Abstract
Class I hydrophobins function in fungal growth and development by self-assembling at hydrophobic-hydrophilic interfaces into amyloid-like fibrils. SC3 of the mushroom-forming fungus Schizophyllum commune is the best studied class I hydrophobin. This protein spontaneously adopts the amyloid state at the water-air interface. In contrast, SC3 is arrested in an intermediate conformation at the interface between water and a hydrophobic solid such as polytetrafluoroethylene (PTFE; Teflon). This finding prompted us to study conditions that promote assembly of SC3 into amyloid fibrils. Here, we show that SC3 adopts the amyloid state at the water-PTFE interface at high concentration (300 μg ml−1) and prolonged incubation (16 h). Moreover, we show that amyloid formation at both the water-air and water-PTFE interfaces is promoted by the cell wall components schizophyllan (β(1–3),β(1–6)-glucan) and β(1–3)-glucan. Hydrophobin concentration and cell wall polysaccharides thus contribute to the role of SC3 in formation of aerial hyphae and in hyphal attachment.
Hydrophobins are a class of surface active proteins that play diverse roles in fungal growth and development. For instance, they allow fungi to escape an aqueous environment, confer hydrophobicity to fungal surfaces in contact with air, and mediate attachment of fungi to hydrophobic surfaces (1, 2). They also play a role in the architecture of the cell wall (3).
Hydrophobins share eight conserved cysteine residues, but otherwise their sequences are diverse (4). Class I and II hydrophobins are distinguished on the basis of differences in hydropathy patterns and biophysical properties (5). SC3 of Schizophyllum commune is the best characterized class I hydrophobin. It self-assembles at interfaces between water and air, water and oil, and water and hydrophobic solids (6–8). The four disulfide bridges of SC3 prevent spontaneous self-assembly in solution and thus account for the controlled assembly at hydrophobic-hydrophilic interfaces (9).
The water-soluble form of SC3 is oligomeric (10) and rich in β-sheet (11). Upon assembly at the water-air interface, SC3 proceeds via an intermediate form that has increased α-helical structure (α-helical state) to a stable end form that has increased β-sheet structure (β-sheet state) (11–13). SC3 in the β-sheet state initially has no clear ultrastructure (β-sheet I state) (12), but after prolonged incubation, the protein forms 10-nm wide amyloid-like fibrils (β-sheet II state) (12–14) that are called rodlets (6, 15). Like other amyloid fibrils (16), rodlets of the hydrophobins SC3 of S. commune and EAS of Neurospora crassa increase fluorescence of thioflavin T and bind Congo red (14, 17, 18). Moreover, x-ray diffraction of rodlets of EAS showed reflections at 4.8 Å (distance between strands in a β-sheet) and 10–12 Å (spacing between β-sheets stacked perpendicular to the fibril long axis) (19), which are indicative for amyloid fibrils.
Notably, SC3 does not spontaneously self-assemble into amyloid fibrils at an interface between water and a hydrophobic solid. Instead, SC3 is arrested in the intermediate α-helical state. Transition to the β-sheet state is observed only by heating the sample in the presence of detergent (11, 12). These observations prompted us to study conditions that promote assembly of SC3 into amyloid fibrils. Here, we show that amyloid formation of SC3 is promoted by increasing its concentration or by the presence of cell wall polysaccharides.
EXPERIMENTAL PROCEDURES
Purification of SC3
S. commune strain D3 (ΔSC15ΔSC4) resulted from a cross between a strain that contains a disruption of the SC15 gene (20) and a strain containing a disruption of the SC4 gene (21). This strain was grown in production medium (22), and SC3 was purified from the culture medium as described (4, 6). Before use, assemblages of SC3 were dissociated with trifluoroacetic acid. After the trifluoroacetic acid was removed with a stream of nitrogen gas, SC3 was dissolved in water or 50 mm sodium phosphate buffer (pH 7). The concentration of the SC3 stock solution was determined by the intensity of a circular dichroism spectrum that was calibrated with quantitative amino acid analysis (for details, see Ref. 11). Electrobubbling was performed by applying a current of 20 mA between two platinum electrodes that had been placed in an aqueous solution of 300 μg ml−1 SC3. The resulting foam was collected and freeze-dried.
Purification of Schizophyllan
S. commune strain 72-3, which contains a deletion of the SC3 gene (7, 23), was grown for 3–5 days in liquid minimal medium (24) at 225 rpm until the glucose was depleted. The mycelium was separated from the medium via filtration over nylon gauze (200-μm mesh). Schizophyllan was precipitated from 300 ml of spent medium by addition of 1.5 volumes of ethanol. After 1 h at room temperature, the precipitated schizophyllan was washed three times for 1 h with 100 ml of ethanol at 50 rpm using a vertical rotary table with a radius of 11 cm. Excess ethanol was removed with filter paper, after which the schizophyllan was taken up in 250 ml of distilled water and dialyzed overnight against water. The concentration of schizophyllan was determined via the anthrone reaction (25) using glucose as a standard.
Preparation of Scleroglucan, Laminarin, and Paramylon Solutions
Scleroglucan of Sclerotium rolfsii (kindly provided by Cargill Inc.) and laminarin of Laminaria digitata (Sigma) were dissolved in Milli-Q water at 80 °C and room temperature, respectively. These β(1–3),β(1–6)-glucans have branching ratios of 3:1 and 10:1, respectively. Unsoluble material was removed by centrifugation (600 × g, 5 min), after which samples were dialyzed against 200 volumes of Milli-Q water (Spectra/Por, 3.5 kDa). Paramylon (β(1–3)-glucan) of Euglena gracilis (Fluka) was dissolved in 0.5 m NaOH (30 min, stirring). The pH of the solutions was adjusted by adding NaOH or HCl.
Circular Dichroism Measurements
CD spectra were recorded over the wavelength region from 190 to 250 nm on an Aviv 62A DS CD spectrometer using a 1-mm quartz cuvette. The temperature was kept at 25 °C, and the sample compartment was continuously flushed with N2. Spectra were recorded using a bandwidth of 1 nm and a 1-s averaging per point and were corrected for the signal of the solvent. SC3 was dissolved in distilled water for spectra of the soluble protein. An aqueous SC3 solution was placed on a vertical rotor at 50 rpm for 1–4 h for spectra of hydrophobin assembled at the water-air interface. On the other hand, 159-nm non-stabilized, colloidal polytetrafluoroethylene (PTFE)2 was added to the hydrophobin solution for spectra of SC3 bound to a hydrophobic support (11). Surface coverage of hydrophobin on PTFE was typically 10%. The surface coverage is defined as the percentage of the surface that can be coated with the amount of hydrophobin used (7).
Interaction of SC3 with Thioflavin T
Thioflavin T (ThT) was used at a final concentration of 3 μm. Assembly at a hydrophobic solid was studied by adding colloidal PTFE to the aqueous hydrophobin solution to a final coverage of the hydrophobic solid of 10 or 1200% (see above) (11). ThT was added to the suspensions, and the emission spectrum was recorded directly after the assembly and after 2 and 16 h. Fluorescence spectra were recorded on an SPF-500C spectrofluorometer (SLM Aminco) operating with a 300-watt xenon lamp (type 300 UV). The excitation wavelength was 450 nm with a 5-nm band pass. Changes in emission intensity were monitored with a 10-nm band pass at 482 nm. The fluorescence values were corrected using a reference solution in the absence of the dye or the protein. Fluorescence microscopy was performed with an Axiophot microscope (Carl Zeiss) using 450–490- and 515–560-nm filters for excitation and emission, respectively.
SDS-PAGE
Samples were taken up in SDS sample buffer after treatment with trifluoroacetic acid and adjusted to pH 6.8 with concentrated ammonia when necessary. SDS-PAGE was performed using 12.5% (w/v) polyacrylamide gels (26). The gels were fixed in 10% trichloroacetic acid for 1 h and stained with colloidal Coomassie Brilliant Blue G-250 for 16 h (27).
Water Contact Angle Measurements
PTFE sheets were incubated at room temperature for 15 min to 16 h in 50 mm sodium phosphate buffer (pH 7) or in water containing 0–300 μg ml−1 SC3. Samples were washed with distilled water with or without a prior extraction for 10 min with 1% SDS at 100 °C. Samples were air-dried or incubated for 15 min in 1.5 ml of 0.1% Tween 20 (pH 7) at 50 rpm using a vertical rotary table with a diameter of 11 cm. In the latter case, this was followed by washing with distilled water and air drying. Water contact angles were measured with a DSA 10-Mk2 drop shape analysis system (Krüss GmbH). 6–10 2-μl drops were measured across each surface.
Immunodetection of SC3-coated PTFE Sheets
PTFE sheets coated with 5 μg ml−1 SC3 in the presence or absence of 1 mg ml−1 paramylon at pH 2 were blocked for 1 h in phosphate-buffered saline containing 5% (w/v) skimmed milk (Sigma). The mouse monoclonal IgG against β(1–3)-glucan (κ light; Biosupplies Australia Pty. Ltd.) was diluted 20,000-fold in blocking buffer (1× phosphate-buffered saline containing 5% (w/v) skimmed milk) for detection of paramylon. Rabbit polyclonal IgG against SC3 (15) was diluted 10,000-fold in blocking buffer for detection of the hydrophobin. After 1 h of incubation at room temperature, the samples were washed three times for 5 min with phosphate-buffered saline. Anti-β(1–3)-glucan and anti-SC3 IgG were detected with 6000-fold diluted goat anti-mouse and goat anti-rabbit IgG, respectively, that were conjugated with alkaline phosphatase (Abcam). After 1 h of incubation at room temperature, the samples were washed three times for 5 min with phosphate-buffered saline and placed in substrate solution containing 400 μm nitro blue tetrazolium and 450 μm 5-bromo-4-chloro-3-indolyl phosphate (Sigma) using alkaline phosphatase buffer (100 mm Tris-HCl (pH 9.5), 100 mm NaCl, and 5 mm MgCl2).
RESULTS
Self-assembly of SC3 at the Water-Gas Interface
The effect of schizophyllan on the assembly of SC3 at a water-gas interface was examined via electrobubbling of an aqueous solution of the hydrophobin (300 μg ml−1). Foam that had formed during electrobubbling in the presence or absence of 1 mg ml−1 schizophyllan was freeze-dried, after which 100 μl of water was added to 200 μg of the freeze-dried material. Insoluble and soluble SC3 were separated by centrifugation for 10 min at 14,000 × g. The supernatants and pellets were dried, treated with trifluoroacetic acid, and analyzed by SDS-PAGE. About one-third of the SC3 that had been electrobubbled in the absence of schizophyllan had become insoluble in water (Fig. 1, lanes 1 and 2). In contrast, almost all of the SC3 had become insoluble in the presence of this polysaccharide (Fig. 1, lanes 3 and 4). The water-soluble SC3 fractions resulted in low ThT fluorescence, whereas the insoluble fraction gave rise to high ThT fluorescence (data not shown). These results show that schizophyllan promotes the conversion of water-soluble SC3 into the β-sheet II amyloid state at the water-gas interface.
FIGURE 1.

Schizophyllan promotes the transition of SC3 to the β-sheet state at the water-gas interface. SC3 (300 μg ml−1) was assembled at the water-gas interface in the absence (lanes 1 and 2) and presence (lanes 3 and 4) of 1 mg ml−1 schizophyllan. SC3 was assembled by electrobubbling (see “Experimental Procedures” for details). The resulting foam was freeze-dried and taken up in water, followed by centrifugation (14,000 × g, 10 min). The pellets (lanes 1 and 3) and supernatants (lanes 2 and 4) were dried and treated with trifluoroacetic acid to dissociate SC3, allowing separation by SDS-PAGE. About one-third of the SC3 became insoluble in water during electrobubbling in the absence of schizophyllan (lanes 1 and 2), whereas almost all of the SC3 became insoluble in the presence of this polysaccharide (lanes 3 and 4).
Self-assembly of SC3 at the Water-PTFE Interface
Assembly of SC3 is arrested in the intermediate α-helical state when confronted with a hydrophobic solid such as PTFE. The β-sheet state can be induced by heating the sample in 1–2% SDS (11, 12).3 Here, we assessed whether conversion to the β-sheet state could also be induced by increasing the concentration of SC3 and/or the incubation time. To this end, PTFE sheets were incubated at room temperature in aqueous solutions containing 5, 50, 100, and 300 μg ml−1 SC3. To assess the state of the hydrophobin at the PTFE surface, we made use of the fact that SC3 adsorbed to PTFE in the α-helical state is removed by 0.1% Tween 20 (pH 7) at room temperature (supplemental Fig. 1A). As a consequence, the water (not shown) contact angle (WCA) increased and became more similar to that of bare PTFE not coated with SC3 (see below). Like heating in SDS, SC3 in the α-helical state can be converted to the β-sheet state by treating it with 0.1% Tween 20 at pH 2 (supplemental Fig. 1B). In contrast to the α-helical state, SC3 in the β-sheet state is not removed by 0.1% Tween 20 at pH 7 (supplemental Fig. 1B). As a result, the WCA does not change upon treatment with 0.1% Tween 20 at pH 7.
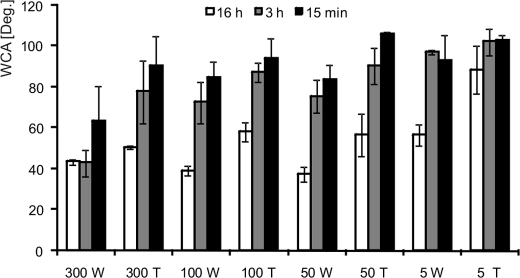
Bare PTFE not coated with SC3 has a WCA of 100 ± 2°. Coating with 300 μg ml−1 SC3 for 16 h decreased the WCA to 43.5 ± 1.2°. The WCA was not affected by treatment with 0.1% Tween 20 at pH 7 (Fig. 2), which is indicative of the β-sheet state of SC3. In contrast, part of the coating was removed by 0.1% Tween 20 (pH 7) when PTFE had been incubated for 16 h in 50 or 5 μg ml−1 SC3. As a result, the WCA and its heterogeneity increased (Fig. 2). Coating with 300 μg ml−1 SC3 for only 15 min or 3 h also did not result in a stable coating (Fig. 2). Experiments performed at pH 2, 5, and 7 gave similar results (data not shown). Microscopic examination showed ThT fluorescence at the surface of PTFE sheets that had been coated with 300 μg ml−1 SC3 for 16 h. This shows that this coating is in the β-sheet II state (data not shown). Fluorescence was not observed at a lower SC3 concentration or a shorter incubation time. Taken together, these results show that transition of SC3 from the α-helical state into the amyloid form is promoted by increasing the hydrophobin concentration and the incubation time.
FIGURE 2.
Increasing the SC3 concentration and incubation time promotes the transition of SC3 to the β-sheet state at the water-PTFE interface. This is indicated by a low WCA after treating the coated PTFE with Tween 20 at pH 7. WCAs were measured on PTFE sheets that had been incubated with 5, 50, 100, and 300 μg ml−1 SC3 at pH 7. This was followed by washing with water (5 W, 50 W, 100 W, and 300 W) or with 0.1% Tween 20 at pH 7 (5 T, 50 T, 100 T, and 300 T). Coating was performed for 16 h (white bars), 3 h (gray bars), or 15 min (black bars). Uncoated PTFE has a WCA of 110 ± 2°, whereas PTFE coated with SC3 in the β-sheet state has a WCA of 48 ± 10° (7). Deg., degrees.
WCA measurements were performed to study the effect of schizophyllan on the assembly of SC3 at the water-PTFE interface. Coating was performed for 2 or 16 h at 5, 50, and 100 μg ml−1 SC3 in the presence or absence of 0.3 mg ml−1 schizophyllan. After 2 h, a clear effect of schizophyllan was observed (Fig. 3). The WCA was lower, and the coating showed an increased stability in 0.1% Tween 20 at pH 7. This effect was more clear at a lower concentration of SC3 (Fig. 3). In fact, stable coatings could be obtained at 1 μg ml−1 SC3 (supplemental Fig. 2). The conversion to the β-sheet state was not observed when the schizophyllan was added after the coating (data not shown) or when the concentration of the polysaccharide was below 0.3 mg ml−1 during the coating process (supplemental Fig. 3). Experiments performed at pH 2, 5, and 7 gave similar results (data not shown).
FIGURE 3.
Schizophyllan promotes the transition of SC3 to the β-sheet state at the water-PTFE interface. WCA measurements were made on PTFE sheets coated with 5, 50, and 100 μg ml−1 SC3 (pH 7) and washed with water (5 W, 50 W, and 100 W) or 0.1% Tween 20 at pH 7 (5 T, 50 T, and 100 T). Coating was performed for 2 h (white and dark gray bars) or 16 h (light gray and black bars) in the absence (white and light gray bars) or presence (dark gray and black bars) of 0.3 mg ml−1 schizophyllan (SCH). The effect of schizophyllan was most clear at a low SC3 concentration. The WCAs of PTFE sheets coated at 5 μg ml−1 SC3 were much lower after treatment with Tween 20 (pH 7) when the coating was performed in the presence of schizophyllan (see 5 T samples). deg., degrees.
ThT measurements were performed to confirm that 0.3 mg ml−1 schizophyllan promotes conversion of SC3 to the amyloid form (β-sheet II state) and not the β-sheet I state. To this end, a suspension of colloidal PTFE was mixed with a solution of soluble SC3 at a final coverage of 1200% in the presence or absence of schizophyllan. ThT was added to the mixtures, and the fluorescence was measured directly or after 2 and 16 h of incubation at room temperature. To determine the maximal fluorescence, Tween 20 was added to the PTFE spheres at a final concentration of 0.1% (pH 2). In the presence of schizophyllan, the ThT fluorescence increased in 16 h to 80% of the maximal value (supplemental Fig. 4). In the absence of schizophyllan, only 25% of the maximal fluorescence was reached. These results confirm that schizophyllan accelerates the formation of the β-sheet II state.
Coating of PTFE sheets with SC3 (5 μg ml−1) was also performed in the presence of other glucans. Like schizophyllan, addition of scleroglucan (β(1–3),β(1–6)-glucan; 3:1) to the coating solution resulted in lowering of the WCA and in stable coatings at pH 2, 5, and 7 (supplemental Fig. 5). However, the amount of scleroglucan needed to induce stable coatings (0.5 mg ml−1) was somewhat higher than that of schizophyllan. The concentration of paramylon (β(1–3)-glucan) needed to obtain stable coatings was also a bit higher (1 mg ml−1). These stable coatings were formed at pH 2 and 5, but not at pH 7 (data not shown). In contrast, 1–5 mg ml−1 laminarin (β(1–3),β(1–6)-glucan; 10:1) did not stabilize the SC3 coating because 0.1% Tween 20 at pH 7 removed the hydrophobin (data not shown). Coatings were also not stabilized when up to 5 mg ml−1 carboxymethylcellulose (β(1–4)-glucan), pustullan (β(1–6)-glucan), glycogen (α(1–4),α(1–6)-glucan), dextran (α(1–6),α(1–3)-glucan), starch (α(1–4)-glucan/α(1–4),α(1–6)-glucan), heparin (2-O-sulfo-α-l-iduronic acid/2-deoxy-2-sulfamino-α-d-glucopyranosyl 6-O-sulfate), or dermatan sulfate (N-acetylgalactosamine/iduronic acid) was used (data not shown). However, stable coatings were obtained at pH 2 with 1 mg ml−1 heparan sulfate (glucuronic acid/N-acetylglucosamine) (data not shown). This shows that the conversion of SC3 to the β-sheet II state is not specific for β(1–3)-glucans.
Immunodetection of β(1–3)-glucan was performed to examine whether paramylon co-immobilized with SC3 at the surface of PTFE. Coating of SC3 in the presence of paramylon resulted in a clear immunosignal for β(1–3)-glucan (Fig. 4, lanes 2 and 4), whereas in the absence of paramylon (lanes 1 and 3) or SC3 (data not shown), no signal was obtained. Immunodetection of SC3 confirmed the presence of the hydrophobin on the PTFE sheets (Fig. 4, lanes 5–8). Treating the samples with hot 1% SDS resulted in partial removal of paramylon (Fig. 4, lane 4) and correlated with a small increase in the WCA. The SC3 coating was unaffected by this treatment (Fig. 4, lanes 7 and 8).
FIGURE 4.
Paramylon co-immobilizes with SC3 at the surface of PTFE. Shown is the immunodetection of β(1–3)-glucan and SC3 on PTFE sheets coated with the hydrophobin in a solution without (W) or with (P) 1 mg ml−1 paramylon. After coating, the PTFE sheets were washed with water (−) or treated with 1% SDS at 95 °C (+).
DISCUSSION
SC3 self-assembles into an amphipathic membrane at the water-air interface (6). Self-assembly proceeds through the α-helical state and the β-sheet I state into the β-sheet II state (11, 12). The latter has an ultrastructure consisting of 10-nm wide amyloid-like fibrils (6, 12–15). These amyloid fibrils, called rodlets, increase the fluorescence of ThT and bind Congo red (14, 17, 18). SC3 also assembles at the interface between water and a hydrophobic solid. However, treatment with hot 1–2% SDS is needed to induce the transition from the α-helical state to the amyloid form (11, 12).3 Here, we have shown that SC3 can spontaneously adopt the β-sheet II state at the interface between water and the hydrophobic solid PTFE when the concentration of the hydrophobin and incubation time are increased. Moreover, we have shown that certain polysaccharides promote the formation of amyloids of SC3. The cell wall components schizophyllan and β(1–3)-glucan are among these polysaccharides. It is expected that a high concentration of hydrophobin or the presence of certain polysaccharides also induces formation of SC3 amyloids at other hydrophobic solids. This is based on the observation that assembly of SC3 depends on hydrophobicity and not on the chemical composition of the surface (7, 8).
PTFE sheets were incubated for 15 min to 16 h in 0–300 μg ml−1 SC3. SDS-PAGE analysis showed that the amount of SC3 that had adsorbed to the surface was not influenced by the concentration of SC3 in the solution (data not shown). ThT staining was performed to address whether the hydrophobin had assembled in the amyloid form. Fluorescence of ThT was not observed at the PTFE surface when this solid was coated for 15 min to 16 h with 0–100 μg ml−1 SC3. The absence of the amyloid form of SC3 correlated with a partial removal of the hydrophobin from the PTFE surface with 0.1% Tween 20 (pH 7). This resulted in an increase in the WCA, which became more similar to that of uncoated PTFE. Interestingly, ThT fluorescence was observed at the PTFE surface after coating for 16 h with 300 μg ml−1 SC3. This was accompanied by a moderate increase in the WCA after 0.1% Tween 20 (pH 7) treatment, indicative of stable SC3 coating. Taken together, these data show that SC3 can spontaneously adopt the amyloid form at the PTFE surface when a high concentration of the protein is used.
SC3 is secreted into the culture medium at a concentration of ∼50 μg ml−1. At this concentration, rodlets can be observed only at the water-air interface after 16 h of incubation (12). This suggests that, under natural conditions, the transition to the β-sheet II state is a very slow process. We have shown here, however, that 0.3 mg ml−1 schizophyllan, a high molecular mass β(1–3),β(1–6)-glucan, stimulates the conversion of SC3 into the amyloid state at concentrations as low as 1 μg ml−1. Schizophyllan is part of the cell wall but is also secreted into the culture medium at a concentration of up to 0.5 mg ml−1. Our data imply that schizophyllan promotes formation of SC3 amyloids at the interface between the medium and the air and at the surface of aerial hyphae. It would thus be instrumental in allowing hyphae to escape the medium and in rendering aerial hyphae hydrophobic. Schizophyllan also promotes formation of amyloids of SC3 at a hydrophobic solid. This stabilizes the hydrophobin coating, as was shown by a reduction in the increase of the surface hydrophobicity after extraction with 0.1% Tween 20 at pH 7. It was reported previously that schizophyllan does not affect the wettability of a coating on a hydrophobic surface (28), which can be explained by the fact that we used a 30-fold higher concentration of the polysaccharide. By stabilizing the SC3 coating at a hydrophobic solid, schizophyllan is expected to function in hyphal attachment. Hydrophobin-mediated attachment is important in fungal pathogenic interactions (29) and probably also in mutual beneficial interactions (1).
Scleroglucan and paramylon also promoted amyloid formation by SC3. Like schizophyllan, scleroglucan is a high molecular mass β(1–3),β(1–6) glucan. Paramylon has a similar molecular mass but lacks β(1–6) branches. This indicates that β(1–3)-glucan in the cell wall of S. commune also promotes conversion of SC3 to the amyloid form. This polysaccharide would thus contribute to surface hydrophobicity of aerial hyphae and to attachment of hyphae to hydrophobic surfaces. The low molecular mass glucan laminarin (104 Da), containing a low degree of β(1–6) branching (1:10), was not capable of inducing the β-sheet II state. Thus, molecular mass and/or the degree of β(1–6) branching influences the ability of β(1–3)-glucan to promote transition of SC3 to the amyloid state. Like laminarin, also other glucose polymers (β(1–4)-glucan, β(1–6)-glucan, α(1–4),α(1–6)-glucan, and α(1–6),α-(1–3)-glucan) failed to induce amyloid formation of SC3. How can schizophyllan, scleroglucan, and paramylon promote formation of amyloids of SC3? It was shown previously that schizophyllan stabilizes SC3 in solution and prevents formation of aggregates larger than tetramers (28). The smaller oligomers could have a higher capacity to assemble at a hydrophobic-hydrophilic interface. It may also be that the polysaccharides aid in the self-assembly process itself. This is suggested by the finding that paramylon co-immobilized with SC3 at the surface of PTFE.
We have reported here for the first time that cell wall polysaccharides can promote formation of a functional microbial amyloid. It may well be that other proteins that form functional amyloids at a microbial surface such as repellents of Ustilago maydis (30), chaplins of Streptomyces coelicolor (31), and curli of Escherichia coli (32) are also stimulated by cell wall polysaccharides to adopt this conformation. This is strengthened by the fact that disease-associated amyloid formation is promoted by glycosaminoglycans (polysaccharides containing repeating units of disaccharides) and proteoglycans (glycosaminoglycans bound to a core protein) (33, 34). The mechanism by which these sugars stimulate amyloid formation is largely unknown (35), but binding of the polysaccharides to the protein has been observed (for references, see Ref. 34). Interestingly, heparan sulfate, a glycosaminoglycan known to accelerate disease-associated amyloidogenesis (33), also induced the β-sheet II state of SC3. In contrast, heparin and dermatan sulfate did not affect the SC3 conformation. It is not clear whether heparan sulfate interacts with SC3 in a similar way as schizophyllan. In disease-associated amyloidogenesis, the interaction between the protein and heparan sulfate is based on electrostatic interactions between positive charges in the protein and negative charges in the polysaccharides (36). The interaction between SC3 and schizophyllan and that between SC3 and heparan sulfate seem not to result from an electrostatic interaction. SC3 is mainly uncharged, and schizophyllan is a neutral polysaccharide. Heparan sulfate induced the β-sheet II state only at pH 2. At such a pH, the sulfate groups will not be charged. Determination of the binding sites between SC3 and amyloid-promoting polysaccharides may result in a better understanding of the nature of the interaction. This will be the subject of further studies.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
K. Scholtmeijer, M. L. de Vocht, R. Rink, G. T. Robillard, and H. A. B. Wösten, unpublished data.
- PTFE
- polytetrafluoroethylene
- ThT
- thioflavin T
- WCA
- water contact angle.
REFERENCES
- 1.Wösten H. A. (2001) Annu. Rev. Microbiol. 55, 625–646 [DOI] [PubMed] [Google Scholar]
- 2.Linder M. B., Szilvay G. R., Nakari-Setälä T., Penttilä M. E. (2005) FEMS Microbiol. Rev. 29, 877–896 [DOI] [PubMed] [Google Scholar]
- 3.van Wetter M. A., Wösten H. A., Sietsma H., Wessels J. G. (2000) Fungal Genet. Biol. 31, 99–104 [DOI] [PubMed] [Google Scholar]
- 4.Wessels J. G. (1997) Adv. Microb. Physiol. 38, 1–45 [DOI] [PubMed] [Google Scholar]
- 5.Wessels J. G. (1994) Annu. Rev. Phytopathol. 32, 413–437 [Google Scholar]
- 6.Wösten H., de Vries O., Wessels J. (1993) Plant Cell 5, 1567–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wösten H. A., Schuren F. H., Wessels J. G. (1994) EMBO J. 13, 5848–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wösten H. A., Ruardy T. G., van der Mei H. C., Busscher H. J., Wessels J. G. (1995) Colloids Surf. B Biointerfaces 5, 189–195 [Google Scholar]
- 9.de Vocht M. L., Reviakine I. R., Wösten H. A., Brisson A., Wessels J. G., Robillard G. T. (2000) J. Biol. Chem. 275, 28428–28432 [DOI] [PubMed] [Google Scholar]
- 10.Wang X., de Vocht M. L., de Jonge J., Poolman B., Robillard G. T. (2002) Protein Sci. 11, 1172–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vocht M. L., Scholtmeijer K., van der Vegte E. W., de Vries O. M., Sonveaux N., Wösten H. A., Ruysschaert J. M., Hadziioannou G., Wessels J. G., Robillard G. T. (1998) Biophys. J. 74, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vocht M. L., Reviakine I., Ulrich W. P., Bergsma-Schutter W., Wösten H. A., Horst V., Brisson A., Wessels J. G., Robillard G. T. (2002) Protein Sci. 11, 1199–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X., Shi F., Wösten H. A., Hektor H., Poolman B., Robillard G. T. (2005) Biophys. J. 88, 3434–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wösten H. A., de Vocht M. L. (2000) Biochim. Biophys. Acta 1469, 79–86 [DOI] [PubMed] [Google Scholar]
- 15.Wösten H. A., Asgeirsdottir S. A., Krook J. H., Drenth J. H., Wessels J. G. (1994) Eur. J. Cell Biol. 63, 122–129 [PubMed] [Google Scholar]
- 16.LeVine H., 3rd (1993) Protein Sci. 2, 404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackay J. P., Matthews J. M., Winefield R. D., Mackay L. G., Haverkamp R. G., Templeton M. D. (2001) Structure 9, 83–91 [DOI] [PubMed] [Google Scholar]
- 18.Butko P., Buford J. P., Goodwin J. S., Stroud P. A., McCormick C. L., Cannon G. C. (2001) Biochem. Biophys. Res. Commun. 280, 212–215 [DOI] [PubMed] [Google Scholar]
- 19.Kwan A. H., Winefield R. D., Sunde M., Matthews J. M., Haverkamp R. G., Templeton M. D., Mackay J. P. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3621–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lugones L. G., de Jong J. F., de Vries O. M., Jalving R., Dijksterhuis J., Wösten H. A. (2004) Mol. Microbiol. 53, 707–716 [DOI] [PubMed] [Google Scholar]
- 21.van Wetter M. A., Wösten H. A., Wessels J. G. (2000) Mol. Microbiol. 36, 201–210 [DOI] [PubMed] [Google Scholar]
- 22.Scholtmeijer K., Janssen M. I., Gerssen B., de Vocht M. L., van Leeuwen B. M., van Kooten T. G., Wösten H. A., Wessels J. G. (2002) Appl. Environ. Microbiol. 68, 1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Wetter M. A., Schuren F. H., Schuurs T. A., Wessels J. G. (1996) FEMS Microbiol. Lett. 140, 265–269 [Google Scholar]
- 24.Dons J. J., de Vries O. M., Wessels J. G. (1979) Biochim. Biophys. Acta 563, 100–112 [DOI] [PubMed] [Google Scholar]
- 25.Fairbairn N. J. (1953) Chem. Ind. 4, 86 [Google Scholar]
- 26.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 27.Neuhoff V., Arold N., Taube D., Ehrhardt W. (1988) Electrophoresis 9, 255–262 [DOI] [PubMed] [Google Scholar]
- 28.Martin G. G., Cannon G. C., McCormick C. L. (2000) Biomacromolecules 1, 49–60 [DOI] [PubMed] [Google Scholar]
- 29.Talbot N. J., Kershaw M. J., Wakley G. E., de Vries O. M., Wessels J. G., Hamer J. E. (1996) Plant Cell 8, 985–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teertstra W. R., van der Velden G. J., de Jong J. F., Kruijtzer J. A., Liskamp R. M., Kroon-Batenburg L. M., Müller W. H., Gebbink M. F., Wösten H. A. (2009) J. Biol. Chem. 284, 9153–9159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claessen D., Stokroos I., Deelstra H. J., Penninga N. A., Bormann C., Salas J. A., Dijkhuizen L., Wösten H. A. (2004) Mol. Microbiol. 53, 433–443 [DOI] [PubMed] [Google Scholar]
- 32.Chapman M. R., Robinson L. S., Pinkner J. S., Roth R., Heuser J., Hammar M., Normark S., Hultgren S. J. (2002) Science 295, 851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexandrescu A. T. (2005) Protein Sci. 14, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellotti V., Chiti F. (2008) Curr. Opin. Struct. Biol. 18, 771–779 [DOI] [PubMed] [Google Scholar]
- 35.Relini A., De Stefano S., Torrassa S., Cavalleri O., Rolandi R., Gliozzi A., Giorgetti S., Raimondi S., Marchese L., Verga L., Rossi A., Stoppini M., Bellotti V. (2008) J. Biol. Chem. 283, 4912–4920 [DOI] [PubMed] [Google Scholar]
- 36.Ancsin J. B., Kisilevsky R. (1999) J. Biol. Chem. 274, 7172–7181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.