Abstract
Galectins are a family of β-galactoside-binding proteins that are widely found among animal species and that regulate diverse biological phenomena. To study the biological function of glycolipid-binding galectins, we purified recombinant Caenorhabditis elegans galectins (LEC-1–11) and studied their binding to C. elegans glycolipids. We found that LEC-8 binds to glycolipids in C. elegans through carbohydrate recognition. It has been reported that Cry5B-producing Bacillus thuringiensis strains can infect C. elegans and that the C. elegans Cry5B receptor molecules are glycolipids. We found that Cry5B and LEC-8 bound to C. elegans glycolipid-coated plates in a dose-dependent manner and that Cry5B binding to glycolipids was inhibited by the addition of LEC-8. LEC-8 is usually expressed strongly in the pharyngeal-intestinal valve and intestinal-rectal valve and is expressed weakly in intestine. However, when C. elegans were fed Escherichia coli expressing Cry5B, intestinal LEC-8::EGFP protein levels increased markedly. In contrast, LEC-8::EGFP expression triggered by Cry5B was reduced in toxin-resistant C. elegans mutants, which had mutations in genes involved in biosynthesis of glycolipids. Moreover, the LEC-8-deficient mutant was more susceptible to Cry5B than wild-type worms. These results suggest that the glycolipid-binding lectin LEC-8 contributes to host defense against bacterial infection by competitive binding to target glycolipid molecules.
Galectins represent a large family of β-galactoside-binding proteins that are widely found among animal species, including vertebrates, nematodes, and sponges. These proteins regulate diverse biological phenomena, including proliferation, apoptosis, and cell-cell interactions (1, 2). Although galectins do not contain a secretory signal peptide, they are often found outside cells (1, 3). Human galectin-4 is expressed in the epithelium of the alimentary tract and is a major component of the detergent-resistant membranes of the intestinal brush border and of colon adenocarcinoma cells (4–6). Galectin-4 binds strongly to glycolipids containing 3-O-sulfated Gal residues (7) and sulfated cholesterol (8). On the surface of human colonic adenocarcinoma cells, it co-localizes with glycolipids containing SO3−→3Galβ1→3(GalNAc)2 residues (9). However, it is not easy to study the biological functions of this glycolipid-binding lectin because complicated mammalian systems are regulated by many factors and because few models exist for in vivo study.
Caenorhabditis elegans is a good model for studying the biological functions of glycolipid-binding galectins because the complete genome sequence is available and gene knock-out technology can be easily applied. The C. elegans genome project revealed the presence of at least 11 galectins, LEC-1–11. LEC-1–5 are tandem repeat-type galectins that contain two carbohydrate recognition domains, whereas LEC-6–11 are prototype galectins that contain one carbohydrate recognition domain each. However, there was no previous evidence for the existence of glycolipid-binding lectin in C. elegans. In this study, we searched for a galectin that binds to glycolipids and that is expressed in the alimentary tract of C. elegans. To do this, we sequentially studied the functional roles of glycolipid-binding galectins in C. elegans.
Many pathogenic bacteria target cell surface glycolipids as receptors (10), and the initial binding to the host cell surface is an essential step in establishing infection in tissues and producing toxic effects. We speculate that glycolipid-binding lectins play a role in host defense by masking glycolipids and preventing binding of bacterial and viral toxins. It has been reported that C. elegans shows toxic effects following infection by Bacillus thuringiensis and that B. thuringiensis producing the crystal toxin Cry5 binds to glycolipids extracted from C. elegans in a carbohydrate-dependent manner (11). We used the Cry5B toxin, which enters C. elegans intestinal cells through glycolipid receptors, to investigate whether a glycolipid-binding lectin could function in host defense against infection in this study.
EXPERIMENTAL PROCEDURES
C. elegans Strains and Culture Conditions
C. elegans strains were cultured using standard techniques. All strains were derived from the wild-type Bristol strain N2. Bre-2(ye31), -4(ye27), and -5(ye17) strains were supplied by the Caenorhabditis Genetics Center. Genetic crosses were carried out with standard genetic protocols using the lec-8-deficient mutant lec-8(tm1477). Deletion alleles were identified by PCR amplification using primers that spanned the deleted region. The following primers were used for PCR screening and genotyping of the deletion allele: 5′-acttggaaacgctgtcctgt-3′ and 5′-tgctcccggttgcgaaccat-3′, and 5′-tgcgattggcatattggtac-3′ and 5′-tactcgaccttagtcatcgt-3′.
Materials
Reagents used in this study were purchased from Sigma. Lactose, GM1, GM2, GM3, SM4, GM4, GalCer, LacCer, Gb3, and nLC4 were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). The pQE9 plasmid, Escherichia coli strain M15[pREP4], and nickel-nitrilotriacetic acid-agarose were obtained from Qiagen. The pGEX-6P-1 plasmid, E. coli BL21, glutathione-Sepharose, horseradish peroxidase (HRP)-conjugated streptavidin, HRP-conjugated anti-GST, and anti-mouse IgG antibodies were purchased from GE Healthcare. E. coli strains JM103[pQE9] and JM103[pQE9(Cry5B)] were kindly provided by Raffi V. Aroian (University of California San Diego).
Preparation of Recombinant C. elegans Galectins
Plasmids and phagemids containing full-length galectin cDNA, yk572f1 (lec-1), yk497f10 (lec-2), yk496c7 (lec-3), yk746b4 (lec-4), yk1735f04 (lec-6), yk377g8 (lec-8), yk145g7 (lec-9), yk1597d9 (lec-10), and yk1642d07 (lec-11) were kindly provided by Yuji Kohara (National Institute of Genetics, Japan). His6-tagged and GST-fused galectins were expressed using the following methods. From plasmids or phagemids containing full-length galectin cDNA, PCR amplification was performed using 5′- and 3′-primers containing the appropriate restriction enzyme sites as follows: lec-1, 5′-tttggatccatgtccgccgaagag-3′ and 5′-tttaagcttttattggatttgaattcc-3′; lec-2, 5′-tttggatccatggcccatgagtccg-3′ and 5′-tttaagcttttaggcgagttggattc-3′; lec-3, 5′-tttggatccatggccgagccgaaatcc-3′ and 5′-tttaagcttttattcctgagcaggttg-3′; lec-4, 5′-tttggatccatgagctctcacgagaac-3′ and 5′-tttaagcttttaatgcgaaaactcaa-3′; lec-5, 5′-tttggatccatgataacaagaacg-3′ and 5′-tttaagcttttaaactcctggagc-3′; lec-6, 5′-tttggatccatgatcggaggagga-3′ and 5′-tttgtcgacttagtgagaaacatgggc-3′; lec-8, 5′-tttggatccatgcataccattaatagcc-3′ and 5′-tttgtcgacttagaagtaggcgt-3′; lec-9, 5′-tttggatccatggccgctccaattctc-3′ and 5′-tttgtcgacttaatggaattgag-3′; lec-10, 5′-tttgaattcatgcactcctatcac-3′ and 5′-tttgtcgacttatctgtatgggttg-3′; and lec-11, 5′-tttggatccatggttcacgtcattgaaaatcc-3′ and 5′-tttgtcgactcaatagtgatggtgacggtg-3′. Amplified fragments containing lec-1–5 were ligated between the BamHI and HindIII sites of pQE9, and those containing lec-6, -8, -9, and -11 or lec-10 were inserted into the BamHI and SalI or EcoRI and SalI sites of pGEX-6p-1, respectively. Constructs generated in pQE9 were transformed into E. coli M15[pREP4], and those in pGEX6p-1 were transformed into E. coli BL21. The nucleic acid sequences were confirmed using an ABI PRISM 310 Genetic Analyzer (PE Biosystems). His6-tagged and GST fusion proteins were expressed and purified according to the manufacturer's instructions. Protein concentration was determined using a Bio-Rad protein assay dye reagent with bovine serum albumin (BSA) as the standard.
Preparation of a Truncated Form of Cry5B
A truncated form of Cry5B (t-Cry5B) was used for assays in solution because full-length Cry5B (1245 amino acids) could not be dissolved easily and was not purified as single band. We PCR-amplified t-Cry5B cDNA encoding amino acids 1–697 using plasmid pQE9(Cry5B) as a template. PCR amplification was performed using the primers 5′-ccggtcggatccgcaacaattaatgagttgtatcc-3′ and 5′-tcgtaagcttgatttttggaacaaactcaatacgatc-3′. An amplified fragment was inserted into the BamHI and Hind III sites of pQE9 and was then transformed in the E. coli M15[pREP4] strain. M15[pREP4, pQE9(t-Cry5B)] was toxic when fed to wild-type N2 worms, whereas M15[pREP4, pQE9] was not. We expressed t-Cry5B protein and purified it using a nickel-nitrilotriacetic acid-agarose column according to the manufacturer's instructions. The purified t-Cry5B was dialyzed into phosphate-buffered saline (PBS) and concentrated using an Amicon Ultra 4 membrane (Millipore). t-Cry5B was biotinylated using a 10-fold molar excess of N-hydroxysuccinimide-biotin (Pierce) according to the manufacturer's protocol.
Preparation of Glycolipids from C. elegans
Glycolipids were prepared from C. elegans according to the methods of Griffitts et al. (11). In brief, nematodes were grown at 20 °C for 4 days in OP50 liquid culture (2 liters) and then washed several times in water followed by centrifugation; a chilled packed pellet (3-ml volume) was then flash-frozen for storage. The pellet was thawed and combined with three pellet volumes of cold water, and the suspension was sonicated until all nematodes were lysed. Lysates were treated with methanol:chloroform (final chloroform:methanol:water ratio, 4:8:3) during 4 h of extraction at 37 °C, and insoluble material was removed by centrifugation (2000 × g, 10 min). For solvent partitioning, the supernatant was combined with 0.173 volume of water, generating a final chloroform:methanol:water ratio of 4:8:5.6. This mixture was shaken vigorously for 5–10 min, and phases were separated by centrifugation (2000 × g, 10 min). The lower phase was evaporated and resuspended in chloroform:methanol (1:1) for analysis as the lipid fraction. The upper phase was desalted with a tC18 cartridge (Waters) preequilibrated with chloroform:methanol:water (2:43:55) and then eluted with chloroform:methanol (1:1). This eluate was evaporated and dissolved in methanol for analysis as the glycolipid fraction. After dissolving in methanol, insoluble material was removed by centrifugation. The solution was spotted onto an HPTLC plate (10 × 10 cm; silica gel 60, Merck) and developed using chloroform:methanol:water (45:18:3) as the solvent for the lower phase and using the same solvent at 4:4:1 for the upper phase. Both glycolipids and lipids were visualized by orcinol sulfate staining (12).
TLC overlay staining was performed using the methods of Hidari et al. (13) with a slight modification. The glycolipid fractions were separated on silica gel-coated HPTLC plates with aluminum backing (Merck) with a solvent system consisting of chloroform:methanol:water (4:4:1). The plate was cut into several pieces, one of which was sprayed with orcinol reagent to determine the positions of the glycolipids. The other pieces used for overlay staining were soaked in 0.1% polyisobutylmethacrylate in cyclohexane for 60 s and dried. For Cry5B overlay staining, the piece was preincubated with blocking solution (0.5% BSA-PBS containing 0.01% Tween 20) for 30 min. Then it was incubated for 2 h with biotinylated-t-Cry5B (bt-Cry5B) (50 nm) in blocking solution. After washing with PBS-T (PBS containing 0.01% Tween 20), the piece was incubated for 1 h with an avidin-biotin-peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) to detect bound bt-Cry5B. For LEC-8, -9, and -10 overlay staining, pieces were blocked with 1% BSA in PBS for 30 min and were then incubated for 1.5 h with GST-LEC-8 (25 nm), -9 (150 nm), or -10 (150 nm) in 1% BSA solution. After washing with PBS-T, they were incubated for 1.5 h with HRP-conjugated anti-GST antibody. After washing, Cry5B- and LEC protein-binding bands were visualized by enhanced chemiluminescence with Super Signal (Pierce) using an LAS-1000 imaging system (Fujifilm, Tokyo, Japan).
ELISA to Detect Binding of Galectins to Lipids and Glycolipids
ELISA to detect binding of His6-tagged galectins to lipids and glycolipids was carried out as described previously (9). Briefly, glycolipid and lipid aliquots were purified as described above and then evaporated and redissolved in methanol (10 × the original volume). Each well of the microtiter plate (Nunc-Immuno Plate Maxisorp Surface, Nalge Nunc International) was coated with 5 μl of the methanol solution and then coated with glycolipids and lipids purified from a 1.2-μl wet volume of C. elegans. After evaporation of the solvent, 100 μl of 1% BSA in PBS was added as a blocking solution, and the plate was left for 30 min at room temperature. After washing with PBS, 50 μl of various concentrations of galectins in blocking solution was added to each well, and the plate was left for 1 h at room temperature. The plate was washed several times with washing buffer (0.01% Tween 20 in PBS), and then anti-His6 antibody (clone BMG-His-1, Roche, Basel, Switzerland) diluted in the washing buffer was added. After incubation for 1 h at room temperature, the plate was washed and then incubated with HRP-conjugated anti-mouse IgG antibody diluted in washing buffer followed by incubation with 0.3% orthophenylenediamine (Nacalai Tesque, Inc., Kyoto, Japan) and 0.03% H2O2 in 10 mm acetate buffer (pH 5.0). ELISA for binding of GST-tagged galectins to various glycolipids was carried out using HRP-conjugated anti-GST antibody instead of anti-His6 and HRP-conjugated anti-mouse IgG antibody.
Inhibition assays between C. elegans galectins and glycolipids were performed using ELISA. Fifty μl of galectins (100 nm) with various concentrations of lactose in 1% BSA in PBS was applied to glycolipid-coated plates, and the relative binding abilities of galectins were measured.
Binding and Inhibition Assays for t-Cry5B
Glycolipid aliquots were coated on plates as described above, and then blocking solution (1% BSA in PBS) was added and allowed to block for 1 h. Wells were probed with 50 μl of bt-Cry5B in blocking solution for 1 h and incubated with HRP-conjugated streptavidin for 1 h after washing with PBS. For inhibition experiments, 50 μl of bt-Cry5B (60 nm) with various concentrations of LEC-8, -9, or -10 in blocking solution was applied to glycolipid-coated plates, and the relative binding ability of bt-Cry5B was measured as described above.
Translational Reporter Constructs and Transgenic Line
The lec-8::gfp translational reporter construct was generated by PCR amplification of a 5563-bp genomic fragment, including a 5-kb region containing a potential promoter. The fragment was amplified by PCR from C. elegans genomic DNA using 5′- and 3′-primers (5′-ttgctgtacttcaccgtttga-3′ and 5′-gaagtaggcgttgtaggagtg-3′, respectively), with the latter located just before the stop codon. This fragment was ligated into the XcmI-digested vector pFX_EGFPT (14), and TA cloning was used to generate a translational fusion with the EGFP coding region. PCR was performed using Platinum Taq high fidelity DNA polymerase (Invitrogen).
We injected N2 and bre-2(ye31), bre-4(ye27), and bre-5(ye17) (15) hermaphrodites with a DNA mixture containing lec-8::gfp (10 ng/μl), pBluescript (100 ng/μl), and pRF4 (90 ng/μl), which expresses a dominant rol-6 gene (16). Germ line transformation was performed as described by Mello et al. (17). Transgenic lines were generated by injecting experimental DNA (10 ng/μl) into the distal gonads. Transgenic worms were selected on the basis of the dominant rol-6 phenotype and the LEC-8::EGFP expression. Live transgenic worms were anesthetized with a 10 mm sodium azide solution and then placed on an agar pad on a glass slide (Matsunami Glass, Japan) and examined using confocal microscopy (Zeiss LSM5 microscope with differential interference contrast and fluorescence optics).
Cry5B Toxicity Assay
For the toxicity assay in solution, young adult stage nematodes were picked into the wells of 96-well microtiter plates that contained various concentrations of purified t-Cry5B in S medium (18), 3 μl of saturated OP50 culture medium as a standard E. coli food source, and tetracycline (30 μg/ml) to prevent spore germination and bacterial growth (19, 20). The final volume in each well was 120 μl. To score for susceptibility or resistance, the worms were incubated at 20 °C and examined under a dissecting microscope.
To assess Cry5B toxicity by feeding, toxin-expressing plates were prepared as follows. A saturated overnight culture of E. coli JM103[pQE9(Cry5B)] was diluted 1:10 in LB containing 50 μg/ml ampicillin, grown for 1 h at 37 °C, and induced with 50 μm isopropyl β-d-thiogalactopyranoside for an additional 3 h at 30 °C. For 100% Cry5B plates, 50 μl of Cry5B bacteria was spread on 35-mm C. elegans high growth plates with 100 μm isopropyl β-d-thiogalactopyranoside and 50 μg/ml ampicillin. For 1 and 10% Cry5B plates, Cry5B-expressing bacteria were diluted with empty vector-containing bacteria before plating (at the same A600). Individual L4 hermaphrodites were transferred to these plates and grown at 20 °C. They were then either examined under a dissecting microscope or transferred to an agarose pad containing 50 mm sodium azide as an anesthetic and visualized with confocal microscopy. As a control, hermaphrodites were fed induced JM103 cells that contained vector alone. For the rescue experiment, homozygous lec-8(tm1477) hermaphrodites were injected with a mixture of plasmids that included the dominant rol-6 marker and the lec-8::gfp-expressing vector. Rolling worms with LEC-8::EGFP expression were transferred to Cry5B toxin plates and scored under a dissecting microscope.
RESULTS
Preparation of Recombinant C. elegans Galectins
To identify a glycolipid-binding galectin in C. elegans, we prepared recombinant C. elegans galectins. We expressed His6-tagged and GST-fused galectins and used these for purification and the glycoconjugate binding assay. In this study, LEC-7 was excluded because it was predicted to be a pseudogene due to the lack of an EST clone in the data base. LEC-5 protein was not purified as a single band and did not have affinity for asialofetuin (data not shown) and was thus excluded from the analysis. Using ELISA and surface plasmon resonance, we first examined the binding ability of purified galectins for asialofetuin, which contains N- and O-linked glycans and represents a good ligand for a mammalian galectin assay. The C. elegans galectins bound asialofetuin to a similar extent, suggesting conservation of lectin-like characteristics (data not shown).
Binding Specificity of LEC-1–11
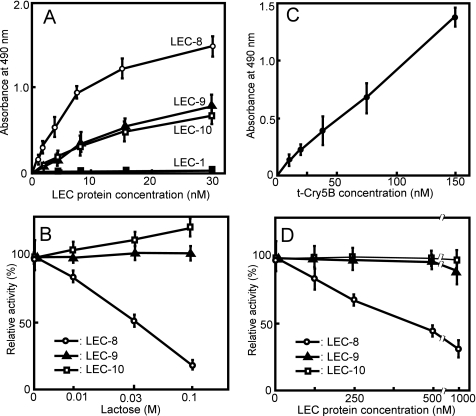
To investigate glycolipid-binding galectins in C. elegans, we performed an extraction using the methods described by Griffitts et al. (11). The lower phase (lipid) (Fig. 1, lane 1) and the upper phase (glycolipid) (Fig. 1, lane 2) fractions were separated by HPTLC and visualized with orcinol sulfate. Using chloroform:methanol:water (4:4:1) as a solvent, the main glycolipid bands were observed in positions similar to those reported previously by Griffitts et al. (11), suggesting that this upper phase fraction contained those same glycolipids (11). We determined which galectins (LEC-1–11) bound to C. elegans glycolipids using ELISA. Among these, only LEC-8, -9, and -10 bound to the glycolipid fraction in a dose-dependent manner (Fig. 2A) whereas LEC-1–4, GST, LEC-6(GST-LEC-6), and LEC-11(GST-LEC-11) did not bind even at the highest concentrations (10 μg/ml, 200–300 nm).
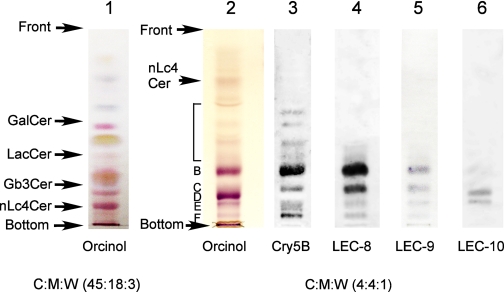
FIGURE 1.
HPTLC of the upper and the lower fractions of lipid extracts from C. elegans. The lower phase (lane 1) and upper phase (lanes 2–6) fractions derived from wild-type worms were separated by HPTLC using chloroform:methanol:water (C:M:W) 45:18:3 for lane 1 and 4:4:1 for lanes 2–6 as the developing solvents. Arrows on the left indicate positions of the glycolipid standards GalCer, LacCer, Gb3, and nLc4Cer. HPTLC plates were stained with orcinol sulfate (lanes 1 and 2) and with bt-Cry5B (lane 3) and GST-LEC-8 (lane 4), -9 (lane 5), and -10 (lane 6) as described under “Experimental Procedures.” The glycolipid components were termed B, C, D, E, and F according to the classification of Griffitts et al. (11).
FIGURE 2.
Specific binding of Cry5B and LEC-8 to C. elegans glycolipids. A, binding of C. elegans galectins to C. elegans glycolipids. Fifty μl of various concentrations of galectins was applied to glycolipid-coated plates as described under “Experimental Procedures.” The bound galectins were measured using an anti-His6 tag antibody followed by an HRP-conjugated anti-mouse IgG (LEC-1–4) or an HRP-conjugated anti-GST antibody (GST, LEC-6–11). ○, LEC-8; ▵, LEC-9; □, LEC-10; and ■, LEC-1. GST, LEC-2, LEC-3, LEC-4, LEC-6, and LEC-11 did not bind to glycolipids, similar to LEC-1. B, Lac-mediated inhibition of glycolipid binding by LEC-8, -9, and -10, respectively. Inhibition assays between C. elegans galectins and glycolipids were performed using ELISA. Fifty μl of galectins (100 nm) with various concentrations of lactose in 1% BSA solution were applied to glycolipid-coated plates, and their relative binding abilities were measured. C, bt-Cry5B binding to C. elegans glycolipids. Various concentrations of bt-Cry5B were applied to glycolipid-coated plates, and bound bt-Cry5B was measured as described under “Experimental Procedures.” D, inhibitory effects of LEC-8, -9, and -10 on bt-Cry5B (60 nm) binding to glycolipid-coated plates. The enzymatic reaction was stopped by the addition of 0.1 n H2SO4 before the absorbance reached 2.0. The absorbance of bound bt-Cry5B and LEC protein was determined by subtracting the values on the nonimmobilized wells from the values on the glycolipid-coated wells. Data points represent mean specific binding from three experiments; error bars denote S.E.M.
Binding Patterns of LEC-8, -9, and -10 Analyzed by TLC Overlay Staining
To study which glycolipid components bind to GST-LEC-8, -9, and -10, we applied TLC overlay staining to the upper phase fraction. From the patterns of orcinol staining, we tentatively identified the glycolipid components using the classifications of Griffitts et al. (11) for comparison. LEC-8 bound to several glycolipid components, including components B, C, E, and F and other minor species (Fig. 1, lane 4) but did not bind to glycolipid component D. LEC-9 showed faint staining to several components, similar to LEC-8 (Fig. 1, lane 5); however, the staining pattern of LEC-10 was different from that of LEC-8 (Fig. 1, lane 6).
Binding Specificity of LEC-8–10 to Glycolipids
To clarify the mechanism of LEC-8, -9, and -10 binding to glycolipids, glycolipid binding of LEC-8, -9, and -10 was studied in the presence of monosaccharides and lactose as haptenic inhibitors. Lactose inhibited LEC-8 binding to the glycolipid fraction in a dose-dependent manner (Fig. 2B), whereas 0.1 m mannose, glucose, fucose, or galactose did not exhibit any inhibitory effect (data not shown). Because the amino acids that bind carbohydrate in the carbohydrate recognition domain are well conserved in C. elegans galectins and because 0.1 m lactose usually effectively inhibits galectin binding to glycoconjugate (21), the ability of LEC-8 to bind glycolipids seems to be mediated by the carbohydrate recognition domain. In the cases of LEC-9 and -10, lactose did not show any inhibitory effect (Fig. 2B), and these galectins also bound to the lipid-rich fraction to some extent (data not shown), suggesting that their binding might be mediated via hydrophobic interactions rather than by a carbohydrate moiety. Furthermore, LEC-8 did not bind mammalian glycolipids (1 μg/well) such as GalCer, GM1, GM2, GM3, GM4, or LacCer (data not shown). These results suggest that LEC-8 specifically binds glycolipids in C. elegans via carbohydrate moieties.
LEC-8 Inhibits Cry5B Binding to Glycolipids
Griffitts et al. (11) reported that crystal toxin Cry5B-producing B. thuringiensis strains can infect C. elegans and that the receptor molecules for Cry5B are glycolipids. As a first step in elucidating the mechanism of inhibition of Cry5B binding in LEC-8, -9, and -10, we examined whether these galectins competitively inhibit Cry5B binding to glycolipids in vitro. For the binding assay, we prepared a truncated form of Cry5B (t-Cry5B) that contained the active protein region (1–697) by comparing the sequences of Cry5B with Cry4Ba, for which the three-dimensional structures and propeptide regions have been reported (22). The binding of biotin-labeled t-Cry5B to the upper phase C. elegans glycolipid fraction was evaluated by ELISA. t-Cry5B binding to glycolipids occurred in a dose-dependent manner (Fig. 2C). t-Cry5B (60 nm) binding to glycolipids was competitively inhibited by LEC-8 at a half-inhibitory concentration of ≅500 nm but not by 500 nm LEC-9 or LEC-10 (Fig. 2D). We also found that t-Cry5B bound to a number of glycolipid species, namely components B, C, E, and F and other minor species, but not component D, as reported previously by Griffitts et al. (11) (Fig. 1, lane 3), similar to LEC-8. The anatomic expression data in WormBase show LEC-8 expression in the intestine, and it has been reported that Cry5B enters C. elegans intestinal cells through glycolipid binding (11). These results suggested that LEC-8 functions by competing with the binding of Cry5B toxin to glycolipids on the surface of intestinal cells.
t-Cry5B Toxin Sensitivity of the LEC-8-deficient Mutant lec-8(tm1477)
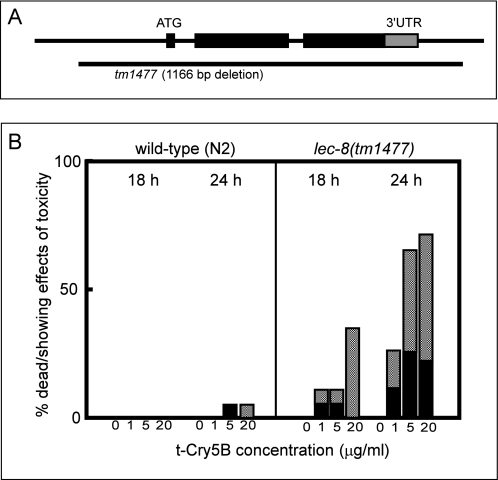
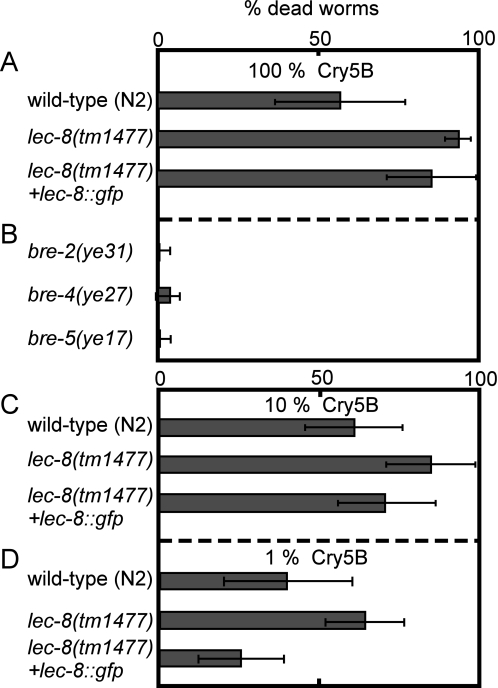
To investigate the functional roles of glycolipid-binding galectins in vivo, we isolated a lec-8 deletion mutant, designated lec-8(tm1477), by PCR-based screening of UV-trimethylpsoralen-mutagenized libraries (23). Because tm1477 lacks the exons containing the ATG translational initiation codon, it was predicted to be a molecular null allele of lec-8 (Fig. 3A). We examined t-Cry5B toxicity, comparing lethality between N2 worms and the lec-8-deficient mutant lec-8(tm1477). In a preliminary t-Cry5B toxicity assay in solution, the LD50 for N2 (5–15 μg/ml) was about 4 times higher than that for lec-8(tm1477) (1–5 μg/ml) (data not shown). On the basis of these data, we compared the vulnerability of N2 and lec-8(tm1477) adult worms to t-Cry5B over 24 h of exposure. At low concentrations of t-Cry5B, the percentage of dead or visibly ill N2 worms was almost negligible, whereas the lec-8(tm1477) mutants were more vulnerable (Fig. 3B). These results suggest that LEC-8 functions in host defense against Cry5B toxin.
FIGURE 3.
Susceptibility of the LEC-8-deficient mutant lec-8(tm1477) to t-Cry5B toxicity. A, genomic structure of the lec-8 locus. The position of the deletion in the mutant allele (tm1477) is indicated (horizontal line). A 3′-untranslated region (3′-UTR) is indicated as a gray box. B, toxicity assay in solution. Young adult N2 and lec-8(tm1477) worms were picked individually and transferred into the wells of 96-well microtiter plates containing various concentrations of purified t-Cry5B as described under “Experimental Procedures.” To score for susceptibility, animals were examined after 18 and 24 h using a dissecting microscope. The percentage of dead worms at each time point was divided by the number of worms at the start of the experiment. Black bars represent the proportion of dead worms. Shaded bars are the proportion of worms that exhibited toxic effects (abnormal movement and morphology, culminating in death within several hours). Assay was performed at 20 °C with ≥20 worms per condition. Data shown represent typical results from three experiments.
Effects of Cry5B on LEC-8 Expression in Wild-type and bre Mutant Worms
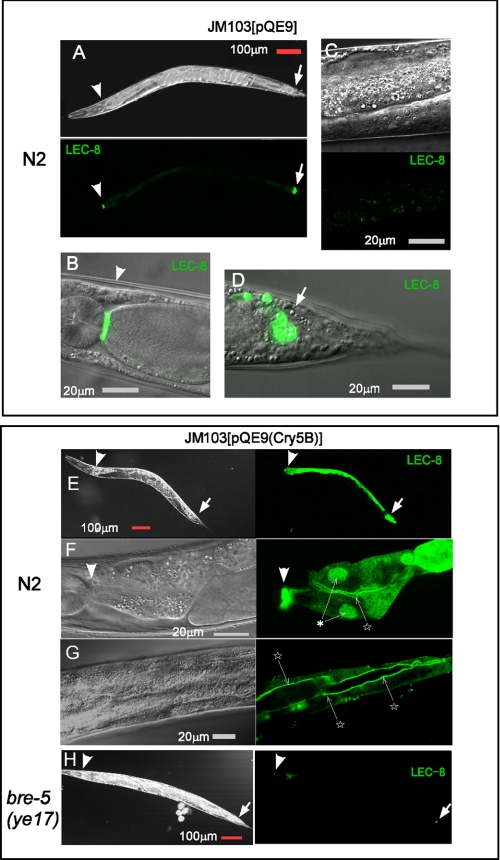
Next, we investigated whether Cry5B affected LEC-8 expression in the intestine. When lec-8::gfp transgenic animals were fed OP50 or E. coli JM103[pQE9] (Fig. 4, A–D), LEC-8::EGFP was expressed predominantly in the pharyngeal-intestinal valve (Fig. 4B) and in a small group of cells in the tail (Fig. 4D), which probably represent the intestinal-rectal valve, and only low level expression was observed in some vesicles of the intestine (Fig. 4C). When we examined worms that were fed E. coli JM103[pQE9(Cry5B)] after 24 h, LEC-8::EGFP was induced strongly in intestinal cells and was observed in the cytoplasm, apical membrane, and nucleus of intestinal cells (Fig. 4, E–G).
FIGURE 4.
Effects of Cry5B on LEC-8::EGFP expression. Images of representative expression patterns of lec-8::gfp transgenic animals that were fed E. coli JM103[pQE9] (A–D) or JM103[pQE9(Cry5B)] (E–H) are shown. A, adult showing EGFP fluorescence in pharyngeal-intestinal valve cells (arrowheads) and in the tail region (arrows). B, head regions of an adult showing EGFP fluorescence in pharyngeal-intestinal valve cells. C, EGFP fluorescence in the intestine. D, adult with EGFP expression in a small group of cells around the intestinal-rectal valve. E, wild-type N2 expressing LEC-8::EGFP was fed E. coli JM103[pQE9(Cry5B)]. F and G, adult showing EGFP fluorescence in intestinal nuclei (asterisk) and membrane (stars). H, bre-5(ye17) worms fed E. coli JM103[pQE9(Cry5B)]. Exposure times were identical for each pair of wild-type N2 and bre-5(ye17) images. Scale bars, 100 μm (A, E, and H) and 20 μm (B–D, F, and G).
B. thuringiensis toxin-resistant mutants, including bre-2(ye31), -4(ye27), and -5(ye17), lack receptor glycolipids for Cry5B (11); they contain mutations in glycosyltransferase genes, which are involved in the biosynthesis of glycolipids acting at the indicated positions in Table 1. When bre mutant worms were fed E. coli expressing Cry5B, they did not exhibit toxic effects and remained alive under the same conditions in which wild-type worms had died. We injected lec-8::gfp into B. thuringiensis toxin-resistant mutants and examined localization of LEC-8::EGFP. When lec-8::gfp transgenic bre mutants were fed E. coli JM103[pQE9(Cry5B)], the localization of LEC-8::EGFP in the head and tail regions (Fig. 4H) was similar to that of wild-type animals fed JM103[pQE9] (Fig. 4, A–D). As summarized in Table 1, the induction of LEC-8::EGFP was low in bre-2(ye31) and -4(ye27) mutants, and almost no induction was observed in the bre-5(ye17) mutants. Glycolipid carbohydrate moieties seem to be important for Cry5B-mediated expression of LEC-8::EGFP because induction of LEC-8::EGFP was most suppressed in the bre-5 mutant. Although the majority (>95%) of wild-type adult worms showed weak LEC-8::EGFP expression in the intestine (Fig. 4C), some worms (<5%) showed enhanced expression (Table 1); strong expression was observed most commonly in sick worms exhibiting signs of constipation (a swollen tail) and/or larvae that had hatched internally. These results suggest that glycolipid-mediated toxicity induces LEC-8 expression in the intestine.
TABLE 1.
Percentage of worms with strong intestinal LEC-8 ::EGFP expression
1) Worms with strong LEC-8 ::EGFP expression in intestine were counted under a dissecting microscope. The percentage of worms with strong expression was calculated by dividing the number of worms expressing the rol-6 marker gene. More than 90 worms were counted per condition.
2) The actual datum was 1/101.
3) Worms with strong LEC-8 ::EGFP expression in intestine were counted after 24 h on a toxin plate. The assay was performed two or three times independently at 20 °C with more than 30 worms with the marker gene per condition.
4) The actual datum was 1/102.
5) Structure of C. elegans glycosphingolipids and linkages in the oligosaccharide headgroup that are synthesized by BRE enzymes are indicated with arrows ((11)). GlcNAc, N-acetylglucosamine.
Cry5B Toxin Sensitivity of the LEC-8-deficient Mutant lec-8(tm1477)
To confirm the vulnerability of lec-8(tm1477) to Cry5B, N2 and lec-8(tm1477) worms and the Cry5B-resistant mutants bre-2(ye31), -4(ye27), and -5(ye17) were fed E. coli expressing full-length Cry5B. We found that lec-8(tm1477) died within 24 h after feeding (Fig. 5A). In contrast, about 60% of N2 died, and most bre-2(ye31), -4(ye27), and -5(ye17) worms were still alive after 24 h (Fig. 5B). When we reexpressed LEC-8 by injecting lec-8::gfp in lec-8-deficient mutant worms, LEC-8::EGFP expression and vulnerability against Cry5B were rescued, and their viability was close to the level of N2 worms (Fig. 5, A, C, and D). Our results indicate that Cry5B triggers expression of LEC-8 in the intestine and that LEC-8 competes with Cry5B for cell surface glycolipids.
FIGURE 5.
Cry5B susceptibility of the LEC-8-deficient mutant, lec-8(tm1477). A and B, for the toxicity assay, young adult N2, lec-8(tm1477), bre-2(ye31), -4(ye27), and -5(ye17) worms were transferred to Cry5B plates as described under “Experimental Procedures.” To score susceptibility, the animals were examined after 24 h using a dissecting microscope. C and D, for 10 and 1% Cry5B plates, Cry5B-expressing bacteria were diluted with empty vector containing bacteria before plating (at the same A600). The percentage of dead worms was divided by the number of worms at the start of the experiment. Shaded bars represent the proportion of dead worms. The assay was performed at 20 °C with ≥30 worms/condition. Data represent mean and ±S.D. from three experiments.
DISCUSSION
We have clearly demonstrated that C. elegans LEC-8 recognizes glycolipids and functions in host defense against bacterial infection by competition. To our knowledge, this is the first study to identify a glycolipid-binding lectin in C. elegans. We have reported previously that human galectin-4 recognizes mammalian sulfated glycolipids, and we speculated that such lectins might function in host defense (9). Because it is not easy to examine these interactions in vivo in mammals, we examined the functional role of galectins in host defense of C. elegans. Because amino acid sequence homology did not enable us to identify a galectin-4 homolog in C. elegans, we attempted to find a functional homolog of galectin-4 through a lectin glycolipid-binding assay.
Although the carbohydrate-binding specificity of C. elegans galectins has been studied with respect to oligosaccharides of mammalian origin (24–26), their physiological ligands have not yet been identified. Because high mannose-type N-glycan is the most abundant in C. elegans-derived glycans (27) and because most of the galactose residues are attached to the nonreducing terminal of O-linked glycans and glycolipids (11, 28), glycolipids were a likely candidate to serve as galectin ligands. As Griffitts et al. (11) reported, glycolipids extracted from C. elegans are structurally different from those of mammalian origin. Although C. elegans glycolipids contain many galactose residues at the nonreducing terminus, only specific galectins bound these glycolipids, even if they exhibited an affinity toward asialofetuin. Thus, even galectins that share some sequence homology have distinct binding specificities, especially for glycolipids. The binding of LEC-8 to glycolipids can be inhibited by lactose, whereas LEC-9 and -10 glycolipid binding was unaffected by lactose (Fig. 2B), suggesting that LEC-8 binds to glycolipids through the carbohydrate moiety. Furthermore, LEC-8 inhibited Cry5B binding to glycolipids, whereas LEC-9 and -10 did not (Fig. 2D).
There are several reports that glycolipids serve as receptors for bacterial and viral toxins. The human respiratory pathogen Bordetella pertussis binds to SM4 and glycolipids containing a GalNAcβ1→4Gal sequence (29). Influenza A viruses bind to SM4 (30), and the neutrophil-activating protein of Helicobacter pylori binds to SM4 and SO3−→3Galβ1→3GalNAcβ1→4Galβ1→4Glcβ1→Cer (31). SM4 and some sulfated glycolipids are expressed in the mammalian gastrointestinal tract (32), and we also found that SM4 and cholesterol sulfate are localized in the intestine (9). Because galectin-4 is expressed specifically in the gastrointestinal tract (33), we speculate that galectin-4 may play a role in host defense by masking sulfated glycolipids to which bacterial or viral toxins bind.
B. thuringiensis is an interesting bacterium both agronomically and scientifically. This bacterium colonizes and kills a large variety of host insects, but each strain attacks with a high degree of specificity. The specificities are determined primarily by the crystal toxins, which the bacterium produces during sporulation. It is of interest that the crystal toxins contain subdomains with galectin-like β-sheet structures and that they bind to the carbohydrate portions of glycoconjugates in target insects (34). These unique lectin-like characteristics define the distinct binding specificities of the toxins. Accordingly, it is important to study the carbohydrate-binding specificity of toxins with lectin-like characteristics because the glycoconjugate sugar chain structures show species specificity (34). Glycolipids extracted from C. elegans were structurally different from those of mammals, and differences in the carbohydrate structures of glycoproteins and glycolipids between insects and mammals make crystal toxin a viable agricultural poison.
Griffitts et al. (11) found that Cry5B is able to bind to a number of glycosphingolipid species, namely components B, C, E, and F and other minor species, but not glycolipid component D (Fig. 1, lane 3). They also determined the chemical structures of components B, C, D, and E and showed that carbohydrates are key mediators of Cry5B binding to glycolipids and that the β-galactose-rich terminus of these receptors is an important binding epitope. LEC-8 also bound to the same C. elegans glycolipids to which Cry5B bound. LEC-8 bound components B and C more strongly than the other components (Fig. 1, lane 4). Griffitts et al. (11) showed that these components are important for toxicity because they are missing in four Cry5B-resistant mutants, bre-2, -3, -4, and -5. Moreover, Cry5B binding to glycolipids was inhibited by LEC-8 in a dose-dependent manner (Fig. 2D). The half-inhibitory concentration was rather high (≅500 nm) because the LEC-8 binding pattern (Fig. 1, lane 4) was similar, but not identical, to that of Cry5B (Fig. 1, lane 3). These results suggest that LEC-8 and Cry5B bind competitively to C. elegans glycolipid components, although their precise binding epitopes may not be identical. This may be a mechanism by which LEC-8 contributes to the host defense against Cry5B infection. Higher concentrations of LEC-9 and LEC-10 also bound glycolipid components weakly, so they may have some redundant function with LEC-8. This question should be resolved in the near future.
It is interesting that Cry5B-induced LEC-8 expression in intestine was suppressed in bre mutants, suggesting that LEC-8 expression in the intestine undergoes toxic effects via glycolipids. Using high density cDNA microarrays, Mallo et al. (35) showed that a marked up-regulation of lec-8 and lysosomal enzyme gene expression in C. elegans is induced by infection with the Gram-negative bacterium Serratia marcescens, indicating that induction of LEC-8 expression is not limited to B. thuringiensis expressing Cry5B, but may be a common defense mechanism against this kind of bacterial infection.
Because it has been reported that p38 MAPK and a c-Jun N-terminal-like MAPK are both transcriptionally up-regulated by Cry5B (36), LEC-8 induction by Cry5B may be related to the MAPK signaling pathway. The question of how LEC-8 functions in host defense signaling pathways remains to be answered.
Recently, it has been reported that ceramide glucosyltransferase genes involved in the biosynthesis of glycosphingolipids are strongly expressed in the pharyngeal-intestinal valve and in a small group of cells in the tail (37), similar to LEC-8::EGFP expression. It is also of interest how LEC-8 might function cooperatively with glycosphingolipids in those cells in C. elegans. The intracellular localization of LEC-8 induced in intestinal cells appears to be similar to that of galectin-4, which also localizes to the cytosol, submembrane region, and nucleus (8). These studies of C. elegans LEC-8 may provide insight into the functional role and localization mechanisms of mammalian galectin-4. Transgenic crops producing insecticidal crystal toxin, Cry1Ac, have been developed commercially to control Heliothis virescens (tobacco budworm) in the field. Cry5B exhibits ∼24% sequence identity to Cry1Ac in the toxin domain containing the three-domain structure common to insecticidal toxins (38, 39). Similarities in toxin sequences and toxic responses suggest that a general mechanism for B. thuringiensis toxicity is conserved between C. elegans and insects. Thus, studies of glycolipid-binding lectins examining endogenous lectins and exogenous toxins in host defense may be useful both for agricultural production and for human health.
Acknowledgments
We appreciate the gifts of JM103[pQE9(Cry5B)] from Dr. Raffi V. Aroian (University of California San Diego) and of galectin plasmids from Dr. Yuji Kohara (National Institute of Genetics, Mishima, Japan). We are also grateful for the helpful technical advice of Dr. Junko Matsuda (Tokai University, Hiratsuka, Japan) with HPTLC analysis.
This work was supported in part by the New Energy and Industrial Technology Development Organization (NEDO) in Japan.
- GalNAc
- N-acetylgalactosamine
- Cer
- ceramide
- EGFP
- enhanced green fluorescent protein
- HRP
- horseradish peroxidase
- t-Cry5B
- a truncated form of Cry5B
- ELISA
- enzyme-linked immunoabsorbent assay
- GST
- glutathione S-transferase
- Lac
- lactose (Galβ1→4Glc)
- LacCer
- Galβ1→4Glcβ1→1Cer
- nLc4Cer
- Galβ1→3GalNAcβ1→3Galβ1→4Glcβ1→1Cer
- Gb3
- Galα1→4Galβ1→4Glcβ1→1Cer
- GM1
- Galβ1→3GalNAcβ1→4(Neu5Acα2→3)Galβ1→4Glcβ1→1Cer
- GM2
- GalNAcβ1→4(Neu5Acα2→3)Galβ1→4Glcβ1→1Cer
- GM3
- Neu5Acα2→3Galβ1→4Glcβ1→1Cer
- GM4
- Neu5Acα2→3Galβ1→Cer
- GalCer
- Galβ1→1Cer
- SM4
- SO3−→3Galβ1→1Cer
- HPTLC
- high performance thin layer chromatography
- PBS
- phosphate-buffered saline
- BSA
- bovine serum albumin
- MAPK
- mitogen-activated protein kinase
- bt
- biotinylated.
REFERENCES
- 1.Barondes S. H., Cooper D. N., Gitt M. A., Leffler H. (1994) J. Biol. Chem. 269, 20807–20810 [PubMed] [Google Scholar]
- 2.Hernandez J. D., Baum L. G. (2002) Glycobiology 12, 127R–136R [DOI] [PubMed] [Google Scholar]
- 3.Hughes R. C. (1999) Biochim. Biophys. Acta 1473, 172–185 [DOI] [PubMed] [Google Scholar]
- 4.Danielsen E. M., Hansen G. H. (2003) Biochim. Biophys. Acta 1617, 1–9 [DOI] [PubMed] [Google Scholar]
- 5.Braccia A., Villani M., Immerdal L., Niels-Christiansen L. L., Nystrom B. T., Hansen G. H., Danielsen E. M. (2003) J. Biol. Chem. 278, 15679–15684 [DOI] [PubMed] [Google Scholar]
- 6.Delacour D., Gouyer J., Zanetta J. P., Drobecq H., Leteurtre E., Grard G., Moreau-Hannedouche O., Maes E., Pons A., André S., Le Bivic A., Gabius H. J., Manninen A., Simons K., Huet G. (2005) J. Cell Biol. 169, 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ideo H., Seko A., Ohkura T., Matta K. L., Yamashita K. (2002) Glycobiology 12, 199–208 [DOI] [PubMed] [Google Scholar]
- 8.Ideo H., Seko A., Yamashita K. (2007) J. Biol. Chem. 282, 21081–21089 [DOI] [PubMed] [Google Scholar]
- 9.Ideo H., Seko A., Yamashita K. (2005) J. Biol. Chem. 280, 4730–4737 [DOI] [PubMed] [Google Scholar]
- 10.Karlsson K. A. (1989) Annu. Rev. Biochem. 58, 309–350 [DOI] [PubMed] [Google Scholar]
- 11.Griffitts J. S., Haslam S. M., Yang T., Garczynski S. F., Mulloy B., Morris H., Cremer P. S., Dell A., Adang M. J., Aroian R. V. (2005) Science 307, 922–925 [DOI] [PubMed] [Google Scholar]
- 12.Skipski V. P. (1975) Methods Enzymol. 35, 396–425 [DOI] [PubMed] [Google Scholar]
- 13.Hidari K. I., Kawashima I., Tai T., Inagaki F., Nagai Y., Sanai Y. (1994) Eur. J. Biochem. 221, 603–609 [DOI] [PubMed] [Google Scholar]
- 14.Izumikawa T., Kitagawa H., Mizuguchi S., Nomura K. H., Nomura K., Tamura J., Gengyo-Ando K., Mitani S., Sugahara K. (2004) J. Biol. Chem. 279, 53755–53761 [DOI] [PubMed] [Google Scholar]
- 15.Marroquin L. D., Elyassnia D., Griffitts J. S., Feitelson J. S., Aroian R. V. (2000) Genetics 155, 1693–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox G. N., Laufer J. S., Kusch M., Edgar R. S. (1980) Genetics 95, 317–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. (1991) EMBO J. 10, 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulston J., Hodgkin J. (1988) in The Nematode Caenorhabditis elegans ( Wood W. B. ed) pp. 587–606, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 19.Borgonie G., Van Driessche R., Leyns F., Arnaut G., De Waele D., Coomans A. (1995) J. Invertebr. Pathol. 65, 61–67 [DOI] [PubMed] [Google Scholar]
- 20.Borgonie G., Claeys M., Leyns F., Arnaut G., De Waele D., Coomans A. (1996) Fund. Appl. Nematol. 19, 391–398 [Google Scholar]
- 21.Knibbs R. N., Agrwal N., Wang J. L., Goldstein I. J. (1993) J. Biol. Chem. 268, 14940–14947 [PubMed] [Google Scholar]
- 22.Boonserm P., Davis P., Ellar D. J., Li J. (2005) J. Mol. Biol. 348, 363–382 [DOI] [PubMed] [Google Scholar]
- 23.Gengyo-Ando K., Mitani S. (2000) Biochem. Biophys. Res. Commun. 269, 64–69 [DOI] [PubMed] [Google Scholar]
- 24.Arata Y., Hirabayashi J., Kasai K. (2001) J. Biol. Chem. 276, 3068–3077 [DOI] [PubMed] [Google Scholar]
- 25.Nemoto-Sasaki Y., Hayama K., Ohya H., Arata Y., Kaneko M. K., Saitou N., Hirabayashi J., Kasai K. (2008) Biochim. Biophys. Acta 1780, 1131–1142 [DOI] [PubMed] [Google Scholar]
- 26.Ahmed H., Bianchet M. A., Amzel L. M., Hirabayashi J., Kasai K., Giga-Hama Y., Tohda H., Vasta G. R. (2002) Glycobiology 12, 451–461 [DOI] [PubMed] [Google Scholar]
- 27.Haslam S. M., Dell A. (2003) Biochimie 85, 25–32 [DOI] [PubMed] [Google Scholar]
- 28.Haslam S. M., Gems D., Morris H. R., Dell A. (2002) Biochem. Soc. Symp. 69, 117–134 [PubMed] [Google Scholar]
- 29.Brennan M. J., Hannah J. H., Leininger E. (1991) J. Biol. Chem. 266, 18827–18831 [PubMed] [Google Scholar]
- 30.Suzuki T., Sometani A., Yamazaki Y., Horiike G., Mizutani Y., Masuda H., Yamada M., Tahara H., Xu G., Miyamoto D., Oku N., Okada S., Kiso M., Hasegawa A., Ito T., Kawaoka Y., Suzuki Y. (1996) Biochem. J. 318, 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teneberg S., Miller-Podraza H., Lampert H. C., Evans D. J., Jr., Evans D. G., Danielsson D., Karlsson K. A. (1997) J. Biol. Chem. 272, 19067–19071 [DOI] [PubMed] [Google Scholar]
- 32.Ishizuka I. (1997) Prog. Lipid Res. 36, 245–319 [DOI] [PubMed] [Google Scholar]
- 33.Oda Y., Herrmann J., Gitt M. A., Turck C. W., Burlingame A. L., Barondes S. H., Leffler H. (1993) J. Biol. Chem. 268, 5929–5939 [PubMed] [Google Scholar]
- 34.de Maagd R. A., Bravo A., Berry C., Crickmore N., Schnepf H. E. (2003) Annu. Rev. Genet. 37, 409–433 [DOI] [PubMed] [Google Scholar]
- 35.Mallo G. V., Kurz C. L., Couillault C., Pujol N., Granjeaud S., Kohara Y., Ewbank J. J. (2002) Curr. Biol. 12, 1209–1214 [DOI] [PubMed] [Google Scholar]
- 36.Huffman D. L., Abrami L., Sasik R., Corbeil J., van der Goot F. G., Aroian R. V. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10995–11000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marza E., Simonsen K. T., Faergeman N. J., Lesa G. M. (2009) J. Cell Sci. 122, 822–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnepf E., Crickmore N., Van Rie J., Lereclus D., Baum J., Feitelson J., Zeigler D. R., Dean D. H. (1998) Microbiol. Mol. Biol. Rev. 62, 775–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crickmore N., Zeigler D. R., Feitelson J., Schnepf E., Van Rie J., Lereclus D., Baum J., Dean D.H. (1998) Microbiol. Mol. Biol. Rev. 62, 807–813 [DOI] [PMC free article] [PubMed] [Google Scholar]