mixture containing 15 mm Hepes, pH 7.3, 3.5 mm phosphate, 50 mm KCl, 80 μm coenzyme-Q10, and the stated amount of Az-LPAF. The reaction mixture was equilibrated at room temperature (3 min) prior to the addition of 0.2 mm NADH to initiate the reaction. NADH oxidation was monitored at 340 nm at 37 °C over time.
Mitochondrial Membrane Potential
Rat liver mitochondria (0.1 mg/ml) were mixed with 0.5 μm TMRM, a positively charged fluorescent dye accumulated by energized mitochondria, and the fluorescence intensity of the organelle was determined using stirred, thermostatically controlled cuvettes. Fluorescence was determined using excitation wavelengths of 546 and 573 nm and an emission wavelength of 590 nm. The ratio between these signals reflects mitochondrial membrane potential. Changes in mitochondrial NADH levels were determined by incubating mitochondria (0.4 mg/ml) at room temperature in medium composed of 250 mm sucrose, 10 mm Hepes (pH 7.4), 25 mm EGTA, and 2 mm phosphate at 24 °C as fluorescence was determined by excitation at 340 nm with emission monitored at 460 nm. Fluorescence measurements used an LPS-200B fluorometer (Photon Technologies International, Lawrenceville, NJ) operated by FeliX32 software with slit widths for both emission and excitation set at 2.5 nm.
Flow Cytometry
HL-60 cells were washed and resuspended in serum-free RPMI 1640 and incubated with the stated amount of Az-LPAF for 4 h. One aliquot of washed cells was irradiated by 400 J/m2 (measured using a IL1700 radiometer and a SED240 UVB detector; International Light, Newburyport, MA) for 5 min, whereas a third aliquot remained unstimulated prior to the 4-h culture.
Apoptosis Quantification
To measure surface phosphatidylserine and the ability to exclude a nuclear dye, HL-60 cells were washed with PBS and stained with Annexin V-Alex 488 (1/100 dilution) and 1 μg/ml propidium iodide in binding buffer (140 mm NaCl, 10 mm Hepes, pH 7.4, and 2.5 mm CaCl2) for 15 min at room temperature. At the end of this incubation the samples were diluted 4-fold with binding buffer and fluorescence was assessed by two-color flow cytometry.
Assessment of Mitochondrial Potential
Mitochondrial membrane potential was detected by incubating the cells with the potentiometric dye JC-1 (10 μg/ml) for 15 min, before the cells were washed twice and resuspended for two-color flow cytometry.
RESULTS
The Truncated Phospholipid Az-LPAF Initiates Apoptosis and Depolarizes Intracellular Mitochondria
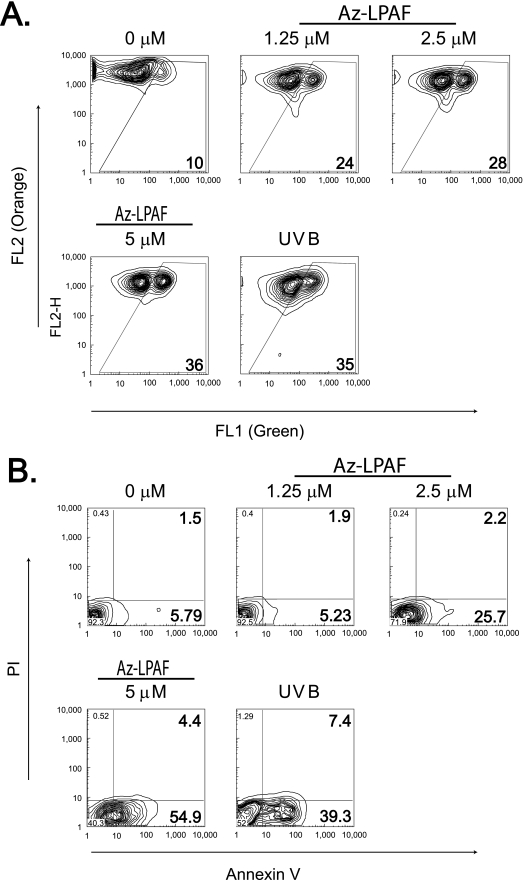
Oxidatively modified phospholipids affect cell function through G-protein coupled and nuclear hormone receptors, but also act in currently undefined, receptor-independent ways. For example, the truncated phospholipid Az-LPAF stimulates PAF receptor signaling (25), but low micromolar concentrations of this oxidatively truncated phospholipid rapidly depolarized HL-60 cell mitochondria (Fig. 1A). Because these cells do not express the PAF receptor (26), mitochondria are one non-receptor dependent target of the oxidized phospholipids. The electrochemical potential across the mitochondrial inner membrane in this experiment was monitored by JC-1 fluorescence. This cationic dye is accumulated by polarized mitochondria where its high concentration promotes aggregation, changing its green fluorescence as a monomer to a reddish orange. Two-color flow cytometry of HL-60 cells showed that as little as 1.25 μm Az-LPAF doubled the number of cells with dysfunctional mitochondria. The number of these cells increased as the concentration was increased to 5 μm Az-LPAF, rivaling that of the positive control, UVB irradiation.
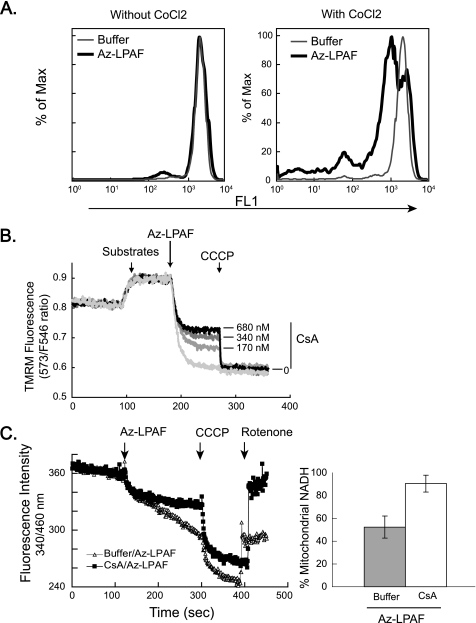
FIGURE 1.
The oxidatively truncated phospholipid Az-LPAF depolarized mitochondria and initiated apoptosis in intact HL-60 cells. A, mitochondrial transmembrane potential is compromised in intact HL-60 cells by Az-LPAF. HL-60 cells were incubated with the stated concentrations of Az-LPAF for 4 h, and then stained with the fluorescent potentiometric dye JC-1 as described under “Experimental Procedures.” The positive control was irradiation with 400 J/m2 UVB for 5 min. Two-color flow cytometry in the green FL1 (monomeric dye) and red FL2 channel (mitochondrial aggregates) are presented as topologic representations (n = 3). B, Az-LPAF initiates early apoptotic events. HL-60 cells were incubated in RPMI with the stated concentrations of Az-LPAF for 6 h in media, or were irradiated with UVB. The washed cells were stained with Alexa 488-conjugated annexin V and propidium iodide to stain nuclei of cells with failed permeability barriers as defined under “Experimental Procedures” (n = 3).
HL-60 cells exposed to these same concentrations of Az-LPAF also lost the phospholipid asymmetry of their plasma membrane, an early event in apoptotic cell death. The data show (Fig. 1B) that over half of the cells exposed to 5 μm Az-LPAF displayed phosphatidylserine on their surface, which was comparable with the 40% of cells that expressed phosphatidylserine on their surface after UVB irradiation. Few cells exposed to either Az-LPAF or UVB at this time had progressed to the point where they were unable to exclude propidium iodide from their nucleus.
Az-LPAF Depolarizes Isolated Mitochondria
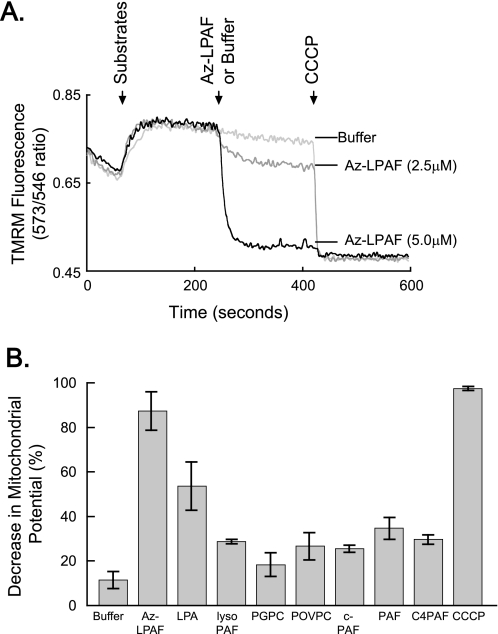
We determined whether Az-LPAF physically affected mitochondrial function using isolated rat liver mitochondria. Isolated mitochondria were provided glutamate and malate to oxidize (shown by the first arrow, Fig. 2A), which increased the transmembrane potential detected by increased TMRM fluorescence. The addition of 2.5 μm Az-LPAF at the time shown by the second arrow produced a rapid, but partial, loss of mitochondrial potential. There was an immediate and extensive loss of fluorescence when this truncated phospholipid was added at a final concentration of 5 μm. Complete depolarization of the isolated mitochondria was achieved by addition of the protonophore CCCP (third arrow, Fig. 2A). This agent had no effect on mitochondria previously exposed to 5 μm Az-LPAF, so this concentration of the truncated phospholipid had completely depolarized the organelle.
FIGURE 2.
Isolated mitochondria exposed to truncated phospholipids rapidly lose their transmembrane potential. A, Az-LPAF rapidly depolarized isolated mitochondria. Mitochondria isolated from rat liver were loaded with the potentiometric dye TMRM as described under “Experimental Procedures,” and then the ratio of fluorescence intensity from excitation at 573 and 546 nm with emission at 590 nm was recorded as a function of time. The oxidizable substrates glutamate and malate were added (first arrow), and then the stated concentration of Az-LPAF or buffer was added (second arrow). Fluorescence by mitochondria in a completely depolarized state was determined by the addition of the protonophore CCCP (5 μm) at the third arrow (n = 5). B, Az-LPAF is the most effective, but not the only, phospholipid to depolarize mitochondria. Isolated mitochondria labeled with TMRM were treated with 2.5 μm of various short-chain phospholipids or lysolipids, and the change in TMRM fluorescence after 200 s of exposure relative to the loss of membrane potential after exposure to CCCP was recorded. The data from three independent experiments are shown with standard error (n = 3). LPA, lysophosphatidic acid; PGPC, palmitoyl glutaroyl phosphatidylcholine; POVPC, palmitoyl oxovaleroyl phosphatidylcholine; c-PAF, carbamoyl platelet-activating factor; C4-PAF; butyroyl platelet-activating factor.
Az-LPAF, although the Most Effective Truncated Phospholipid, Is Not the Sole Lipid to Depolarize Mitochondria
We first determined whether the sn-1 ether bond of Az-LPAF was required for mitochondrial depolarization. We found that Az-LPAF was about twice as effective as its diacyl homolog 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine (95 ± 2% depolarization by 5 μm Az-LPAF versus 40 ± 15% for diacyl palmitoyl azelaoyl glycerophosphocholine). We next tested related phospholipids and lysophospholipids, and many of these also caused a rapid loss of the energy gradient across intact mitochondria (Fig. 2B). Phospholipids with very short sn-2 residues (e.g. those with the 2-carbon acetyl residue of PAF and carbamoyl-PAF, and the 4-carbon long residues of butyroyl-LPAF) reduced the transmembrane potential by about one-third, which was similar to the effect of lyso-PAF that lacks any sn-2 residue. Phospholipids containing sn-2 residues 5 carbon atoms long (e.g. palmitoyl glutaroyl and palmitoyl oxovaleroyl phosphatidylcholines) that, like Az-LPAF, possess ω-oxy functions on the fragmented sn-2 residue, were also no more than one-third as effective as Az-LPAF. Apparently, the combination of an ω-acidic function in conjunction with a medium length sn-2 acyl chain is particularly devastating to mitochondrial function. Only the strongly acidic phospholipid lysophosphatidic acid approached Az-LPAF effectiveness in depolarizing mitochondria.
Mitochondria Are Not Irretrievably Damaged by Az-LPAF
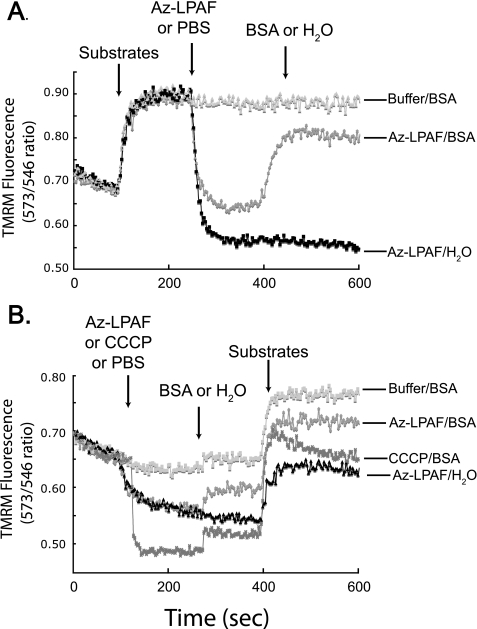
Amphipathic truncated phospholipids bearing a carboxyl function are detergents that disrupt membrane structure (14, 27), and they physically bind to cytochrome c (28). We therefore determined whether Az-LPAF irreversibly damaged mitochondrial function, as would be the case after solubilization, by depleting mitochondria of this lipid by sequestering it with excess albumin. Addition of Az-LPAF to mitochondria oxidizing glutamate and malate resulted, as before, in an immediate loss of transmembrane potential (Fig. 3A). Addition of BSA to the Az-LPAF de-energized mitochondria produced an immediate recovery of most, although not all, of the lost potential energy. Mass spectrometry (not shown) confirmed that an albumin wash removed 80 to 90% of the Az-LPAF associated with mitochondria. These data show that Az-LPAF must be continuously present to fully depolarize mitochondria.
FIGURE 3.
The effect of Az-LPAF on isolated mitochondria is reversible. A, sequestration of Az-LPAF from energized mitochondria allows rapid repolarization. Isolated mitochondria were loaded with TMRM and energized by the addition of glutamate and malate, and 2.5 μm Az-LPAF was added at the time shown by the arrow. Subsequently, either PBS or 100 μg/ml BSA in PBS was added (third arrow) and fluorescence intensity was recorded as described in the legend to Fig. 2. B, Az-LPAF pre-treatment reversibly suppressed subsequent polarization by substrate oxidation. Mitochondria were loaded with TMRM and fluorescence in the absence of oxidizable substrate was recorded before the addition of Az-LPAF, PBS, or CCCP (first arrow). At the second arrow, PBS or 100 μg/ml BSA was added, and 2.5 min after that the oxidizable substrates glutamate and malate were added as shown by the third arrow (n = 3).
We determined whether a transmembrane potential gradient, perhaps aiding internalization of the lipid, was essential for depolarization by Az-LPAF by treating mitochondria with Az-LPAF in the absence of oxidizable substrates. We found (Fig. 3B) that the resting transmembrane potential was rapidly and fully depolarized by CCCP and by about half with Az-LPAF. Albumin alone did not affect organelle function, but the introduction of albumin induced a small increase in transmembrane potential of mitochondria exposed to the lipids Az-LAPF or CCCP. Substrate addition after albumin addition induced a rapid increase in polarization, although this increase was diminished in mitochondria that had previously been exposed to Az-LPAF or CCCP. These results show that the effect of Az-LPAF on mitochondrial function is aided by a large transmembrane potential as in Fig. 3A, but that, again, the bulk of the effect of Az-LPAF on mitochondria was reversible.
We determined whether complex I composition or function was altered by Az-LPAF using Blue Native electrophoresis, which recovers intact electron transport lipoprotein complexes (29), to find all macromolecular complexes were still present after exposure to Az-LPAF (not shown). Staining these gels with the oxidizable substrate nitro blue tetrazolium dye indicated that complex I function was normal after Az-LPAF exposure (data not shown).
Mitochondria Metabolize Az-LPAF
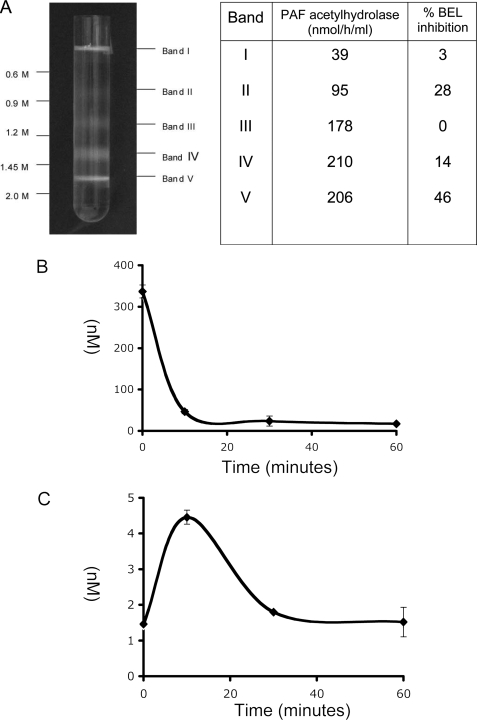
Mitochondria contain iPLA2 that protects against staurosporine-induced apoptosis (30). Mitochondria also express the calcium-dependent phospholipase A2-γ that hydrolyzes phosphatidylcholine and then can also reacylate the resulting lysophosphatidylcholine (31). We determined whether mitochondria also displayed PAF acetylhydrolase-like activity, that is, the ability to hydrolyze PAF in the absence of Ca2+. We found that the fraction in a sucrose density gradient containing intact mitochondria (fraction V) effectively hydrolyzed PAF, and that this activity was displayed by the other subcellular fractions (Fig. 4A). The mechanism-based iPLA2 inhibitor BEL (32) reduced PAF hydrolysis in fraction V by half, indicating that mitochondrial iPLA2 was able to hydrolyze short-chain phospholipids. Plasma (33) and type II (34) PAF acetylhydrolases additionally will hydrolyze oxidized phospholipids such as Az-LPAF. The ability to hydrolyze this truncated phospholipid was also present in mitochondria. Thus, isolated mitochondria completely catabolized Az-LPAF by 10 min (Fig. 4B), with an increase in lyso-PAF (Fig. 4C). Lyso-PAF was not stable in isolated mitochondria and its concentration fell over the subsequent 10 min.
FIGURE 4.
Mitochondria catabolize Az-LPAF. A, PAF acetylhydrolase activity and iPLA2 contribution in liver subcellular organelles separated in a density gradient. Rat liver homogenates were separated on a sucrose density gradient and the apparent specific activity for [acetyl-3H]PAF hydrolysis determined without or with preincubation with the mechanism based iPLA2 inhibitor BEL as described under “Experimental Procedures.” B, mitochondria hydrolyze Az-LPAF. Mitochondria were incubated with 400 nm Az-LPAF for the stated times before the reaction was stopped by lipid extraction. The recovered lipids were separated and quantified by mass spectrometry as described under “Experimental Procedures.” C, lyso-PAF transiently accumulates from Az-LPAF hydrolysis by isolated mitochondria. Lipid samples used in panel B were analyzed for lyso-PAF by mass spectrometry as described under “Experimental Procedures.” Panels B and C report values with their standard errors (n = 3). Endogenous levels of lyso-PAF were 5.8 ± 0.43 and 85 ± 45 pg/mg for Az-LPAF.
Az-LPAF Opens the Mitochondrial Permeability Transition Pore
One route to loss of membrane potential, with subsequent irreversible effects, is through the opening of the mitochondrial permeability transition pore that allows molecules below ∼2000 daltons to move across the inner membrane. To determine whether Az-LPAF opened this channel, we loaded HL-60 cells with the fluorescent dye calcein, which distributes between mitochondria and cytoplasm, and treated them with either cobalt chloride or buffer. The cobalt cation quenches calcein fluorescence, but cannot do so when an intact permeability barrier excludes the ion from the mitochondrial matrix. We found that, first, this dye was retained by cells exposed to Az-LPAF, which shows that the plasma membrane barrier function was uncompromised by the truncated phospholipid. Second, we found that the internalized dye content was the same between control and Az-LPAF-treated cells (Fig. 5A, left). We also found that the cobalt ion had no effect on the fluorescence of control cells, but that fluorescence in the majority of cells exposed to Az-LPAF was sharply reduced by cobalt exposure (Fig. 5A, right). This means the cobalt ion had access to the inner mitochondrial compartment, which in turn implies that the mitochondrial permeability barrier had been breached.
FIGURE 5.
The mitochondrial permeability barrier is compromised by Az-LPAF. A, mitochondria of whole cells are compromised by exogenous Az-LPAF. HL-60 cells were loaded with the fluorescent dye calcein-AM and then exposed (right panel) or not (left panel) to CoCl2 before the cells were treated with 5 μm Az-LPAF or buffer for 30 min. Fluorescence intensity recorded during flow cytometry shows the cobalt cation, which does not penetrate the mitochondrial permeability barrier, had no effect on the fluorescence of control cells, but quenched the fluorescence of the majority of cells that had been exposed to Az-LPAF. B, blockade of the mitochondrial permeability transition pore with cyclosporin A partially protects isolated mitochondria from Az-LPAF-induced depolarization. Mitochondria from rat liver were labeled with TMRM as described in the legend to Fig. 2 and then treated with the stated concentrations of cyclosporin A for 5 min and then provided with glutamate and malate (first arrow). Az-LPAF was added (second arrow) and, as before, the mitochondria were completely depolarized by the addition of CCCP (third arrow) (n = 3). C, Az-LPAF induces NADH loss through the permeability transition pore. Mitochondria were treated or not with 720 nm cyclosporin A and then with 5 μm Az-LPAF (first arrow). CCCP (1 μm) was added to maximally stimulate flux through the electron transport chain (second arrow), and finally 1 μm rotenone was added (third arrow) to block electron transport to complex II as fluorescence at 340 nm was continuously recorded. The right panel quantitates the amount of NADH, determined by fluorescence at 340 nm, remaining in mitochondria recovered by centrifugation at the termination of the experiment. Values are shown with their standard error (n = 3).
We determined whether the permeability transition pore participated in the loss of barrier function using isolated mitochondria treated with varied concentrations of cyclosporin A to block the cyclophilin D component of the pore. This inhibitor produced a partial, concentration-dependent increase (Fig. 5B) in mitochondrial potential in mitochondria that would have been completely depolarized by 5 μm Az-LPAF. Cyclosporin A also reduced mitochondrial swelling secondary to collapse of the electrical potential, but again was only partially effective in this (not shown).
Opening of the permeability transition pore allows NADH to leach from mitochondria, compromising their function. To test for the effect of Az-LPAF on mitochondrial NADH content we treated mitochondria with cyclosporin A, or not, and then with 5 μm Az-LPAF. We found (Fig. 5C, left, first arrow) that there was a time-dependent loss of NADH fluorescence, and that this change was less severe when the permeability transition pore was blocked by cyclosporin A. We then added CCCP (second arrow) to stimulate maximal electron transport and this, as expected, pulled electrons from NADH causing a rapid loss of fluorescence at 340 nm as non-fluorescent NAD+ accumulated. Finally, we added rotenone (third arrow) to block the flow of electrons from complex I to complex II. In this case, NADH produced by glutamate and malate oxidation cannot be reduced by the electron transport chain. Thus, rotenone addition allowed a full recovery of NADH fluorescence when the permeability transition pore was blocked by cyclosporin A, but allowed only a partial recovery of NADH fluorescence in mitochondria exposed to Az-LPAF when the permeability transition pore was not blocked.
We determined whether NADH had been in fact released from Az-LPAF-treated mitochondria by physically recovering them by centrifugation from the foregoing experiment. The data (Fig. 5C, right) showed that cyclosporin A-treated mitochondria retained a greater portion of their NADH after Az-LPAF exposure than did those organelles exposed to just the truncated phospholipid.
Az-LPAF-induced Mitochondrial Dysfunction Is Regulated by Bcl-2 Family Members
Bcl-XL is a member of the Bcl-2 family that physically interacts with the mitochondrial outer membrane and suppresses apoptosis (35). Overexpressing Bcl-XL in HL-60 cells conferred a significant protection from Az-LPAF-induced apoptosis as shown by the reduced abundance of cell surface phosphatidylserine (Fig. 6A). Bcl-XL overexpression also reduced the loss of mitochondrial membrane potential in cells exposed to Az-LPAF, although Bcl-XL overexpression itself modestly decreased mitochondrial function (Fig. 6B).
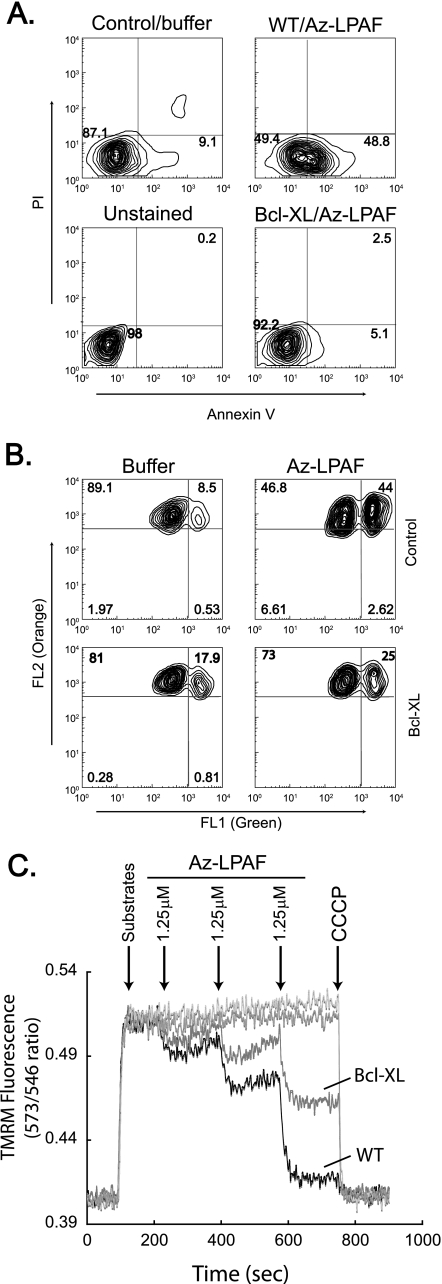
FIGURE 6.
The anti-apoptotic protein Bcl-XL protects cells and isolated mitochondria from Az-LPAF-induced depolarization and apoptosis. A, Bcl-XL suppresses Az-LPAF-induced surface expression of phosphatidyl-serine. HL-60 cells stably transfected with human Bcl-XL or empty vector were treated with 5 μm Az-LPAF or buffer for 6 h as described in the legend to Fig. 1. The cells were then stained with Alexa 488-conjugated annexin V to detect externalized phosphatidylserine and propidium iodide before cellular fluorescence was quantified by flow cytometry also as described in the legend to Fig. 1 (n = 3). B, Bcl-XL suppressed Az-LPAF-induced depolarization of mitochondria within HL-60 cells. HL-60 cells stably transfected with human Bcl-XL or empty vector were incubated with 5 μm Az-LPAF for 4 h, and then loaded with the dye JC-1 for 30 min before monomeric fluorescence in FL1 (x axis) and mitochondria internalized dye was assessed in FL2 (y axis) (n = 3). C, Bcl-XL protects isolated mitochondria from Az-LPAF-induced depolarization. Mitochondria isolated from HL-60 cells expressing Bcl-XL or empty vector were labeled with TMRM as described in the legend to Fig. 2 and given glutamate and malate at the times shown by the first arrow. Az-LPAF was then added in 1.25 μm increments at the times shown by subsequent arrows, and then the mitochondria were completely depolarized by the addition of CCCP (n = 3).
The protection afforded by overexpressing Bcl-XL was a direct effect on mitochondria because the protective effect remained with mitochondria isolated from the overexpressing cells. Exposing mitochondria isolated from control HL-60 cells to Az-LPAF in 1.25 μm increments produced a progressive loss of transmembrane potential with each addition of Az-LPAF (Fig. 6C). This loss of transmembrane potential was greatly reduced when the mitochondria were obtained from Bcl-XL overexpressing cells. For example, when mitochondria from control cells were nearly completely depolarized by 3.75 μm Az-LPAF, mitochondria from Bcl-XL expressing cells still retained about 50% of their charge.
Bid is a pro-apoptotic member of the Bcl-2 family that uniquely possesses phospholipid transferase activity. Accordingly, we found that recombinant murine Bid (rBid) sensitized the mitochondria isolated from wild type animals to the effects of small amounts of Az-LPAF (Fig. 7A). Bid itself had no effect on mitochondrial potential, nor did 1 μm Az-LPAF, but the combination of the two resulted in a steady loss of the accumulated charge.
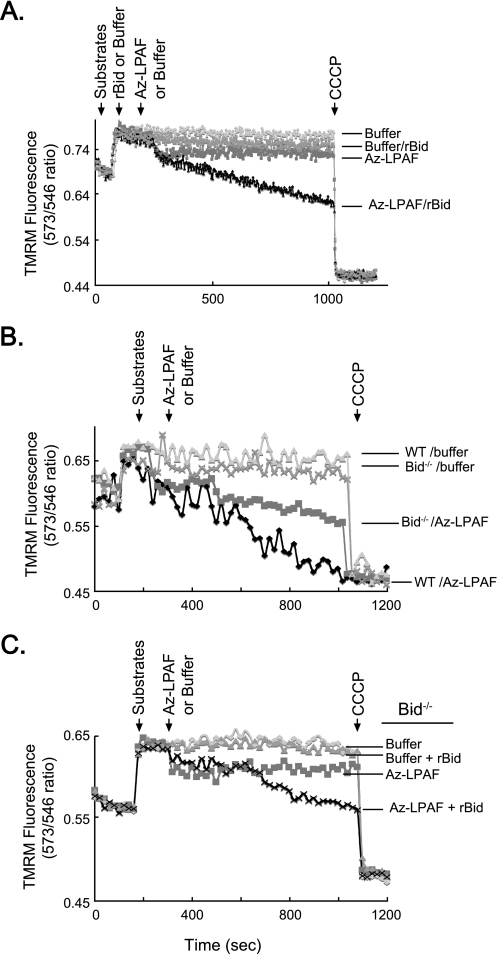
FIGURE 7.
Az-LPAF-induced mitochondrial dysfunction is enhanced by the pro-apoptotic protein Bid. A, Bid and Az-LPAF synergize to depolarize mitochondria. Isolated rat liver mitochondria were loaded with TMRM, the organelles were provided with glutamate and malate, and changes to the transmembrane potential were recorded as described in the legend to Fig. 2. Recombinant murine Bid (2 μg) was added, or not, as shown by the second arrow, and 2 min later 1 μm Az-LPAF was introduced into the cuvette. CCCP was added at the final arrow to achieve complete depolarization (n = 3). B, mitochondria from Bid−/− animals were less sensitive to Az-LPAF than mitochondria from the parental strain. Mitochondria isolated from Bid−/− or BL6 murine livers were loaded with TMRM and changes in transmembrane potential with or without the addition of 5 μm Az-LPAF (second arrow) were recorded as in the proceeding panel (n = 3). C, recombinant Bid restores sensitivity of Bid−/− mitochondria to Az-LPAF. Mitochondria isolated from the livers of Bid null mice were incubated with 2 μg of recombinant Bid for 5 min before they were loaded with TMRM, energized with glutamate and malate (first arrow), treated or not with 5 μm Az-LPAF (second arrow), and finally depolarized with CCCP (third arrow) (n = 3).
Conversely, a lack of Bid suppressed mitochondrial sensitivity to Az-LPAF. Mitochondria isolated from the livers of Bid knock-out animals behaved like mitochondria from control mice when given oxidizable substrates, but were depolarized at a much slower rate than their normal counterparts when exposed to Az-LPAF (Fig. 7B). Ultimately, when mitochondria from wild type animals had been completely depolarized by Az-LPAF, mitochondria from Bid null animals still retained half their original membrane potential. Mitochondria from Bid knock-out animals remained sensitive to Bid, however, because the addition of recombinant Bid sensitized these organelles to low concentrations of Az-LPAF (Fig. 7C). Here, the combination of Az-LPAF and recombinant Bid initiated a time-dependent loss of membrane potential by mitochondria isolated from Bid−/− animals that did not occur when just Az-LPAF was present.
DISCUSSION
The truncated phospholipid Az-LPAF rapidly enters cells, and a significant portion of this internalized phospholipid associates with mitochondria (13). Here, we find that mitochondria exposed to this oxidatively truncated phospholipid rapidly lose their transmembrane potential at least partially through opening of the permeability transition pore. As with other apoptotic stimuli, Bcl-XL protected mitochondria from Az-LPAF, whereas Bid augmented this mitochondrial dysfunction.
What we did not find in this study was that truncated phospholipids “ruined” the membrane structure (36). The critical site of bilayer perturbation by the various truncated phospholipids was at the mitochondrial membrane, and not at the plasma membrane (14) with (15) or without (18) the participation of acid sphingomyelinase that hydrolyzes sphingomyelin to non-bilayer forming ceramide. We conclude this because cells exposed to Az-LPAF retained cytoplasmic fluorescent dyes, and did not allow entry of extracellular Ca2+ (not shown). Although the barrier to NADH or cobalt cations presented by mitochondrial membranes was functionally compromised by Az-LPAF, the mitochondrial membrane organization was not impaired to the extent that the electron transport chain was compromised. Moreover, washing the organelle with albumin to remove this phospholipid allowed the mitochondria to rapidly recover most of their membrane potential. This shows that mitochondrial depolarization primarily was an ongoing process that required the continual presence of Az-LPAF to reduce transmembrane potential. The small amount of non-reversible change induced by Az-LPAF might result from the release of a portion of matrix NADH.
Model systems show that truncated phospholipids like Az-LPAF greatly perturb the membrane bilayer structure because the ionizable carboxylate function orients the residue toward the solvent (8–10) rather than into the hydrophobic phase. This extension of the sn-2 residue into the solvent disrupts phospholipid packing, distorts the bilayer, causes phase separation of normal and truncated phospholipids (8, 9, 37), and changes membrane surface potential (8). Phospholipid oxidation products that disrupt membrane structure and function in these ways are physiologically relevant because these types of damaged phospholipids are present in mitochondria isolated from the livers of animals oxidatively stressed by CCl4 intoxication (36), and are generated by mitochondria themselves during state IV respiration (38).
Az-LPAF was the most effective depolarizing phospholipid we examined, and, interestingly, was more effective than glutaroyl phosphatidylcholine derived from oxidative fragmentation of sn-2 arachidonoyl residues. The sn-2 glutaroyl fragment, like the azelaoyl fragment derived from oxidative cleavage of the more abundant linoleoyl residues, contains an ionizable carboxyl function at the ω end of the shortened sn-2 fatty acyl fragment. The glutaroyl fragment is shorter than the azelaoyl fragment by 4 methylene molecules, and the decreased hydrophobicity of the glutaroyl residue had a less severe effect on bilayer organization than the longer azelaoyl fragment (39). Still, glutaroyl phosphatidylcholine will induce apoptotic cell death (40) and, like Az-LPAF, is internalized (12, 41). However unlike Az-LPAF that was primarily found in mitochondria (13), fluorescent glutaroyl phosphatidylcholine preferentially accumulates in lysosomes (12). Whether this disparate localization reflects differences in the length of the sn-2 fragment or structural differences, e.g. the sn-1 ether bond of Az-LPAF or the presence of a bulky fluorescent group of the glutaroyl phosphatidylcholine probe, remains to be determined.
Disruption of phospholipid packing by Az-LPAF intercalation into mitochondrial membranes was not the sole agent of mitochondrial dysfunction because opening of the mitochondrial permeability transition pore had a role in extending the loss of the transmembrane potential. Thus we found that addition of cyclosporin A, which occludes the pore through its interaction with cyclophilin D (42), protected a portion of the transmembrane potential from the effect of Az-LPAF. Free fatty acids, which like Az-LPAF contain a full negative charge on a carboxylate function, depolarize mitochondria, and do so in part by opening the permeability transition pore in a cyclosporin-dependent way (43).
In fact, the charge on these lipids may not be the only, or even the major, element in opening the pore because ceramide also depolarizes mitochondria in a fashion that is reduced by about half by the presence of cyclosporin A (44). This suggests the common element of these lipids is their ability to physically intercalate into mitochondrial membranes and change the highly ordered nature of these membranes. The central effect of membrane organization on pore opening is reinforced by the observation that the membrane intercalating peptide mastoparan also opens a cyclosporin-sensitive permeability transition pore (45). Indeed gangliosides GD3 and GM3, which normally reside in cholesterol- and sphingomyelin-rich microdomains at the plasma membrane, move to the mitochondrial inner membrane in response to extrinsic apoptotic signals where they again organize into cholesterol-rich microdomains, disrupt membrane structure, and collapse the electrochemical gradient (46).
Mitochondria have the ability to protect themselves and limit their exposure to oxidatively truncated phospholipids because they were able to hydrolyze short-chain phospholipids PAF and Az-LPAF. PAF acetylhydrolases specifically hydrolyze short-chain phospholipids (33, 34). Whether iPLA2 also can hydrolyze these lipids has not been tested, but specific inhibition of this enzyme showed that almost half of the PAF hydrolase activity of mitochondria could be ascribed to this enzyme. iPLA2 reduces peroxide-initiated cell death (47) and protects mitochondria against oxidative damage (30). This suggests turnover of oxidatively damaged phospholipids, including truncated phospholipids, and preserves mitochondrial function and cell viability.
The permeability transition pore (48) and mitochondrial transmembrane potential are regulated by Bcl-2 family members (49–52), and we found that Bcl-XL and the BH3-only protein Bid exerted opposing effects on the sensitivity of mitochondria to Az-LPAF. We observed that neither intact Bid nor low concentrations of Az-LPAF affected mitochondrial transmembrane potential, but their combination did so. Because Bid interacts with microsomal lipid microdomains (46), it is well positioned to promote the intercalation of Az-LPAF into mitochondrial membranes. It is notable that Bid, at least after proteolytic cleavage to truncated Bid, induces cytochrome c escape in a way that is not dependent on the permeability transition pore (53). Potentially, this unidentified mechanism also participates in the pore-independent effect of Az-LPAF.
Bid, alone among Bcl-2 family members, displays weak homology to plant lipid transfer proteins (54), and in fact functions as a lipid transporter. Bid enhances the mobility of acidic phospholipids (55, 56) and lysophosphatidic acid (22), but not unesterified fatty acids. A mutated Bid that does not bind lysophospholipids also is not pro-apoptotic (22). Lipid transfer activity is present whether or not Bid has been cleaved to its truncated form (22), and we found that full-length Bid promoted mitochondrial dysfunction by Az-LPAF. This then means that sensitivity to oxidatively truncated phospholipids is not regulated by Bid cleavage. Instead, it is the accumulation of oxidatively truncated phospholipids, e.g. during CCl4 intoxication (36), that determines whether mitochondria depolarize, swell, and release cytochrome c to complete the apoptosome under conditions that promote formation of phospholipid oxidation products.
These studies focused on levels of truncated phospholipid sufficient to evoke large changes in mitochondrial function associated with apoptotic events. Our data, however, also show that the effect of these lipids is progressive and that lower levels of exposure still induce noticeable decreases in mitochondrial polarization. Truncated phospholipids, and lysophosphatidic acid, are uncoupling agents that are more effective in this than other lipid uncoupling agents such as fatty acids, hydroperoxy fatty acids, or their Co-A thioesters (57, 58). Mitochondrial uncoupling reduces ATP generation that can compromise cellular function, but also reduces superoxide production and so may be protective during some pathologic challenges (59).
Oxidatively truncated phospholipids are specifically catabolized by PAF acetylhydrolase, either within or outside cells, to soluble fatty acid fragments and lysophospholipids (60, 61). This is a critical protective mechanism against exogenous oxidized phospholipids, tert-butyl hydroperoxide oxidative stress, or Ca2+ overloading of neuronal cells because overexpression of PAF acetylhydrolase protects cells from all these apoptotic insults (13, 16, 34, 62, 63). This means that for these oxidative insults it is the intact oxidized phospholipid that is responsible for the apoptotic event. Conversely, neither the lysophospholipid nor the fragmented acyl chain products of the phospholipase reaction, or even proteinaceous components such as Bid, are sufficient to initiate the apoptotic cascade in these circumstances. We now understand that the target of these pro-apoptotic phospholipids are the mitochondria, and that Bid and Bcl-XL are co-factors that act in opposing directions to alter mitochondrial sensitivity to oxidatively truncated phospholipids.
Acknowledgments
We thank Jessica Cemate and Gopal Marathe for analysis of PAF acetylhydrolase activity and Jinbo Liu and Renliang Zhang and the Mass Spectrometry Core II for aid with these analyses. We thank Michael Berk for aid with animal husbandry, and greatly appreciate the generous gift of Bid−/− mice from Dr. Xiao-Ming Yin (University of Pittsburgh).
This work was supported, in whole or in part, by National Institutes of Health Grants R01AA017748, R01HL092747, and 1R01DK076852 and National Center for Research Resources Grant CTSA 1UL1RR024989 (Cleveland, OH).
- TMRM
- tetramethylrhodamine methyl ester
- BEL
- bromoenol lactone
- CCCP
- carbonyl cyanide 3-chlorophenylhydrazone
- PAF
- platelet-activating factor
- iPLA2
- calcium-independent phospholipase A2
- PBS
- phosphate-buffered saline
- BSA
- bovine serum albumin
- Az-LPAF
- alkyl azelaoyl glycerophosphocholine (azelaoyl lysoPAF).
REFERENCES
- 1.Buettner G. R. (1993) Arch. Biochem. Biophys. 300, 535–543 [DOI] [PubMed] [Google Scholar]
- 2.Morrow J. D., Hill K. E., Burk R. F., Nammour T. M., Badr K. F., Roberts L. J., 2nd (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 9383–9387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salomon R. G., Miller D. B. (1985) Adv. Prostaglandin Thromboxane Leukot. Res. 15, 323–326 [PubMed] [Google Scholar]
- 4.Tokumura A., Toujima M., Yoshioka Y., Fukuzawa K. (1996) Lipids 31, 1251–1258 [DOI] [PubMed] [Google Scholar]
- 5.Reis A., Domingues P., Ferrer-Correia A. J., Domingues M. R. (2004) Rapid Commun. Mass Spectrom. 18, 2849–2858 [DOI] [PubMed] [Google Scholar]
- 6.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Gugiu B., Fox P. L., Hoff H. F., Salomon R. G., Hazen S. L. (2002) J. Biol. Chem. 277, 38503–38516 [DOI] [PubMed] [Google Scholar]
- 7.Chen X., Zhang W., Laird J., Hazen S. L., Salomon R. G. (2008) J. Lipid Res. 49, 832–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabatini K., Mattila J. P., Megli F. M., Kinnunen P. K. (2006) Biophys. J. 90, 4488–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong-Ekkabut J., Xu Z., Triampo W., Tang I. M., Tieleman D. P., Monticelli L. (2007) Biophys. J. 93, 4225–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg M. E., Li X. M., Gugiu B. G., Gu X., Qin J., Salomon R. G., Hazen S. L. (2008) J. Biol. Chem. 283, 2385–2396 [DOI] [PubMed] [Google Scholar]
- 11.Kugiyama K., Sakamoto T., Misumi I., Sugiyama S., Ohgushi M., Ogawa H., Horiguchi M., Yasue H. (1993) Circ. Res. 73, 335–343 [DOI] [PubMed] [Google Scholar]
- 12.Moumtzi A., Trenker M., Flicker K., Zenzmaier E., Saf R., Hermetter A. (2007) J. Lipid Res. 48, 565–582 [DOI] [PubMed] [Google Scholar]
- 13.Chen R., Yang L., McIntyre T. M. (2007) J. Biol. Chem. 282, 24842–24850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itabe H., Kushi Y., Handa S., Inoue K. (1988) Biochim. Biophys. Acta 962, 8–15 [PubMed] [Google Scholar]
- 15.Loidl A., Sevcsik E., Riesenhuber G., Deigner H. P., Hermetter A. (2003) J. Biol. Chem. 278, 32921–32928 [DOI] [PubMed] [Google Scholar]
- 16.Umemura K., Kato I., Hirashima Y., Ishii Y., Inoue T., Aoki J., Kono N., Oya T., Hayashi N., Hamada H., Endo S., Oda M., Arai H., Kinouchi H., Hiraga K. (2007) Stroke 38, 1063–1068 [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez J., Ballinger S. W., Darley-Usmar V. M., Landar A. (2006) Circ. Res. 99, 924–932 [DOI] [PubMed] [Google Scholar]
- 18.Kogure K., Nakashima S., Tsuchie A., Tokumura A., Fukuzawa K. (2003) Chem. Phys. Lipids 126, 29–38 [DOI] [PubMed] [Google Scholar]
- 19.Smith E. L., Schuchman E. H. (2008) FASEB J. 22, 3419–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chipuk J. E., Green D. R. (2008) Trends Cell Biol. 18, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji H., Shekhtman A., Ghose R., McDonnell J. M., Cowburn D. (2006) Magn. Reson. Chem. 44, S101–S107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goonesinghe A., Mundy E. S., Smith M., Khosravi-Far R., Martinou J. C., Esposti M. D. (2005) Biochem. J. 387, 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marathe G. K., Zimmerman G. A., McIntyre T. M. (2003) J. Biol. Chem. 278, 3937–3947 [DOI] [PubMed] [Google Scholar]
- 24.Bligh E. G., Dyer W. J. (1959) Can J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 25.Chen R., Chen X., Salomon R. G., McIntyre T. M. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller E., Dupuis G., Turcotte S., Rola-Pleszczynski M. (1991) Biochem. Biophys. Res. Commun. 181, 1580–1586 [DOI] [PubMed] [Google Scholar]
- 27.Megli F. M., Russo L., Conte E. (2009) Biochim. Biophys. Acta 1788, 371–379 [DOI] [PubMed] [Google Scholar]
- 28.Mattila J. P., Sabatini K., Kinnunen P. K. (2008) Biochim. Biophys. Acta 1778, 2041–2050 [DOI] [PubMed] [Google Scholar]
- 29.Brookes P. S., Pinner A., Ramachandran A., Coward L., Barnes S., Kim H., Darley-Usmar V. M. (2002) Proteomics 2, 969–977 [DOI] [PubMed] [Google Scholar]
- 30.Seleznev K., Zhao C., Zhang X. H., Song K., Ma Z. A. (2006) J. Biol. Chem. 281, 22275–22288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamashita A., Tanaka K., Kamata R., Kumazawa T., Suzuki N., Koga H., Waku K., Sugiura T. (2009) Biochim. Biophys. Acta, in press [DOI] [PubMed] [Google Scholar]
- 32.Hazen S. L., Zupan L. A., Weiss R. H., Getman D. P., Gross R. W. (1991) J. Biol. Chem. 266, 7227–7232 [PubMed] [Google Scholar]
- 33.Stremler K. E., Stafforini D. M., Prescott S. M., McIntyre T. M. (1991) J. Biol. Chem. 266, 11095–11103 [PubMed] [Google Scholar]
- 34.Kono N., Inoue T., Yoshida Y., Sato H., Matsusue T., Itabe H., Niki E., Aoki J., Arai H. (2008) J. Biol. Chem. 283, 1628–1636 [DOI] [PubMed] [Google Scholar]
- 35.Kroemer G. (1999) Biochem. Soc. Symp. 66, 1–15 [DOI] [PubMed] [Google Scholar]
- 36.Megli F. M., Sabatini K. (2004) FEBS Lett. 573, 68–72 [DOI] [PubMed] [Google Scholar]
- 37.Megli F. M., Russo L., Sabatini K. (2005) FEBS Lett. 579, 4577–4584 [DOI] [PubMed] [Google Scholar]
- 38.Megli F. M., Sabatini K. (2003) FEBS Lett. 550, 185–189 [DOI] [PubMed] [Google Scholar]
- 39.Megli F. M., Russo L. (2008) Biochim. Biophys. Acta 1778, 143–152 [DOI] [PubMed] [Google Scholar]
- 40.Fruhwirth G. O., Moumtzi A., Loidl A., Ingolic E., Hermetter A. (2006) Biochim. Biophys. Acta 1761, 1060–1069 [DOI] [PubMed] [Google Scholar]
- 41.Rhode S., Grurl R., Brameshuber M., Hermetter A., Schütz G. J. (2009) J. Biol. Chem. 284, 2258–2265 [DOI] [PubMed] [Google Scholar]
- 42.Waldmeier P. C., Zimmermann K., Qian T., Tintelnot-Blomley M., Lemasters J. J. (2003) Curr. Med. Chem. 10, 1485–1506 [DOI] [PubMed] [Google Scholar]
- 43.Wieckowski M. R., Brdiczka D., Wojtczak L. (2000) FEBS Lett. 484, 61–64 [DOI] [PubMed] [Google Scholar]
- 44.Novgorodov S. A., Szulc Z. M., Luberto C., Jones J. A., Bielawski J., Bielawska A., Hannun Y. A., Obeid L. M. (2005) J. Biol. Chem. 280, 16096–16105 [DOI] [PubMed] [Google Scholar]
- 45.Pfeiffer D. R., Gudz T. I., Novgorodov S. A., Erdahl W. L. (1995) J. Biol. Chem. 270, 4923–4932 [DOI] [PubMed] [Google Scholar]
- 46.Garofalo T., Giammarioli A. M., Misasi R., Tinari A., Manganelli V., Gambardella L., Pavan A., Malorni W., Sorice M. (2005) Cell Death Differ. 12, 1378–1389 [DOI] [PubMed] [Google Scholar]
- 47.Kinsey G. R., Blum J. L., Covington M. D., Cummings B. S., McHowat J., Schnellmann R. G. (2008) J. Lipid Res. 49, 1477–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vander Heiden M. G., Thompson C. B. (1999) Nat. Cell Biol. 1, E209–E216 [DOI] [PubMed] [Google Scholar]
- 49.Shimizu S., Eguchi Y., Kamiike W., Funahashi Y., Mignon A., Lacronique V., Matsuda H., Tsujimoto Y. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 1455–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuyama S., Llopis J., Deveraux Q. L., Tsien R. Y., Reed J. C. (2000) Nat. Cell Biol. 2, 318–325 [DOI] [PubMed] [Google Scholar]
- 51.Shimizu S., Shinohara Y., Tsujimoto Y. (2000) Oncogene 19, 4309–4318 [DOI] [PubMed] [Google Scholar]
- 52.Vander Heiden M. G., Chandel N. S., Williamson E. K., Schumacker P. T., Thompson C. B. (1997) Cell 91, 627–637 [DOI] [PubMed] [Google Scholar]
- 53.Kim T. H., Zhao Y., Barber M. J., Kuharsky D. K., Yin X. M. (2000) J. Biol. Chem. 275, 39474–39481 [DOI] [PubMed] [Google Scholar]
- 54.Degli Esposti M. (2002) Biochim. Biophys. Acta 1553, 331–340 [DOI] [PubMed] [Google Scholar]
- 55.Esposti M. D., Erler J. T., Hickman J. A., Dive C. (2001) Mol. Cell Biol. 21, 7268–7276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhai D., Miao Q., Xin X., Yang F. (2001) Eur. J. Biochem. 268, 48–55 [DOI] [PubMed] [Google Scholar]
- 57.Korge P., Honda H. M., Weiss J. N. (2003) Am. J. Physiol. Heart Circ. Physiol. 285, H259–H269 [DOI] [PubMed] [Google Scholar]
- 58.Jaburek M., Miyamoto S., Di Mascio P., Garlid K. D., Jezek P. (2004) J. Biol. Chem. 279, 53097–53102 [DOI] [PubMed] [Google Scholar]
- 59.Echtay K. S. (2007) Free Radic. Biol. Med. 43, 1351–1371 [DOI] [PubMed] [Google Scholar]
- 60.Stafforini D. M. (2009) Cardiovasc. Drugs Ther. 23, 73–83 [DOI] [PubMed] [Google Scholar]
- 61.McIntyre T. M., Prescott S. M., Stafforini D. M. (2009) J. Lipid Res. 50, (Suppl.) S255–S259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuzawa A., Hattori K., Aoki J., Arai H., Inoue K. (1997) J. Biol. Chem. 272, 32315–32320 [DOI] [PubMed] [Google Scholar]
- 63.Hirashima Y., Ueno H., Karasawa K., Yokoyama K., Setaka M., Takaku A. (2000) Brain Res. 885, 128–132 [DOI] [PubMed] [Google Scholar]