Abstract
ATP synthase uses a unique rotary mechanism to couple ATP synthesis and hydrolysis to transmembrane proton translocation. As part of the synthesis mechanism, the torque of the rotor has to be converted into conformational rearrangements of the catalytic binding sites on the stator to allow synthesis and release of ATP. The γ subunit of the rotor, which plays a central role in the energy conversion, consists of two long helices inside the central cavity of the stator cylinder plus a globular portion outside the cylinder. Here, we show that the N-terminal helix alone is able to fulfill the function of full-length γ in ATP synthesis as long as it connects to the rest of the rotor. This connection can occur via the ϵ subunit. No direct contact between γ and the c ring seems to be required. In addition, the results indicate that the ϵ subunit of the rotor exists in two different conformations during ATP synthesis and ATP hydrolysis.
F1Fo-ATP synthase is responsible for the bulk of ATP synthesis from ADP and Pi in most organisms. F1Fo-ATP synthase consists of the membrane-embedded Fo subcomplex with, in most bacteria, a subunit composition of ab2cn (with n = 10–15) and the peripheral F1 subcomplex, with a subunit composition of α3β3γδϵ. The energy necessary for ATP synthesis is derived from an electrochemical transmembrane proton (or, in some organisms, sodium ion) gradient. Proton flow, down the gradient, through Fo is coupled to ATP synthesis on F1 by a unique rotary mechanism. The protons flow through channels at the interface of a and c subunits, which drives rotation of the ring of c subunits. The cn ring, together with F1 subunits γ and ϵ, forms the rotor. Rotation of γ leads to conformational changes in the catalytic nucleotide-binding sites on the β subunits, where ADP and Pi are bound. The conformational changes result in formation and release of ATP. Thus, ATP synthase converts electrochemical energy, the proton gradient, into mechanical energy in the form of subunit rotation and back into chemical energy as ATP. In bacteria, under certain physiological conditions, the process can run in reverse. ATP is hydrolyzed to generate a transmembrane proton gradient that the bacterium requires for such functions as nutrient import and locomotion (for reviews, see Refs. 1–6).
F1 (or “F1-ATPase”) has three catalytic nucleotide-binding sites, located on the β subunits, at the interface with the adjacent α subunit. The catalytic sites have pronounced differences in their nucleotide-binding affinity. During rotational catalysis, the sites switch their affinities in a synchronized manner; the position of γ determines which catalytic site is the high affinity site (Kd1 in the nanomolar range), which site is the medium affinity site (Kd2 ≈ 1 μm), and which site is the low affinity site (Kd3 ≈ 30–100 μm; see Refs. 7, 8). Only the high affinity site is catalytically active (9). In the original crystal structure of bovine mitochondrial F1 (10), one of the three catalytic sites was filled with the ATP analog AMPPNP,3 a second one with ADP (plus azide; see Ref. 11), and the third site was empty. Hence, the β subunits are referred to as βTP, βDP, and βE, respectively. The high affinity site is located on the βTP subunit (12).
The coupling process between ATP synthesis or hydrolysis on β and rotation of γ is not yet fully understood on a residue level. A number of point mutations at the interfaces between α or β and γ and between γ, ϵ, and c have been described that result in varying degrees of uncoupling (13–17). Some mutations at these interfaces were found that abolish ATP synthesis or hydrolysis activity or both (18–20). Considering the pronounced effect of these point mutations, some of which were even conservative substitutions, it came as a surprise when it was recently shown that an “axle-less” γ, consisting just of the globular portion, with the portions of the N- and C-terminal helices that reach into the α3β3 cylinder removed, displayed ATP-driven rotation in the correct direction (21).
Some reports have implicated the ϵ subunit (corresponding to the δ subunit in the mitochondrial enzyme) as being involved in coupling (15, 22–25). It was shown that ϵ exists in different conformations that vary in the folding and positioning of the C-terminal domain. The x-ray structure of the mitochondrial enzyme (26) shows the two helices of the C-terminal domain folded back on each other like a hairpin and positioned close to the interface between γ and the c ring (“down” conformation). In the crystal structure of a γϵ complex from Escherichia coli the hairpin is unfolded (27); when integrated into F1 or F1Fo, the C terminus would reach “up,” coming close to the DELSEED-loop of the α and/or β subunits. While in this up conformation the angle between both helices of the C-terminal domain of ϵ is ∼90°, it has been postulated that this domain might also exist in a fully extended up conformation, stretching close to the N terminus of γ, with helical regions replacing the turn between the two helices (28). Fixing ϵ in either up conformation by cross-linking to γ has been shown to impair ATP hydrolysis but not synthesis. Freezing ϵ in the down position inhibited neither reaction (29, 30).
Here, we report a finding that is arguably just as surprising as the rotation of an axle-less γ. In ATP synthase from the thermophilic bacterium Bacillus PS3, enzymes with γ subunit constructs where the globular domain and the C-terminal helix were eliminated, consisting of just the N-terminal 35 or 42 residues, TF1Fo(γQ36stop)4 and TF1Fo(γP43stop), were able to catalyze significant rates of ATP synthesis. According to the crystal structure (26), the shorter of the two γ constructs should not make any contact either with c or with ϵ in the down conformation. Thus, the fact that ATP synthesis was observed suggests that ϵ in an up conformation forms a complex with the truncated γ, which is able to catalyze ATP synthesis. Indeed, when the γQ36stop truncation was combined with an ϵ truncation where the C-terminal domain was removed, ATP synthesis was abolished. The functions of the γ and ϵ subunits will be discussed in light of the new findings.
MATERIALS AND METHODS
Bacterial Strains and Plasmids
Plasmid pTR19-ASDS, which carries the genes for the F1Fo-ATP synthase from thermophilic Bacillus PS3 (31), was used to generate the mutations γK7stop, γQ36stop, γP43stop, and ϵI88stop. In initial experiments to generate γ-less TF1Fo (TF1FoΔγ) by introducing the γK7stop mutation, we observed that the E. coli host produced small amounts of full-length PS3 γ, probably caused by a termination suppressor mutation in a tRNA. To eliminate this problem, an NheI site was introduced downstream of the stop codon. This allowed removal of the remainder of the gene for γ on an NheI-NheI fragment (the second NheI site is between the genes for γ and β). For construction of the γQ36stop and γP43stop truncation mutants, the same strategy was used. The mutagenic oligonucleotides were designed in such a way that, in addition to the desired mutation, a restriction site would be eliminated or generated to facilitate screening. Deletions were introduced by PCR using the QuikChange II XL mutagenesis kit (Stratagene). Wild-type and mutated plasmids were transformed into E. coli strain DK8, which does not express E. coli ATP synthase (32).
Isolation of Inverted Membrane Vesicles, Determination of F1Fo Content in E. coli Membranes
E. coli strain DK8, harboring wild-type or mutated pTR19-ASDS plasmids, was aerobically cultivated at 37 °C for 18 h in 2× YT medium containing 100 μg/ml ampicillin. Inverted membrane vesicles from E. coli cells expressing thermophilic F1Fo were prepared as described (31, 33). The amount of wild-type F1Fo in E. coli membrane preparations was determined by SDS-PAGE, as visualized by staining with Coomassie Brilliant Blue. The relative amount of mutant F1Fo the membranes was estimated via Western blotting, using an anti-β antibody (Agrisera, Vännäs, Sweden) or an anti-α/anti-β antibody (a kind gift from Bill Brusilow, Wayne State University, Detroit, MI). The staining intensity was quantified using a Photodyne imaging system and ImageJ acquisition software (National Institutes of Health). The antibody against the globular portion of γ, used to confirm the absence of this portion of the protein in the truncation mutants, was a kind gift from Toshiharu Suzuki and Masasuke Yoshida (Japan Science and Technology Agency, Tokyo).
Functional Analysis of Mutant Strains and Enzymes
Growth of strains expressing wild-type or mutant PS3 ATP synthase in limiting glucose was determined as described previously (34). ATPase activities were measured as described previously (35).
ATP synthesis activity in vitro was measured as follows. The batch assay used to obtain the data in Table 1 allowed running of the assay at a different temperature (42 °C) than the luciferin/luciferase reaction (room temperature) because the luciferase turned out to be very unstable at 42 °C. For this assay, inverted membrane vesicles were suspended in a solution containing 20 mm Hepes/KOH, 100 mm KCl, 5 mm MgCl2, 2 mm ADP, 5 mm KPi, 2 μm Ap5A, and 10% glycerol (pH 7.5) and incubated at 42 °C; Ap5A was included to suppress adenylate kinase activity of the membranes (36). The reaction was initiated by the addition of 2 mm NADH. After 10, 40, and 70 s, aliquots of the reaction mixture (each containing 20 μg of membrane protein) were transferred into boiling buffer of 100 mm Tris/H2SO4, 4 mm EDTA (pH 7.75) for heat denaturation. The samples were incubated at 100 °C for 2 min, cooled on ice, and centrifuged for 1 min at 1000 × g. The amount of ATP produced was determined by the luciferin/luciferase method (CLS II ATP bioluminescence kit, Roche Applied Science). Light emission was measured at 562 nm in a spectrofluorometer type Fluorolog 3 (HORIBA Jovin Yvon, Edison, NY). In control experiments in the presence of the inhibitor oligomycin (20 μg/ml), no ATP synthesis was observed (Table 1).
TABLE 1.
Oxidative phosphorylation in vivo and ATP synthesis activities in vitro
Oxidative phosphorylation in vivo and ATP synthesis activities of membrane preparations of mutants and controls were measured as described under “Materials and Methods.” When indicated, 20 μg/ml oligomycin was present in the ATP synthesis assay. The given values represent the average of at least four independent measurements ± S.D. The positive control was strain pTR19-ASDS/DK8, which expresses Bacillus PS3 ATP synthase in E. coli; this strain served as background for the deletions. The negative control was strain pUC118/DK8, which expresses neither PS3 ATP synthase nor the endogenous E. coli enzyme. The amount of F1Fo in the membrane preparations was measured by quantitative immunoblot analysis, as described under “Materials and Methods.” Turnover rates were calculated using a molecular mass of 531 kDa for the holoenzyme, taking into account the differing amounts of ATP synthase in the individual membrane preparations.
| Strain/mutation | Growth yields in limiting glucose | Amount of F1Fo on membranes | NADH-driven ATP synthesis ctivity | Turnover rate kcat |
|---|---|---|---|---|
| % of wild-type | % of total protein | milliunits/mg | s−1 | |
| Wild-type | 100 ± 4 | 20 ± 5 | 60 ± 22 | 2.7 |
| + oligomycin | 1.35 ± 0.50 | <0.1 | ||
| ϵI88stop | 101 ± 4 | 21 ± 6 | 151 ± 33 | 6.4 |
| Δγ | 65 ± 2 | 9 ± 4 | 0.62 ± 0.97 | <0.1 |
| pUC118/DK8 (unc−) | 65 ± 3 | 0 | 0.17 ± 0.85 | |
| γQ36stop | 75 ± 2 | 9 ± 2 | 7.5 ± 2.6 | 0.7 |
| + oligomycin | 0.13 ± 0.93 | <0.1 | ||
| γQ36stop/ϵI88stop | 62 ± 2 | 10 ± 2 | 1.03 ± 0.86 | <0.1 |
| γP43stop | 76 ± 3 | 13 ± 4 | 14.1 ± 3.4 | 1.0 |
| + oligomycin | −1.05 ± 1.26 | <0.1 | ||
| γP43stop/ϵI88stop | 71 ± 2 | 12 ± 4 | 9.5 ± 4.5 | 0.7 |
| + oligomycin | 0.04 ± 0.90 | <0.1 |
The real time ATP synthesis assay was performed in a buffer containing 20 mm Hepes/KOH, 100 mm potassium acetate, 5 mm magnesium acetate, 2 mm ADP, 5 mm KPi, and 10% glycerol (pH 7.5) at 37 °C. Membrane vesicles (containing 100 μg of membrane protein/ml) and 1/20 volume of the luciferin/luciferase mix were added. The reaction was initiated by the addition of 5 mm succinate. The slow but steady decrease in the signal of the negative control is caused by instability of the luciferase at 37 °C.
NADH- and ATP-driven H+-pumping in membrane vesicles was measured via fluorescence quenching of ACMA at 42 °C. To a buffer of 10 mm Hepes/KOH, 100 mm KCl, 5 mm MgCl2 (pH 7.5), 0.5 mg/ml membrane vesicles and 0.3 μg/ml ACMA were added. Proton pumping was initiated by adding NADH or ATP to a final concentration of 1 mm and terminated by adding carbonyl cyanide m-chlorophenylhydrazone (final concentration 1 μm). The excitation wavelength was 410 nm, and the emission wavelength was 480 nm. Protein concentrations of membrane vesicles were determined by the Lowry method (37) using bovine serum albumin as standard.
RESULTS
Overview of γ and ϵ Truncation Mutants
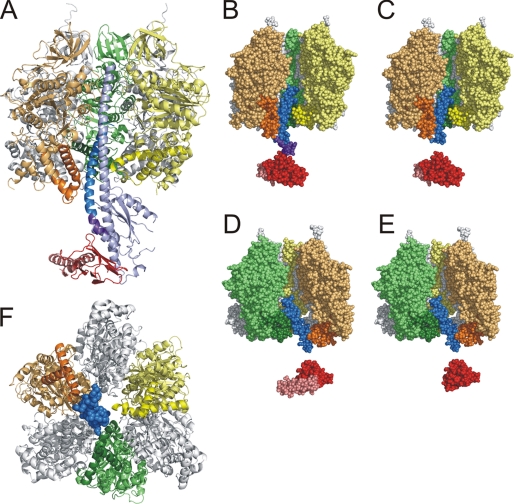
The γQ36stop and γP43stop truncation mutants remove the globular portion of γ and the C-terminal helix, leaving just a large fragment of the N-terminal helix (Fig. 1). With γP43stop, the contact site between the truncated γ (abbreviated γ′ in the following) and the N-terminal domain of ϵ would be reduced to a small patch; with γQ36stop, there would be no interaction between γ′ and the N-terminal domain of ϵ (Fig. 1, B–E). Neither γ truncation mutant should be able to reach the ring of c subunits (which would be located directly below γ and ϵ in Fig. 1A). To obtain an ϵ subunit consisting of just the N-terminal domain, the C-terminal domain was removed using an ϵI88stop mutation.
FIGURE 1.
Structure of the F1 portion of ATP synthase. A, side view. The α subunit in the foreground of the α3β3 stator cylinder has been omitted for clarity. The remaining α subunits are shown in light gray. βTP, βDP, and βE are colored yellow, green, and orange, respectively, with the DELSEED-loop (see under “Discussion”) in the C-terminal domain in a darker shade than the rest of the molecule. Up to residue 35, γ is colored medium blue; this portion of γ is preserved in the γQ36stop truncation mutant. Residues 36–42 are colored purple, the rest of the subunit is light blue. The medium blue and purple portions together correspond to the γP43stop truncation mutant. The ϵ subunit (δ in the mitochondrial enzyme) is depicted in reddish tones, the N-terminal domain (corresponding to the ϵI88stop truncation mutant) in bright red, the C-terminal domain in pink. It should be noted that in the structures shown here the ϵ subunit is in the down conformation. B, γP43stop mutant. Color coding is as in A. C, γQ36stop mutant. D, γQ36stop mutant, rotated by 120° from the position in C. Again, the α subunit in the foreground has been omitted. E, γQ36stop/ϵI88stop mutant. F, view of F1 with γQ36stop, as seen from the membrane. Residues 11–35 of γ′, which interact with the DELSEED-loop of the β subunits, are shown in spacefill. ϵ is omitted for clarity. A 120° rotation in synthesis direction (clockwise) will bring γ′ in contact with βTP and convert it into βE. All pictures were generated using PyMol (DeLano Scientific).
Expression and Purification of Truncation Mutants
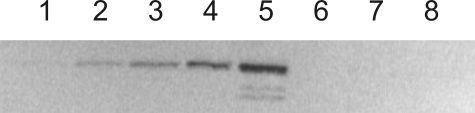
E. coli membrane vesicles containing TF1Fo, TF1FoΔγ, TF1Fo(γQ36stop), TF1Fo(γP43stop), TF1Fo(ϵI88stop), TF1Fo(γQ36stop/ϵI88stop), and TF1Fo(γP43stop/ϵI88stop) were prepared as described under “Materials and Methods.” Western blotting using an anti-γ antibody raised against a peptide from the globular domain of γ confirmed that this domain was absent from the γ deletion and truncation mutants (for TF1FoΔγ, see Fig. 2). Western blotting using an anti-β or an anti-α/anti-β antibody was used to quantify the amount of TF1Fo in the membranes; the results are included in Table 1. The data (Fig. 2) show that it is possible to generate a γ-less ATP synthase. In contrast, it has been reported, based on in vitro experiments, that δ and ϵ subunits are required for binding of TF1 to TFo (38).
FIGURE 2.
Quantification of γ in TF1Fo. E. coli membrane preparations containing wild-type TF1Fo (lanes 1–5), TF1FoΔγ (lane 7), and no TF1Fo (pUC118/DK8; lane 8) were run on an SDS gel and developed by Western blotting using an antibody against the globular portion of γ. The amount of wild-type TF1Fo in the membranes loaded into lanes 1–5 was as follows: lane 1, 40 ng; lane 2, 100 ng; lane 3, 200 ng; lane 4, 400 ng; lane 5, 1 μg. Lane 6 was empty. Lane 7 contained membranes with 1 μg of TF1FoΔγ. Lane 8 contained the same amount of total membrane protein as lane 7 but without any TF1Fo. The nature of the two faint bands below γ that are visible at the highest concentration of wild-type TF1Fo is not known.
Oxidative Phosphorylation in Vivo and ATP Synthesis in Vitro
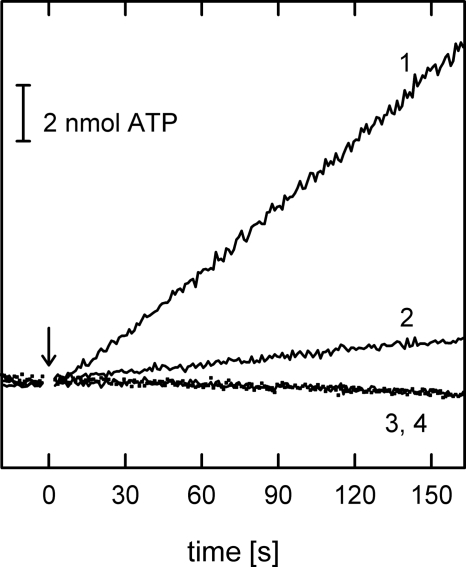
To analyze the ability of the truncation mutants to catalyze ATP synthesis in vivo, we measured growth yields on limiting glucose of E. coli cultures containing strain pTR19-ASDS/DK8 with the desired mutation. As expected, growth of the strain expressing a γ-less enzyme was the same as that of the negative control strain, pUC118/DK8, which does not express ATP synthase. In contrast, very surprisingly, TF1Fo containing either truncation mutant, γQ36stop or γP43stop, supported significantly higher growth than the negative control (Table 1), demonstrating that γ requires only its N-terminal helix to effect the conformational changes in β (and perhaps α) required to catalyze ATP synthesis. To confirm these findings, we measured ATP synthesis by membrane vesicles in vitro. Again, although the γ-less enzyme did not show any synthesis activity, both γ truncation mutants displayed significant ATP synthesis rates, corresponding to 25–35% of the activity of the wild-type enzyme (Table 1; for γQ36stop, see also Fig. 3).
FIGURE 3.
Time course of ATP synthesis. The light output at 562 nm from the luciferin/luciferase reaction is shown as a function of time. The reaction was started at t = 0 s (indicated by the arrow) by the addition of succinate.1, wild-type TF1Fo; 2, TF1Fo(γQ36stop); 3 (solid line), TF1Fo(γQ36stop/ϵI88stop); 4 (dotted line), pUC118/DK8 (no F1Fo). The bar in the upper left shows the light output of a 2-nmol ATP standard. Details of the reaction conditions are described under “Materials and Methods.”
Given the small size of the contact area between ϵ in the down conformation and γ′ in the γP43stop mutant, and the complete absence of any interaction with γ′ in the γQ36stop mutant, it seemed possible that a complex between γ′ and the C-terminal domain of ϵ in the up conformation might be responsible for the observed ATP synthesis in the truncation mutants. To test this possibility, we combined the γ truncation mutants with a truncation of the C-terminal domain of ϵ, using an ϵI88stop mutation. As shown before (25), in wild-type enzyme with full-length γ the C-terminal domain of ϵ is not necessary for ATP synthesis; actually, removal of the domain in the ϵI88stop mutant resulted in increased ATP synthesis rates (Table 1). TF1Fo(γP43stop/ϵI88stop) was able to catalyze ATP synthesis, demonstrating that in this case the interaction between γ′ and the N-terminal domain of ϵ is sufficiently strong to couple proton flux-driven rotation of the c ring to ATP synthesis. In contrast, TF1Fo(γQ36stop/ϵI88stop) had lost the ability to catalyze ATP synthesis (Table 1 and Fig. 3). The fact that the TF1Fo(γQ36stop) single mutant catalyzed ATP synthesis showed that, in the absence of any direct interaction between γ′ and the N-terminal domain of ϵ, the C-terminal domain of ϵ can connect the N-terminal domain and γ′ so that γ′ can perform its functional role in ATP synthesis.
ATPase Activity and ATP- and NADH-driven Proton Pumping
Compared with their ATP synthase activity, the ATP hydrolysis activity of the truncation mutants was low. In membrane vesicles the ATPase activity was less than 10% of that of the wild-type control. Still, at least in the case of the γP43stop mutant, this was somewhat higher than found for the γ-less enzyme (Table 2). Membrane vesicles containing the truncation mutants did not exhibit any ATP-driven proton pumping (data not shown). The ATPase activity is too low to build up a proton gradient against the natural leak rate of the membranes. NADH-driven proton pumping in vesicles containing mutant ATP synthase resulted in the same degree of quenching as observed for the wild-type enzyme, indicating that truncation or elimination of the γ subunit and/or truncation of the ϵ subunit does not cause assembly problems associated with increased proton leak rates of the membranes.
TABLE 2.
ATPase activities of membrane vesicles of deletion mutants
ATPase activities of membrane preparations of mutants and controls were measured as described under “Materials and Methods.” The given values represent the average of at least three independent measurements ± S.D. The positive control was strain pTR19-ASDS/DK8, which expresses Bacillus PS3 ATP synthase in E. coli; this strain served as the background for the deletions. The negative control was strain pUC118/DK8, which expresses neither PS3 ATP synthase nor the endogenous E. coli enzyme. Turnover rates were calculated as described in the legend to Table 1.
| Strain/mutation | Membrane ATPase activity | Turnover rate kcat |
|---|---|---|
| units/mg | s−1 | |
| Wild type | 2.4 ± 0.6 | 102 |
| ϵI88stop | 2.5 ± 0.3 | 106 |
| Δγ | 0.051 ± 0.020 | 5 |
| pUC118/DK8 (unc−) | 0.012 ± 0.011 | |
| γQ36stop | 0.075 ± 0.052 | 7 |
| γQ36stop/ϵI88stop | 0.076 ± 0.043 | 7 |
| γP43stop | 0.102 ± 0.020 | 7 |
| γP43stop/ϵI88stop | 0.106 ± 0.012 | 8 |
DISCUSSION
The results of the present study demonstrate that the globular portion and the C-terminal helix of γ, together accounting for about 80% of the molecular mass of γ, are not required for ATP synthesis activity. The N-terminal domain of γ alone is sufficient to generate the conformational changes in the catalytic sites necessary for ATP synthesis and release. If γ is completely absent, in TF1FoΔγ, ATP synthesis is abolished.
Recently it was shown that another radically reduced γ construct, this one consisting of just the globular portion, thus missing both the N-terminal and the C-terminal helix inside the α3β3 cylinder (γ-ΔN22C43), did indeed rotate in the correct direction upon ATP hydrolysis (21). ATPase activity of α3β3γ′ subcomplex containing γ-ΔN22C43 was not higher than that of α3β3 (21), and the torque generated by ATP hydrolysis seemed rather low. When a small portion of both helices inside the α3β3 cylinder was preserved, in the γ-ΔN11C32 construct, the torque reached 50% of that of wild-type (39). This prompted the authors to conclude that “neither helix in the coiled-coil region of the axle of F1-ATPase plays a significant role in torque production” (39). The results presented here contradict this conclusion because a significant amount of torque is required to effect the conformational changes in the β (and perhaps α) subunits necessary to synthesize and release ATP, and this torque is transmitted just using the N-terminal helix of γ. Combining the outcome of the mentioned studies (21, 39) and the results presented here, it appears that the portion of γ that is most involved in coupling of catalysis and rotation is the stretch of the N-terminal helix between residues ∼20 and ∼40.
According to the x-ray structures (10, 26, 40), the contacts of the N-terminal helix of γ with the α3β3 ring are with the DELSEED-loop, a helix-turn-helix structure named after the conserved DELSEED motif, in the C-terminal domains of βDP and βE, as well as with the αE subunit. The finding that the γ truncation mutants catalyze ATP synthesis underlines the importance of the DELSEED-loop for mechanochemical coupling of rotation and catalysis. There is very little, if any, contact between the N-terminal helix of γ and βTP, which carries the catalytically active high affinity nucleotide-binding site (9, 12, 41). In some models of the enzyme mechanism (5, 42, 43), a major function of the γ subunit in ATP synthesis is to convert the torque produced by proton gradient-driven rotation to force the βTP subunit open, to release the newly formed ATP. Our results offer strong support for this hypothesis. Rotation of the N-terminal helix of γ by 120° in ATP synthesis direction will bring the convex portion of the helix in contact with the βTP subunit and press against it (Fig. 1F). This should rotate the DELSEED-loop and push it downward, to open the subunit and bring it into βE conformation, thereby releasing product ATP. No other parts of γ except for the N-terminal helix seem to be required for this step, just as observed here.
Another important outcome of the present study concerns the role of the ϵ subunit. It had been shown before that the C-terminal domain of ϵ can assume different conformations, with different properties (see the Introduction). Transition between the different conformations was found to be regulated by nucleotides (44). Here, we present evidence that ϵ is indeed in an up position during ATP synthesis. γ′ in the γQ36stop truncation mutant makes no contact with the N-terminal domain of ϵ (Fig. 1, C–E). Still, the TF1Fo(γQ36stop) enzyme can synthesize ATP, suggesting that the C-terminal domain of ϵ in the up conformation might connect γ′ to the rest of the rotor. Indeed, removal of the C-terminal domain of ϵ abolished ATP synthesis. Of the two up conformations of the C-terminal domain of ϵ observed or postulated, the half-extended conformation seen by x-ray structure analysis (27) is less likely to form a complex with γ′. Unless the N-terminal domain of γ by itself assumes a different position than in the presence of the rest of γ, it seems to have very little contact with the C-terminal domain of ϵ in this conformation. In contrast, cross-linking experiments (30) support the notion that the C terminus of ϵ in a fully extended conformation comes close to the extreme N terminus of γ. Very recently, an antiparallel coiled-coil between the C-terminal helix of ϵ and the N-terminal helix of γ has been detected in the E. coli enzyme (45). As mentioned above, the experiments with TF1FoΔγ indicate that ϵ alone, in the absence of any portion of γ, cannot catalyze ATP synthesis.
It is more difficult to assess whether the C-terminal domain of ϵ also stabilizes the interaction between γ′ and the N-terminal domain of ϵ in the γP43stop mutant. TF1Fo(γP43stop/ϵI88stop) synthesizes ATP at rates slightly lower than those for TF1Fo(γP43stop). However, it should be taken into account that for the wild-type enzyme removal of the C-terminal domain of ϵ actually accelerates ATP synthesis, at least in the in vitro assay. While TF1Fo(γP43stop) has about 35% of the synthesis activity of the wild-type enzyme, TF1Fo(γP43stop/ϵI88stop) has ∼10% of the activity of TF1Fo(ϵI88stop). Thus, one could argue that in this case as well the C-terminal domain of ϵ contributes to the stability of the rotor construct. Based on the crystal structure of the E. coli γϵ complex (28), direct contacts between γ′ and the N-terminal domain of ϵ might involve residues γN37, γS40, γF41, ϵP11, ϵD12, and ϵP14. Formation of hydrogen bonds appears possible between the hydroxyl group of γS40 and the main chain oxygen of ϵD12, and between the amide nitrogen of γN37 and the carboxyl group of ϵD12. It should be noted that the γ truncations remove two residues, γF205 and γE206, which were identified as important for high affinity binding of γ to the c ring (46). As noted before for other protein-protein interactions in ATP synthase (47), the high binding affinity between full-length γ and the c ring appears to be not absolutely necessary, but an example of “overengineering.”
ATP stabilizes the down conformation of ϵ, probably by direct binding to ϵ (28). Fixating ϵ in this position by cross-linking eliminates inhibition of the ATPase activity by ϵ (29). Thus, the down conformation appears to be the one that ϵ assumes during ATP hydrolysis. Having two different structures of ϵ for ATP synthesis and hydrolysis has far reaching consequences. Above all, it means that the pathways for ATP synthesis and hydrolysis do not necessary have to be the exact reversal of each other (48, 49). Among others, this could solve one of the main problems encountered during ATP synthesis, the necessity to bind ADP in the presence of an excess of ATP (2, 50). Under ATP hydrolysis conditions, there is no catalytic site that binds ADP with a higher affinity than ATP, at least in the absence of Pi (51). ϵ in the up position might change the affinities for nucleotides, generating a site that preferentially binds ADP.
It is sometimes stated that ϵ does not inhibit ATP synthesis by F1Fo (28, 52), although it is difficult to find experimental data in support of this claim. As discussed above, we show here that TF1Fo(ϵI88stop) has significantly higher ATP synthesis activity than wild-type TF1Fo, suggesting that the C-terminal domain of ϵ, which is removed in the mutant, is indeed inhibiting ATP synthesis. In general, one difficulty in assessing the role of the ϵ subunit is the fact that ϵ from different organisms behaves differently. Even among bacterial ATP synthases, the function of ϵ in the enzymes from E. coli and Bacillus PS3 is not the same. For instance, in E. coli ATP synthase or F1-ATPase the degree of inhibition of ATPase activity is constant over a large range of ATP concentrations, up to millimolar (25, 53). In contrast, in the PS3 enzyme the inhibition is much more effective at low ATP concentrations (54).
Finally, it should be noted that the results presented recently (21) and here support the notion of ATP synthase being evolved from RNA helicases and RNA or protein translocases (55, 56). Both studies demonstrate that ATP synthase maintains some functionality with fragments of γ, showing that proteins of much simpler structure than full-length γ can serve as a rotor in ATP synthase.
This work was supported, in whole or in part, by National Institutes of Health Grant GM071462 (to J. W.).
Bacillus PS3 numbering is used throughout.
- AMPPNP
- 5′-adenylyl-β,γ-imidodiphosphate
- ACMA
- 9-amino-6-chloro-2-methoxyacridine
- Ap5A
- P1,P5-di(adenosine-5′)pentaphosphate; TF1 or TF1Fo (instead of F1 and F1Fo, respectively) refer specifically to the enzyme from the thermophilic bacterium Bacillus PS3
- γ′
- truncated γ subunit.
REFERENCES
- 1.Noji H., Yoshida M. (2001) J. Biol. Chem. 276, 1665–1668 [DOI] [PubMed] [Google Scholar]
- 2.Weber J., Senior A. E. (2003) FEBS Lett. 545, 61–70 [DOI] [PubMed] [Google Scholar]
- 3.Wilkens S. (2005) Adv. Protein Chem. 71, 345–382 [DOI] [PubMed] [Google Scholar]
- 4.Weber J. (2006) Biochim. Biophys. Acta 1757, 1162–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamoto R. K., Baylis Scanlon J. A., Al-Shawi M. K. (2008) Arch. Biochem. Biophys. 476, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Junge W., Sielaff H., Engelbrecht S. (2009) Nature 459, 364–370 [DOI] [PubMed] [Google Scholar]
- 7.Weber J., Wilke-Mounts S., Lee R. S., Grell E., Senior A. E. (1993) J. Biol. Chem. 268, 20126–20133 [PubMed] [Google Scholar]
- 8.Weber J., Senior A. E. (2004) Methods Enzymol. 380, 132–152 [DOI] [PubMed] [Google Scholar]
- 9.Baylis Scanlon J. A., Al-Shawi M. K., Le N. P., Nakamoto R. K. (2007) Biochemistry 46, 8785–8797 [DOI] [PubMed] [Google Scholar]
- 10.Abrahams J. P., Leslie A. G., Lutter R., Walker J. E. (1994) Nature 370, 621–628 [DOI] [PubMed] [Google Scholar]
- 11.Bowler M. W., Montgomery M. G., Leslie A. G., Walker J. E. (2007) J. Biol. Chem. 282, 14238–14242 [DOI] [PubMed] [Google Scholar]
- 12.Mao H. Z., Weber J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 18478–18483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Shawi M. K., Nakamoto R. K. (1997) Biochemistry 36, 12954–12960 [DOI] [PubMed] [Google Scholar]
- 14.Ketchum C. J., Nakamoto R. K. (1998) J. Biol. Chem. 273, 22292–22297 [DOI] [PubMed] [Google Scholar]
- 15.Peskova Y. B., Nakamoto R. K. (2000) Biochemistry 39, 11830–11836 [DOI] [PubMed] [Google Scholar]
- 16.Andrews S. H., Peskova Y. B., Polar M. K., Herlihy V. B., Nakamoto R. K. (2001) Biochemistry 40, 10664–10670 [DOI] [PubMed] [Google Scholar]
- 17.Lowry D. S., Frasch W. D. (2005) Biochemistry 44, 7275–7281 [DOI] [PubMed] [Google Scholar]
- 18.Greene M. D., Frasch W. D. (2003) J. Biol. Chem. 278, 51594–51598 [DOI] [PubMed] [Google Scholar]
- 19.Boltz K. W., Frasch W. D. (2005) Biochemistry 44, 9497–9506 [DOI] [PubMed] [Google Scholar]
- 20.Boltz K. W., Frasch W. D. (2006) Biochemistry 45, 11190–11199 [DOI] [PubMed] [Google Scholar]
- 21.Furuike S., Hossain M. D., Maki Y., Adachi K., Suzuki T., Kohori A., Itoh H., Yoshida M., Kinosita K., Jr. (2008) Science 319, 955–958 [DOI] [PubMed] [Google Scholar]
- 22.Xiao Y., Metzl M., Mueller D. M. (2000) J. Biol. Chem. 275, 6963–6968 [DOI] [PubMed] [Google Scholar]
- 23.Duvezin-Caubet S., Caron M., Giraud M. F., Velours J., di Rago J. P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13235–13240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rondelez Y., Tresset G., Nakashima T., Kato-Yamada Y., Fujita H., Takeuchi S., Noji H. (2005) Nature 433, 773–777 [DOI] [PubMed] [Google Scholar]
- 25.Cipriano D. J., Dunn S. D. (2006) J. Biol. Chem. 281, 501–507 [DOI] [PubMed] [Google Scholar]
- 26.Gibbons C., Montgomery M. G., Leslie A. G., Walker J. E. (2000) Nat. Struct. Biol. 7, 1055–1061 [DOI] [PubMed] [Google Scholar]
- 27.Rodgers A. J., Wilce M. C. (2000) Nat. Struct. Biol. 7, 1051–1054 [DOI] [PubMed] [Google Scholar]
- 28.Yagi H., Kajiwara N., Tanaka H., Tsukihara T., Kato-Yamada Y., Yoshida M., Akutsu H. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11233–11238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsunoda S. P., Rodgers A. J., Aggeler R., Wilce M. C., Yoshida M., Capaldi R. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6560–6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki T., Murakami T., Iino R., Suzuki J., Ono S., Shirakihara Y., Yoshida M. (2003) J. Biol. Chem. 278, 46840–46846 [DOI] [PubMed] [Google Scholar]
- 31.Suzuki T., Ueno H., Mitome N., Suzuki J., Yoshida M. (2002) J. Biol. Chem. 277, 13281–13285 [DOI] [PubMed] [Google Scholar]
- 32.Klionsky D. J., Brusilow W. S., Simoni R. D. (1984) J. Bacteriol. 160, 1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senior A. E., Latchney L. R., Ferguson A. M., Wise J. G. (1984) Arch. Biochem. Biophys. 228, 49–53 [DOI] [PubMed] [Google Scholar]
- 34.Senior A. E., Langman L., Cox G. B., Gibson F. (1983) Biochem. J. 210, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mnatsakanyan N., Krishnakumar A. M., Suzuki T., Weber J. (2009) J. Biol. Chem. 284, 11336–11345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goelz S. E., Cronan J. E., Jr. (1982) Biochemistry 21, 189–195 [DOI] [PubMed] [Google Scholar]
- 37.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 38.Yoshida M., Okamoto H., Sone N., Hirata H., Kagawa Y. (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 936–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hossain M. D., Furuike S., Maki Y., Adachi K., Suzuki T., Kohori A., Itoh H., Yoshida M., Kinosita K., Jr. (2008) Biophys. J. 95, 4837–4844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menz R. I., Walker J. E., Leslie A. G. (2001) Cell 106, 331–341 [DOI] [PubMed] [Google Scholar]
- 41.Yang W., Gao Y. Q., Cui Q., Ma J., Karplus M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 874–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y. Q., Yang W., Karplus M. (2005) Cell 123, 195–205 [DOI] [PubMed] [Google Scholar]
- 43.Mao H. Z., Abraham C. G., Krishnakumar A. M., Weber J. (2008) J. Biol. Chem. 283, 24781–24788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iino R., Murakami T., Iizuka S., Kato-Yamada Y., Suzuki T., Yoshida M. (2005) J. Biol. Chem. 280, 40130–40134 [DOI] [PubMed] [Google Scholar]
- 45.Duncan T. M., Cingolani G. (2009) ASBMB Annual Meeting, New Orleans, LA, April 18–22, 2009, Abstract 504.6 [Google Scholar]
- 46.Pogoryelov D., Nikolaev Y., Schlattner U., Pervushin K., Dimroth P., Meier T. (2008) FEBS J. 275, 4850–4862 [DOI] [PubMed] [Google Scholar]
- 47.Weber J., Wilke-Mounts S., Senior A. E. (2003) J. Biol. Chem. 278, 13409–13416 [DOI] [PubMed] [Google Scholar]
- 48.Vinogradov A. D. (2000) J. Exp. Biol. 203, 41–49 [DOI] [PubMed] [Google Scholar]
- 49.Feniouk B. A., Suzuki T., Yoshida M. (2006) Biochim. Biophys. Acta 1757, 326–338 [DOI] [PubMed] [Google Scholar]
- 50.Weber J., Senior A. E. (2000) Biochim. Biophys. Acta 1458, 300–309 [DOI] [PubMed] [Google Scholar]
- 51.Mao H. Z., Gray W. D., Weber J. (2006) FEBS Lett. 580, 4131–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feniouk B. A., Suzuki T., Yoshida M. (2007) J. Biol. Chem. 282, 764–772 [DOI] [PubMed] [Google Scholar]
- 53.Weber J., Dunn S. D., Senior A. E. (1999) J. Biol. Chem. 274, 19124–19128 [DOI] [PubMed] [Google Scholar]
- 54.Kato-Yamada Y., Bald D., Koike M., Motohashi K., Hisabori T., Yoshida M. (1999) J. Biol. Chem. 274, 33991–33994 [DOI] [PubMed] [Google Scholar]
- 55.Walker J. E., Cozens A. L. (1986) Chem. Scr. 268, 263–272 [Google Scholar]
- 56.Mulkidjanian A. Y., Makarova K. S., Galperin M. Y., Koonin E. V. (2007) Nat. Rev. Microbiol. 5, 892–899 [DOI] [PubMed] [Google Scholar]